Abstract
Background
Late-stage chronic kidney disease (LS-CKD) can be defined by glomerular filtration rate (GFR) 0–30 mL/min. It is a period of risk for medication discrepancies because of frequent hospitalizations, fragmented medical care, inadequate communication and polypharmacy. In this study, we sought to characterize medication discrepancies in LS-CKD.
Methods
We analyzed all patients enrolled in Northwell Health’s Healthy Transitions in LS-CKD program. All patients had estimated GFR 0–30 mL/min, not on dialysis. Medications were reviewed by a nurse at a home visit. Patients’ medication usage and practice were compared with nephrologists’ medication lists, and discrepancies were characterized. Patients were categorized as having either no discrepancies or one or more. Associations between patient characteristics and number of medication discrepancies were evaluated by chi-square or Fisher’s exact test for categorical variables, and two-sample t-test or Wilcoxon text for continuous variables.
Results
Seven hundred and thirteen patients with a median age of 70 (interquartile range 58–79) years were studied. There were 392 patients (55.0% of the study population) with at least one medication discrepancy. The therapeutic classes of medications with most frequently occurring medication discrepancies were cardiovascular, vitamins, bone and mineral disease agents, diuretics, analgesics and diabetes medications. In multivariable analysis, factors associated with higher risk of discrepancies were congestive heart failure [odds ratio (OR) 2.13; 95% confidence interval (CI) 1.44–3.16; P = 0.0002] and number of medications (OR 1.29; 95% CI 1.21–1.37; P < 0.0001).
Conclusions
Medication discrepancies are common in LS-CKD, affect the majority of patients and include high-risk medication classes. Congestive heart failure and total number of medications are independently associated with greater risk for multiple drug discrepancies. The frequency of medication discrepancies indicates a need for great care in medication management of these patients.
Keywords: chronic renal failure, chronic renal insufficiency, CKD, diuretics, medications
Introduction
Medication discrepancies can be defined as a mismatch between a treating physician’s understanding of patients’ current medications (including dose and frequency) and the actual medications that the patient takes. Discrepancies have the potential to impair the effectiveness and safety of medication treatment. Ideally, the physician conducts a medication reconciliation with the patient and educates patients about medications. In addition, the physician should maintain good communication with other medical providers regarding medication changes. In actual practice, however, medication discrepancies are frequent, occurring in 34–95% of patients at the time of admission for acute hospitalizations or psychiatric clinics [1, 2]. When discrepancies occur there may be failure to recognize symptoms and signs as medication side effects and increased risk for adverse events [3]. A systematic review of acute hospitalizations found that 11–59% of medication discrepancies were clinically important and 39% had potential to cause moderate-to-severe patient harm [1].
To date there have been no studies of medication discrepancies in late-stage chronic kidney disease (LS-CKD). LS-CKD can be defined as CKD Stages 4–5, prior to the initiation of renal replacement therapy. There are several reasons to suspect that medication discrepancies may be common in LS-CKD. Patients often take multiple medications, reaching a mean of 11.8 by the time of dialysis need [4]. Polypharmacy and conflicting dosing schedules are never easy for patients to manage. This is probably more true in LS-CKD, where patients are usually older and have comorbidities including cognitive decline. In addition, patients with LS-CKD often have multiple different physicians involved in their care. The result can sometimes be care fragmentation with confusion created by numerous drug and dosing changes. Frequent hospitalizations among patients with LS-CKD result in medication and dose changes, often without adequate reconciliation and communication among providers at hospital discharge. In general, communication problems are a recurring theme throughout the care system, contributing to medication risk.
Since 2012, we have operated the Healthy Transitions care management program for patients with LS-CKD. Nephrologist treatment is supplemented by nursing care management services and advanced informatics. All program patients have an initial home visit, which offers a unique opportunity for medication review. The visit occurs as the initial program contact, so the medication reviews are free from any interventional effect. At the visit, the patient and key caregivers show the bottles of medications being used and explain how many pills are taken and how frequently. Because this occurs in the patient’s home, with the actual drugs consumed, it is a rigorous form of medication review. In this analysis, we sought to utilize this uniquely clear view of medication use to better understand and characterize the frequency, type and risk factors for medication discrepancies in LS-CKD. We are aware of no previous studies of this subject in CKD and studies in other populations have often relied on health record reviews rather than direct patient interviews [5, 6].
Materials and methods
Patient population
The Healthy Transitions in Late Stage Kidney Disease program began operation in October 2012. The program provides nursing care management services to patients with LS-CKD, in an effort to facilitate care processes and improve preparation for end-stage kidney disease (all such patients were eligible for participation in the program). The program includes patients with CKD Stages 4–5 defined as estimated glomerular filtration rate (eGFR) 0–30 mL/min, and excludes patients (i) on dialysis and (ii) with significant cognitive impairment, defined as need for assistance with activities of daily living. The program began as a pilot, entered a full implementation phase and subsequently had an imbedded randomized controlled trial (RCT) [7] and a 3-year period of funding by Centers for Medicare and Medicaid Innovation program Health Care Innovations Award.
All patients enrolled throughout all program phases from October 2012 to December 2016 were included in the current analysis except for patients from the RCT control group. The justification for inclusion of all of these program phase patients is that all had exactly the same initial home visit with medication review conducted prior to any other interventions. The program functions and patients were enrolled, from Nassau, Suffolk, Queens, Kings and New York counties of New York State. There have been seven different participating nephrology groups. Three of the offices were private practices, four were academic nephrology practices. There was no standardized method for medication history-taking among the nephrologists.
Medication review
Medication discrepancies were defined from the perspective of the nephrologist. Most patients had a recent visit with the nephrologist at the time of program enrollment. After enrollment, a home visit was conducted by a program nurse. The nephrologist’s medication list was printed from the electronic health record (EHR) prior to the home visit. All program nephrologists’ EHRs prompt for medication reconciliation at all patient visits.
Medication reviews were all conducted by one of the program’s registered nurses. All took place during an initial home visit that occurred prior to any other program interventions. The purpose of the home visit is to begin to build a trusting relationship, to assess the patient’s knowledge regarding their kidney disease, to initiate education regarding modality options for renal replacement therapy and diet and most relevant to the current analysis, to conduct a complete medication review.
The medication portion of the visit begins with the nurse asking the patient and key care giver where in the home medications are kept, and how the patient carries medications if needed during the day. Next, the patient was asked to remove their currently used medication jars from their medicine cabinet or other storage area. In many cases, the nurse helped the patient with this task. Each medication jar was reviewed individually with attention to accuracy. The patient was questioned on (i) if the medication was currently being taken, (ii) how many pills, (iii) how many times a day and (iv) were there any other medications, including over-the-counter agents, herbals or vitamins, that the patient took. The patient was questioned on other medications that were on the nephrologist’s medication list but that the patient was not taking at that time. For any difference between the nephrologist’s list of medications and the patient’s actual medication consumption, a discrepancy and its characteristics were recorded. Although all patients had subsequent medication reviews as part of the program, only the baseline review was included in the current analysis to avoid potential bias introduced by program interventions.
Statistical analyses
Baseline patient demographics (age, gender, race, ethnicity, hypertension, diabetes, congestive heart failure, insurance type, smoking status and eGFR) were described using categorical or continuous variables as appropriate. In the entire patient cohort, the frequency, proportion and type of medication discrepancy was described. Categorization of medication discrepancy type was determined a priori, and included therapeutic class, and any difference between the nephrologist’s list of medications and the patient’s actual medication consumption (i.e. patient taking medication not on nephrologist’s list, patient not taking medication on nephrologist’s list, different dose and/or different frequency, drug discontinued and patient taking, drug discontinued and still on nephrologist’s list or patient not taking medication as instructed).
To evaluate patient characteristics associated with risk for multiple medication discrepancies, we first divided patients into two categories, one with no medication discrepancies and one with one or more discrepancies. We decided on categorization prior to any inspection of results by category. The variables chosen for study were based on availability and then on those with potential causal pathways. In an initial univariable analysis, we used chi-square or Fisher’s exact test as appropriate for categorical variables, and two-sample t-test or Wilcoxon text as appropriate for continuous variables, to evaluate the association of each patient characteristic with the outcome of number of medication discrepancies (no discrepancies versus one or more). In subsequent multivariable logistic regression analysis, factors that were found statistically significant in the univariable analysis plus age and gender were taken as the candidate variables for the final model. We conducted all analyses using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
A total of 713 patients were reviewed who were enrolled from October 2012 to December 2016. In the entire cohort, the median age was 70 [interquartile range (IQR) 58–79] years, 56.5% were men, 24.4% were black, 60.9% were white, 9% were Hispanic, 51.1% were diabetic and 92.8% had hypertension. Patient characteristics are displayed in Table 1. The distribution of eGFR was <10 mL/min, 7.3%; 10–15 mL/min, 26.3%; 15.1–20 mL/min, 25%; > 20 mL/min, 41.4%. Six patients had previously undergone kidney transplantation. The median time from last nephrologist medication reconciliation to nurse visit was 3 (IQR 1–6) days.
Table 1.
Patient characteristics
| Characteristic | n (% or SD) |
|---|---|
| Mean age (years) | 67.5 (15.9) |
| Male | 403 (56.5) |
| Race | |
| White | 434 (60.9) |
| Black | 174 (24.4) |
| Other or multiracial | 67 (9.4) |
| Asian | 38 (5.3) |
| Hispanic ethnicity | 64 (9.0) |
| Hypertension | 662 (92.8) |
| Diabetes | 364 (51.1) |
| Congestive heart failure | 181 (25.3) |
| Primary insurance type | |
| Medicare | 400 (56.1) |
| Private | 239 (33.5) |
| Medicaid | 67 (9.4) |
| No insurance | 7 (1) |
| Active smoker | |
| Yes | 41 (5.8) |
| No | 666 (93.4) |
| Not specified | 6 (0.8) |
| Mean eGFR (mL/min) | 18.6 ± 6.4 |
| eGFR category (mL/min) | |
| <10 | 52 (7.3) |
| 10–15 | 188 (26.4) |
| 15.1–20 | 178 (25) |
| >20 | 295 (41.4) |
| Mean number of medications | 8.1 (3.4) |
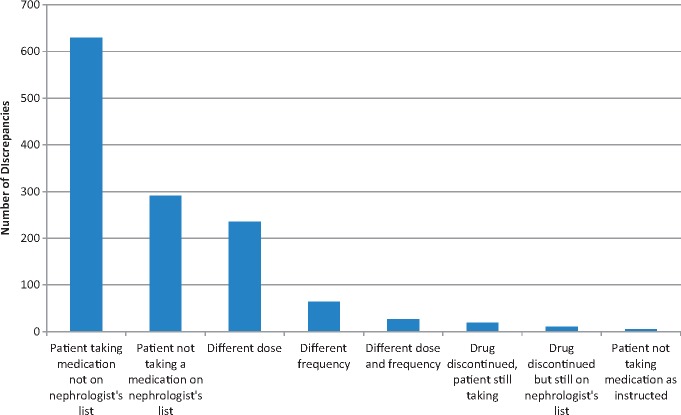
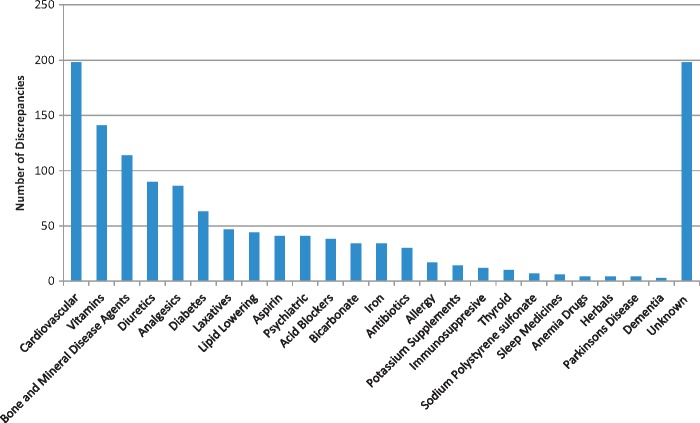
There were 392 patients (55.0% of the study population) with at least one medication discrepancy. The total number of discrepancies was 1280, and the median number of discrepancies per patient (in patients with at least 1 discrepancy) was 2 (IQR 1–4). The distribution of discrepancies per patient is displayed in Table 2. There were 321 patients with zero discrepancies (45%), 125 patients with one discrepancy and 83 patients with two discrepancies. There were 12.1% of patients with five or more discrepancies. The most common type of discrepancies were patients taking a medication not on nephrologist’s list (49.1% of discrepancies), patients not taking a medication on the nephrologist’s list (22.7%), different dose (18.4%) and different frequency (5%) (Figure 1). The therapeutic medication classes with the greatest number of discrepancies were cardiovascular (15.5% of discrepancies), vitamins (11.0%), bone and mineral disease agents (8.9%), diuretics (7.0%), analgesics (6.7%) and diabetes medications (5.0%) (Figure 2). The types of discrepancies were similar for different drug classes. Among cardiovascular drugs, the subcategories with the greatest number of discrepancies were renin angiotensin aldosterone system inhibitors (66% of cardiovascular discrepancies) and beta blockers (24%).
Table 2.
Frequency distribution of number of medication discrepancies per patient
| Medication discrepancies per patient | Number | Percentage |
|---|---|---|
| 0 | 321 | 45.0 |
| 1 | 125 | 17.5 |
| 2 | 83 | 11.6 |
| 3 | 57 | 8.0 |
| 4 | 41 | 5.8 |
| 5 | 19 | 2.7 |
| 6 | 24 | 3.4 |
| 7 | 14 | 2.0 |
| 8 | 4 | 0.6 |
| 9 | 5 | 0.7 |
| 10 | 8 | 1.1 |
| 11 | 3 | 0.4 |
| 12 | 3 | 0.4 |
| 13 | 1 | 0.1 |
| 14 | 2 | 0.3 |
| 15 | 1 | 0.1 |
| 16 | 1 | 0.1 |
| 17 | 0 | 0.0 |
| 18 | 1 | 0.1 |
Fig. 1.
Frequency distribution of types of medication discrepancy, from most common to least common.
Fig. 2.
Frequency of medication discrepancies by therapeutic class.
When assessing the outcome of multiple medication discrepancies, there were 321 patients with no discrepancies, and 392 patients with one or more discrepancies. In a univariable analysis, the following patient characteristics were found to be associated with medication discrepancies (P < 0.05): congestive heart failure, white race, hypertension and number of medications taken (Table 3). In subsequent multivariable logistic regression analysis, factors that were found statistically significant in the univariable analysis plus age and gender were taken as the candidate variables for the final model. After backward variable selection, factors in the final model independently associated with higher risk of one or more medication discrepancies compared with zero discrepancy were congestive heart failure [odds ratio (OR) 2.13; 95% confidence interval (CI) 1.44–3.16; P = 0.0002] and number of medications (OR 1.29; 95% CI 1.21–1.37; P < 0.0001) (Table 4).
Table 3.
Univariate association of patient characteristics and number of medication discrepancies
| Number of medication discrepancies |
|||
|---|---|---|---|
| Patient characteristics | Zero discrepancies (n = 321) | ≥1 discrepancy (n = 392) | P-value |
| Mean number of discrepancies (SD) | 0 | 3.3 (2.8) | NA |
| Mean (±SD) age (years) | 68.0 ± 15.8 | 67.3 ± 16.1 | 0.40 |
| Gender, n (%) | 0.15 | ||
| Male | 172 (42.7) | 231 (57.3) | |
| Female | 149 (48.1) | 161 (51.9) | |
| Race, n (%) | 0.03 | ||
| White | 177 (40.8) | 257 (59.2) | |
| Black | 89 (51.2) | 85 (48.9) | |
| Other or multiracial | 33 (49.3) | 34 (50.8) | |
| Asian | 22 (57.9) | 16 (42.1) | |
| Hispanic ethnicity, n (%) | 0.27 | ||
| Yes | 33 (51.6) | 31 (48.4) | |
| No | 288 (44.4) | 361 (55.6) | |
| Hypertension, n (%) | 0.01 | ||
| Yes | 289 (43.7) | 373 (56.3) | |
| No | 32 (62.8) | 19 (37.2) | |
| Diabetes mellitus, n (%) | 0.90 | ||
| Yes | 163 (44.8) | 201 (55.2) | |
| No | 158 (45.3) | 191 (54.7) | |
| Congestive heart failure, n (%) | 0.0004 | ||
| Yes | 61 (33.7) | 120 (66.3) | |
| No | 260 (48.9) | 272 (51.1) | |
| Current smoker, n (%) | 0.82 | ||
| Yes | 20 (48.8) | 21 (51.2) | |
| No | 298 (44.7) | 368 (55.3) | |
| Not specified | 3 (50.0) | 3 (50.0) | |
| Insurance status, n (%) | 0.23 | ||
| Medicaid | 37 (55.2) | 30 (44.8) | |
| Medicare | 180 (45.0) | 220 (55.0) | |
| No insurance | 4 (57.1) | 3 (42.9) | |
| Private | 100 (41.8) | 139 (58.2) | |
| Mean (±SD) eGFR (mL/min) | 19.1 ± 6.6 | 18.3 ± 6.2 | 0.08 |
| Mean (±SD) number of medications | 6.8 ± 2.3 | 9.2 ± 3.8 | <0.0001 |
Table 4.
Multivariable analysis for medication discrepancy (logistic regression)
| Multivariable analysis for medication discrepancy | ||
|---|---|---|
| Variables | Odds ratio (95% confidence interval) | P-value |
| CHF (yes versus no) | 2.13 (1.44–3.16) | 0.0002 |
| Number of medications | 1.29 (1.21–1.37) | <0.0001 |
CHF, congestive heart failure.
Discussion
We found that more than half of patients with LS-CKD had at least one discrepancy between the nephrologist’s medication record and the medications actually being consumed. Approximately one-third of patients had two or more discrepancies. Most discrepancies were related to medications being taken that were not on the nephrologist’s list, medications on the nephrologist’s list that were not being taken, and dose and frequency differences.
Effective medication management is achieved by optimizing the benefits and reducing risks of treatment. Ongoing medication review and reconciliation by the provider is essential for longitudinal maintenance of medication safety. When discrepancies develop between the medications that the provider intends for the patient to be taking and those actually being used, there is increased risk for loss of efficacy or adverse events. Discrepancies can be highly relevant, with a recent study finding that in intensive care units, 17.1% of discrepancies were serious or potentially life-threatening [8].
In the treatment of patients with LS-CKD, the medication management process is fraught with polypharmacy, extensive comorbidity and altered medication pharmacology due to reduced renal function, fragmented medical care and frequent hospitalizations. The nephrologist plays a key and often principal role in maintenance of medication safety in these patients. Our finding of a high rate of medication discrepancies signifies an important gap in performance and opportunity for improvement in LS-CKD. The fact that more than one-third of patients had two or more discrepancies is a strong indicator of the magnitude of the problem.
It is concerning that cardiovascular medications were the most commonly discrepant class of medication and diuretics the fourth most common, because of the hemodynamic and metabolic effects of these agents. This aligns with the finding that congestive heart failure as comorbidity was a key independent predictor of multiple discrepancies. It is likely that concordant cardiac disease creates important vulnerabilities in medication management in LS-CKD. The diseases complicate and confound treatment of each other. Medications such as renin angiotensin aldosterone system inhibitors and diuretics are adjusted frequently in response to cardiac, renal or electrolyte disturbances. When the medications are changed, communication between physicians may often be suboptimal. This complex, chaotic setting is fertile ground for medication discrepancies and complications.
We also found that the number of medications that patients were taking was associated with greater risk for discrepancies. This is an intuitive finding and consistent with existing knowledge [9]. In LS‐CKD, patients take a large number of medications and it is wise to review ongoing need for all chronic medications.
Reduction in medication discrepancies in LS-CKD requires a more intensive approach to medication reconciliation. Sharing of medication information between healthcare providers and accurate is a prerequisite. Incorporation of pharmacy information on medication fill histories into EHRs holds great promise [10]. Ideally, the physician’s medication record should reflect the time of the last patient visit or other communication, but annotated with subsequent activity from other providers and pharmacies. Improved patient education is important as well, as it is probable that better understanding of medications and their purpose would enhance patient adherence and limit errors. Polypharmacy is certainly an issue as well. The mean number of medications taken by LS-CKD patients is substantial. Elimination of unnecessary medications should be an ongoing effort to improve patient safety.
Strengths of our study include the large numbers of patients in different types of practice settings. In addition the determination of medication discrepancies was by a highly rigorous approach with home visits and review of actual medication bottles and usage descriptions by the patient and key caregivers. Limitations include the geographically limited region covered, the metropolitan New York counties of Nassau, Suffolk, Queens, Kings and Manhattan. In addition, there was underrepresentation of individuals with Hispanic ethnicity. Hispanic patients made up only 9% of the study population, compared with ∼17% of the US population. The dataset had a low proportion of missing data in that ∼15% of discrepancies were accompanied by insufficient documentation of the specific involved medication. In addition, we were unable to determine which discrepancies might create a higher level of risk to patients as no available classification scheme is reliable. Additionally, we were not able to identify potential intentional discrepancies, for example, if a second physician had changed a medication. Finally, our dataset also did not allow for determination of the underlying reasons for unintentional medication discrepancies.
In conclusion, we found a high rate of medication discrepancies in LS-CKD. There is a need to better understand the subject to help improve medication management and reduce risk for medication errors. Future research efforts should focus on whether discrepancies relate to patient outcomes and on causative factors for medication discrepancies.
Funding
Part of this work was funded by the Centers for Medicare and Medicaid Innovation Program.
Conflict of interest statement
None declared.
References
- 1. Tam VC, Knowles SR, Cornish PL. et al. Frequency, type and clinical importance of medication history errors at admission to hospital: a systematic review. CMAJ 2005; 173: 510–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Prins MC, Drenth-van Maanen AC, Kok RM. et al. Use of a structured medication history to establish medication use at admission to an old age psychiatric clinic: a prospective observational study. CNS Drugs 2013; 27: 963–969 [DOI] [PubMed] [Google Scholar]
- 3. Simoons M, Mulder H, Risselada AJ. et al. Medication discrepancies at outpatient departments for mood and anxiety disorders in the Netherlands: risks and clinical relevance. J Clin Psychiatry 2016; 77: 1511–1518 [DOI] [PubMed] [Google Scholar]
- 4. Manley HJ, Cannella CA, Bailie GR. et al. Medication-related problems in ambulatory hemodialysis patients: a pooled analysis. Am J Kidney Dis 2005; 46: 669–680 [DOI] [PubMed] [Google Scholar]
- 5. Patel CH, Zimmerman KM, Fonda JR. et al. Medication complexity, medication number, and their relationships to medication discrepancies. Ann Pharmacother 2016; 50: 534–540 [DOI] [PubMed] [Google Scholar]
- 6. Hale J, Neal EB, Myers A. et al. Medication discrepancies and associated risk factors identified in home health patients. Home Healthcare Now 2015; 33: 493–499 [DOI] [PubMed] [Google Scholar]
- 7. Fishbane S, Agoritsas S, Bellucci A. et al. Augmented nurse care management in CKD stages 4 to 5: a randomized trial. Am J Kidney Dis 2017; 70: 498–505 [DOI] [PubMed] [Google Scholar]
- 8. Wills BM, Darko W, Seabury R. et al. Pharmacy impact on medication reconciliation in the medical intensive care unit. J Res Pharm Pract 2016; 5: 142–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hias J, Van der Linden L, Spriet I. et al. Predictors for unintentional medication reconciliation discrepancies in preadmission medication: a systematic review. Eur J Clin Pharmacol 2017; 73: 1355–1377 [DOI] [PubMed] [Google Scholar]
- 10. Pevnick JM, Palmer KA, Shane R. et al. Potential benefit of electronic pharmacy claims data to prevent medication history errors and resultant inpatient order errors. J Am Med Inform Assoc 2016; 23: 942–950 [DOI] [PMC free article] [PubMed] [Google Scholar]