Abstract
Background and Aims
More intense droughts under climate change threaten species resilience. Hydraulic strategies determine drought survival in woody plants but have been hardly studied in herbaceous species. We explored the intraspecific variability of hydraulic and morphological traits as indicators of dehydration tolerance in a perennial grass, cocksfoot (Dactylis glomerata), which has a large biogeographical distribution in Europe.
Methods
Twelve populations of cocksfoot originating from Mediterranean, Temperate and Northern European areas were grown in a controlled environment in pots. Dehydration tolerance, leaf and stem anatomical traits and xylem pressure associated with 88 or 50 % loss of xylem conductance (P88, P50) were measured.
Key Results
Across the 12 populations of cocksfoot, P50 ranged from –3.06 to – 6.36 MPa, while P88 ranged from –5.06 to –11.6 MPa. This large intraspecific variability of embolism thresholds corresponded with the biogeographical distribution and some key traits of the populations. In particular, P88 was correlated with dehydration tolerance (r = –0.79). The dehydration-sensitive Temperate populations exhibited the highest P88 (–6.1 MPa). The most dehydration-tolerant Mediterranean populations had the greatest leaf dry matter content and leaf fracture toughness, and the lowest P88 (–10.4 MPa). The Northern populations displayed intermediate trait values, potentially attributable to frost resistance. The thickness of metaxylem vessel walls in stems was highly correlated with P50 (r = –0.92), but no trade-off with stem lignification was observed. The relevance of the linkage between hydraulic and stomatal traits is discussed for drought survival in perennial grasses.
Conclusions
Compared with woody species, the large intraspecific variability in dehydration tolerance and embolism resistance within cocksfoot has consequences for its sensitivity to climate change. To better understand adaptive strategies of herbaceous species to increasing drought and frost requires further exploration of the role of hydraulic and mechanical traits using a larger inter- and intraspecific range of species.
Keywords: Cavitation resistance, Dactylis glomerata L, dehydration tolerance, drought survival, embolism threshold, plant mortality, leaf fracture toughness, vessel wall thickness, stem anatomy, intraspecific variability, perennial grass
INTRODUCTION
Higher temperatures due to climate change have led to more frequent extreme climate events around the world (Orlowsky and Seneviratne, 2012; IPCC, 2014; Seneviratne et al., 2014). Extreme events, including intense droughts and heat waves, will become important drivers of future ecosystem dynamics and function (Smith, 2011). Resilience, defined as the capacity of a population or community to recover following disturbance, may therefore be the key to the persistence of ecosystems (Hodgson et al., 2015; Nimmo et al., 2015).
Forests and grasslands are major terrestrial ecosystems, covering a quarter and a third of the total land area, respectively. They provide many important ecosystem services including timber and forage supply, carbon storage and biodiversity preservation, but their persistence is threatened under more frequent extreme climate events (Pilgrim et al., 2010; Anderegg et al., 2013). Climate-induced forest dieback is an increasing global concern (Allen et al., 2010), and major research efforts have been carried out to identify strategies for drought survival among woody plant species (Pivovaroff et al., 2015). In parallel, declines in grassland productivity (Brookshire and Weaver, 2015) and long-term degradation from drought may gradually become more common in the future (Ciais et al., 2005). Drought reduces plant productivity and induces plant mortality in grasslands under extreme events (Van Peer et al., 2001; Hodgkinson and Muller, 2005; Griffin and Hoffmann, 2012; Poirier et al., 2012; Moran et al., 2014). Although the ability of woody species to survive intense drought was strongly related to their embolism resistance, i.e. the vulnerability to embolism (McDowell et al., 2008), the traits and processes involved in the survival and recovery of herbaceous species after extreme stress are still poorly characterized (Craine et al., 2013; Hoover et al., 2014).
Recent methodological developments have enabled interspecific study of the vulnerability to embolism in stems of herbaceous plants (Lens et al., 2013; Tixier et al., 2013), opening the door for explorations of the role of hydraulic traits in stress survival of non-woody species. Building on these recent results showing large interspecific variability in embolism resistance in grasses distributed along an aridity gradient (Lens et al., 2016), the present study aimed to explore the extent of intraspecific variability in the perennial grass Dactylis glomerata L. This model species was chosen since it has a large biogeographical distribution over Europe and Asia (Borrill, 1991) and a high genetic variation within both populations and geographical origins (Lumaret, 1988; Xie et al., 2010).
Intraspecific variation with respect to embolism resistance is still poorly understood (Martinez-Vilalta et al., 2009). In woody trees, phenotypic plasticity in resistance to embolism was found to be low and buffered against environmental variation in a large range of Pinus pinaster (Lamy et al., 2011, 2014) but was not different across populations of Fagus sylvatica (Herbette et al., 2010; Wortemann et al., 2011); however, not a single study has assessed the intraspecific variation within a herbaceous grass species. Perennial species exhibit various functional strategies to cope with moderate to severe drought (Volaire, 2008; Pérez-Ramos et al., 2013; Zwicke et al., 2015), since they undergo many successive periods of stress and recovery during their life cycle. Under severe drought, the basal aerial meristems and roots are the key organs for plant survival and recovery, while most leaves of perennial grasses senesce to reduce water loss (Volaire et al., 2009). As a result, no significant correlation was found between vulnerability of leaf hydraulic conductance and whole-plant survival of drought in grasses (Ocheltree et al., 2016). The role of hydraulic traits in herbaceous species should therefore be analysed through a whole-plant perspective.
Survival in increasingly drought-prone environments depends on a range of traits which include sufficient mechanical strengthening to avoid irreversible effects of tissue dehydration. Embolism-resistant tree species are often characterized by a high wood density (Hacke et al., 2001; Lens et al., 2013; Ogasa et al., 2013) although no strong evidence was found that denser woods conferred higher survival, or that the risk of embolism caused by wide conduits increased mortality (Russo et al., 2010). In addition, increased tolerance to hydraulic dysfunction implies increased carbon costs for leaf construction and water use (Nardini et al., 2012). Low embolism threshold values were associated with narrower and denser vein conduits, increased thickness of the conduit wall, increased vein density and with reduced leaf area (Nardini et al., 2012). In herbaceous species too, arabidopsis plants with increased wood formation in their stems were shown to be significantly more embolism resistant (Lens et al., 2013; Tixier et al., 2013), and grass species from the most arid environments had more lignified stems compared with the vulnerable species native to wetter habitats (Lens et al., 2016).
The main objective of this study is to identify relationships between embolism resistance, whole-plant dehydration survival, leaf traits and stem anatomy amongst 12 populations of Dactylis glomerata L. originating from Scandinavia to Morocco. Given the contrasting biogeographic origins of the studied populations, we expected that they differ strongly in hydraulic and anatomical traits, which would subsequently contribute to their dehydration tolerance. To determine ecological responses of grassland species under climate extremes (Smith, 2011a), this study therefore aims to (1) identify the role of embolism thresholds and associated traits for drought survival and mechanical resistance in a perennial herbaceous species; and (2) determine the intraspecific variability of these traits as indicators of potential drought survival.
MATERIALS AND METHODS
Plant material
Twelve populations of Dactylis glomerata L. (cocksfoot), including native populations and cultivars, were selected from germplasm banks to be representative from three climatic regions: four Mediterranean, four Temperate and four Northern populations (Table 1). Cocksfoot is a tetraploid (2n = 4x = 28) species from the genus Dactylis whose taxonomic constitution is debated, but broadly consists of a complex of diploids and tetraploids (Borrill, 1991). The 12 populations tested were all verified to be tetraploid (P. Barre, pers. comm).
Table 1.
List of the 12 populations of Dactylis glomerata
| Population | Origin | Country | Latitude | Longitude | Temperature (°C) | Rainfall mean (mm) | Aridity index | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Annual mean | Min of the coldest month | Max of the warmest month | Annual | Wettest quarter | Driest quarter | ||||||
| Maroc 1(ma1) | Mediterranean | Morocco | 33.10 | –8.08 | 18.0 | 7.0 | 30.2 | 346 | 7 | 4 | 0.27 |
| Maroc 9 (ma9) | Mediterranean | Morocco | 29.71 | –8.96 | 16.2 | 1.9 | 31.1 | 246 | 13 | 5 | 0.17 |
| Kasbah (kas) | Mediterranean | Morocco | 32.35 | –6.16 | 11.7 | 3.9 | 39.6 | 270 | 215 | 17 | 0.31 |
| Sicily (sic) | Mediterranean | Italy | 37.48 | 14.50 | 15.4 | 4.0 | 27.6 | 572 | 237 | 40 | 0.47 |
| Carnac (car) | Temperate | France | 47.58 | –3.07 | 11.7 | 3.5 | 21.6 | 893 | 179 | 153 | 0.86 |
| Fourchette (fou) | Temperate | France | 48.17 | –2.75 | 10.8 | 2.3 | 20.8 | 857 | 174 | 153 | 0.74 |
| St Michel (stm) | Temperate | France | 48.63 | –1.51 | 11.5 | 2.6 | 22.3 | 731 | 169 | 149 | 0.78 |
| Ludac (lud) | Temperate | France | 46.58 | 0.33 | 11.5 | 9 | 25.0 | 724 | 152 | 152 | 0.90 |
| Danemark (dan) | Northern | Denmark | 56.46 | 8.66 | 7.8 | -3.2 | 19.4 | 809 | 201 | 134 | 1.48 |
| Sweden (swe) | Northern | Sweden | 60.50 | 24.22 | 4.8 | -9.8 | 21.4 | 613 | 199 | 93 | 1.10 |
| Loke (lok) | Northern | Sweden | 62.98 | 14.80 | 2.6 | -12.6 | 18.9 | 563 | 213 | 87 | 1.14 |
| Tammisto (tam) | Northern | Finland | 60.23 | 23.75 | 4.9 | -9.3 | 21.3 | 609 | 196 | 92 | 1.08 |
Climatic data associated either with their origin sites of collection or sites of breeding (for cultivars in Temperate and Northern areas) from the WorldClim data set (http://www.worldclim.org; Hijmans et al., 2005). Seeds from Northern populations were provided by the Nordic Genetic Resource Center (NordGen). Temperate populations were provided by the plant genetic resources information system of the National Institute for Agronomical Research (INRA, Lusignan, France). The Sicilian ecotype came from University of Catania, Italy. The Moroccan ecotypes came from INRA Rabat, Morocco.
Experimental sites and design
Three pot experiments were carried out to determine dehydration tolerance, embolism resistance as well as anatomy and leaf traits, at the CEFE-CNRS research centre in Montpellier, France (43°6’N, 3°8’E). Seeds from each D. glomerata population were sown in seedling trays in February 2013 and the adult plants (>1 year old) were used for all subsequent experiment.
Experiment 1 was carried out to determine plant dehydration tolerance measured as the plant survival rate in plants growing in short pots, after rewatering following an intense drought (Volaire, 2018). Plants were transplanted into 4 L, 18 cm diameter pots in May 2013. As roots were equally limited in depth for all the populations in pots, plant drought survival mirrored a plant’s dehydration tolerance under a similar soil water availability (Volaire, 2008). It allowed us to discount the effect of rooting depth associated mainly with dehydration avoidance (Volaire and Lelievre, 2001; Pérez-Ramos et al., 2013). For this experiment, the quantity of dry substrate was measured to be identical for each of the 4 L pots so that the time dynamics of soil moisture could be accurately assessed and compared simply by pot weight during the period of intense water deficit in summer 2014.
Experiment 2 was carried out to measure the embolism threshold and stem anatomy. Individuals started from tillers taken from the adult plants sown in 2013 were transplanted in September 2014 into 10 L (24 cm diameter) pots in order to obtain reproductive tillers from spring 2015.
Experiment 3 was carried out to measure biomechanical and leaf traits. Individuals taken from the initial adult plants were transplanted into new 4 L pots in September 2015.
Each 4 and 10 L pot contained four clones from a single population, to ensure soil cover close to field conditions, with ten and four replicates for 4 and 10 L pots, respectively, per population. Pots were filled with a substrate composed of 65 % local loamy-clay soil and 35 % compost.
For all experiments, the plants were grown under an open-ended hoop-house and they were kept fully irrigated and fertilized (20 kg ha–1 of P and K at establishment and every autumn, then 40 kg ha–1 nitrogen after each cut) except during the imposition of the severe drought in Experiment 1 during summer 2014. Pesticides were applied to prevent the development of diseases caused by insects (aphids) or fungi (rust). For all experiments, pots were distributed on a bench according to a full randomization design. In addition, the position of pots was modified multiple times during the experiment to minimize placement effects.
In addition, plants of one of the Mediterranean populations (Kasbah), that was grown in a nearby field in a 1.30 m deep loamy soil since 2011 (Barkaoui et al., 2016) and without irrigation or fertilization for 2 years, were used for stem sampling in order to assess the plasticity of embolism resistance in one population. Twenty stems of this field-grown ‘Kasbah’ population were collected in spring 2015 and processed for the measurement of stem embolism (see below).No other measurement was carried out on these plants.
Mean temperatures were 9.5 °C in winter, 16.6 °C in spring and 24.2 °C in summer, with 63 % mean air relative humidity and mean radiation of 800 µmol m–2 s–1 from spring to summer.
Measurements
Dehydration tolerance under severe summer drought (Experiment 1).
Plants of Experiment 1 were kept fully irrigated and fertilized until June 2014 when the severe summer drought started to be imposed, as follows. In June 2014, the soil water content was raised to field capacity (60 % soil water content for the substrate used) and irrigation was withheld thereafter. Pots were weighed at frequent intervals (every 2–5 d) throughout the experiment to monitor the decrease in soil water content. Once full leaf senescence was reached for all populations, and when soil water content in the pots decreased to near 12 % (27–35 d after withholding irrigation), plants were harvested at 3 cm from the soil surface and the pot was put back under full irrigation (field capacity), thus ending the severe drought. All pots were re-watered after they reached the same soil water content to ensure that plant survival corresponded to an identical soil final dehydration for all populations irrespective of their growth potential. The plants were irrigated for 15 d, after which dehydration survival was measured. Plants that did not produce new shoots after 15 d were counted as dead. The survival rate was measured as the number of living plants divided by the total number of plants (plant survival; SURV %).
Embolism resistance (Experiment 2).
In late spring 2015, three pots for each population were maintained under irrigated conditions and allowed to flower. Stems were then collected and processed using the static centrifuge technique (Alder et al., 1997). A negative pressure was applied to stem pieces in a standard centrifuge with a custom-built, 26 cm rotor. Each stem segment was only spun once in the centrifuge. After centrifugation, a 2 cm long segment containing a node was excised under water from the middle of the stem segment. The segment was connected to the XYL’EM apparatus (Bronkhorst, Montigny-les-Cormeilles, France) to measure its hydraulic conductance using a solution of 10 mm KCl and 1 mm CaCl2 in deionized ultrapure water. The sample was then resaturated with pressurized water to determine the percentage loss of conductance caused by embolized conduits. For each pressure point in the vulnerability curve (VC), one to seven grass stems were used with 14–36 pieces per curve (minimum–maximum), and one S-shaped curve per population was fitted (Supplementary DataFig. S1) according to a sigmoid function (Pammenter and Vander Willigen, 1998). Embolism resistance was expressed by the 50 or 88 % percentage loss of hydraulic conductance (P50, P88). P50 is the metric most used to compare hydraulic thresholds between species and populations, while P88 was shown to be the embolism threshold leading to irreversible drought damage in angiosperm trees (Urli et al., 2013)
Stem anatomy.
To evaluate which stem characters contribute to the variation in embolism resistance observed among the D. glomerata populations, light microscopy observations were performed on cross-sections of stems – previously used for the measurement of embolism resistance – at the level of internodes (from three stems per population). After vulnerability curve determination, samples were frozen until processed for anatomy. Samples were defrosted in water, stored in 50 % ethanol and then were embedded with LR White (hard grade, London Resin, UK) following Hamann et al. (2011), and sectioned with a rotary microtome (Leica RM 2265) equipped with disposable blades. Transverse sections of 4 µm were made, heat-attached to the slides, stained with toluidine blue and mounted in Entellan®. The slides were observed with a Leica DM2500 light microscope equipped with a Leica DFC-425C digital camera (Leica Microscopes, Wetzlar, Germany). A range of stem anatomical characters (Lens et al., 2016), such as total stem area (AS), area of lignified outer stem tissue (ALIG), surface area of the fibre wall (AFW) and fibre lumen (AFL), thickness of the metaxylem vessel wall (TVW), hydraulically weighted diameter of vessels (DHV) and traits derived from these measurements, such as the proportion of lignified tissue per total stem area (PLIG), the proportion of cell wall per fibre (PCWF), the proportion of total fibre wall area in the lignified area (PFWF × ALIG) and the proportion of total fibre wall in the lignified area per stem area [(PFWF × ALIG):AS]. Observations were made based on three individuals per population and at least 30 measurements per feature using ImageJ v 1.43 (National Institutes of Health, Bethesda, MD, USA).
The diameter of the vessels (DV) was calculated based on the lumen area that was considered to be a circle following the equation:
| (1) |
where D is the vessel diameter and A is the area of the vessel. The hydraulically weighted vessel diameter (DHv; weights diameters of vessels according to their hydraulic conductance) was calculated following the equation (Sperry et al., 1994):
| (2) |
where D is the vessel diameter as measured in eqn (1).
Leaf traits under full optimum conditions (Experiment 3).
In grasses, leaf tissue density (dry mass per volume) can be assessed both by leaf dry matter content (Garnier and Laurent, 1994; Garnier et al., 2001) and by the resistance of the leaf to mechanical fracture, that can be measured in a variety of ways (Aranwela et al., 1999; Ang et al., 2008; Onoda et al., 2011). For Experiment 3, in early spring of 2016, at the time before the reproductive stage, one fully extended leaf was collected from one plant per pot (four replicates). Fracture toughness was measured with a portable mechanical measuring device (Instron® Inspec 2200) adapted for cutting with a single razor cut (Ang et al., 2008). Leaf blades were placed on the cutting platform across a gap 2 mm wide, permitting the passage of the diagonally angled blade in the middle of each leaf blade (single-edged blade, ‘Personna GEM, 0.23 mm thickness, Cat.# 71962). Leaf toughness, the energy required to shear the leaf cross-section in J m–2, was calculated after subtracting initial elastic loading and final elastic unloading, as well as friction energy from the force–distance curves. As in Ang et al. (2008), the initial cut was followed by a second pass that measured just the friction force generated by the blade against the cut surfaces of the leaf. Leaves were cut with a razor angle of 20° and at a cross-head speed of 0.1 mm s–1. Leaf thickness and width were measured with a binocular at the central vein of the leaf and at the widest part of the leaf, respectively. The leaf was then immediately placed into a tube with the cut end submerged in deionized water and stored in the dark at 4 °C for 24 h. The fresh and dry leaf biomass (g) were weighed to obtain leaf dry matter content [LDMC; the oven-dry mass (mg) of a leaf divided by its water-saturated fresh mass (g), expressed in mg g–1] following Perez-Harguindeguy et al. (2013). This trait is used as a proxy for leaf density which is a trait more difficult to measure (Shipley and Vu, 2002).
Statistical analysis
All statistical analyses were performed with Rstudio (version 0.98.501). The effect of biogeographical origin was tested with anova (‘lmtest’ package) followed with Tukey’s post-hoc tests. A principal component analysis (PCA; ‘ade4’ package) was performed on the 12 variables showing significant differences between origins, scaled and centred, to visualize their covariations. The four bioclimatic variables (www.worldclim.org) chosen to characterize the drought and frost occurrence in the sites of origins of the populations [annual precipitations, precipitations of the driest quarter, aridity index (Trabucco et al., 2008) and minimum temperature of the coldest months] were used as supplementary variables in the PCA. Pairwise correlations between all measured traits for each season were evaluated using Pearson’s method (‘Hmisc’ package). We tested the relationships between key variables using simple linear regressions.
RESULTS
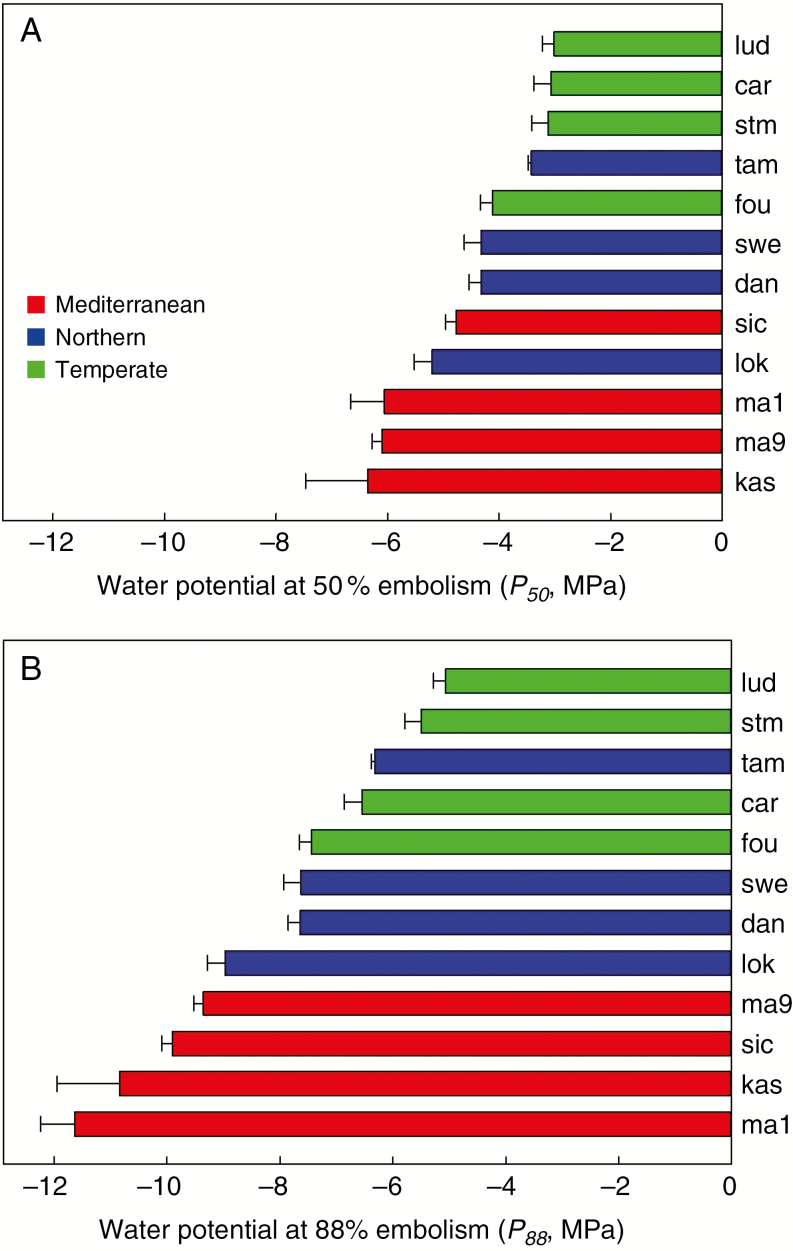
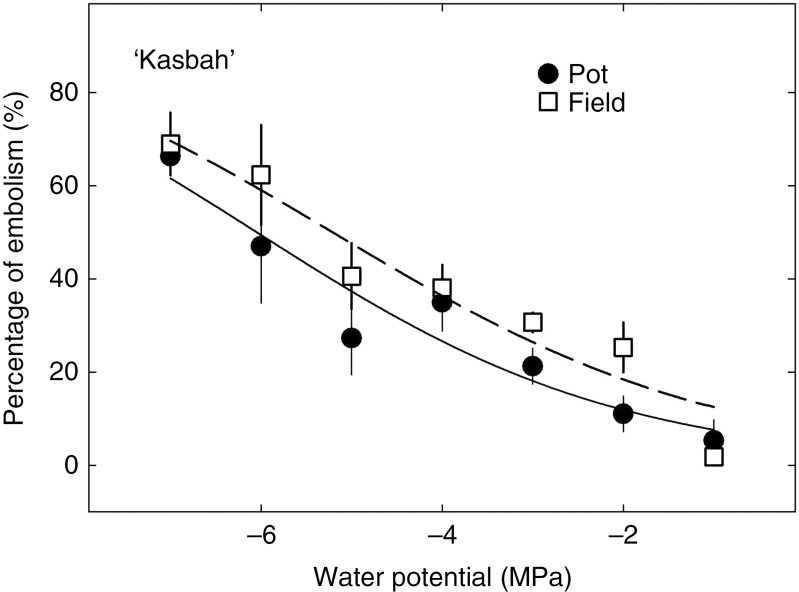
Across the 12 populations of D. glomerata studied, P50 ranged from –3.06 to – 6.36 MPa (Fig. 1A), while P88 ranged from –5.06 to –11.6 MPa (Fig. 1B). The Mediterranean populations had significantly lower P50 and P88 (–5.8 and –10.4 MPa, respectively) than the Temperate populations (–3.3 and –6.1 MPa, respectively), while the Northern populations showed intermediate embolism thresholds (Table 2). In addition, P50 of the Mediterranean population Kasbah significantly differed according to the growing environment (Fig. 2), either in pots (P50 = –6.05 ± 0.31) or in the field (P50 = –5.21 ± 0.29).
Fig. 1.
Thresholds of embolism resistance in cocksfoot. Means and standard errors of water potentials at 50 % embolism (A, P50) and at 88% embolism (B, P88) in stems of 12 populations of Dactylis glomerata of contrasting origins.
Table 2.
Results of variance analysis and Tukey test between means of variables for the three origins of Dactylis glomerata populations
| Biogeographic origin of D. glomerata populations | ||||
|---|---|---|---|---|
| P-value | Mediterranean | Northern | Temperate | |
| SURV, plant dehydration survival (%) | <0.001*** | 58.2a | 13.1b | 6.9b |
| P 50, embolism pressure threshold for 50 % embolism (MPa) | 0.0016** | –5.8a | –4.3ab | –3.3b |
| P 88, embolism pressure threshold for 88 % embolism (MPa) | <0.001*** | –10.4a | –7.6ab | –6.1b |
| Lwidth, leaf width (mm) | <0.001*** | 4.28a | 7.97c | 6.22b |
| Lthick, leaf thickness (mm) | 0.13n.s. | 0.30 | 0.36 | 0.35 |
| LDMC, leaf dry matter content (mg g DM–1) | <0.001*** | 232.72a | 185.75b | 188.41b |
| LFrac, leaf fracture resistance (J m–2) | <0.001*** | 478.6a | 188.47b | 225.55b |
| A S, total stem area (mm2) | 0.006** | 2.07a | 3.09b | 2.9b |
| A LIG, lignified area (μm2) | 0.02* | 0.27a | 0.38b | 0.33ab |
| P LIG, proportion of lignified area per total stem area | 0.02* | 0.1308b | 0.1258ab | 0.115a |
| A F, fibre cell area (μm2) | 0.34n.s. | 70.61 | 77.21 | 77.56 |
| A FL, fibre lumen area (μm2) | 0.50n.s. | 15.95 | 14.16 | 14.76 |
| A FW, fibre wall area (μm2) | 0.12n.s. | 54.66 | 63.04 | 62.79 |
| P CW F, proportion of cell wall per fibre | 0.029* | 0.77 a | 0.82b | 0.81ab |
| P FW F × ALIG, proportion of total fibre wall area in lignified area | 0.037* | 0.30 ab | 0.33b | 0.29a |
| (PFWF × ALIG):AS, proportion of total fibre wall in lignified area per stem area | 0.002** | 0.156a | 0.117b | 0.102b |
| D HV, hydraulically weighted diameter of vessels (μm) | 0.17n.s. | 23.04 | 21.77 | 21.18 |
| T VW, thickness of metaxylem vessel wall (μm) | <0.001*** | 1.426c | 1.226b | 1.109a |
***P < 0.001; **P < 0.01; *P < 0.05; n.s., non-significant. For each variable, different superscript letters indicate significant differences between origins.
Fig. 2.
Embolism resistance at increasing water potentials in stems of the Mediterranean Dactylis glomerata population ‘Kasbah’ growing either in the pot or in the field. Mean water potentials at 50 % embolism (P50) are –6.1 and –5.2 MPa for the population growing in the pot and in the field, respectively.
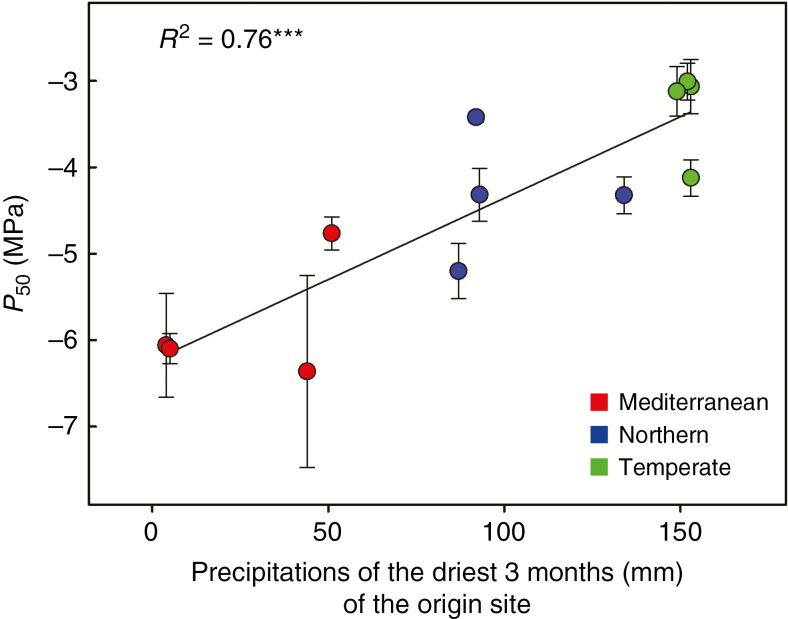
Both P50 and P88 measured on potted irrigated plants were highly and more correlated (r = 0.88 and 0.89, P < 0.001; Table 3) with the mean precipitation of the driest quarter of the year (Fig. 3) than with the annual precipitation of the origin site. Both embolism thresholds were, however, less strongly correlated with the aridity index of the site of origin (r = 0.60, P = 0.039). The mean aridity index was 1.20 for Northern populations (less arid) compared with 0.82 (more arid) for Temperate populations despite the greater rainfall in Temperate areas. In addition, no correlation was detected between cavitation resistance and minimum temperatures of the coldest months of the sites studied.
Table 3.
Pearson coefficients of correlations (lower part of the table) and P-values (upper part of the table) between traits measured on 12 populations of Dactylis glomerata
| P 88 | P 50 | SURV | Lwidth | Lthick | LDMC | LFrac | A S | A LIG | P LIG | A F | A FL | A FW | P CW F | P FW F × ALIG | (PFWF × ALIG):As |
D HV | T VW | P SUM | P YEAR | T MIN | Aridity | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P 88 | 1 | 0.00 | 0.00 | 0.10 | 0.19 | 0.00 | 0.00 | 0.13 | 0.18 | 0.89 | 0.45 | 0.67 | 0.38 | 0.37 | 0.88 | 0.14 | 0.09 | 0.00 | 0.00 | 0.00 | 0.76 | 0.04 |
| P 50 | 0.94 | 1 | 0.02 | 0.21 | 0.25 | 0.02 | 0.03 | 0.24 | 0.45 | 0.58 | 0.51 | 0.47 | 0.38 | 0.27 | 0.73 | 0.18 | 0.33 | 0.00 | 0.00 | 0.00 | 0.92 | 0.04 |
| SURV | –0.79 | –0.65 | 1 | 0.01 | 0.02 | 0.00 | 0.00 | 0.02 | 0.03 | 0.85 | 0.09 | 0.73 | 0.06 | 0.13 | 0.80 | 0.04 | 0.07 | 0.11 | 0.00 | 0.01 | 0.25 | 0.01 |
| Lwidth | 0.50 | 0.39 | –0.71 | 1 | 0.01 | 0.00 | 0.00 | 0.00 | 0.02 | 0.40 | 0.14 | 0.87 | 0.16 | 0.35 | 0.15 | 0.09 | 0.71 | 0.36 | 0.10 | 0.21 | 0.00 | 0.00 |
| Lthick | 0.40 | 0.36 | –0.66 | 0.72 | 1 | 0.02 | 0.02 | 0.20 | 0.42 | 0.53 | 0.08 | 0.48 | 0.04 | 0.07 | 0.30 | 0.73 | 0.81 | 0.78 | 0.06 | 0.12 | 0.03 | 0.01 |
| LDMC | –0.82 | –0.68 | 0.87 | –0.85 | –0.65 | 1 | 0.00 | 0.01 | 0.07 | 0.50 | 0.23 | 0.66 | 0.18 | 0.22 | 0.64 | 0.07 | 0.13 | 0.07 | 0.00 | 0.04 | 0.09 | 0.01 |
| LFrac | –0.78 | –0.61 | 0.92 | –0.84 | –0.68 | 0.97 | 1 | 0.02 | 0.03 | 0.81 | 0.21 | 0.62 | 0.16 | 0.18 | 0.62 | 0.08 | 0.10 | 0.11 | 0.01 | 0.05 | 0.08 | 0.00 |
| A S | 0.46 | 0.37 | –0.66 | 0.83 | 0.40 | –0.70 | –0.67 | 1 | 0.00 | 0.24 | 0.15 | 0.22 | 0.30 | 0.99 | 0.14 | 0.00 | 0.90 | 0.30 | 0.11 | 0.19 | 0.08 | 0.13 |
| A LIG | 0.41 | 0.24 | –0.61 | 0.65 | 0.26 | –0.54 | –0.62 | 0.78 | 1 | 0.36 | 0.24 | 0.24 | 0.42 | 0.88 | 0.06 | 0.08 | 0.71 | 0.34 | 0.30 | 0.49 | 0.17 | 0.13 |
| P LIG | –0.04 | –0.18 | 0.06 | –0.27 | –0.20 | 0.22 | 0.08 | –0.37 | 0.29 | 1 | 0.81 | 0.80 | 0.87 | 0.96 | 0.67 | 0.18 | 0.37 | 0.92 | 0.53 | 0.42 | 0.52 | 0.99 |
| A F | 0.24 | 0.21 | –0.52 | 0.45 | 0.53 | –0.37 | –0.39 | 0.44 | 0.37 | –0.08 | 1 | 0.66 | 0.00 | 0.06 | 0.09 | 0.99 | 0.87 | 0.96 | 0.19 | 0.23 | 0.41 | 0.32 |
| A FL | –0.14 | –0.23 | 0.11 | 0.05 | –0.22 | 0.14 | 0.16 | 0.38 | 0.37 | –0.08 | 0.14 | 1 | 0.64 | 0.01 | 0.05 | 0.76 | 0.21 | 0.64 | 0.26 | 0.17 | 0.92 | 0.10 |
| A FW | 0.28 | 0.28 | –0.55 | 0.43 | 0.59 | –0.41 | –0.44 | 0.33 | 0.26 | –0.05 | 0.96 | –0.15 | 1 | 0.00 | 0.28 | 0.92 | 0.60 | 0.93 | 0.09 | 0.10 | 0.43 | 0.13 |
| P CWF | 0.28 | 0.35 | –0.46 | 0.29 | 0.53 | –0.38 | –0.41 | 0.00 | –0.05 | –0.02 | 0.56 | –0.73 | 0.77 | 1 | 0.60 | 0.90 | 0.21 | 0.65 | 0.06 | 0.03 | 0.49 | 0.02 |
| P FW F × ALIG | –0.05 | –0.11 | –0.08 | 0.44 | 0.33 | –0.15 | –0.16 | 0.45 | 0.56 | 0.14 | 0.52 | 0.59 | 0.34 | –0.17 | 1 | 0.60 | 0.11 | 0.68 | 0.69 | 0.53 | 0.20 | 0.78 |
| (PFWF × ALIG):As | –0.45 | –0.42 | 0.59 | –0.50 | –0.11 | 0.53 | 0.53 | –0.76 | –0.52 | 0.41 | 0.00 | –0.10 | 0.03 | 0.04 | 0.17 | 1 | 0.59 | 0.16 | 0.08 | 0.08 | 0.47 | 0.24 |
| D HV | –0.51 | –0.31 | 0.53 | –0.12 | –0.08 | 0.46 | 0.50 | 0.04 | –0.12 | –0.28 | –0.05 | 0.39 | –0.17 | –0.39 | 0.49 | 0.17 | 1 | 0.44 | 0.19 | 0.37 | 0.97 | 0.36 |
| T VW | –0.85 | –0.92 | 0.48 | –0.29 | –0.09 | 0.53 | 0.49 | –0.33 | –0.30 | 0.03 | 0.02 | 0.15 | –0.03 | –0.14 | 0.13 | 0.44 | 0.25 | 1 | 0.01 | 0.01 | 0.96 | 0.09 |
| P SUM | 0.88 | 0.89 | –0.84 | 0.50 | 0.55 | –0.77 | –0.74 | 0.48 | 0.32 | –0.20 | 0.40 | –0.35 | 0.51 | 0.56 | –0.13 | –0.53 | –0.40 | –0.71 | 1 | 0.00 | 0.83 | 0.01 |
| P YEAR | 0.75 | 0.86 | –0.71 | 0.39 | 0.48 | –0.61 | –0.57 | 0.40 | 0.22 | –0.26 | 0.38 | –0.42 | 0.50 | 0.61 | –0.20 | –0.52 | –0.29 | –0.70 | 0.94 | 1 | 0.83 | 0.01 |
| T MIN | 0.10 | –0.03 | 0.36 | –0.82 | –0.62 | 0.51 | 0.53 | –0.53 | –0.42 | 0.21 | –0.26 | –0.03 | –0.25 | –0.22 | –0.40 | 0.23 | 0.01 | –0.02 | –0.07 | –0.07 | 1 | 0.03 |
| Aridity | 0.60 | 0.60 | –0.71 | 0.76 | 0.73 | –0.74 | –0.77 | 0.47 | 0.46 | 0.01 | 0.32 | –0.49 | 0.46 | 0.65 | 0.09 | –0.37 | –0.29 | –0.51 | 0.70 | 0.70 | –0.62 | 1 |
Climatic variables of the origin sites (or breeding sites) of the populations: PDRY. mean precipitations of the driest three 3 months; PYEAR. mean annual precipitations; TMIN. mean minimum temperatures of the coldest months; aridity, aridity index.
P < 0.05 (italics. bold). P < 0.01 (bold). P < 0.001 (bold underlined).
Fig. 3.
Embolism resistance and aridity of the site of origin in cocksfoot. Linear relationships between mean precipitation of the driest quarter at the site of origin and water potential at 50 % embolism (P50) in 12 populations of Dactylis glomerata of contrasting origins. Bars correspond to standard errors.
Plant dehydration survival in pots was significantly greater (Table 2) for the Mediterranean populations (58 %) than for the group of Northern and Temperate populations (7–13 %).
Although leaf thickness did not discriminate the populations according to their origin, leaf width almost doubled with increasing latitude, from on average 4.3 mm in Mediterranean populations to 8 mm in Northern populations. Mediterranean populations also had a higher LDMC (20 % greater) and a very significantly greater leaf fracture toughness (2.5-fold greater) than the Northern and Temperate populations.
The Mediterranean populations were distinguished from the others by having thinner stems with a higher fibre wall proportion in lignified area per stem (Table 2). The traits for Northern and Temperate populations were mostly not significantly different although the proportion of total fibre wall area in lignified area was greater in Northern populations. The thickness of metaxylem vessel wall highly discriminated all origins and spanned 1.4, 1.2 and 1.1 μm in Mediterranean Northern and Temperate populations, respectively (Table 2).
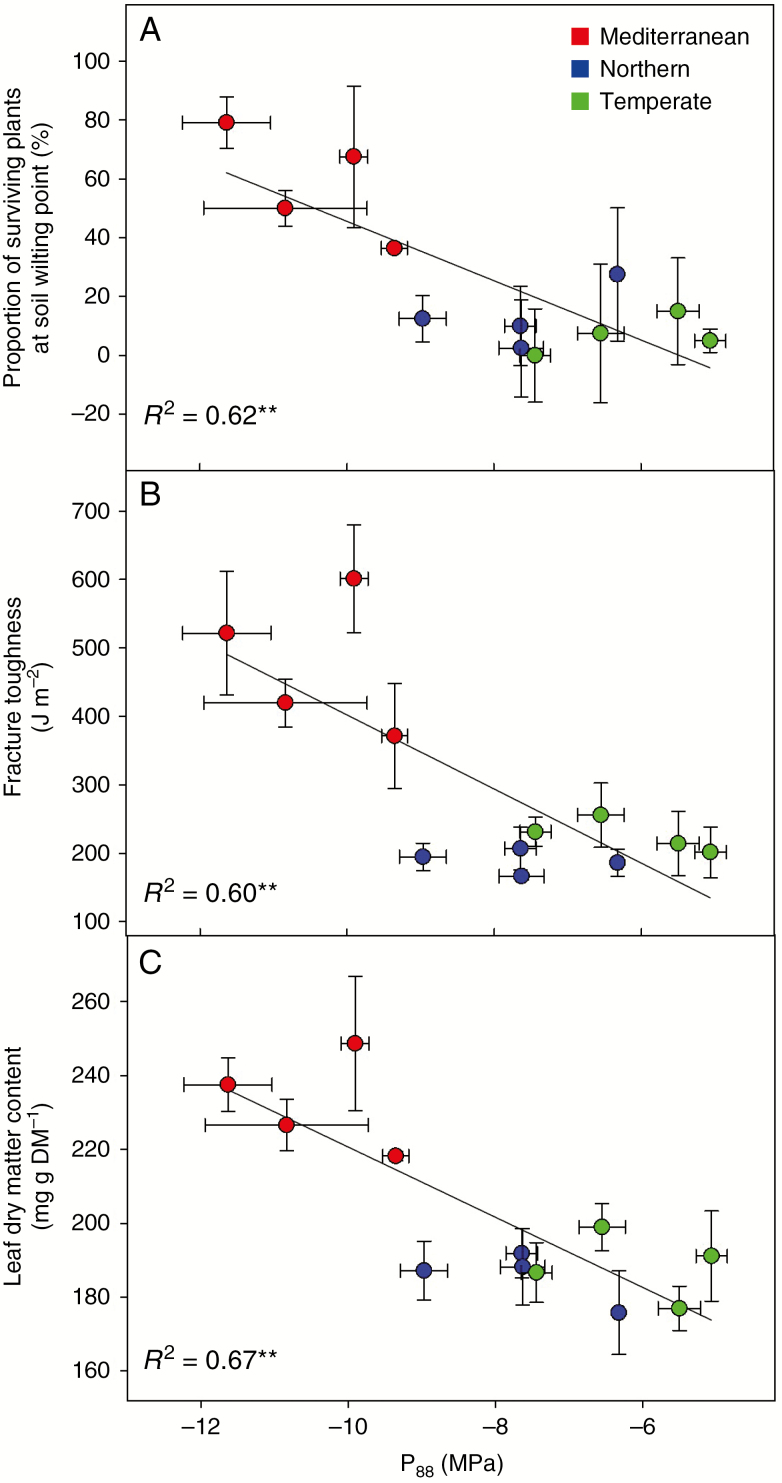
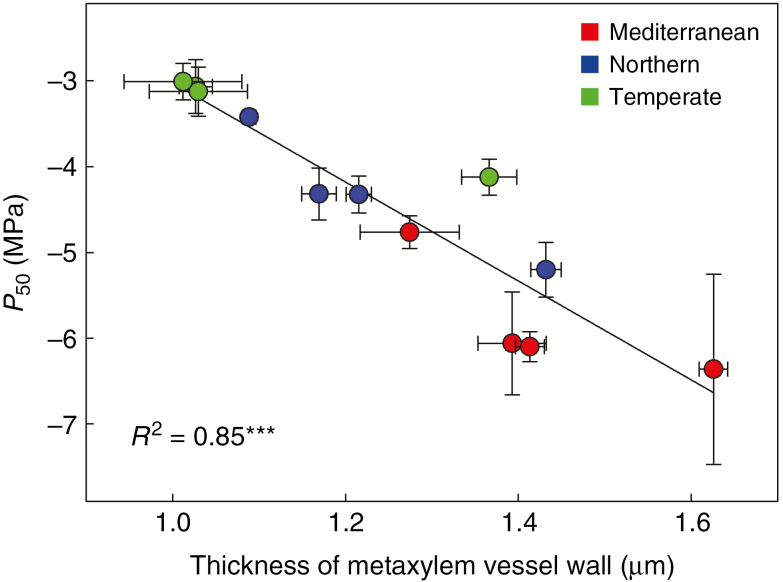
The value of P88 was highly and better correlated to dehydration survival (r = –0.79; Table 3; Fig. 4A) and leaf traits such as fracture toughness (r = –0.78; Table 3; Fig. 4B) and LDMC (r = –0.82; Table 3; Fig. 3C), than P50. A highly significant correlation was found between both embolism thresholds (Table 3; Figs 5 and 6) and the thickness of metaxylem vessel wall in stems (TVW, r = –0.92 for P50). Notably, no other stem anatomical trait was correlated with either embolism threshold. In contrast, plant dehydration survival was correlated to the total stem area (AS, r = –0.66) and therefore with the area of lignified outer stem tissue (ALIG, r = –0.61) as well as with the proportion of the fibre wall in the lignified area over the entire stem area [(PFWF × ALIG):AS, r = 0.59).
Fig. 4.
Embolism resistance and (A) dehydration tolerance, (B) leaf fracture toughness and (C) leaf dry matter content in cocksfoot. Linear relationships between water potential at 88% embolism (P88) and (A) the proportion of surviving plants at soil wilting point in short pots (dehydration tolerance), (B) leaf fracture toughness of irrigated plants and (C) leaf dry matter content (LDMC) of irrigated plants in 12 populations of Dactylis glomerata of contrasting origins. Bars correspond to standard errors.
Fig. 5.
Embolism resistance and stem anatomy in cocksfoot. Linear relationships between water potential at 50% embolism (P50) and thickness of metaxylem vessel walls in stems in 12 populations of Dactylis glomerata of contrasting origins. Bars correspond to standard errors.
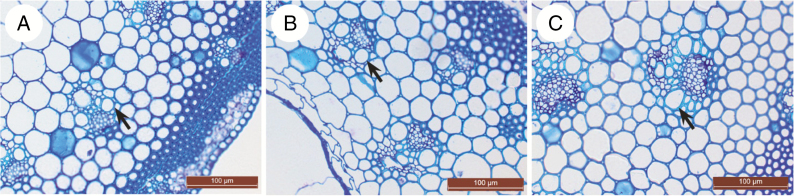
Fig. 6.
Thickness of metaxylem vessel wall in stems of cocksfoot: three populations of Dactylis glomerata of contrasting origins: (A) ‘car’ (Temperate); (B) ‘swe’ (Northern); (C) ‘ma1’ (Mediterranean). The arrows point to the wall of the metaxylem vessels.
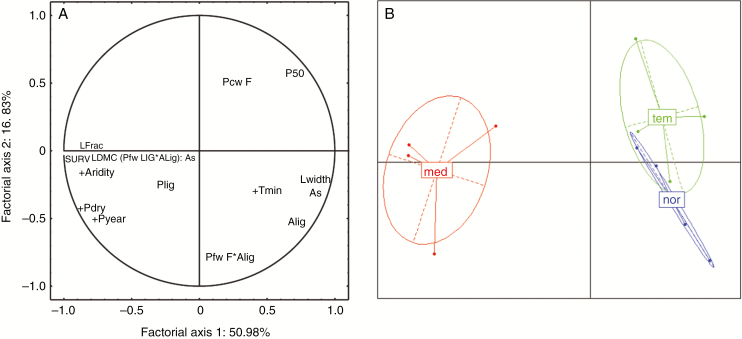
Overall, the covariation between LDMC, leaf fracture toughness, embolism thresholds and dehydration tolerance was very significant (Table 3). Unexpectedly, leaf toughness was not correlated with any trait associated with stem lignification over the entire data set. However, a PCA (Fig. 7A, B) clearly illustrates that high embolism resistance and dehydration survival associated with small and more lignified lamina and lignified stems discriminate the group of Mediterranean populations. In contrast, Temperate and Northern populations were characterized by low dehydration survival and embolism resistance, bigger leaves, and stems with thicker walled fibres, especially for Northern populations.
Fig. 7.
PCA of 12 plant traits and 12 populations of cocksfoot. Principal component analysis (factorial axes 1 and 2) showing (A) the distribution of the 12 traits significantly discriminating origins of populations (only P50 was included since it is highly correlated to P88; see Table 2) and (B) 12 populations of Dactylis glomerata of contrasting origins. Climatic data of the origin or breeding site: annual precipitations (Pyear), precipitations of the driest quarter (Pdry), aridity index (aridity) and minimum temperature of the coldest months (Tmin) were included as supplementary variables (+).
DISCUSSION
This study is the first to explore intraspecific variability of embolism resistance, whole-plant dehydration tolerance and associated traits in a herbaceous grass species. Our results show a huge range of embolism thresholds amongst the 12 D. glomerata populations studied, reflecting its biogeographical distribution and corresponding to some key traits.
Large intraspecific variability in cocksfoot
If we compare the interspecific range for P50 (Lens et al., 2016) observed within 18 Poaceae species native to similar mesic and xeric environments (–3.2 to –7.5 MPa) with the intraspecific variability in D. glomerata found here (–3.06 to –6.36 MPa), the overlap is striking. Only Stipa pennata, a grass native to arid rangelands, exhibited a lower P50 (–7.5 MPa) compared with the Mediterranean populations of D. glomerata. In addition, this intraspecific variation is much greater than that reported for a dicot herbaceous perennial Solidago canadensis sampled from dry to humid sites and showing P50 ranging from –2.37 to –3.08 MPa (Nolf et al., 2014). The intraspecific range in P50 variation within cocksfoot matches with most of the woody species studied in angiosperms (–2 to –4 MPa) and gymnosperms (–4 to –8 MPa), although far from the extreme embolism threshold (–18.8 MPa) found so far in the gymnosperm Callitris tuberculata (Larter et al., 2015). This intraspecific variability is strikingly large in comparison with former results on woody species. For instance, across 17 populations of Fagus sylvatica originating from sites in a large European latitudinal gradient with annual precipitations spanning from 563 to 1362 mm, P50 was relatively constant from –2.8 to –3.2 MPa (Wortemann et al., 2011). No significant differences in embolism resistance (–3.9 to –4.1 MPa) were found either across 24 populations of Pinus pinaster from Northern African and French sites (Lamy et al., 2011). Nevertheless, plasticity in vulnerability to embolism (from –3 to –6 MPa) was shown in the very dry limits of Pinus canariensis under a strong aridity gradient (Lopez et al., 2016). It could be interesting to explore whether the high embolism resistance of D. glomerata from the most arid sites (P50 less than –5 MPa) could be correlated to morphological and anatomical differences in the rooting system compared with woody species, by analogy with shrubs that were found to be more embolism tolerant than trees in the same habitat, probably reflecting differences in rooting depth and drought tolerance (Brendel and Cochard, 2011). The remarkable range in embolism resistance could be added to the list of factors contributing to explain why cocksfoot covers a greater span of biomes that that of most woody species.
Our intraspecific comparison is based on plants grown in similar optimum pot conditions for 2 years, underlining that the large differences within populations are genetically determined. Yet, our results also show a high plasticity of embolism threshold since for the tested Kasbah population, P50 significantly varied between potted and field-grown plants of the same population. Many factors differ, such as plant age at sampling date as well as many biotic and abiotic factors (soil depth, plant density, etc.) between these two growing conditions for the same population. However, it may be hypothesized that a less negative P50 of field-growing plants may be ascribed in particular to a deeper rooting system (130 cm in the field vs. 20 cm in pots) which enhances dehydration avoidance vs. dehydration tolerance and may have impacted hydraulic adaptations (Scholz et al., 2012). It could be interesting to measure embolism resistance in the same populations of cocksfoot all growing in a similar deep soil in order to analyse the impact of the expression of full rooting depth on embolism thresholds. However, it can therefore be assumed that if sampling was done using populations growing in their natural environment, our results would have probably generated stronger genotype × environments interactions, and even further increased the range of intraspecific variability in embolism thresholds. Significant site × population interactions were, for instance, found in beech (Herbette et al., 2010; Wortemann et al., 2011), underlining the high phenotypic plasticity of embolism resistance. Further work is necessary to assess intraspecific P50 and P88 plasticity as a response to environments (e.g. soil depth, soil nutrients and water availability).
Relationships between embolism resistance and environmental limiting factors
Our results show a correlation between the annual and driest quarter precipitations of the sites studied with embolism thresholds measured on plants grown in a common garden under optimum irrigation. As a general pattern, dehydration survival in pots was greater for the populations coming from the driest sites, with P50 and P88 more negative than –4.50 and –9.3 MPa, respectively. Plant mortality under severe drought when the resource is limited (shallow soils) can then be predicted by the hydraulic failure model assuming that a significant loss of conductivity can trigger plant mortality (McDowell et al., 2013). Our results are in line with a meta-analysis of tree species showing that hydraulic traits capture key mechanisms determining tree death (Anderegg et al., 2016). However, embolism resistance was less strongly correlated with the aridity index of the site of origin in contradiction to a former study dealing with a range of Temperate to semi-arid grass species that showed a strong correlation between P50 and aridity (Lens et al., 2016). It would be interesting to test whether this interspecific correlation would hold when including herbaceous species from the highest latitudes. In D. glomerata, Northern populations showed a trend to more negative P50 values than Temperate populations and are more frost tolerant, suggesting that the Northern populations could have also developed greater embolism resistance as a mechanism of frost tolerance (Lens et al., 2013). Both drought and freezing–thawing of stems induce a loss of hydraulic conductivity in woody plants (Feng et al., 2015). Frost ‘drought’ had a large effect on plant water transport, adaptations in hydraulic safety and related anatomical parameters (Mayr et al., 2006), and the propensity for freeze-induced embolism increases as the conduit diameter increases (Zanne et al., 2014). The relationships between recovery from winter embolism and anatomical vessel traits also varied according to functional groups of tree species (Niu et al., 2017). The role of hydraulic dysfunction under frost remains to be explored in herbaceous species.
Tissue lignification and embolism resistance
In pseudostems, partly constituted with leaf sheaths, we found a strong positive correlation between the thickness of the metaxylem vessel wall and embolism resistance, while lignification characters are not correlated with P50 or P88. Despite a significant positive correlation (Lens et al., 2016) between embolism resistance and stem lignification amongst 19 grass species (in terms of the thickness of the fibre walls and the relative proportion of lignified tissue per stem area), this trade-off is lacking within populations of D. glomerata. This lack of correlation is noteworthy given the huge range in P50 variation within cocksfoot. There are slight indications that stem lignification may contribute to differences in embolism resistance. For instance, Fig. 6A and B shows that the proportion of lignified area per stem area clusters with other key traits for the embolism-resistant Mediterranean populations. Furthermore, the two most resistant Mediterranean populations have more lignified fibres inside and surrounding the vascular bundles (Supplementary DataFig. S2) compared with the other cocksfoot populations studied. However, this character is difficult to quantify and seemingly shows random variation amongst the remaining populations. Since we have sectioned internodes, we had to restrict ourselves to measurements of solitary vessels. It is plausible that our observations also reflect a correlation between more negative P50/P88 values and increased intervessel wall thickness compared with vessel diameter (so-called thickness to span ratio) in the case of vessel multiples that occur much more in the nodal parts of the stem. Vessel wall reinforcement was suggested to be required for embolism resistance in order to prevent wall implosion when xylem pressure is very negative (Hacke et al., 2001). However, vessels have never been observed to implode in stems, probably because embolism events occur before the critical vessel implosion threshold is reached. Whatever the exact mechanism(s) operating during xylem embolism dysfunction in D. glomerata, it appears that plants with more fracture-resistant leaves have stems that are more resistant to embolism than plants with less resistant leaves. In addition possibly to reducing the probability of lethal embolism (Hacke and Sperry, 2001), mechanically robust leaf architecture will also play a role in mechanically supporting water-stressed leaves and protecting leaf meristems, as discussed below. This perhaps at least partly explains the tendency towards narrower, more compact, denser, tougher leaves in the Mediterranean ecotypes that are more likely to be exposed to more drought conditions. Of course leaf lamina traits are anatomically and functionally graded into the more proximal leaf sheath (Bell, 1991) which is also important for mechanical robustness and resistance to dehydration of the self-supporting meristems of D. glomerata.
Drought survival in perennial herbaceous species: hydraulic leaf traits may not be crucial
A general tenet of ecophysiology of drought adaptation is based on the assumption of a strong linkage between hydraulic and stomatal traits especially in woody species (Skelton et al., 2015; Bartlett et al., 2016) and also in herbaceous species (Holloway-Phillips and Brodribb, 2011; Nardini et al., 2012; Lens et al., 2016). Regarding the extreme case of drought plant mortality in both annual and perennial grasses, basal leaf meristems are the tissues surviving longer and able to regenerate when the mature blades are dead (Van Peer et al., 2004). These leaf meristems enclosed in mature leaf sheaths are protected from intense evaporation and can survive more intense water deficits than older tissues (Munns et al., 1979; Barlow et al., 1980). As they accumulate carbohydrates such as fructans (Schnyder and Nelson, 1989; Volaire et al., 1998), apices exhibit the greatest osmotic adjustment relative to other tissues during drought (Matsuda and Riazi, 1981; West et al., 1990). In addition, the strategy of summer dormancy (Volaire and Norton, 2006; Gillespie and Volaire, 2017) found in some species and populations of perennial species triggers leaf senescence and meristem dehydration to reduce plant metabolism and consequently enhance drought survival. Our results show that the Mediterranean populations of D. glomerata which are the most summer dormant, ‘sacrifice’ their lamina in summer (irrespective of the water supply) but also have the highest embolism resistance and dehydration tolerance. In frost-prone areas too, perennial herbaceous species avoid freezing by senescing above-ground tissues and overwintering as underground storage organs (Zanne et al., 2014). For dehydration tolerance under frost or drought (Gillespie and Volaire, 2017), these model species can then raise the relevance of the paradigm of hydraulic safety margins in leaves for stress survival. The leaf (lamina)-level drought tolerance (Nardini and Luglio, 2014; Ocheltree et al., 2016) may not be the key level to explore drought survival in all species. However, studies linking hydraulic conductivity and lamina anatomy (Scoffoni et al., 2017) could be fruitful provided they take into account the continuum between expanded blades, sheaths and meristematic tissues. The co-variation between hydraulic traits and anatomical traits in meristems but also in root tissues should be explored further in grasses.
In conclusion, this study investigated dehydration survival amongst 12 populations of D. glomerata from a latitudinal gradient from Mediterranean to Scandinavian areas, representing only a restricted range of its much larger biogeographical distribution. Dactylis glomerata represents an ‘ideal’ species for analysing the genotype × environment interactions because its biogeographical distribution is huge and spans over Europe and Asia (Borrill, 1991), and its phylogenetic relationships between populations are being investigated and show high genetic variation within both populations and geographical origins (Lumaret, 1988; Xie et al., 2010). Compared with woody species, the large degree of intraspecific variability in dehydration tolerance and embolism resistance within cocksfoot has consequences for its sensitivity to climate shifts, possible migrations and future biogeographical distribution (Anderegg, 2015). In addition, given that the measurement of plant drought survival is experimentally difficult to standardize, our study demonstrating the key role of embolism threshold and tissue lignification as proxy for dehydration tolerance provides highly relevant knowledge to screen genetic resources and new breeding material.
Finally, this study calls for extending the numerous studies on mortality factors and lethal embolism thresholds in woody species to a large range of herbaceous species, at both inter- and intraspecific levels, under contrasting environmental constraints.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Figure S1: S curves for determination of embolism resistance in cocksfoot. Figure S2: overview of the stem anatomy of the 12 populations of Dactylis glomerata and P88 (MPa).
ACKNOWLEDGEMENTS
We thank Pascal Chapon and Mathieu Ogier for excellent technical assistance, the ‘Terrain d’expérience platform’ and the ‘Plateforme d’Analyses Chimiques en Ecologie’ both at CEFE-CNRS (Labex CEMEB). We also thank Mark Norton, Jeffrey Clary, Rajae Kallida, N. Shaimi, A. Palmé, S. Fourtier, B. Reid, G. Test, and V. Copani, for providing seeds. This study was supported by the European Research Council (ERC) Starting Grant Project ‘Ecophysiological and biophysical constraints on domestication in crop plants’ [grant ERC-StG-2014-639706-CONSTRAINTS], and by the Agence National de la Recherche [PRAISE project, ANR-13-BIOADAPT-0015]. P.B. was financially supported by INRA (EA department).
LITERATURE CITED
- Alder NN, Pockman WT, Sperry JS, Nuismer S. 1997. Use of centrifugal force in the study of xylem cavitation. Journal of Experimental Botany 48: 665–674. [Google Scholar]
- Allen CD, Macalady AK, Chenchouni H, et al. 2010. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. Forest Ecology and Management 259: 660–684. [Google Scholar]
- Anderegg WRL. 2015. Spatial and temporal variation in plant hydraulic traits and their relevance for climate change impacts on vegetation. New Phytologist 205: 1008–1014. [DOI] [PubMed] [Google Scholar]
- Anderegg WRL, Kane JM, Anderegg LDL. 2013. Consequences of widespread tree mortality triggered by drought and temperature stress. Nature Climate Change 3: 30–36. [Google Scholar]
- Anderegg WRL, Klein T, Bartlett M et al. 2016. Meta-analysis reveals that hydraulic traits explain cross-species patterns of drought-induced tree mortality across the globe. Proceedings of the National Academy of Sciences, USA 113: 5024–5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang KY, Lucas PW, Tan HTW. 2008. Novel way of measuring the fracture toughness of leaves and other thin films using a single inclined razor blade. New Phytologist 177: 830–837. [DOI] [PubMed] [Google Scholar]
- Aranwela N, Sanson G, Read J. 1999. Methods of assessing leaf fracture properties. New Phytologist 144: 369–383. [Google Scholar]
- Barkaoui K, Roumet C, Volaire F. 2016. Mean root trait more than root trait diversity determines drought resilience in native and cultivated Mediterranean grass mixtures. Agriculture Ecosystems & Environment 231: 122–132. [Google Scholar]
- Barlow EWR, Munns RE, Brady DJ. 1980. Drought responses of apical meristems. In: Turner NC, Kramer PJ, eds. Adaptation of plants to water and high temperature stress. New York, USA: John Wiley & Sons, 191–205. [Google Scholar]
- Bartlett M, Klein T, Jansen S, Choat B, Sack L. 2016. The correlations and sequence of plant stomatal, hydraulic and wilting responses to drought. Proceedings of the National Academy of Sciences, USA 113: 13098–13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell A. 1991. Plant form. Portland, OR: Timber Press. [Google Scholar]
- Borrill M. 1991. Evolution and genetic resources in cocksfoot. Amsterdam: Elsevier Science Publishers. [Google Scholar]
- Brendel O, Cochard H. 2011. How plant species cope with water stress. In: Birot Y, Gracia C, Palahi M, eds. Water for forest and people in the Mediterranean: a challenging balance. Joensuu, Finland: European Forest Institute, 76–80. [Google Scholar]
- Brookshire ENJ, Weaver T. 2015. Long-term decline in grassland productivity driven by increasing dryness. Nature Communications 6: 7148. doi: 10.1038/ncomms8148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciais P, Reichstein M, Viovy N et al. 2005. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 437: 529–533. [DOI] [PubMed] [Google Scholar]
- Craine JM, Ocheltree TW, Nippert JB et al. 2013. Global diversity of drought tolerance and grassland climate-change resilience. Nature Climate Change 3: 63–67. [Google Scholar]
- Feng F, Ding F, Tyree MT. 2015. Investigations concerning cavitation and frost fatigue in clonal 84K poplar using high-resolution cavitron measurements. Plant Physiology 168: 144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier E, Laurent G. 1994. Leaf anatomy, specific mass and water-content in congeneric annual and perennial grass species. New Phytologist 128: 725–736. [Google Scholar]
- Garnier E, Shipley B, Roumet C, Laurent G. 2001. A standardized protocol for the determination of specific leaf area and leaf dry matter content. Functional Ecology 15: 688–695. [Google Scholar]
- Gillespie L, Volaire F. 2017. Are winter and summer dormancy symmetrical seasonal adaptive strategies? The case of temperate herbaceous perennials. Annals of Botany 119: 311–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin PC, Hoffmann AA. 2012. Mortality of Australian alpine grasses (Poa spp.) after drought: species differences and ecological patterns. Journal of Plant Ecology 5: 121–133. [Google Scholar]
- Hacke U, Sperry J. 2001. Functional and ecological xylem anatomy. Perspectives in Plant Ecology, Evolution and Systematics 4: 97–115. [Google Scholar]
- Hacke UG, Sperry JS, Pockman WT, Davis SD, McCulloch KA. 2001. Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia, 126: 457–461. [DOI] [PubMed] [Google Scholar]
- Hamann TD, Smets E, Lens F. 2011. A comparison of paraffin and resin-based techniques used in bark anatomy. Taxon 60: 841–851. [Google Scholar]
- Herbette S, Wortemann R, Awad H, Huc R, Cochard H, Barigah TS. 2010. Insights into xylem vulnerability to cavitation in Fagus sylvatica L.: phenotypic and environmental sources of variability. Tree Physiology 30: 1448–1455. [DOI] [PubMed] [Google Scholar]
- Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. 2005. Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology 25: 1965–1978. [Google Scholar]
- Hodgkinson KC, Muller WJ. 2005. Death model for tussock perennial grasses: a rainfall threshold for survival and evidence for landscape control of death in drought. Rangeland Journal 27: 105–115. [Google Scholar]
- Hodgson D, McDonald JL, Hosken DJ. 2015. What do you mean, ‘resilient’?Trends in Ecology and Evolution 30: 503–506. [DOI] [PubMed] [Google Scholar]
- Holloway-Phillips MM, Brodribb TJ. 2011. Minimum hydraulic safety leads to maximum water-use efficiency in a forage grass. Plant, Cell and Environment 34: 302–313. [DOI] [PubMed] [Google Scholar]
- Hoover DL, Knapp AK, Smith MD. 2014. Resistance and resilience of a grassland ecosystem to climate extremes. Ecology 95: 2646–2656. [Google Scholar]
- IPCC 2014. International Panel of Climatic changes. Fifth assessment report (AR5). https://www.ipcc.ch/report/ar5/ [Google Scholar]
- Lamy JB, Bouffier L, Burlett R, Plomion C, Cochard H, Delzon S. 2011. Uniform selection as a primary force reducing population genetic differentiation of cavitation resistance across a species range. PLoS One 6: e23476. doi: 10.1371/journal.pone.0023476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamy JB, Delzon S, Bouche PS et al. 2014. Limited genetic variability and phenotypic plasticity detected for cavitation resistance in a Mediterranean pine. New Phytologist 201: 874–886. [DOI] [PubMed] [Google Scholar]
- Lens F, Tixier A, Cochard H, Sperry JS, Jansen S, Herbette S. 2013. Embolism resistance as a key mechanism to understand adaptive plant strategies. Current Opinion in Plant Biology 16: 287–292. [DOI] [PubMed] [Google Scholar]
- Larter M, Brodribb TJ, Pfautsch S, Burlett R, Cochard H, Delzon S. 2015. Extreme aridity pushes trees to their physical limits. Plant Physiology 168: 804–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lens F, Picon-Cochard C, Delmas C et al. 2016. Herbaceous angiosperms are not more vulnerable to drought-induced embolism than angiosperm trees. Plant Physiology 172: 661–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez R, Cano FJ, Choat B, Cochard H, Gil L. 2016. Plasticity in vulnerability to cavitation of Pinus canariensis occurs only at the driest end of an aridity gradient. Frontiers in Plant Science 7: 769. doi: 10.3389/fpls.2016.00769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumaret R. 1988. Cytology, genetics, and evolution in the genus Dactylis. Critical Reviews in Plant Sciences 7: 55–91. [Google Scholar]
- Martinez-Vilalta J, Cochard H, Mencuccini M et al. 2009. Hydraulic adjustment of Scots pine across Europe. New Phytologist 184: 353–364. [DOI] [PubMed] [Google Scholar]
- Matsuda K, Riazi A. 1981. Stress-induced osmotic adjustment in growing regions of barley leaves. Plant Physiology 68: 571–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr S, Hacke U, Schmid P, Schwienbacher F, Gruber A. 2006. Frost drought in conifers at the alpine timberline: xylem dysfunction and adaptations. Ecology 87: 3175–3185. [DOI] [PubMed] [Google Scholar]
- McDowell N, Pockman WT, Allen CD et al. 2008. Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought?New Phytologist 178: 719–739. [DOI] [PubMed] [Google Scholar]
- McDowell NG, Fisher RA, Xu CG et al. 2013. Evaluating theories of drought-induced vegetation mortality using a multimodel-experiment framework. New Phytologist 200: 304–321. [DOI] [PubMed] [Google Scholar]
- Moran MS, Ponce-Campos GE, Huete A et al. 2014. Functional response of U.S. grasslands to the early 21st-century drought. Ecology 95: 2121–2133. [DOI] [PubMed] [Google Scholar]
- Munns R, Brady CJ, Barlow EWR. 1979. Solute accumulation in the apex and leaves of wheat during water-stress. Australian Journal of Plant Physiology 6: 379–389. [Google Scholar]
- Nardini A, Luglio J. 2014. Leaf hydraulic capacity and drought vulnerability: possible trade-offs and correlations with climate across three major biomes. Functional Ecology 28: 810–818. [Google Scholar]
- Nardini A, Peda G, La Rocca N. 2012. Trade-offs between leaf hydraulic capacity and drought vulnerability: morpho-anatomical bases, carbon costs and ecological consequences. New Phytologist 196: 788–798. [DOI] [PubMed] [Google Scholar]
- Nimmo DG, MacNally R, Cunningham SC, et al. 2015. Vive la resistance: reviving resistance for 21st century conservation. Trends in Ecology and Evolution 30: 516–523. [DOI] [PubMed] [Google Scholar]
- Niu C-Y, Meinzer FC, Hao G-Y. 2017. Divergence in strategies for coping with winter embolism among co-occurring temperate tree species: the role of positive xylem pressure, wood type and tree stature. Functional Ecology 31: 1550–1560. [Google Scholar]
- Nolf M, Pagitz K, Mayr S. 2014. Physiological acclimation to drought stress in Solidago canadensis. Physiologia Plantarum 150: 529–539. [DOI] [PubMed] [Google Scholar]
- Ocheltree TW, Nippert JB, Prasad PVV. 2016. A safety vs efficiency trade-off identified in the hydraulic pathway of grass leaves is decoupled from photosynthesis, stomatal conductance and precipitation. New Phytologist 210: 97–107. [DOI] [PubMed] [Google Scholar]
- Ogasa M, Miki NH, Murakami Y, Yoshikawa K. 2013. Recovery performance in xylem hydraulic conductivity is correlated with cavitation resistance for temperate deciduous tree species. Tree Physiology 33: 335–344. [DOI] [PubMed] [Google Scholar]
- Onoda Y, Westoby M, Adler P et al. 2011. Global patterns of leaf mechanical properties. Ecology Letters 14: 301–312. [DOI] [PubMed] [Google Scholar]
- Orlowsky B, Seneviratne SI. 2012. Global changes in extreme events: regional and seasonal dimension. Climatic Change 110: 669–696. [Google Scholar]
- Pammenter NW, Vander Willigen C. 1998. A mathematical and statistical analysis of the curves illustrating vulnerability of xylem to cavitation. Tree Physiology 18: 589–593. [DOI] [PubMed] [Google Scholar]
- Perez-Harguindeguy N, Diaz S, Garnier E et al. 2013. New handbook for standardised measurement of plant functional traits worldwide. Australian Journal of Botany 61: 167–234. [Google Scholar]
- Pérez-Ramos IM, Volaire F, Fattet M, Blanchard A, Roumet C. 2013. Tradeoffs between functional strategies for resource-use and drought-survival in Mediterranean rangeland species. Environmental and Experimental Botany 87: 126–136. [Google Scholar]
- Pilgrim ES, Macleod CJA, Blackwell MSA et al. 2010. Interactions among agricultural production and other ecosystem services delivered from European temperate grassland systems. Advances in Agronomy 109: 117–154. [Google Scholar]
- Pivovaroff AL, Pasquini S, De Guzman ME, Alstad KP, Stemke JS, Santiago LS. 2015. Multiple strategies for drought survival among woody plant species. Functional Ecology 30: 1–10. [Google Scholar]
- Poirier M, Durand JL, Volaire F. 2012. Persistence and production of perennial grasses under water deficits and extreme temperatures: importance of intraspecific vs. interspecific variability. Global Change Biology 18: 3632–3646. [Google Scholar]
- Russo SE, Jenkins KL, Wiser SK, Uriarte M, Duncan RP, Coomes DA. 2010. Interspecific relationships among growth, mortality and xylem traits of woody species from New Zealand. Functional Ecology 24: 253–262. [Google Scholar]
- Schnyder H, Nelson CJ. 1989. Growth rates and assimilate partitioning in the elongation zone of tall fescuue leaf blades at high and low irradiance. Plant Physiology 90: 1201–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz FG, Bucci SJ, Arias N, Meinzer FC, Goldstein G. 2012. Osmotic and elastic adjustments in cold desert shrubs differing in rooting depth: coping with drought and subzero temperatures. Oecologia 170: 885–897. [DOI] [PubMed] [Google Scholar]
- Scoffoni C, Albuquerque C, Brodersen CR et al. 2017. Leaf vein xylem conduit diameter influences susceptibility to embolism and hydraulic decline. New Phytologist 213: 1076–1092. [DOI] [PubMed] [Google Scholar]
- Seneviratne SI, Donat MG, Mueller B, Alexander LV. 2014. No pause in the increase of hot temperature extremes. Nature Climate Change 4: 320–320. [Google Scholar]
- Shipley B, Vu TT. 2002. Dry matter content as a measure of dry matter concentration in plants and their parts. New Phytologist 153: 359–364. [Google Scholar]
- Skelton RP, West AG, Dawson TE. 2015. Predicting plant vulnerability to drought in biodiverse regions using functional traits. Proceedings of the National Academy of Sciences, USA 112: 5744–5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MD. 2011. The ecological role of climate extremes: current understanding and future prospects. Journal of Ecology 99: 651–655. [Google Scholar]
- Sperry JS, Nichols KL, Sullivan JEM, Eastlack SE. 1994. Xylem embolism in ring-porous, diffuse-porous, and coniferous trees of northern Utah and interior Alaska. Ecology 75: 1736–1752. [Google Scholar]
- Tixier A, Cochard H, Badel E, Dusotoit-Coucaud A, Jansen S, Herbette S. 2013. Arabidopsis thaliana as a model species for xylem hydraulics: does size matter?Journal of Experimental Botany 64: 2295–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabucco A, Zomer RJ, Bossio DA, van Straaten O, Verchot LV. 2008. Climate change mitigation through afforestation/reforestation: a global analysis of hydrologic impacts with four case studies. Agriculture Ecosystems & Environment 126: 81–97. [Google Scholar]
- Urli M, Porte AJ, Cochard H, Guengant Y, Burlett R, Delzon S. 2013. Xylem embolism threshold for catastrophic hydraulic failure in angiosperm trees. Tree Physiology 33: 672–683. [DOI] [PubMed] [Google Scholar]
- Van Peer L, Nijs I, Bogaert J, Verelst I, Reheul D. 2001. Survival, gap formation, and recovery dynamics, in grassland ecosystems exposed to heat extremes: the role of species richness. Ecosystems 4: 797–806. [Google Scholar]
- Van Peer L, Nijs I, Reheul D, De Cauwer B. 2004. Species richness and susceptibility to heat and drought extremes in synthesized grassland ecosystems: compositional vs physiological effects. Functional Ecology 18: 769–778. [Google Scholar]
- Volaire F. 2008. Plant traits and functional types to characterise drought survival of pluri-specific perennial herbaceous swards in Mediterranean areas. European Journal of Agronomy 29: 116–124. [Google Scholar]
- Volaire F. 2018. A unified framework for plant drought adaptive strategies: across scales and disciplines. Global Change Biology (in press). [DOI] [PubMed] [Google Scholar]
- Volaire F, Lelievre F. 2001. Drought survival in Dactylis glomerata and Festuca arundinacea under similar rooting conditions in tubes. Plant and Soil 229: 225–234. [Google Scholar]
- Volaire F, Norton M. 2006. Summer dormancy in perennial temperate grasses. Annals of Botany 98: 927–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volaire F, Thomas H, Bertagne N, Bourgeois E, Gautier MF, Lelievre F. 1998. Survival and recovery of perennial forage grasses under prolonged Mediterranean drought: water status, solute accumulation, abscisic acid concentration and accumulation of dehydrin transcripts in bases of immature leaves. New Phytologist 140: 451–460. [DOI] [PubMed] [Google Scholar]
- Volaire F, Seddaiu G, Ledda L, Lelievre F. 2009. Water deficit and induction of summer dormancy in perennial Mediterranean grasses. Annals of Botany 103: 1337–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West CP, Oosterhuis DM, Wullschleger SD. 1990. Osmotic adjustment in tissues of tall fescue in response to water deficit. Environmental and Experimental Botany 30: 149–156. [Google Scholar]
- Wortemann R, Herbette S, Barigah TS et al. 2011. Genotypic variability and phenotypic plasticity of cavitation resistance in Fagus sylvatica L. across Europe. Tree Physiology 31: 1175–1182. [DOI] [PubMed] [Google Scholar]
- Xie WG, Zhang XQ, Ma X et al. 2010. Diversity comparison and phylogenetic relationships of cocksfoot (Dactylis glomerata L.) germplasm as revealed by SSR markers. Canadian Journal of Plant Science 90: 13–21. [Google Scholar]
- Zanne AE, Tank DC, Cornwell WK et al. 2014. Three keys to the radiation of angiosperms into freezing environments. Nature 506: 89–92. [DOI] [PubMed] [Google Scholar]
- Zwicke M, Picon-Cochard C, Morvan-Bertrand A, Prud’homme M, Volaire F. 2015. What functional strategies drive drought survival and recovery of perennial species from upland grassland?Annals of Botany 116: 1001–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.