Abstract
Background
Metabolism of glutamine by glutaminase 1 (GLS1) plays a key role in tumor cell proliferation via the generation of ATP and intermediates required for macromolecular synthesis. We hypothesized that glutamine metabolism also plays a role in proliferation of autosomal-dominant polycystic kidney disease (ADPKD) cells and that inhibiting GLS1 could slow cyst growth in animal models of ADPKD.
Methods
Primary normal human kidney and ADPKD human cyst-lining epithelial cells were cultured in the presence or absence of two pharmacologic inhibitors of GLS1, bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide 3 (BPTES) and CB-839, and the effect on proliferation, cyst growth in collagen and activation of downstream signaling pathways were assessed. We then determined if inhibiting GLS1 in vivo with CB-839 in the Aqp2-Cre; Pkd1fl/fl and Pkhd1-Cre; Pkd1fl/fl mouse models of ADPKD slowed cyst growth.
Results
We found that an isoform of GLS1 (GLS1-GAC) is upregulated in cyst-lining epithelia in human ADPKD kidneys and in mouse models of ADPKD. Both BPTES and CB-839 blocked forskolin-induced cyst formation in vitro. Inhibiting GLS1 in vivo with CB-839 led to variable outcomes in two mouse models of ADPKD. CB-839 slowed cyst growth in Aqp2-Cre; Pkd1fl/fl mice, but not in Pkhd1-Cre; Pkd1fl/fl mice. While CB-839 inhibited mammalian target of rapamycin (mTOR) and MEK activation in Aqp2-Cre; Pkd1fl/fl, it did not in Pkhd1-Cre; Pkd1fl/fl mice.
Conclusion
These findings provide support that alteration in glutamine metabolism may play a role in cyst growth. However, testing in other models of PKD and identification of the compensatory metabolic changes that bypass GLS1 inhibition will be critical to validate GLS1 as a drug target either alone or when combined with inhibitors of other metabolic pathways.
Keywords: ADPKD, glutaminase 1, glutamine, metabolism, Warburg effect for ESRD
INTRODUCTION
Autosomal-dominant polycystic kidney disease (ADPKD) affects more than 12 million people worldwide and is a common cause of end-stage kidney disease. In the majority of cases, ADPKD is caused by mutations in one of two genes, PKD1 or PKD2, which encode polycystin 1 (PC1) and polycystin 2 (PC2), respectively [1, 2]. Loss of both copies of PKD1 or PKD2 is associated with cyst initiation and cyst enlargement by stimulating the enhanced growth of renal epithelia as well as the stimulation of apical chloride secretion via the cystic fibrosis transmembrane conductance regulator (CFTR).
Altered regulation of a number of different signaling pathways and transcription factors has been identified in ADPKD that may account for proliferation and cyst formation [3, 4]. Interestingly, many of these signaling pathways mirror changes found in cancer cells [4]. Recent evidence has indicated that tumor cells undergoing increased growth and division exhibit marked changes in cellular metabolism which intersect with known signaling pathways that have been implicated in the proliferation of ADPKD cells [3, 5, 6]. In this regard, two recent papers have demonstrated an increase in glycolytic flux in PKD cells and kidneys, and inhibiting this by the administration of 2-deoxy-d-glucose slowed cyst growth in several animal models of ADPKD [7, 8].
Glutamine is the most abundant amino acid in plasma and has emerged as one of the key nutrients required to support growth of some cancer cells [9–12]. Glutaminase catalyzes the first step in glutamine metabolism, converting glutamine to glutamate. Glutamate is subsequently catabolized to α-ketoglutarate (αKG), a key intermediate in the TCA cycle to generate ATP as well as the carbon backbone for cellular anabolic processes [9]. There are two distinct but related Gls genes, GLS1 and GLS2 [13–15]. GLS2 is expressed highly in the liver, whereas GLS1 is more broadly expressed. GLS1 has two spliced variants: KGA, which is the kidney-specific isoform that mediates ammonia generation for acid secretion, and GAC, which has been shown to be upregulated in cancer cells and is thought to play a role in glutaminolysis [16–18].
GLS1 has emerged as a promising therapeutic drug target to treat a variety of different cancers [19]. Studies have shown that interfering with GLS1 activity by siRNA knockdown, genetically, or by pharmacologic inhibition slows cancer cell growth in vitro and in vivo in murine models of cancer [16, 17, 20, 21]. bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide 3 (BPTES) is an allosteric specific GLS1 inhibitor that on crystal structure was shown to dock at the tetrameric interfaces and locks GLS1 in an inactive state [22, 23]. CB-839 is a selective oral GLS1 inhibitor that has shown improved potency and bioavailability in animal models compared with BPTES [16, 20] and is currently in Phase 1 clinical trial for cancer treatment. We hypothesized that glutamine metabolism also plays a critical role in proliferation of ADPKD cells and that inhibitors of glutamine metabolism and more specifically GLS1 could be a potential new target to slow cyst growth in patients with ADPKD.
MATERIALS AND METHODS
Cell culture
Primary cultures of ADPKD cells were generated by the PKD Biomaterials and Cellular Models Core at the University of Kansas Medical Center, as previously described [24, 25]. Briefly, primary cell cultures were derived from normal regions of nephrectomy specimens confirmed by histological examination, or surface cysts of ADPKD kidneys. These cell lines have characteristics of collecting duct cells, in that they stain positive for cytokeratin and Dolichus biflorus agglutinin. Cells were maintained in DMEM/F12 supplemented with 10% FBS, insulin (5 μg/mL)/transferrin (5 μg/mL)/selenium (5 ng/mL) (Gibco) and used up to Passage 4.
Cyst growth in collagen gel and cyst number
ADPKD and normal human kidney (NHK) cells were seeded within collagen and stimulated with forskolin (10 μmol) as described [24–26]. Briefly, 800 cells were mixed with 0.4 mL of ice-cold PureCol collagen (3.0 mg/mL collagen; Advanced Biomatrix) supplemented with 10% (v/v) 10× minimum essential medium, HEPES (10 mM), NaHCO3 (27 mM), penicillin and streptomycin and plated into 24-well plates. Cells were stimulated with forskolin (10 μM) in the presence or absence of the GLS1 inhibitor BPTES or CB-839 and cyst formation was assessed on Day 16. On Day 16 of culture, the 24-well plate was placed on the stage of an inverted microscope and the number of cysts in each well was counted; the diameter of each cyst was measured using an ocular scale at 40× as previously described [26]. Only cysts larger than 100 μm were clearly identified and counted as previously reported [26].
siRNA knockdown of GLS1 was performed by transfecting NHK and ADPKD cells with either of the two siRNA to GLS1 (25 nM) [(i) ATGGTGGTTTCTGCCCAATTA, Qiagen; (ii) ACCAUUACAACAAUCCAUC, Sigma] and Lipofectamine RNAiMAX (Invitrogen). Control cells were transfected with equal concentration of scrambled siRNA (25 nM). Cyst number was assessed as above in the absence or presence of dimethyl-α-ketoglutarate (8 mM). GLS1 messenger RNA knockdown was quantitated on Day 3 and Day 8 post-transfection by real time polymerase chain reaction using 5′ primer CGAAGATTTGCTTTGTCA and 3′ primer CTCTGCAGCAGCTACAT. GLS1 protein expression was assessed on Day 3 and Day 8 post-transfection by western blot and immunoblotting with anti-GLS1 antibody.
Mice
Aqp2-Cre mice were purchased from Jackson Labs and crossed with Pkd1fl/fl mice to generate Aqp2-Cre; Pkd1fl/+ mice. Pkhd1-Cre; Pkd1fl/fl mice have been previously reported [27]. Pkhd1-Cre; Pkd1fl/+ were crossed with Pkd1fl/fl mice to generate Pkhd1-Cre; Pkd1fl/fl mice. Pkhd1-Cre; Pkd1fl/fl and Aqp2-Cre; Pkd1fl/fl mice from the same litter were treated with vehicle or CB-839 (200 mg/kg) by gavage 2×/day starting on Day 10. Pkhd1-Cre; Pkd1fl/flmice were sacrificed on Day 28 and Aqp2-Cre; Pkd1fl/fl mice were sacrificed on Day 24. We report only the results of GLS1 inhibition in Aqp2-Cre; Pkd1fl/fl female mice. In contrast to Aqp2-Cre; Pkd1fl/fl male mice, the clinical course of Aqp2-Cre; Pkd1fl/fl female mice was reproducible with all mice demonstrating large cysts and an elevated blood urea nitrogen (BUN) at 28 days of age. Aqp2-Cre; Pkd1fl/fl male mice frequently had only minimal cysts at this time and progressed to renal failure at a much slower and more unpredictable rate overall. The reason for the differences are not apparent but is likely related to differences in Cre expression between female and male mice.
All animals were used in accordance with scientific, humane and ethical principles and in compliance with regulations approved by the New York University School of Medicine Institutional Animal Care and Use Committee.
Cystic index and kidney immunohistochemistry
Kidneys from both models were harvested, fixed in 4% paraformaldehyde for 4 h at 4 °C, and sagittal kidney sections were stained with hematoxylin and eosin. Sections were then photographed under the same magnification and cystic index was calculated using ImageJ analysis software on two sagittal sections/kidney as described [28]. Cystic index was calculated as the cumulative cyst volume per total area of kidney.
Kidney immunohistochemistry was performed on ADPKD and NHKs obtained from nephrectomy, and kidneys from WT (Pkd1fl/fl) and Aqp2-Cre; Pkd1fl/fl and Pkhd1-Cre; Pkd1fl/fl mice. Immunohistochemistry was performed on 4-µm formalin-fixed, paraffin-embedded kidney sections using antibodies as indicated. Chromogenic immunohistochemistry was performed on a Ventana Medical Systems Discovery XT platform with online deparaffinization, antigen retrieval and using Ventana’s reagents and detection kits. In brief, heat-mediated antigen retrieval was performed using either CC1 (Tris–Borate–EDTA, pH 8.5) or RCC2 (sodium citrate pH 6.0) as required. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide. Primary antibodies were diluted in Dulbecco’s phosphate-buffered saline (Life Technologies) 3 h at 37 °C and detected using anti-rabbit or anti-mouse HRP-labeled multimers incubated for 8 min. The complex was visualized with 3,3 diaminobenzidene and enhanced with copper sulfate.
Seahorse oxygen consumption rates
Cells were seeded (10 000–15 000 cells/well) on XFe24 plates (Seahorse Biosciences) in complete medium in the presence or absence of CB-839 (1 μM) and forskolin (10 μg/mL) and allowed to attach overnight. The cells were then placed in fresh medium lacking FBS, incubated without CO2 for 1 h, and oxygen consumption rates was then determined at baseline and following the sequential addition of oligomycin (1 mg/mL), carbonyl cyanide-4 (trifluoromethoxy) phenylhydrazone (FCCP) (125 nM) and antimycin (1 mmol/L) as per the manufacturer’s protocol.
Glutamine starvation, anti-phospho-S6 staining and immunofluorescence
NHK and ADPKD cells were cultured for 24 h in media lacking glutamine (Q) and leucine (L). L is added together with Q as L is required to activate glutamine dehydrogenase, which converts glutamate to α- KG [30]. Cells were then placed in media containing Q and L for 1 h, lysed and immunoblotted with anti-phospho-S6 antibodies or fixed in 4% paraformaldehyde and then stained with primary anti-mTOR and anti-LAMP2 antibodies followed by secondary anti-mouse fluorescein isothiocyanate (FITC) or CY3 antibodies. Cells were then imaged with a Zeiss 710 confocal microscope.
Western analysis
Kidneys harvested at time of sacrifice were flash frozen in liquid nitrogen, homogenized in lysis buffer, separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS/PAGE), immunoblotted with primary antibody as indicated and detected with an Li-cor IRdye secondary antibody as described [29].
RESULTS
The GLS1 GAC isoform is upregulated in cyst-lining epithelia in kidneys from patients with ADPKD
To assess the expression of GLS1 isoforms, NHK and kidneys from patients with ADPKD were stained with specific antibodies to GLS1 KGA or GLS1 GAC. We found that KGA is the predominant isoform expressed in tubule cells in NHK, while GAC expression was much more limited (Figure 1). In contrast, >70% of cyst-lining epithelia in human ADPKD kidneys had upregulated GAC protein expression (Figure 1A), indicating that increased GLS1 GAC expression was associated with renal cyst formation. Increased GLS1 GAC expression was also seen in two mouse models of ADPKD (Figure 1B and C).
FIGURE 1.
Expression of GLS1 KGA and GAC isoforms. (A) NHK and ADPKD kidneys were probed with anti-GLS1 KGA (upper) or anti-GLS1 GAC (lower) as shown. Slides are scanned at 40× magnification on SlidePath. Images were captured at 10× and 20× viewing magnification and scale bars represent 100 μm. (B) Lysates from WT (Pkd1fl/fl), AQP-2-Cre; PKD1fl/fl and PKHD1-Cre; PKD1fl/fl kidneys were immunoblotted with anti-GLS1 GAC or KGA antibodies as shown. (C) Representative immunohistochemistry of kidneys from (B) with anti-GLS1 GAC or KGA antibodies. Slides are scanned at 40× magnification on SlidePath. Images were captured at 10× viewing magnification and scale bars represent 100 μm.
GLS1 is required for cyst growth by human ADPKD cells in collagen gels
Primary human ADPKD cells have previously been found to be a good model for investigating cellular mechanism involved in cyst growth [24, 25]. In contrast to the studies in Figure 1, we found that both the GAC and KGA isoforms of GLS1 are expressed in roughly equal amounts in primary ADPKD and NHK cells isolated from cyst lining (Figure 2A). In addition, treatment with the potent cyclic AMP agonist forskolin did not affect the expression of either GLS1 isoform in either NHK or ADPKD cells (Figure 2A). We next tested if GLS1 inhibition with BPTES and CB-839-affected cell proliferation and cyst formation of NHK and ADPKD cells. BPTES and CB-839 inhibited cell proliferation (Figure 2B) and the size and number of cysts of both ADPKD cells (Figure 2C–E) and NHK (data not shown) cells when cells were seeded within a collagen matrix and stimulated with forskolin to form cysts [24–26]. Cyst formation was also inhibited by growing cells in glutamine-deprived medium (Figure 2F).
FIGURE 2.
GLS1 inhibition slows in vitro cyst growth by ADPKD cells. (A) Immunoblot of primary human ADPKD and NHK cells with an amino-terminal anti-GLS1 antibody and anti-actin antibody as a loading control after forskolin stimulation (B) ADPKD cells were placed in 0.02% FBS and either untreated or treated with the Gls inhibitor BPTES alone or together with dimethyl-αKG and cell proliferation was assessed 72 h later by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay as described in Supplementary data. Shown are relative units compared with control cells. (C) ADPKD cells were plated in collagen and stimulated with forskolin (10 μM) in the presence or absence of BPTES (8 μM) as described in the ‘Materials and methods’ section. Images were taken at 10× magnification 2 weeks following plating. Scale bar represents 200 μm. (D) Cyst number and cyst size are shown. (E) ADPKD cells were treated with a Dimethyl sulfoxide (DMSO) control or CB-839 and cyst number and size were assessed as described in (C). (F) Cyst number and size when grown in glutamine free medium. (G, H) ADPKD cells were treated with two different siRNA to GLS1 or scrambled siRNA (control) and cyst number were determined in the presence or absence of α-KG. (I) siRNA to GLS1 showed >60% inhibition of GLS1 messenger RNA as evaluated by real time polymerase chain reaction (qPCR) on Days 3 and 8 post-transfection. (J) siRNA to GLS1 decreased protein expression of GLS1 on Days 3 and 8 post-transfection shown as quantification derived from ratio of net GLS1 to net loading control actin. Differences were evaluated by one-way analysis of variance (ANOVA) using Bonferroni test (*P < 0.05, **P < 0.01, ***P < 0.001). All results are representative of three independent experiments of primary ADPKD cells isolated from three independent patients.
To confirm that GLS1 was the drug target and is essential for cyst growth, ADPKD cells were transfected with two different siRNA to GLS1 (Figure 2G and H). siRNA knockdown of GLS1 also led to a statistically significant decrease in cyst formation (Figure 2G and H). The inhibition of cyst formation by GLS1 siRNA (Figure 2G and H) and proliferation by BPTES (Figure 2B) was specific to GLS1 inhibition and not an off-target effect as addition of dimethyl-αKG to the medium, which functions downstream of GLS1, resulted in a significant rescue of cyst formation in siRNA-transfected cells and proliferation in BPTES-treated ADPKD or NHK cells (data not shown).
To address the mechanisms whereby GLS1 inhibition limits cyst growth in vitro, Seahorse analysis was performed to assess mitochondrial respiration. Forskolin stimulation led to an increase in both the basal and maximal mitochodrial O2 consumption in ADPKD and NHK cells (Figure 3A and B) and treatment with CB-839 led to a statistically significant inhibition of basal and maximal mitochodrial O2 consumption of both unstimulated and forskolin-stimulated ADPKD and NHK cells (Figure 3A and B). Glutamine metabolism via generation of α-KG has previously been shown to mediate amino acid activation of mTORC1 in some cancer cells by simulating the recruitment of mTORC1 to the lysosome [30]. Treatment with BPTES or CB-839 also inhibited amino acid activation of mTORC1 in ADPKD and NHK cells as assessed by pS6 immunoblot (Figure 3C and D). In additon, inhibition of mTORC1 was associated with decreased recruitment of mTOR to the lysosome (Figure 3E).
FIGURE 3.
Seahorse analysis and mTOR activation in ADPKD and NHK cells treated with BPTES or CB-839. Seahorse analysis of ADPKD and NHK cells treated with DMSO control or CB-839 in the presence or absence of forskolin. (A, B) Shown is basal and maximal (Max) mitochondrial oxygen consumption assessed according to manufacturers protocols (Seahorse Biosciences). Results are representative of three independent experiments of primary ADPKD and NHK cells isolated from three independent patients. Differences were evaluated by two-way ANOVA (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001). (C, D) ADPKD and NHK cells were deprived of glutamine (Q) or leucine (L) for 24 h and then incubated with Q and L for 60 min in the absence or presence of BPTES (20 μM), CB-839 (1 μM) or rapamycin, and lysates were immunoblotted with anti-pS6 antibodies. L is added together with Q as L is required to activate glutamate dehydrogenase which converts glutamate to αKG [30]. To control for protein loading, cell lysates were also probed with anti-actin antibody. Western blot of phospho-S6 to loading control actin was quantified using ImageJ. The quantification values shown below each band are the ratio of net phospho S6 to net actin. (E) Cells were treated as in (C) and (D), fixed in 4% paraformaldehyde and stained with mouse anti-mTOR and rabbit anti-LAMP2 antibodies followed by Cy3 anti-mouse or FITC anti-rabbit secondary antibodies. Images were captured using a Zeiss 710 confocal microscope. Scale bar represents 100 μm.
Inhibition of GLS1 with CB-839 slows cyst growth in aquaporin-2 but not Pkhd1-Cre polycystin 1 knockout mice
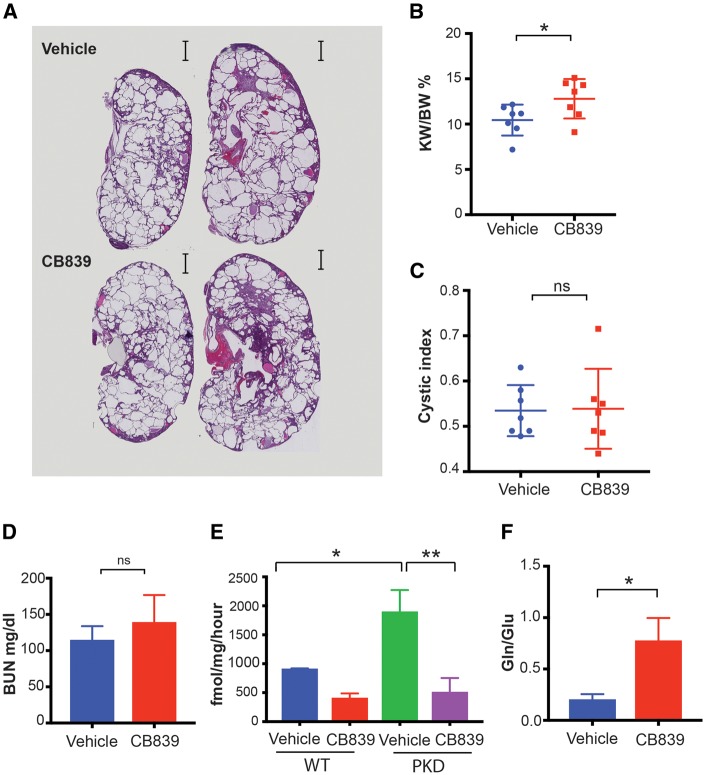
Aquaporin-2 (Aqp2) promoter driven Cre selectively deletes Pkd1 in collecting duct principal cells. Female Aqp2-Cre; Pkd1fl/flmice were treated with vehicle or CB-839 (200 mg/kg) twice a day by gavage as previously reported [16] starting on postnatal Day 10, and mice were sacrificed on Day 28. Treated mice had a statistically significant decrease in cyst volume as determined by kidney weight/body weight ratios (Figure 4A and B) and cystic index (Figure 4A and C) and significantly lower serum levels of BUN (Figure 4D). To determine if CB-839 also slows cyst growth in another model of PKD, we next assessed whether treatment with CB-839 also slows growth in the Pkhd1-Cre; Pkd1fl/fl mice [27] (Figure 5). While Pkhd1-Cre also expresses predominantly in collecting duct principal cells, this model has been shown to give a robust and a very reproducible phenotype [27]. However, in contrast to Aqp2-Cre; Pkd1fl/fl mice, CB-839 treatment did not slow cyst growth or preserve renal function in Pkhd1-Cre; Pkd1fl/fl mice (Figure 5A–D) despite >75% inhibition of GLS1 enzyme activity in treated kidneys (Figure 5E). Consistent with the inhibition of GLS1, CB-839-treated kidneys had elevated glutamine to glutamate ratio compared with vehicle-treated Pkhd1-Cre; Pkd1fl/flkidneys (Figure 5F). Moreover CB-839 levels in Pkhd1-Cre; Pkd1fl/fl kidneys were 15.4 ± SEM 3.18 nmol/g, which is significantly >1.5 nmol/g of CB-839 found in tumors and which led to a slowing of tumor growth in vivo [16]. Thus, the failure of CB-839 to block cyst growth in Pkhd1-Cre; Pkd1fl/flkidneys was not due to under-dosing of drug or the failure to inhibit kidney GLS1 in vivo.
FIGURE 4.
Pharmacologic inhibition of GLS1 with CB-839 slows cyst growth in female Aqp2-Cre; Pkd1fl/fl mice. Female Aqp2-Cre; Pkd1fl/fl mice were treated with vehicle or CB-839 (200 mg/kg) b.i.d. by gastric gavage starting on Day 10 and mice were sacrificed on Day 28. (A) Representative kidney histology from vehicle and CB-839 treated mice, (B) kidney/body weight (KW/BW) ratio, (C) cystic index and (D) BUN are shown. n = 7 mice/group. Differences were evaluated by two-tailed t-tests (**P < 0.01, ***P < 0.001).
FIGURE 5.
Pharmacologic inhibition of GLS1 with CB-839 does not slow cyst growth in Pkhd1-Cre; Pkd1fl/fl mice. Four female and four male pairs of mice were treated with vehicle control or CB-839 as described in Figure 4. (A) Representative kidney histology from vehicle- and CB-839-treated mice, (B) kidney/body weight (KW/BW) ratio, (C) cystic index and (D) BUN are shown, n = 7 mice/group. (E) GLS1 activity in WT (Pkd1fl/fl) or Pkhd1-Cre; Pkd1fl/fl kidneys treated with vehicle or 2 h following treatment with CB-839. (F) Glutamine (Gln) to Glutamate (Glu) ratio in Pkhd1-Cre; Pkd1fl/fl kidneys treated with vehicle or 2 h following treatment with CB-839. Differences were evaluated by two-tailed t-tests (*P < 0.05, **P < 0.01; ns, not significant).
As shown in Figure 1B and C, increased GLS1 GAC expression in cyst-lining epithelia was seen in both models and, therefore, differences in GLS1 GAC protein expression do not account for the differential response. Consistent with the increase in GAC expression, GLS1 enzyme activity was approximately two times greater in Pkhd1-Cre; Pkd1fl/fl kidneys compared with wild type (WT) kidneys (Figure 5E). Activation of mTOR and B-RAF-ERK-MAPK mediate cyst growth in some models of ADPKD [3, 31]. We found that mTOR activity was increased in most kidneys from vehicle-treated Aqp2-Cre; Pkd1fl/fl mice when compared with kidneys from vehicle-treated WT (Pkd1fl/fl) mice and treatment with CB-839 inhibited mTOR activation in Aqp2-Cre; Pkd1fl/fl kidneys as assessed by phospho-S6 expression (Figure 6). In addition, phospho-MEK and phospho-ERK were also increased in most kidneys from vehicle-treated Aqp2-Cre; Pkd1fl/fl mice and the increase in phospho-MEK, but not phospho-ERK, was inhibited by CB-839 (Figure 6). In contrast, phospho-S6 was not increased in kidneys from both vehicle and CB-839-treated Pkhd1-Cre; Pkd1fl/fl mice when compared with kidneys from vehicle-treated WT (Pkd1fl/fl) mice (Figure 6). In addition, while phospho-MEK and phospho-ERK were increased in vehicle-treated Pkhd1-Cre; Pkd1fl/fl kidneys when compared with vehicle-treated Pkd1fl/fl mice kidneys, treatment with CB-839 did not inhibit the increase in either phospho-MEK or phospho-ERK (Figure 6). These results suggest that differential activation and inhibition of mTOR and MEK may account for slowing of cyst growth by CB-839 in Aqp2-Cre; Pkd1fl/fl but not in Pkhd1-Cre; Pkd1fl/fl mice.
FIGURE 6.
Activation of mTOR and MEK-ERK pathways in kidneys from Aqp2-Cre; Pkd1fl/fl, Pkhd1-Cre; Pkd1fl/fl mice. (A) Representative immunoblot of kidney lysates from WT (Pkd1fl/fl) mice, and Aqp2-Cre; Pkd1fl/fl and Pkhd1-Cre; Pkd1fl/fl mice that were either treated with a vehicle control or CB-839, and immunoblotted with anti-pS6, anti-S6, anti-p-MEK, anti-MEK, anti-pERK and anti-ERK antibodies as indicated. (B, C) Quantification of western blots using ImageJ. The quantification values are the ratio of net band to net loading control as shown in the graph (n = 5). Differences were evaluated by two-tailed t-tests (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; ns, not significant).
DISCUSSION
Although ADPKD cells depend on many of the same signaling pathways as cancer cells for proliferation and cyst formation, the role for altered metabolism in ADPKD pathogenesis has been largely unexplored. Recent studies have indicated that altered glucose and lipid metabolism may contribute to cyst formation in PKD [7, 8, 32, 33]. To begin to address whether PKD cells may be uniquely dependent upon glutamine metabolism via GLS1 for cell growth and cyst formation, we assessed whether GLS1 inhibition slows cyst growth in vitro in primary human ADPKD and NHK cells and in vivo in two mouse models of PKD.
We found that inhibiting GLS1 slowed cyst growth in vitro, although we did not detect differences between ADPKD and NHK cells. In addition, we found that GLS1 inhibition in vitro was associated with impaired mitochondrial respiration and decreased activation of mTOR by amino acids, suggesting that inhibiting glutamine metabolism has the potential to impair cyst growth by simultaneously blocking several non-overlapping pathways proposed to play a role in proliferation of cyst-lining epithelia. While ideally we would have liked to see differences between ADPKD and NHK cells, the finding that GLS1 inhibition slowed growth of kidney tubule cells is not insignificant given that many primary cells, cell lines and cancer cells are not dependent upon glutamine metabolism via GLS1 for cell growth as they are able to utilize other nutrients and pathways to generate intermediates of the tricarboxylic acid (TCA) cycle, ATP and carbon and nitrogen intermediates for macromolecular synthesis and cell growth [16, 20, 34, 35].
Despite the failure to see differences between ADPKD and NHK cells in culture, we felt it was still critical to explore whether inhibition of GLS1 slows cyst growth in animal models of PKD for the following reasons. We found marked upregulation of the GLS1 GAC isoform in cyst-lining epithelia in kidneys from both human ADPKD and several mouse models of PKD; increased expression of GLS1 GAC isoform in cancer cells is associated with glutamine dependency and response to GLS1 inhibition in many studies [16, 17, 36]. The increase in GLS1 GAC in tumors has been linked to upregulation of Myc, which is consistent with previous findings that Myc is upregulated in PKD kidneys and plays a role in pathogenesis [33, 37–39]. In addition, changes in cell culture may not reflect the local environment in vivo that cyst-lining epithelia is subjected to as a result of altered kidney morphology and blood supply, which is supported by many studies of cancer cells demonstrating that metabolic changes observed in vitro do not reflect changes in vivo [40].
We found that inhibiting GLS1 in vivo with the GLS1 inhibitor CB-839 led to variable outcomes in two mouse models of ADPKD. While inhibiting GLS1 slowed cyst growth in the less aggressive cystic disease in Aqp2-Cre; Pkd1fl/fl mice, it did not slow cyst growth in the more aggressive Pkhd1-Cre; Pkd1fl/fl cystic model despite >75% inhibition of GLS1 enzyme activity in Pkhd1-Cre; Pkd1fl/fl kidneys, indicating that failure to inhibit GLS1 or to achieve significant levels of CB-839 in Pkhd1-Cre; Pkd1fl/fl kidney does not account for negative results. The reasons for these differences are not apparent, although we have identified differences in signaling pathways activated in the two models and in differences in GLS1 inhibition of these pathways. mTOR was activated in kidneys from Aqp2-Cre; Pkd1fl/fl, but not Pkhd1-Cre; Pkd1fl/fl mice, as assessed by increase in phospho-S6, and the increase in phospho-S6 in Aqp2-Cre; Pkd1fl/fl kidneys was inhibited by CB-839. In addition, an increase in phospho-MEK and phospho-ERK was observed in most kidneys in both models, although CB-839 inhibited the increase in phospho-MEK only in kidneys from Aqp2-Cre; Pkd1fl/fl mice suggesting that inhibition of MEK by CB-839 may also contribute to slowing of cyst growth in Aqp2-Cre; Pkd1fl/fl mice.
Previously, Pkd1–/– MEFs were shown to undergo aerobic glycolysis similar to cancer cells, as described by Warburg [7, 41]. These cells had increased glucose uptake and metabolism, elevated ATP generation and mTOR activity and inhibition of glycolysis in vivo with 2-deoxyglucose in both early rapid mouse models of ADPKD [7] and more recently in a more slowly progressive and late-onset model [8] slowed cyst growth. Another recent study identified alterations in lipid metabolism in PKD kidneys and slowing of cyst growth when mice were placed on a diet containing a lower lipid content [32].
Our findings provide support that alteration in other metabolic pathways, such as glutamine metabolism, may play a role in cyst growth and targeting these pathways either alone or together with inhibitors of other pathways [42] has the potential to slow cyst growth in patients with ADPKD. This is consistent with a previous report demonstrating increased 2-hydroxyglutarate in kidneys from cpk mice, a mouse model of autosomal recessive kidney disease [43]. However, our results highlight the variability in response of different mouse models of PKD to inhibition of the same metabolic drug target, which would be consistent with the broad and variable range of metabolic changes so far identified in cancer cells and the variability in response to GLS1 inhibition in vivo and in vitro due to upregulation of adaptive metabolic networks that bypass GLS1 inhibition [42, 44, 45].
It remains to be determined which adaptive changes bypass GLS1 inhibition in PKD. Previous studies have demonstrated that glutamine metabolism via GLS1 serves as an important carbon source for the TCA cycle and, consistent with this, treatment with GLS1 inhibitors led to decreased oxidative phosphorylation (OXPHOS) in a variety of tumor cell lines [15, 34]; α-KG, which is generated from glutamate by either glutamate dehydrogenase or aminotransferases, feeds directly into the TCA cycle [46]. In addition, increase in reactive oxygen species (ROS) following GLS1 inhibition has been shown to indirectly decrease OXPHOS by impairing carbohydrate flux and thereby glucose flux into the TCA cycle via inhibition of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) [34, 47]. We found a similar decrease on OXPHOS by inhibiting GLS1 in ADPKD cells in vitro. The failure of CB-839 to inhibit cyst growth in vivo in Pkhd1-Cre; Pkd1fl/fl mice as opposed to Aqp2-Cre; Pkd1fl/fl mice could be due to glycolysis playing a predominant role in the former as has been reported for carcinomas that do not respond to GLS1 inhibition [42]. Tumor cells also adapt to GLS1 inhibition via upregulation of arginine metabolism. Two recent papers have found that PKD cells have decreased OXPHOS when compared with WT cells [33, 48]. Hajarnis et al. found that the decrease in OXPHOS is mediated by upregulation of the messenger RNA miR-17, which then secondarily led to the downregulation of peroxisome proliferator-activated receptor (PPAR), and decreasing miR-17 with an anti-miR-17 slowed cyst growth in vivo and in vitro [33]. In contrast to our findings reported here, the beneficial effect of inhibiting miR17 was associated with increased OXPHOS, which the authors proposed could impede cyst growth by limiting the amount of carbon available to be shuttled into alternative metabolic pathways. Inhibiting glutamine metabolism will similarly limit the available carbon and nitrogen atoms for other metabolic pathways, albeit with a divergent effect on OXPHOS.
Validation of GLS1 as potential therapeutic drug targets will require broadly profiling the metabolic changes in kidneys from additional models of PKD and from patients to determine which rodent models best reflect changes in human ADPKD kidneys. These studies will also be critical to identify the compensatory metabolic changes that bypass GLS1 inhibition so that rationale combination therapies may be tested.
FUNDING
The authors are grateful for funding from the PKD foundation to I.S. and E.Y.S., and to Darren Wallace (Univ. of Kansas Medical Center) for the ADPKD and NHK cell lines. The authors are also thankful to the Experimental Pathology Research Laboratory at the New York University Langone Medical Center for Histopathology and IHC staining and NYUCI Center Support Grant, ‘NIH/NCI 5 P30CA16087’ that provides partial support to the Experimental Pathology Research Laboratory.
SUPPLEMENTARY DATA
Supplementary data are available at ndt online.
CONFLICT OF INTEREST STATEMENT
None declared.
Supplementary Material
REFERENCES
- 1. Chapman AB. Approaches to testing new treatments in autosomal dominant polycystic kidney disease: insights from the CRISP and HALT-PKD studies. Clin J Am Soc Nephrol 2008; 3: 1197–1204 [DOI] [PubMed] [Google Scholar]
- 2. Torres VE. Treatment strategies and clinical trial design in ADPKD. Adv Chronic Kidney Dis 2010; 17: 190–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Harris PC, Torres VE.. Genetic mechanisms and signaling pathways in autosomal dominant polycystic kidney disease. J Clin Invest 2014; 124: 2315–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Seeger-Nukpezah T, Geynisman DM, Nikonova AS. et al. The hallmarks of cancer: relevance to the pathogenesis of polycystic kidney disease. Nat Rev Nephrol 2015; 11: 515–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Altman BJ, Dang CV.. Normal and cancer cell metabolism: lymphocytes and lymphoma. FEBS J 2012; 279: 2598–2609 [DOI] [PubMed] [Google Scholar]
- 6. Locasale JW, Cantley LC.. Metabolic flux and the regulation of mammalian cell growth. Cell Metab 2011; 14: 443–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rowe I, Chiaravalli M, Mannella V. et al. Defective glucose metabolism in polycystic kidney disease identifies a new therapeutic strategy. Nat Med 2013; 19: 488–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chiaravalli M, Rowe I, Mannella V. et al. 2-deoxy-d-glucose ameliorates PKD progression. J Am Soc Nephrol 2016; 27: 1958–1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hensley CT, Wasti AT, DeBerardinis RJ.. Glutamine and cancer: cell biology, physiology, and clinical opportunities. J Clin Invest 2013; 123: 3678–3684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang JB, Erickson JW, Fuji R. et al. Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Cancer Cell 2010; 18: 207–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. DeBerardinis RJ, Thompson CB.. Cellular metabolism and disease: what do metabolic outliers teach us? Cell 2012; 148: 1132–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wise DR, Thompson CB.. Glutamine addiction: a new therapeutic target in cancer. Trends Biochem Sci 2010; 35: 427–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aledo JC, Gómez-Fabre PM, Olalla L. et al. Identification of two human glutaminase loci and tissue-specific expression of the two related genes. Mamm Genome 2000; 11: 1107–1110 [DOI] [PubMed] [Google Scholar]
- 14. Mates JM, Segura JA, Campos-Sandoval JA. et al. Glutamine homeostasis and mitochondrial dynamics. Int J Biochem Cell Biol 2009; 41: 2051–2061 [DOI] [PubMed] [Google Scholar]
- 15. Mates JM, Segura JA, Martin-Rufian M. et al. Glutaminase isoenzymes as key regulators in metabolic and oxidative stress against cancer. Curr Mol Med 2013; 13: 514–534 [DOI] [PubMed] [Google Scholar]
- 16. Gross MI, Demo SD, Dennison JB. et al. Antitumor activity of the glutaminase inhibitor CB-839 in triple-negative breast cancer. Mol Cancer Ther 2014; 13: 890–901 [DOI] [PubMed] [Google Scholar]
- 17. van den Heuvel AP, Jing J, Wooster RF. et al. Analysis of glutamine dependency in non-small cell lung cancer: GLS1 splice variant GAC is essential for cancer cell growth. Cancer Biol Ther 2012; 13: 1185–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wilson KF, Erickson JW, Antonyak MA. et al. Rho GTPases and their roles in cancer metabolism. Trends Mol Med 2013; 19: 74–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vander Heiden MG. Targeting cancer metabolism: a therapeutic window opens. Nat Rev Drug Discov 2011; 10: 671–684 [DOI] [PubMed] [Google Scholar]
- 20. Jacque N, Ronchetti AM, Larrue C. et al. Targeting glutaminolysis has anti-leukemic activity in acute myeloid leukemia and synergizes with BCL-2 inhibition. Blood 2015; 126: 1346–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xiang Y, Stine ZE, Xia J. et al. Targeted inhibition of tumor-specific glutaminase diminishes cell-autonomous tumorigenesis. J Clin Invest 2015; 125: 2293–2306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. DeLaBarre B, Gross S, Fang C. et al. Full-length human glutaminase in complex with an allosteric inhibitor. Biochemistry 2011; 50: 10764–10770 [DOI] [PubMed] [Google Scholar]
- 23. Robinson MM, McBryant SJ, Tsukamoto T. et al. Novel mechanism of inhibition of rat kidney-type glutaminase by bis-2-(5-phenylacetamido-1, 2, 4-thiadiazol-2-yl)ethyl sulfide (BPTES). Biochem J 2007; 406: 407–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yamaguchi T, Hempson SJ, Reif GA. et al. Calcium restores a normal proliferation phenotype in human polycystic kidney disease epithelial cells. J Am Soc Nephrol 2006; 17: 178–187 [DOI] [PubMed] [Google Scholar]
- 25. Yamaguchi T, Nagao S, Wallace DP. et al. Cyclic AMP activates B-Raf and ERK in cyst epithelial cells from autosomal-dominant polycystic kidneys. Kidney Int 2003; 63: 1983–1994 [DOI] [PubMed] [Google Scholar]
- 26. Albaqumi M, Srivastava S, Li Z. et al. KCa3.1 potassium channels are critical for cAMP-dependent chloride secretion and cyst growth in autosomal-dominant polycystic kidney disease. Kidney Int 2008; 74: 740–749 [DOI] [PubMed] [Google Scholar]
- 27. Ma M, Tian X, Igarashi P. et al. Loss of cilia suppresses cyst growth in genetic models of autosomal dominant polycystic kidney disease. Nat Genet 2013; 45: 1004–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Patel V, Williams D, Hajarnis S. et al. miR-17∼92 miRNA cluster promotes kidney cyst growth in polycystic kidney disease. Proc Natl Acad Sci USA 2013; 110: 10765–10770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Srivastava S, Cai X, Li Z. et al. 3-kinase C2beta and TRIM27 function to positively and negatively regulate IgE receptor activation of mast cells. Mol Cell Biol 2012; 32: 3132–3139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Duran RV, Hall MN.. Glutaminolysis feeds mTORC1. Cell Cycle 2012; 11: 4107–4108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Torres VE, Harris PC.. Mechanisms of disease: autosomal dominant and recessive polycystic kidney diseases. Nat Rev Nephrol 2006; 2: 40–55; quiz 55 [DOI] [PubMed] [Google Scholar]
- 32. Menezes LF, Lin CC, Zhou F. et al. Fatty acid oxidation is impaired in an orthologous mouse model of autosomal dominant polycystic kidney disease. EBioMedicine 2016; 5: 183–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hajarnis S, Lakhia R, Yheskel M. et al. microRNA-17 family promotes polycystic kidney disease progression through modulation of mitochondrial metabolism. Nat Comms 2017; 8: 14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ulanet DB, Couto K, Jha A. et al. Mesenchymal phenotype predisposes lung cancer cells to impaired proliferation and redox stress in response to glutaminase inhibition. PLoS One 2014; 9: e115144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sellers K, Fox MP, Bousamra M. et al. Pyruvate carboxylase is critical for non-small-cell lung cancer proliferation. J Clin Invest 2015; 125: 687–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Timmerman LA, Holton T, Yuneva M. et al. Glutamine sensitivity analysis identifies the xCT antiporter as a common triple-negative breast tumor therapeutic target. Cancer Cell 2013; 24: 450–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Le A, Lane AN, Hamaker M. et al. Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells. Cell Metab 2012; 15: 110–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Trudel M. c-Myc signalling in the genetic mechanism of polycystic kidney disease In: X Li. (ed). Polycystic Kidney Disease. Brisbane: Codon Publications, 2015; doi: 10.15586/codon.pkd.2015.ch10 [PubMed] [Google Scholar]
- 39. Stine ZE, Walton ZE, Altman BJ. et al. MYC, metabolism, and cancer. Cancer Discov 2015; 5: 1024–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mayers JR, Vander Heiden MG.. Famine versus feast: understanding the metabolism of tumors in vivo. Trends Biochem Sci 2015; 40: 130–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rowe I, Boletta A.. Defective metabolism in polycystic kidney disease: potential for therapy and open questions. Nephrol Dial Transplant 2014; 29: 1480–1486 [DOI] [PubMed] [Google Scholar]
- 42. Momcilovic M, Bailey ST, Lee JT. et al. Targeted inhibition of EGFR and glutaminase induces metabolic crisis in EGFR mutant lung cancer. Cell Rep 2017; 18: 601–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hwang VJ, Kim J, Rand A. et al. The cpk model of recessive PKD shows glutamine dependence associated with the production of the oncometabolite 2-hydroxyglutarate. Am J Physiol Renal Physiol 2015; 309: F492–F498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pavlova NN, Thompson CB.. The emerging hallmarks of cancer metabolism. Cell Metab 2016; 23: 27–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Biancur DE, Paulo JA, Małachowska B. et al. Compensatory metabolic networks in pancreatic cancers upon perturbation of glutamine metabolism. Nat Comms 2017; 8: 15965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Altman BJ, Stine ZE, Dang CV.. From Krebs to clinic: glutamine metabolism to cancer therapy. Nat Rev Cancer 2016; 16: 749. [DOI] [PubMed] [Google Scholar]
- 47. Grant CM. Metabolic reconfiguration is a regulated response to oxidative stress. J Biol 2008; 7: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Padovano V, Kuo IY, Stavola LK. et al. The polycystins are modulated by cellular oxygen-sensing pathways and regulate mitochondrial function. Mol Biol Cell 2017; 28: 261–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.