Abstract
STUDY QUESTION
Are the mean numbers of blastocysts obtained from sibling cohorts of oocytes recruited after follicular phase and luteal phase stimulations (FPS and LPS) in the same ovarian cycle similar?
SUMMARY ANSWER
The cohorts of oocytes obtained after LPS are larger than their paired-FPS-derived cohorts and show a comparable competence, thus resulting in a larger mean number of blastocysts.
WHAT IS KNOWN ALREADY
Three theories of follicle recruitment have been postulated to date: (i) the ‘continuous recruitment’ theory, (ii) the ‘single recruitment episode’ theory and (iii) the ‘wave’ theory. Yet, a clear characterization of this crucial biological process for human reproduction is missing. Recent advances implemented in in vitro fertilization (IVF), such as blastocyst culture, aneuploidy testing and vitrification, have encouraged clinicians to maximize the exploitation of the ovarian reserve through tailored stimulation protocols, which is crucial especially for poor prognosis patients aiming to conceive after IVF. LPS has been already successfully adopted to treat poor prognosis or oncological patients through Duostim, LPS-only or random-start ovarian stimulation approaches. Nevertheless, little, and mainly retrospective, evidence has been produced to support the safety of LPS in general. Feasibility of the LPS approach would severely question the classic ‘single recruitment episode’ theory of follicular development.
STUDY DESIGN, SIZE, DURATION
This case-control study was conducted with paired follicular phase- and luteal phase-derived cohorts of oocytes collected after stimulations in the same ovarian cycle (DuoStim) at two private IVF clinics between October 2015 and December 2017.
PARTICIPANTS/MATERIALS, SETTING, METHODS
The study included 188 poor prognosis patients undergoing DuoStim with preimplantation genetic testing for aneuploidies (PGT-A). FPS and LPS were performed with the same daily dose of recombinant-gonadotrophins in an antagonist protocol. Blastocyst culture, trophectoderm biopsy, vitrification and frozen-warmed euploid single blastocyst transfers were performed. The primary outcome was the mean number of blastocysts obtained per oocyte retrieval from paired-FPS- and LPS-derived cohorts (required sample size = 165 patients; power = 90%). Mean blastulation and euploidy rates were monitored, along with the number of oocytes, euploid blastocysts and clinical outcomes.
MAIN RESULTS AND THE ROLE OF CHANCE
Significantly fewer blastocysts were obtained after FPS than LPS (1.2 ± 1.1 vs. 1.6 ± 1.6, P < 0.01), due to fewer oocytes collected (3.6 ± 2.1 vs. 4.3 ± 2.8, P < 0.01) and a similar mean blastocyst rates per retrieval (33.1% ± 30.3% vs. 37.4% ± 30.8%, P = NS). The number of oocytes collected were correlated (R = 0.5, P < 0.01), while the blastocyst rates were uncorrelated among paired-FPS- and LPS-derived cohorts. Overall, a significantly lower chance of producing blastocyst(s) was reported after FPS than after LPS: 67.6% (n = 127/188, 95%CI: 60.3–74.1) vs. 77.1% (n = 145/188, 95%CI: 70.3–82.8; P = 0.05). The mean euploidy rates per retrieval were similar between FPS- and LPS-derived cohorts of oocytes (13.6% ± 22.8% vs. 16.3% ± 23.4%, P = NS). Therefore, on average fewer euploid blastocysts (0.5 ± 0.8 vs. 0.7 ± 1.0, P = 0.02) resulted from FPS. Similar ongoing-pregnancy/delivery rates were reported, to date, after FPS- and LPS-derived euploid single blastocyst transfers: 42.4% (n = 28/66, 95%CI: 30.5–55.2) vs. 53.8% (n = 35/65, 95%CI: 41.1–66.1; P = NS).
LIMITATIONS, REASONS FOR CAUTION
More studies need to be conducted in the future to confirm the safety of LPS, especially in terms of ovarian and follicular environment, as well as the clinical, peri-natal and post-natal outcomes. Here, we showed preliminary data suggesting a similar ongoing implantation/delivery rate (>22 weeks) between FPS- and LPS-derived euploid blastocysts, that need to be extended in the future, to populations other than poor prognosis patients and using approaches other than DuoStim together with a constant monitoring of the related peri-natal and post-natal outcomes.
WIDER IMPLICATIONS OF THE FINDINGS
These data, from a paired study design, highlight that LPS-derived oocytes are as competent as FPS-derived oocytes, thereby adding some evidence to support the use of LPS for poor prognosis and oncological patients and to question the ‘single recruitment episode’ theory of follicle recruitment. These findings also encourage additional studies of the basics of folliculogenesis, with direct clinical implications for the management of ovarian stimulation in IVF.
TRIAL REGISTRATION
None.
STUDY FUNDING/COMPETING INTEREST(S)
No external funds were used for this study and there are no conflicts of interest.
Keywords: oocyte competence, luteal phase, DuoStim, blastocyst, ovarian stimulation, follicle recruitment
Introduction
The worldwide prevalence of primary and secondary infertility are estimated at ~2% and 10.5%, respectively, among women aged 20–44 years and attempting to conceive (Mascarenhas et al., 2012). These women may enter an IVF program and be classified as poor prognosis patients for several distinct causes (e.g. advanced maternal age, low ovarian reserve), and proper tailoring of the ovarian stimulation protocol to maximize the number of oocytes collected represents a crucial step for them to eventually conceive (Briggs et al., 2015; Drakopoulos et al., 2016). In this scenario, the enhancement of validated oocyte and embryo cryopreservation protocols (Rienzi et al., 2017) has represented a game-changer for the management of in vitro fertilization (IVF) treatment, thereby paving the way to the introduction of a cycle segmentation strategy. In fact, oocytes and embryos may be safely cryopreserved and consecutive frozen-warmed embryo transfers (ET) can be performed on a non-stimulated endometrium during a following uterine cycle (Devroey et al., 2011; Evans et al., 2014). In this context, the target number of oocytes to retrieve and the related ovarian stimulation protocol required should consider all the factors that may affect oocyte quality (Meldrum et al., 2016), among which maternal age has the highest impact (Hassold and Hunt, 2001; Heffner, 2004). In the attempt to properly balance oocyte quantity and competence, a model has been recently proposed by a panel of experts, known as the POSEIDON (Patient-Oriented Strategies Encompassing IndividualizeD Oocyte Number) group (Poseidon et al., 2016). These specialists, starting from the evidence that blastocyst culture and aneuploidy testing provide efficient criteria to predict embryo competence and conduct embryo selection (Dahdouh et al., 2015; Glujovsky et al., 2016), have claimed that the short-term goal of ovarian stimulation should be the production of at least one euploid blastocyst after IVF.
In the last decade, a novel ovarian stimulation protocol has been introduced in IVF: double stimulation in the same ovarian cycle (DuoStim) (Kuang et al., 2014; Ubaldi et al., 2016; Vaiarelli et al., 2017). This approach, by combining conventional follicular phase stimulation (FPS) with a stimulation conducted also in the luteal phase (LPS), provides the unprecedented opportunity to increase the cohort of oocytes retrieved in a single ovarian cycle and, as we suggested in a recent proof-of-concept study (Ubaldi et al., 2016), the chance to identify at least one euploid blastocyst. Similarly, a random-start approach has long been used for urgent fertility preservation needs (e.g. in cancer patients) (von Wolff et al., 2009; Sonmezer et al., 2011; Nayak and Wakim, 2011; Cakmak et al., 2013) and an LPS-only protocol has been also proposed (Buendgen et al., 2013; Martinez et al., 2014; Wang et al., 2016; Li et al., 2016) in IVF.
Intriguingly, the ability to retrieve metaphase II (MII) oocytes from the luteal phase has provided important evidence to question the classic theory of human reproductive biology claiming that oocytes can be collected only after FPS. Indeed, at present, novel theories have been developed suggesting that multiple follicular waves can arise during an ovarian cycle and anovulatory follicles may also be exploited to maximize the yield in terms of oocyte retrieval. Nonetheless, LPS-derived oocytes still need to be thoroughly characterized biologically and clinically to outline the safety of DuoStim, random-start and LPS-only approaches. In fact, only limited, and mainly retrospective, data have been published to date of investigations into the equivalence (in terms of both quantity and quality) of FPS- and LPS-derived cohorts of oocytes, as underlined by Boots et al. (2016) in their review and meta-analysis.
In this study, we defined the mean number of blastocysts produced per oocyte retrieval after paired-FPS and LPS conducted in 188 poor prognosis patients undergoing DuoStim and preimplantation genetic testing for aneuploidies (PGT-A) at two IVF centers (Rome and Naples, Italy). This intra-patient paired design allowed us to exclude any putative confounder upon the primary outcome as well as to provide an unbiased overview on the competence of LPS-derived oocytes in comparison to FPS-derived oocytes from the same ovarian cycle; rates of fertilization, blastocyst development and euploidy per oocyte retrieval were included together with preliminary clinical outcomes after single euploid blastocyst transfer.
Material and methods
Study period, population of patients and design
This is a paired case-control study on sibling cohorts of oocytes conducted between October 2015 and December 2017 at two IVF centers in Italy (Rome and Naples). All consecutive consenting patients who underwent a DuoStim protocol with PGT-A were candidates to be included. The DuoStim approach was proposed, after extensive counseling, to poor prognosis women defined according to the following criteria: anti-mullerian hormone (AMH) ≤1.5 ng/ml and/or antral follicle count (AFC) ≤6 and/or ≤5 oocytes retrieved from a previous cycle and/or ≥35 year (at least two out of these conditions should have been satisfied). Patients whose male partner was azoospermic were excluded, since azoospermia may have per se an impact upon embryo developmental competence (Mazzilli et al., 2017). Patients who did not respond to either FPS or LPS were included as stimulation cycles resulting in zero oocytes retrieved. The paired design prevented the risk of confounders upon the outcomes of the study. Likewise, after oocyte retrieval, the same media and incubation system was adopted to conduct blastocyst culture for FPS- and LPS-derived inseminated MII oocytes. The Institutional Review Board of the clinics approved the study.
Ovarian stimulation and IVF procedures
All consenting patients underwent a DuoStim protocol. Luteal estradiol priming (4 mg/die of estradiol valerate, Progynova, Bayer, Germany) was performed in the previous menstrual cycle (Reynolds et al., 2013). After the scan and basal assessment of the ovaries, FPS was started on Day 2 of the menstrual cycle with a fixed dose of rec-FSH (300 IU/die; Gonal-F, Merck-Serono, Germany; Puregon, MSD, USA) and rec-LH (75 IU/die; Luveris, Merck-Serono) for 4 days. Follicular growth was monitored on Day 5 and then every 2 days. GnRH antagonist (cetrorelix, Cetrotide, Merck-Serono; ganirelix, Orgalutran, MSD) was administered daily after the identification of a leading follicle with a diameter ≥13–14 mm and until the day of ovulation trigger (conducted with a single subcutaneous bolus of buserelin at the dose of 0.5 ml; Suprefact, Hoechst Marion Roussel, Germany), namely when at least two follicles reached a diameter ≥17–18 mm. Oocyte retrieval was performed 35 h after the trigger. After 5 days from the first retrieval, namely when complete luteolysis is attained (Fatemi et al., 2013), LPS was started with the same protocol and the same daily dose as FPS, regardless the number of antral follicles counted at the scan. In case of no response to the FPS, the LPS was started between the 18th and the 20th day of the same ovarian cycle.
The procedures for oocyte retrieval, intra-cytoplasmic sperm injection, embryo culture, trophectoderm biopsy and vitrification have been described in detail previously (Rienzi et al., 1998; Cobo et al., 2008; Capalbo et al., 2014; Ubaldi et al., 2015). Briefly, cumulus–oocyte complexes were collected from the follicular fluid after transvaginal ultrasound-guided aspiration, and cultured in continuous single culture media (CSCM, Irvine Scientific, Australia) in a controlled humidified atmosphere (37°C, 6%CO2 and 5%O2) for 2–3 h. Then, they were denuded in Hepes-buffered medium (Irvine Scientific) and inseminated. Fertilization was assessed 16–20 h after insemination, by the presence of two equally-sized pronuclei. Embryo culture was conducted in single 25-μl micro-drops of CSCM in a benchtop incubator (MINC, Cook Medical, USA) in a controlled humidified atmosphere up to the fully-expanded blastocyst stage (Day 5–7). Trophectoderm biopsy was performed through a method that does not entail any hatching procedure at the cleavage stage, since laser-assisted zona opening and trophectoderm fragment retrieval were conducted sequentially. All embryos that developed as viable blastocysts were biopsied, independently of their morphological quality and/or day of full-expansion. Vitrification was performed after trophectoderm biopsy on collapsed blastocysts through Cryotop devices and solutions (Kitazato BioPharma Co., Japan).
Comprehensive chromosomal testing was conducted by quantitative polymerase chain reaction according to a protocol published by Treff et al. (2012), and validated in our lab in the following study (Capalbo et al., 2015). The method was designed to specifically identify constitutive whole-chromosome but not segmental aneuploidies.
After warming, only single euploid blastocyst transfers were performed. Endometrial preparation and transfer procedures were described previously (Ubaldi et al., 2015).
Outcome measures, sample size and statistical analysis
The primary outcome measure was defined as the mean number of blastocysts obtained per oocyte retrieval after FPS versus paired LPS. The sample size was calculated to determine a significantly different mean number of blastocysts produced per stimulation at a level 5% (power = 90%; α = 5%). The software G*power v3.1 was used to this end and the following settings were adopted: a priori required sample size computation for a Wilcoxon signed-rank test (matched pairs), given a 0.9-power, a 0.05-alpha error probability and 0.27 effect size. Thus, 165 matched pairs were required.
The secondary outcome measures were the mean number of MII oocytes and euploid blastocysts, as well as the mean blastocyst and euploidy rates per oocytes retrieval among the paired-cohorts of oocytes collected.
At last, we monitored the clinical outcomes after the euploid frozen single blastocyst transfers conducted to date. Clinical pregnancy was defined from ultrasonographical evidence of a gestational sac with fetal heartbeat. A pregnancy loss earlier than the 22nd gestational week was considered a miscarriage. The clinical pregnancy rate was calculated upon the number of transfers performed, the miscarriage rate was calculated upon the number of clinical pregnancies and the ongoing-pregnancy rate (>22 weeks) was calculated again upon the number of transfers performed (Zegers-Hochschild et al., 2017a, 2017b).
All data were collected in a relational database (Fertilab Manager, FLM, Italy). Categorical variables are presented as percentages with 95%CI. Continuous variables are presented as mean ± standard deviation and range. Shapiro–Wilk tests were conducted to investigate whether the data followed a normal (Gaussian) distribution. Wilcoxon signed ranks tests for related samples were performed to investigate putative differences among the two paired arms of the study.
The rate of patients producing blastocyst(s) only after FPS, only after LPS, after both FPS and LPS, or after none of the stimulations were calculated and then compared through a McNemar’s test.
The software R was used for statistics.
Results
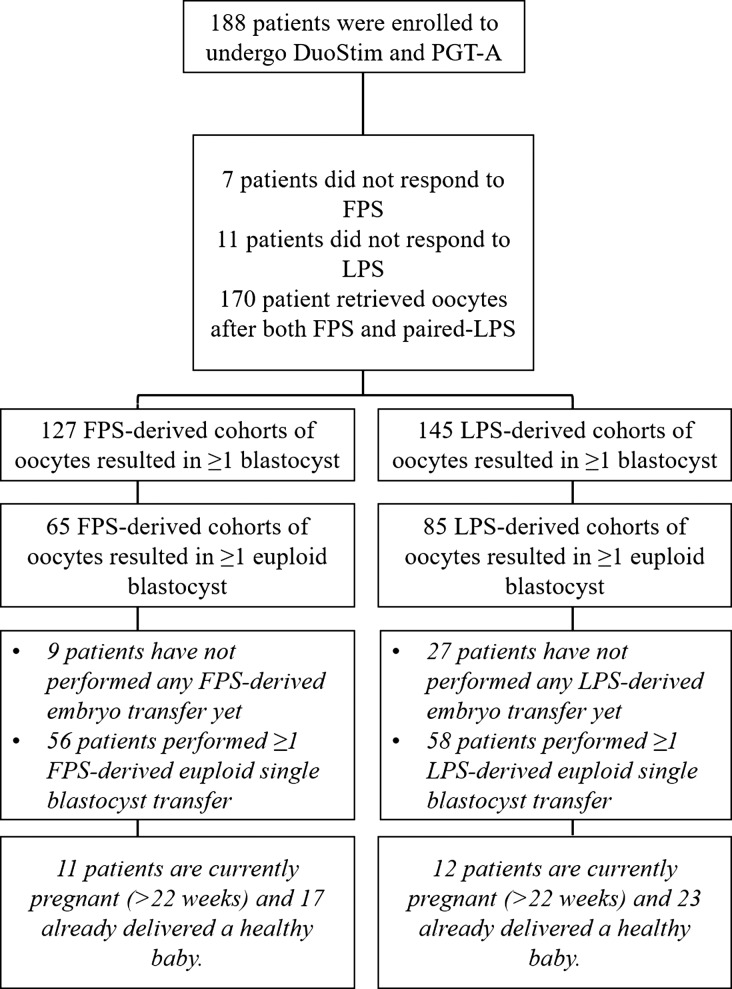
During the study period, 188 poor prognosis women underwent DuoStim and PGT-A. Among them, 7 (3.7%) and 11 (5.9%) patients did not respond to FPS and LPS, respectively, and were included as stimulation cycles resulting in zero oocytes produced. Therefore, of the 188 couples under investigation, 170 couples collected at least one oocyte from both FPS and LPS (Fig. 1). This sample size outnumbers the one required (see Material and methods section). Supplementary Table S1 outlines the patients’ population. Of note, the mean maternal age was 40.0 ± 2.9, the AFC was 5.0 ± 2.5, the AMH was 0.9 ± 0.6 and they had already undergone 1.0 ± 1.3 previous IVF cycles.
Figure 1.
Flowchart of the study. DuoStim, double stimulation in the same ovarian cycle; PGT-A, preimplantation genetic testing for aneuploidies; FPS, follicular phase stimulation; LPS, luteal phase stimulation.
On average, FPS was 1 day shorter than LPS (10.1 ± 1.9 vs. 11.0 ± 1.9; P < 0.01) and the total dose of rec-FSH (3039.8 ± 558.9 IU, 2100–4500 vs. 3288.1 ± 584.8 IU, 2100–4500; P < 0.01) and gonadotrophins in general (3799.7 ± 698.7 IU, 2625–5625 vs. 4110.2 ± 731.0 IU, 2625–5625; P < 0.01) was significantly lower. However, the two lengths were not correlated (R = 0.14, P = NS) with 27.1% (n = 46/170) and 54.7% (n = 93/170) of the patients responding to both stimulations undergoing a longer FPS or LPS, respectively. The number of vials of antagonist administrated was similar between FPS and LPS, namely 3.9 ± 0.9 (3–6) and 4.0 ± 1.0 (3–7), respectively.
Overall, 684 and 804 MII oocytes were retrieved after FPS and LPS, respectively. On average, significantly fewer oocytes were collected after FPS than after LPS (3.6 ± 2.1 vs. 4.3 ± 2.8, P < 0.01) and the two sets of data were significantly correlated (R = 0.5, P < 0.01) (Table I). Of note, the mean blastocyst rate per oocyte retrieval was similar among the two paired groups (33.1% ± 30.3% vs. 37.4% ± 30.8%, P = NS). Therefore, due to the lower mean number of MII oocytes collected, also fewer blastocysts on average were obtained after FPS than after LPS (1.2 ± 1.1 vs. 1.6 ± 1.6, P < 0.01; primary outcome of the study). Interestingly, both the blastocyst rate and number of blastocysts obtained did not show any correlation among FPS and LPS, suggesting that the two cohorts of oocytes retrieved are equivalent from a global analysis but uncorrelated from an intra-patient analysis of each DuoStim cycle (Table I). At last, the mean euploid blastocyst rate per oocyte retrieval among the two paired study groups was similar (13.6% ± 22.8% vs. 16.3% ± 23.4%, P = NS) and did not show any correlation (R = 0.08, P = NS) (Table I). This resulted in a slightly lower, yet significantly different, mean number of euploid blastocysts obtained after FPS than after LPS (0.5 ± 0.8 vs. 0.7 ± 1.0, P = 0.02) (Table I).
Table I.
Embryological data after follicular phase stimulation (FPS) and luteal phase stimulation (FPS) conducted from the 188 couples included in the study.
| FPS mean ± SD (range) | LPS mean ± SD (range) | z-value | P-value | Correlation between LPS and FPS (R) P-value | |
|---|---|---|---|---|---|
| Number of MII oocytes | n = 684 | n = 804 | −2.8 | P < 0.01 | 0.50 |
| 3.6 ± 2.1 (0–9) | 4.3 ± 2.8 (0–10) | P < 0.01 | |||
| Number of fertilized oocytes | n = 485 | n = 595 | −2.8 | P < 0.01 | 0.34 |
| 2.6 ± 1.9 (0–9) | 3.2 ± 2.4 (0–10) | P < 0.01 | |||
| Mean fertilization rate per oocyte retrieval | 68.2% ± 3 3.0% (0–100%) | 70.0% ± 30.8% (0–100%) | −0.5 | NS | 0.01 |
| NS | |||||
| Number of blastocysts | n = 227 | n = 308 | −2.7 | P < 0.01 | 0.09 |
| 1.2 ± 1.1 (0–4) | 1.6 ± 1.6 (0–9) | NS | |||
| Mean blastocyst rate per oocyte retrieval | 33.1% ± 30.3% (0–100%) | 37.4% ± 30.8% (0–100%) | −1.2 | NS | −0.03 NS |
| Number of euploid blastocysts | n = 93 | n = 133 | −2.4 | P = 0.02 | 0.17 |
| 0.5 ± 0.8 (0–4) | 0.7 ± 1.0 (0–5) | P = 0.02 | |||
| Mean euploidy rate per oocyte retrieval | 13.6% ± 22.8% (0–100%) | 16.3% ± 23.4% (0–100%) | −1.1 | NS | 0.08 NS |
Wilcoxon Signed Ranks tests between related samples were conducted, P < 0.05 was considered significant. All the mean rates were calculated upon the number of metaphase II (MII) oocytes collected after either FPS or LPS from each patient within the same ovarian cycle.
The mean blastocyst rates per oocyte retrieval were also shown according to ranges of MII oocytes collected after FPS and LPS (1–4 or 5–10). Supplementary Figure S1 displays the results of this sub-analysis, which involved 109 out the 188 patients (58.0%) included in this study, namely those women achieving a similar ovarian response between FPS and LPS. In the groups of patients collecting 1–4 MII oocytes after both FPS and LPS (n = 76 patients), the mean blastocyst rates were, respectively, 34.3% ± 34.1% versus 40.7% ± 35.4% (z = −1.18, P = NS); in the group of patients collecting 5–10 MII oocytes after both FPS and LPS (n = 33 patients), the mean blastocyst rates were, respectively, 25.3% ± 17.5% versus 36.4% ± 23.0% (z = −2, P = NS).
Overall, 6.9% (n = 13/188, 95%CI: 3.9–11.8) of the patients did not obtain blastocysts after DuoStim. Conversely, among the patients who obtained blastocyst(s) after FPS and/or LPS, the McNemar’s test highlighted a significantly lower chance in the former rather than in the latter: 127/188, 67.6% (95%CI: 60.3–74.1) and 145/188, 77.1% (95%CI: 70.3–82.8; P = 0.05), respectively (Supplementary Table SII).
To date, 66 (from 56 patients) and 65 (from 58 patients) single euploid frozen blastocyst transfers have been performed after FPS and LPS, respectively. The clinical outcomes in terms of clinical pregnancy, miscarriage and ongoing-pregnancy (>22 weeks) rates were comparable between FPS- and LPS-derived euploid blastocysts. Specifically, the ongoing-pregnancy (>22 weeks)/delivery rates were 42.4% (n = 28/66) and 53.8% (n = 35/65, P = NS), respectively (Supplementary Table SIII). To date, 17 FPS- and 23 LPS-derived healthy babies have been already delivered (Fig. 1) and did not show any peri-natal or post-natal severe complication/malformation. Specifically, the mean gestational weeks after FPS- and LPS-derived pregnancies were 38.3 ± 1.0 (range 37–40) and 37.8 ± 1.6 (34–41), respectively (P = NS); one and two cases of gestational diabetes were reported, respectively; the mean birth weights of the newborns were 3390.0 ± 483.3 g (2840–4300) and 3284.0 ± 489.4 g (2400–4152), respectively (P = NS); and the mean lengths of the newborns were 50.0 ± 1.2 cm (48–52) and 50.7 ± 2.2 cm (47–56), respectively (P = NS).
Discussion
In this study, we provided evidence that on average more MII oocytes can be retrieved after LPS than after FPS conducted in a DuoStim approach in poor prognosis patients. LPS-derived embryos then showed similar competence as FPS-derived ones. Therefore, a higher probability of obtaining at least one blastocyst, as well as a higher mean number of blastocysts produced, was reported from LPS- than FPS-derived cohorts of oocytes. Of note, although the mean number of MII oocytes collected showed a significant correlation between FPS- and LPS-derived cohorts, their blastulation and euploidy rates were clearly unrelated. The two cohorts were globally-equivalent in terms of competence, but they were mostly independent in an intra-patient-based analysis (correlation between FPS and LPS in the same ovarian cycle).
Dealing with the number of MII oocytes collected, the correlation between the ovarian response after FPS and LPS outlines a patient-specific pattern. Therefore, the multi-marker evaluation of the ovarian reserve (Al-Azemi et al., 2011) may have a comparable power on both FPS and LPS. This evidence then supports our practice of disregarding the AFC performed before LPS, since it might be highly correlated with the AFC as conventionally performed before FPS (Massin et al., 2015). Furthermore, the presence of multiple corpora lutea before LPS impacts the intra- and inter-operator variability and compromises the reliability of the AFC.
A significantly higher number of oocytes collected after LPS than after FPS has already been reported from previous studies (Kuang et al., 2014; Liu et al., 2017). The higher number may have been influenced by the DuoStim approach itself, since LPS is conducted soon after FPS is ended. Therefore, the high level of estradiol and progesterone reached after FPS may synchronize the cohort of antral follicles that will grow during LPS, as well as boost the proliferation of FSH receptors in their granulosa cells (Fanchin et al., 2003a, 2003b; Reynolds et al., 2013; Messinis et al., 2014), resulting overall in a better response to the stimulation. Of note, the DuoStim protocol used here entailed estradiol priming also in the luteal phase preceding FPS, thus limiting this putative bias. In general, however, FPS may positively affect the subsequent LPS modifying the ovarian micro-environment. For instance, in animal models, an increase in angiogenic factors has been suggested due to the hormonal status after FPS above physiological levels. This may, in turn, enhance the sensitivity of the granulosa cells to FSH within the follicles recruited in the anovulatory wave (Macchiarelli et al., 2006). Another hypothesis for a better ovarian response after LPS in a DuoStim approach is a possible flare-up effect derived from the GnRH agonist trigger in the FPS, which might induce a down-regulation in the expression of AMH in the follicles from the anovulatory wave, thereby increasing the number of follicles with a 3–4 mm diameter recruited in the LPS (Yang et al., 2013). However, all these speculations must be confirmed, as well as the role of endocrine and paracrine factors better unveiled, to understand the mechanisms modulating the recruitment of follicles growing in the anovulatory wave of the ovarian cycle. Previous investigations have also reported a generally higher mean number of MII oocytes after LPS-only stimulation protocols compared to conventional FPS (Wang et al., 2016; Li et al., 2016), supporting a better response in the luteal phase per se. Still, the design of those reports is limited not only by their retrospective nature but also by the lack of an internal paired control, which is intrinsic to the application of DuoStim.
The core evidence of this study is the overall equivalence in terms of mean blastocyst rate per oocyte retrieval after FPS and LPS, which, due to the higher number of MII oocytes collected, resulted in a higher number of blastocysts produced after the latter phase of the same ovarian cycle. This paired design allowed us to outline a feature which might be pivotal to support the clinical use of oocytes collected from waves arising in the luteal phase, a reproductive option valuable not only for poor prognosis patients undergoing IVF, but also for oncological patients requiring an urgent stimulation for fertility preservation purposes.
These data suggest that existing high progesterone levels during ovarian stimulation do not impact oocyte (and possibly embryo) competence, as already suggested by a review recently published (Massin, 2017). Furthermore, they fit in an international debate on the basics of human folliculogenesis with direct clinical implications. To date, three theories of follicle recruitment have been postulated and elegantly synthesized by Baerwald et al. (2012): (i) the continuous recruitment theory, according to which follicles start growing and regress continuously during the inter-ovulatory interval and the dominant one is randomly chosen after luteal regression; (ii) the single recruitment episode theory, according to which a single cohort of follicles starts growing following luteal regression, among which the dominant follicle is selected and (iii) the wave theory, according to which at least two cohorts of antral follicles are recruited per ovarian cycle, and the dominant follicle originates only from the major (or ovulatory) wave. Here, the absence of correlation between the average competence of the MII oocytes recruited by each patient from the follicular and the luteal phase of the same ovarian cycle suggests that the two cohorts are independent. Furthermore, the possibility of producing euploid blastocysts in either of the two phases of the ovarian cycle suggests that non-dominant follicles could be competent and develop following a random fashion. In other words, possibly the dominance of a follicle does not automatically mirror its competence. These two pieces of information add some clues in favor of both the continuous recruitment and the wave theories of follicle development and certainly prompt future investigations on such an intriguing topic, which may revolutionize the way we currently conceive human ovarian physiology.
More studies need to be conducted in the future, not only in populations of poor prognosis patients, to confirm the safety of LPS, in terms of ovarian (and follicular) environment as well as clinical, peri-natal and post-natal outcomes. Here, we showed preliminary data suggesting a similar ongoing implantation/delivery rate (>22 weeks) between FPS- and LPS-derived euploid blastocysts, that will be extended in the future together with a constant monitoring of their related peri-natal and post-natal outcomes. In this regard, in 2015, Chen et al. (2015) compared the data from 587 live births resulting from LPS versus conventional stimulation protocols. The authors supported that there is no difference in terms of gestational age, birth weight, length and congenital birth defects. Yet, their study was retrospective and lacked a proper control. Therefore, more evidence should be provided from future investigations.
Conclusion
This study provides evidence that the follicles recruited during the anovulatory phase of the ovarian cycle may be rescued through LPS. Of note, LPS-derived cohorts of oocytes were also larger than paired-FPS-derived cohorts and the oocytes showed comparable competence. These data support the putative benefits of LPS in poor prognosis and oncological patients. Furthermore, they encourage additional clinical and basic research studies on this topic, which may revolutionize the basics of human folliculogenesis, as well as the future concept of approaches to ovarian stimulation in IVF.
Supplementary Material
Funding
No external funds were used for this study.
Conflict of interest
The authors have no conflict of interest to declare related to this study.
Author contributions
D.C., F.M.U. and L.R. designed the study. D.C. and A.V. analyzed the data and drafted the manuscript. All authors contributed to the interpretation and discussion of the data.
References
- Al-Azemi M, Killick SR, Duffy S, Pye C, Refaat B, Hill N, Ledger W. Multi-marker assessment of ovarian reserve predicts oocyte yield after ovulation induction. Hum Reprod 2011;26:414–422. [DOI] [PubMed] [Google Scholar]
- Baerwald AR, Adams GP, Pierson RA. Ovarian antral folliculogenesis during the human menstrual cycle: a review. Hum Reprod Update 2012;18:73–91. [DOI] [PubMed] [Google Scholar]
- Boots CE, Meister M, Cooper AR, Hardi A, Jungheim ES. Ovarian stimulation in the luteal phase: systematic review and meta-analysis. J Assist Reprod Genet 2016;33:971–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs R, Kovacs G, MacLachlan V, Motteram C, Baker HW. Can you ever collect too many oocytes? Hum Reprod 2015;30:81–87. [DOI] [PubMed] [Google Scholar]
- Buendgen NK, Schultze-Mosgau A, Cordes T, Diedrich K, Griesinger G. Initiation of ovarian stimulation independent of the menstrual cycle: a case-control study. Arch Gynecol Obstet 2013;288:901–904. [DOI] [PubMed] [Google Scholar]
- Cakmak H, Katz A, Cedars MI, Rosen MP. Effective method for emergency fertility preservation: random-start controlled ovarian stimulation. Fertil Steril 2013;100:1673–1680. [DOI] [PubMed] [Google Scholar]
- Capalbo A, Rienzi L, Cimadomo D, Maggiulli R, Elliott T, Wright G, Nagy ZP, Ubaldi FM. Correlation between standard blastocyst morphology, euploidy and implantation: an observational study in two centers involving 956 screened blastocysts. Hum Reprod 2014;29:1173–1181. [DOI] [PubMed] [Google Scholar]
- Capalbo A, Treff NR, Cimadomo D, Tao X, Upham K, Ubaldi FM, Rienzi L, Scott RT Jr. Comparison of array comparative genomic hybridization and quantitative real-time PCR-based aneuploidy screening of blastocyst biopsies. Eur J Hum Genet 2015;23:901–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Wang Y, Lyu Q, Ai A, Fu Y, Tian H, Cai R, Hong Q, Chen Q, Shoham Z et al. Comparison of live-birth defects after luteal-phase ovarian stimulation vs. conventional ovarian stimulation for in vitro fertilization and vitrified embryo transfer cycles. Fertil Steril 2015;103:1194–1201 e1192. [DOI] [PubMed] [Google Scholar]
- Cobo A, Bellver J, Domingo J, Perez S, Crespo J, Pellicer A, Remohi J. New options in assisted reproduction technology: the Cryotop method of oocyte vitrification. Reprod Biomed Online 2008;17:68–72. [DOI] [PubMed] [Google Scholar]
- Dahdouh EM, Balayla J, Garcia-Velasco JA. Comprehensive chromosome screening improves embryo selection: a meta-analysis. Fertil Steril 2015;104:1503–1512. [DOI] [PubMed] [Google Scholar]
- Devroey P, Polyzos NP, Blockeel C. An OHSS-free clinic by segmentation of IVF treatment. Hum Reprod 2011;26:2593–2597. [DOI] [PubMed] [Google Scholar]
- Drakopoulos P, Blockeel C, Stoop D, Camus M, de Vos M, Tournaye H, Polyzos NP. Conventional ovarian stimulation and single embryo transfer for IVF/ICSI. How many oocytes do we need to maximize cumulative live birth rates after utilization of all fresh and frozen embryos? Hum Reprod 2016;31:370–376. [DOI] [PubMed] [Google Scholar]
- Evans J, Hannan NJ, Edgell TA, Vollenhoven BJ, Lutjen PJ, Osianlis T, Salamonsen LA, Rombauts LJ. Fresh versus frozen embryo transfer: backing clinical decisions with scientific and clinical evidence. Hum Reprod Update 2014;20:808–821. [DOI] [PubMed] [Google Scholar]
- Fanchin R, Cunha-Filho JS, Schonauer LM, Kadoch IJ, Cohen-Bacri P, Frydman R. Coordination of early antral follicles by luteal estradiol administration provides a basis for alternative controlled ovarian hyperstimulation regimens. Fertil Steril 2003. a;79:316–321. [DOI] [PubMed] [Google Scholar]
- Fanchin R, Salomon L, Castelo-Branco A, Olivennes F, Frydman N, Frydman R. Luteal estradiol pre-treatment coordinates follicular growth during controlled ovarian hyperstimulation with GnRH antagonists. Hum Reprod 2003. b;18:2698–2703. [DOI] [PubMed] [Google Scholar]
- Fatemi HM, Polyzos NP, van Vaerenbergh I, Bourgain C, Blockeel C, Alsbjerg B, Papanikolaou EG, Humaidan P. Early luteal phase endocrine profile is affected by the mode of triggering final oocyte maturation and the luteal phase support used in recombinant follicle-stimulating hormone-gonadotropin-releasing hormone antagonist in vitro fertilization cycles. Fertil Steril 2013;100:742–747. [DOI] [PubMed] [Google Scholar]
- Glujovsky D, Farquhar C, Quinteiro Retamar AM, Alvarez Sedo CR, Blake D. Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. Cochrane Database Syst Rev 2016:CD002118 doi:10.1002/14651858.CD002118.pub5. [DOI] [PubMed] [Google Scholar]
- Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet 2001;2:280–291. [DOI] [PubMed] [Google Scholar]
- Heffner LJ. Advanced maternal age—how old is too old? N Engl J Med 2004;351:1927–1929. [DOI] [PubMed] [Google Scholar]
- Kuang Y, Chen Q, Hong Q, Lyu Q, Ai A, Fu Y, Shoham Z. Double stimulations during the follicular and luteal phases of poor responders in IVF/ICSI programmes (Shanghai protocol). Reprod Biomed Online 2014;29:684–691. [DOI] [PubMed] [Google Scholar]
- Li Y, Yang W, Chen X, Li L, Zhang Q, Yang D. Comparison between follicular stimulation and luteal stimulation protocols with clomiphene and HMG in women with poor ovarian response. Gynecol Endocrinol 2016;32:74–77. [DOI] [PubMed] [Google Scholar]
- Liu C, Jiang H, Zhang W, Yin H. Double ovarian stimulation during the follicular and luteal phase in women ≥38 years: a retrospective case-control study. Reprod Biomed Online 2017;35:678–684. [DOI] [PubMed] [Google Scholar]
- Macchiarelli G, Jiang JY, Nottola SA, Sato E. Morphological patterns of angiogenesis in ovarian follicle capillary networks. A scanning electron microscopy study of corrosion cast. Microsc Res Tech 2006;69:459–468. [DOI] [PubMed] [Google Scholar]
- Martinez F, Clua E, Devesa M, Rodriguez I, Arroyo G, Gonzalez C, Sole M, Tur R, Coroleu B, Barri PN. Comparison of starting ovarian stimulation on day 2 versus day 15 of the menstrual cycle in the same oocyte donor and pregnancy rates among the corresponding recipients of vitrified oocytes. Fertil Steril 2014;102:1307–1311. [DOI] [PubMed] [Google Scholar]
- Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med 2012;9:e1001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massin N. New stimulation regimens: endogenous and exogenous progesterone use to block the LH surge during ovarian stimulation for IVF. Hum Reprod Update 2017;23:211–220. [DOI] [PubMed] [Google Scholar]
- Massin N, Armand G, Villette C, Dessapt A, Pietin Vialle C, Bry H, Pasquier M, Haddad B. Comparison of antral follicle count across the menstrual cycle with antimullerian hormone and ovarian response to controlled hyperstimulation related to ovarian reserve. Fertil Steril 2015;105:e248. [Google Scholar]
- Mazzilli R, Cimadomo D, Vaiarelli A, Capalbo A, Dovere L, Alviggi E, Dusi L, Foresta C, Lombardo F, Lenzi A et al. Effect of the male factor on the clinical outcome of intracytoplasmic sperm injection combined with preimplantation aneuploidy testing: observational longitudinal cohort study of 1,219 consecutive cycles. Fertil Steril 2017;108:961–972.e3. [DOI] [PubMed] [Google Scholar]
- Meldrum DR, Casper RF, Diez-Juan A, Simon C, Domar AD, Frydman R. Aging and the environment affect gamete and embryo potential: can we intervene? Fertil Steril 2016;105:548–559. [DOI] [PubMed] [Google Scholar]
- Messinis IE, Messini CI, Dafopoulos K. Novel aspects of the endocrinology of the menstrual cycle. Reprod Biomed Online 2014;28:714–722. [DOI] [PubMed] [Google Scholar]
- Nayak SR, Wakim AN. Random-start gonadotropin-releasing hormone (GnRH) antagonist-treated cycles with GnRH agonist trigger for fertility preservation. Fertil Steril 2011;96:e51–e54. [DOI] [PubMed] [Google Scholar]
- Poseidon G, Alviggi C, Andersen CY, Buehler K, Conforti A, De Placido G, Esteves SC, Fischer R, Galliano D, Polyzos NP et al. A new more detailed stratification of low responders to ovarian stimulation: from a poor ovarian response to a low prognosis concept. Fertil Steril 2016;105:1452–1453. [DOI] [PubMed] [Google Scholar]
- Reynolds KA, Omurtag KR, Jimenez PT, Rhee JS, Tuuli MG, Jungheim ES. Cycle cancellation and pregnancy after luteal estradiol priming in women defined as poor responders: a systematic review and meta-analysis. Hum Reprod 2013;28:2981–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rienzi L, Gracia C, Maggiulli R, LaBarbera AR, Kaser DJ, Ubaldi FM, Vanderpoel S, Racowsky C. Oocyte, embryo and blastocyst cryopreservation in ART: systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum Reprod Update 2017;23:139–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rienzi L, Ubaldi F, Anniballo R, Cerulo G, Greco E. Preincubation of human oocytes may improve fertilization and embryo quality after intracytoplasmic sperm injection. Hum Reprod 1998;13:1014–1019. [DOI] [PubMed] [Google Scholar]
- Sonmezer M, Turkcuoglu I, Coskun U, Oktay K. Random-start controlled ovarian hyperstimulation for emergency fertility preservation in letrozole cycles. Fertil Steril 2011;95:2125 e2129–2111. [DOI] [PubMed] [Google Scholar]
- Treff NR, Tao X, Ferry KM, Su J, Taylor D, Scott RT Jr.. Development and validation of an accurate quantitative real-time polymerase chain reaction-based assay for human blastocyst comprehensive chromosomal aneuploidy screening. Fertil Steril 2012;97:819–824. [DOI] [PubMed] [Google Scholar]
- Ubaldi FM, Capalbo A, Colamaria S, Ferrero S, Maggiulli R, Vajta G, Sapienza F, Cimadomo D, Giuliani M, Gravotta E et al. Reduction of multiple pregnancies in the advanced maternal age population after implementation of an elective single embryo transfer policy coupled with enhanced embryo selection: pre- and post-intervention study. Hum Reprod 2015;30:2097–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubaldi FM, Capalbo A, Vaiarelli A, Cimadomo D, Colamaria S, Alviggi C, Trabucco E, Venturella R, Vajta G, Rienzi L. Follicular versus luteal phase ovarian stimulation during the same menstrual cycle (DuoStim) in a reduced ovarian reserve population results in a similar euploid blastocyst formation rate: new insight in ovarian reserve exploitation. Fertil Steril 2016;105:1488–1495 e1481. [DOI] [PubMed] [Google Scholar]
- Vaiarelli A, Venturella R, Vizziello D, Bulletti F, Ubaldi FM. Dual ovarian stimulation and random start in assisted reproductive technologies: from ovarian biology to clinical application. Curr Opin Obstet Gynecol 2017;29:153–159. [DOI] [PubMed] [Google Scholar]
- von Wolff M, Thaler CJ, Frambach T, Zeeb C, Lawrenz B, Popovici RM, Strowitzki T. Ovarian stimulation to cryopreserve fertilized oocytes in cancer patients can be started in the luteal phase. Fertil Steril 2009;92:1360–1365. [DOI] [PubMed] [Google Scholar]
- Wang N, Wang Y, Chen Q, Dong J, Tian H, Fu Y, Ai A, Lyu Q, Kuang Y. Luteal-phase ovarian stimulation vs conventional ovarian stimulation in patients with normal ovarian reserve treated for IVF: a large retrospective cohort study. Clin Endocrinol (Oxf) 2016;84:720–728. [DOI] [PubMed] [Google Scholar]
- Yang DZ, Yang W, Li Y, He Z. Progress in understanding human ovarian folliculogenesis and its implications in assisted reproduction. J Assist Reprod Genet 2013;30:213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, Rienzi L, Sunde A, Schmidt L, Cooke ID et al. The International Glossary on Infertility and Fertility Care, 2017. Fertil Steril 2017. a;108:393–406. [DOI] [PubMed] [Google Scholar]
- Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, Rienzi L, Sunde A, Schmidt L, Cooke ID et al. The International Glossary on Infertility and Fertility Care, 2017. Hum Reprod 2017. b;32:1786–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.