Abstract
Neuronal synapse formation and remodeling is essential to central nervous system (CNS) development and is dysfunctional in neurodevelopmental diseases. Innate immune signals regulate tissue remodeling in the periphery, but how this impacts CNS synapses is largely unknown. Here we show that the IL-1 family cytokine Interleukin-33 (IL-33) is produced by developing astrocytes and is developmentally required for normal synapse numbers and neural circuit function in the spinal cord and thalamus. We find that IL-33 signals primarily to microglia under physiologic conditions, that it promotes microglial synapse engulfment, and that it can drive microglial-dependent synapse depletion in vivo. These data reveal a cytokine-mediated mechanism required to maintain synapse homeostasis during CNS development.
One sentence summary:
The astrocyte-encoded cytokine Interleukin-33 promotes microglial synapse remodeling during CNS development.
Neuronal synapse formation depends on a complex interplay between neurons and their glial support cells. Astrocytes provide structural, metabolic, and trophic support for neurons (1, 2). Gray matter astrocytes are in intimate contact with neuronal synapses and are poised to sense local neuronal cues. In contrast, microglia are the primary immune cells of the CNS parenchyma. Microglia regulate multiple phases of developmental circuit refinement (3, 4), both inducing synapse formation (5, 6) and promoting synapse engulfment (7, 8), in part via complement, an effector arm of the innate immune system(9). Excess complement activity has been implicated in schizophrenia, a neurodevelopmental disorder that includes cortical gray matter thinning and synapse loss(10), suggesting that microglial synapse engulfment may have broad implications for neuropsychiatric disease.
Despite the emerging roles of astrocytes and microglia in neuronal synapse formation and remodeling, how they coordinate synaptic homeostasis in vivo remains obscure. Interleukin-33 (IL-33) is an IL-1 family member with well described roles as a cellular alarmin released from nuclear stores following tissue damage, including in spinal cord injury (11, 12), stroke(13) and Alzheimer’s disease(14). Whereas many cytokines are primarily defined by their roles in inflammation and disease (e.g. IL-1, TNF-α or IL-6), IL-33 also promotes homeostatic tissue development and remodeling (15). The CNS undergoes extensive synapse remodeling during postnatal brain development, but a role for IL-33 or other stromal-derived cytokines is unknown. Here we report that IL-33 is produced postnatally by synapse-associated astrocytes, is required for synaptic development in the thalamus and spinal cord, and signals to microglia to promote increased synaptic engulfment. These findings reveal a physiologic requirement for cytokine-mediated immune signaling in brain development.
We previously developed methods to identify functionally heterogeneous astrocytes by expression profiling of distinct CNS regions (16). In a RNA sequencing screen of developing forebrain astrocytes (P9; flow sorted using an Aldh1l1-eGFP reporter) we identified the cytokine Interleukin-33 (IL-33) as a candidate that is both astrocyte-enriched and heterogeneously expressed by astrocytes throughout the CNS (Fig. S1A-C). We confirmed astrocyte-specific developmental expression of IL-33 in spinal cord and thalamus using a nuclear localized Il33 reporter (Il33mCherry/+ Fig. 1A) and validated these findings with flow cytometry and protein immunostaining (Fig. S2). By adulthood, a subset of oligodendrocytes also co-labeled with IL-33 (Fig. S2C-E), consistent with prior reports(11). Thus, astrocytes are the primary source of IL-33 during postnatal synapse maturation.
Figure 1: IL-33 is developmentally induced in synapse-associated astrocytes.

(A) Representative image of Il33mCherry with Aldh1l1-eGFP+ astrocytes and oligodendrocyte marker CC1 in spinal cord ventral horn (scale = 50 µm).(B) Gray matter restricted expression of Il33lacZ in the spinal cord at P30 (scale = 0.5 mm). (C, D) Il33lacZ increases in the visual thalamus (dLGN) during eye opening, normalized to sensorimotor thalamus (VB) (scale = 0.5 mm). (E) Representative images of Il33lacZ in P21 thalamus in littermate controls and after perinatal enucleation (scale = 0.5 mm). (F) Il33lacZ mean pixel intensity in dLGN. (G) Representative flow plot of spinal cord from Il33mCherry/Aldh1l1-eGFP mice at P15 with sorting gates indicated. (H) Heatmap of the top 444 differentially expressed genes in Il33-mCherry+ vs. mCherry− astrocytes in spinal cord and thalamus (FC>2, pAdj<0.05), select candidates highlighted. Statistics: One-way ANOVA with Tukey’s post hoc comparison or student’s t-test. All points represent independent biological replicates. * p<0.05, **** p<0.0001.
Although most IL-33 positive cells were astrocytes, not all developing astrocytes expressed IL-33, and this number increased in the early postnatal period (Fig. S3;(17)). In fact, IL-33 was detected only in gray matter, where most synapses are located (Fig. 1B, Fig. S2H, Fig. S3D). In the thalamus, which receives regionally distinct sensory synaptic inputs, IL-33 expression in the visual nucleus (dLGN) increased sharply coincident with eye opening (P12-P14, Fig. 1C, D). Removal of afferent sensory synapses by enucleation at birth prevented this developmental increase in IL-33 expression (Fig. 1E, F), whereas dark rearing, in which synapse maturation is largely preserved (18) had no effect. Molecular profiling of IL-33 positive astrocytes in both thalamus and spinal cord (Fig. 1G, H), revealed a negative correlation with white matter astrocyte markers (GFAP, Vimentin), enrichment for genes involved in astrocyte synaptic functions (connexin-30/Gjb6(19)), and enrichment in G-protein coupled and neurotransmitter receptors (e.g. Adora2b, Adra2a; Table S1-S3). Together, these data demonstrate that IL-33 expression is correlated with synaptic maturation and marks a subset of astrocytes potentially sensitive to synaptic cues, raising the question of whether IL-33 plays a role in synapse development.
To test whether IL-33 regulates neural circuit development and function, we examined the effect of IL-33 deletion on synapse numbers and circuit activity. In the thalamus, a region with high IL-33 expression, an intrathalamic circuit between the ventrobasal nucleus (VB) and the reticular nucleus of the thalamus (RT) displays spontaneous oscillatory activity that can also be evoked by stimulating the internal capsule that contains cortical afferents (20, 21). We quantified this oscillatory activity in slices from young adult mice (P30-P40) which revealed enhanced evoked activity in response to stimulation (Fig. 2A, B; Fig. S4A-B) as well as elevated spontaneous firing in the absence of IL-33 (Fig. 2C; Fig. S4C). This increase could result at least in part from enhanced numbers of glutamatergic synapses. To investigate this hypothesis, we performed whole-cell patch-clamp recordings of VB neurons to quantify miniature excitatory postsynaptic currents (mEPSCs; Fig. 2D). We found that the frequency of mEPSCs was enhanced in VB neurons from IL-33-deficient mice, whereas the amplitude and the kinetics were unchanged (Fig. S4D). Together, these results suggest that IL-33 deficiency leads to excess excitatory synapses and a hyperexcitable intrathalamic circuit.
Figure 2: IL-33 deficiency leads to excess synapses and abnormal thalamic and sensorimotor circuit function.
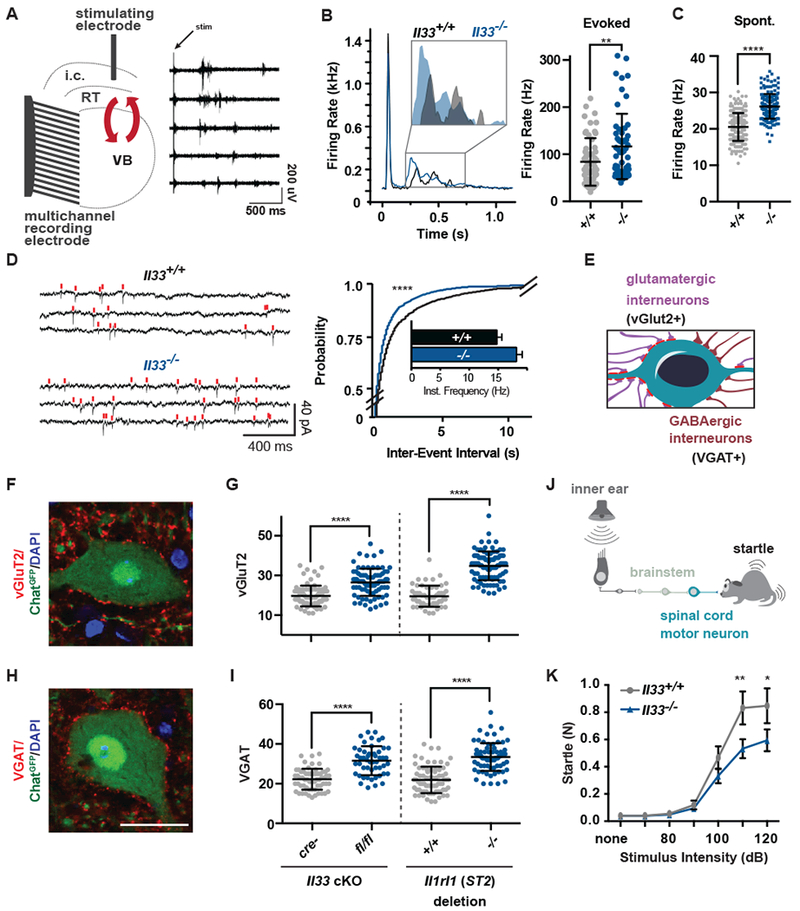
(A) Schematic of extracellular recording setup to measure circuit activity between ventrobasal (VB) and reticular thalamic nuclei (RT) with representative recording showing activity in five channels after stimulation of the internal capsule (i.c.) that contains cortical afferents. Red arrows indicate reciprocal VB-RT connections. (B) Average traces and quantification of mean firing rates reveal higher evoked firing in Il33−/−. (C) Quantification of mean firing rates in the absence of stimulation reveals increased spontaneous firing in Il33−/−. (D) Representative traces and quantification of intracellular patch-clamp recordings from neurons in the VB show increased miniature excitatory postsynaptic currents (mEPSCs) in Il33−/−. (E) Schematic of motor neuron synaptic afferents. (F, G) Representative image and quantification of excitatory inputs per motor neuron after conditional deletion of Il33 (hGFAPcre) or global deletion of Il1rl1. (H, I) Inhibitory (VGAT+) inputs in the same mice (scale = 25 µm). (J) Schematic of startle pathway. (K) Impaired sensorimotor startle in Il33−/− animals. Statistics: Data in B-C from WT: n=6-8 slices, 2 mice. KO: n=14-15 slices, 3 mice, points are individual recordings. Data in B analyzed by Mann-Whitney and C with student’s t test. Data in D from n=23-25 cells and 3-4 mice/group analyzed by Kolmogorov-Smirnov test. Data in F - I from n=3 animals, >75 neurons per genotype, student’s t-test; points are individual neurons. K is n=12/group, two-way ANOVA with Sidak’s multiple comparisons. *p<0.05, **p<0.01, ****p<.0001. B-I are mean±SD, K is mean±SEM.
In the spinal cord, α-motor neurons (α-MN) are the primary outputs of the sensorimotor circuit and receive inputs from excitatory (vGlut2+) and inhibitory (VGAT+) interneurons (Fig. 2E (22)). We conditionally deleted IL-33 from astrocytes (hGFAPcre (16), Fig. S5A), and found increased numbers of excitatory and inhibitory inputs onto α-MN at P30; global deletion of Il1rl1 (ST2) (Fig. 2F-I) or Il33 (Fig. S5B, C) phenocopied this finding. Neuronal soma size, interneuron numbers, and oligodendrocyte numbers were unchanged (Fig. S5D-F). However, by adulthood, IL-33 deficiency led to increased gray matter expression of glial fibrillary acidic protein (GFAP; Fig. S5G-H), a marker of tissue stress. We also found that Il33−/− animals had deficits in acoustic startle response, a sensorimotor reflex mediated by motor neurons in the brainstem and spinal cord (Fig. 2J, K; (23)). Auditory acuity and gross motor performance were normal (Fig. S5 I-J). Taken together, these data demonstrate that IL-33 is required for normal synapse numbers and circuit function in the thalamus and spinal cord.
To determine the cellular targets of IL-33 signaling we first quantified expression of its obligate co-receptor IL1RL1 (ST2)(15). We detected Il1rl1 in microglia by RNA sequencing (7.1± 2.1 FPKM), and by quantitative PCR, in contrast to astrocytes, neurons, or the lineage negative fraction (Fig. 3A). The transcriptome of acutely isolated microglia from Il33−/− animals revealed 483 significantly altered transcripts, including reduced expression of NF-κB targets (e.g. Tnf, Nfkbia, Nfkbiz, Tnfaip3; Fig. 3B-C; Fig. S6A, Additional data table S2), consistent with diminshed NF-κB signaling (24). The transcriptome of Il33−/− astrocytes was unchanged (Fig. S6B), strongly arguing against cell autonomous roles of IL-33 in this context. These data demonstrate physiologic signaling by IL-33 to microglia during brain development, raising the question of whether it promotes physiologic microglial functions.
Figure 3: IL-33 drives microglial synapse engulfment during development.
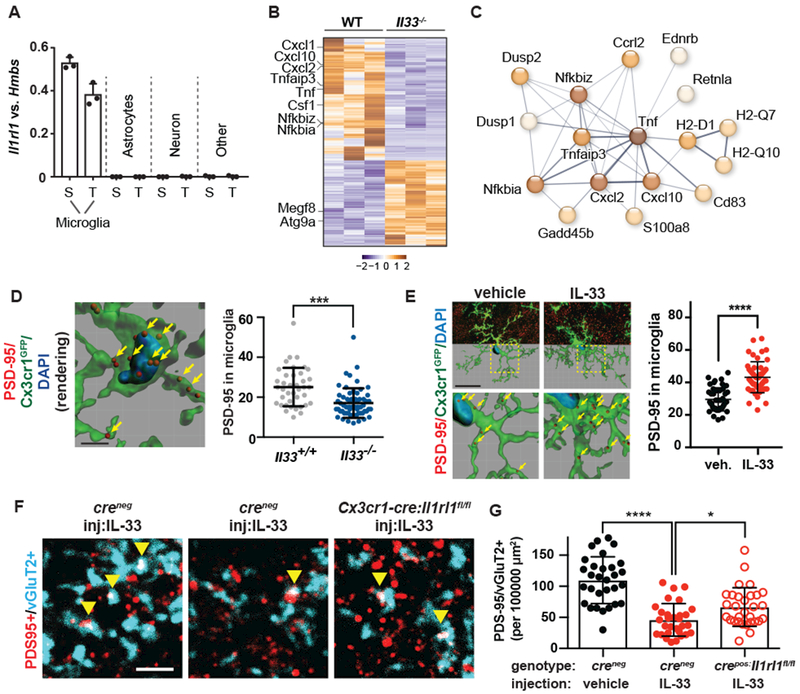
(A) Expression of Il1rl1 by qPCR of flow-sorted populations (S=spinal cord, T=thalamus.) (B) 484 differentially expressed genes in spinal cord microglia at pAdj<0.05. (C) Functionally associated gene clustering (STRING) identifies immune genes enriched in wild-type vs Il33−/− microglia. (D) PSD-95 puncta within microglia (yellow arrows) after IL-33 deletion (scale = 4 μM). (E) Representative image and quantification of engulfed PSD-95 in vehicle or IL-33 injected spinal cord (Scale= 20 µm). (F-G) Colocalized pre-and postsynaptic puncta in spinal cord ventral horn at P14 (yellow arrows) after IL-33 injection into control mice or in littermates with conditional deletion of Il1rl1 (Cx3cr1cre ; scale = 3 µm). Statistics: Points in A represent mice, in D-G individual microglia from N=3-5 animals/group, in G images from N=5 mice/group. In D, E student’s t-test and in G a one-way ANOVA with Tukey’s post hoc comparison, *p<0.05, ***p<0.001, ****p<0.0001, all data are mean±SD.
Given the increased synapse numbers in IL-33 deficient animals, we tested whether IL-33 is required for microglial synapse engulfment. We detected engulfed PSD-95+ synaptic puncta within spinal cord microglia throughout development, as in other CNS regions (7, 8), and found decreased engulfment in microglia from Il33−/− animals (P15; Fig. 3D). This was further validated by dye labeling of spinal cord motor neurons, which revealed fewer dye filled microglia in Il33−/− (Fig. S7). Conversely, local injection of IL-33 increased PSD-95 within microglia in both spinal cord (Fig. 3E) and thalamus (Fig. S8A-B), and altered markers consistent with microglial activation, including IL1RL1-dependent downregulation of P2Y12 (Fig. S9A-C (25)). In vitro, IL-33 promoted synaptosome engulfment by purified microglia, whereas the canonical IL-1 family member IL-1β had no effect (Fig. S9D-E). In vivo, injection of IL-33 into the developing spinal cord led to two-fold depletion of excitatory synapses (colocalized vGlut2/PSD-95) whereas conditional deletion of IL1RL1 from microglia partly reversed this effect (Cx3cr1cre: Il1rl1fl/fl, Fig. 3F, G). In comparison, global loss of Il1rl1 completely reversed IL-33 dependent synapse depletion in spinal cord (Fig. S10) and thalamus (Fig. S8C-D), suggesting that non-microglial sources of ST2 could also contribute. These data indicate that IL-33 regulates synapse numbers in vivo at least in part via IL1RL1 receptor-mediated signaling in microglia.
Our data reveal a mechanism of astrocyte-microglial communication that is required for synapse homeostasis during CNS development. We propose that astrocyte-derived IL-33 serves as a rheostat, helping to tune microglial synapse engulfment during neural circuit maturation and remodeling (Fig. S11). Key unanswered questions include the nature of the cues that induce astrocyte Il33 expression, the mechanism of IL-33 release, and the signals downstream of IL-33 that promote microglial function. These data also raise the broader question of how this process impacts neural circuit function. Synapses are the most tightly regulated variable in the developing CNS (26) and are a primary locus of dysfunction in neurodevelopmental diseases. Il33 is one of five genes that molecularly distinguishes astrocytes from neural progenitors in developing human forebrain (27), suggesting possibly conserved roles in the human CNS. Defining whether signals like IL-33 are permissive or instructive, promiscuous or synapse specific, is a first step towards understanding how neural circuits remodel during development and under stress.
Supplementary Material
Acknowledgements:
We are grateful to the Molofsky labs, Poskanzer lab, R.M. Locksley, and J.R. Chan for helpful comments on the manuscript. Thanks to the J. Huguenard lab for the thalamic network analysis code, to J.P Girard for Il33lacZ mice, R.T. Lee for Il33fl/fl and Il1rl1fl/fl, M. Colonna and the Mucosal Immunology Studies Team (MIST) for Il33H2B-mCherry, D. Julius for the P2Y12 antibody, and the Gladstone Genomics and Behavioral Cores (P30NS065780) for technical contributions.
Funding: A.V.M is supported by a Pew Scholars Award, NIMH (K08MH104417), the Brain and Behavior Research Foundation, and the Burroughs Wellcome Fund. A.B.M is supported by the NIDDK (K08DK101604) and the Larry L. Hillblom Foundation. J.T.P is supported by NINDS (R01NS096369). S.A.L. is supported by the Australian National Health and Medical Research Council (GNT1052961), and the Glenn Foundation Glenn Award. F.S.C (NSF #1144247) and P.T.N. were supported by Graduate Student Fellowships from the National Science Foundation.
Footnotes
Competing interests: None declared.
Data and materials availability: Supplement contains additional data. All data needed to evaluate the conclusions in the paper are present in the paper or the Supplementary Materials. RNA sequencing data is available through GEO #[submission in progress].
Supplementary materials:
Materials and Methods
Figs. S1 to S11
Tables S1 to S3
Additional databases S1 to S2
References (28-50)
References and notes:
- 1.Molofsky AV et al. , Astrocytes and disease: a neurodevelopmental perspective. Genes Dev. 26, 891–907 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clarke LE, Barres BA, Emerging roles of astrocytes in neural circuit development. Nat Rev Neurosci. 14, 311–321 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ransohoff RM, Cardona AE, The myeloid cells of the central nervous system parenchyma. Nature. 468, 253–262 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Salter MW, Beggs S, Sublime microglia: expanding roles for the guardians of the CNS. Cell. 158, 15–24 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Miyamoto A et al. , Microglia contact induces synapse formation in developing somatosensory cortex. Nat Commun. 7, 12540 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parkhurst CN et al. , Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 155, 1596–1609 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paolicelli RC et al. , Synaptic pruning by microglia is necessary for normal brain development. Science. 333, 1456–1458 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Schafer DP et al. , Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 74, 691–705 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stevens B et al. , The classical complement cascade mediates CNS synapse elimination. Cell. 131, 1164–1178 (2007). [DOI] [PubMed] [Google Scholar]
- 10.Sekar A et al. , Schizophrenia risk from complex variation of complement component 4. Nature. 530, 177–183 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gadani SP, Walsh JT, Smirnov I, Zheng J, Kipnis J, The Glia-Derived Alarmin IL-33 Orchestrates the Immune Response and Promotes Recovery following CNS Injury. Neuron, 1–8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pomeshchik Y et al. , Interleukin-33 treatment reduces secondary injury and improves functional recovery after contusion spinal cord injury. Brain Behav Immun. 44, 68–81 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Luo Y et al. , Interleukin-33 ameliorates ischemic brain injury in experimental stroke through promoting Th2 response and suppressing Th17 response. Brain Res. 1597, 86–94 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Fu AKY et al. , IL-33 ameliorates Alzheimer’s disease-like pathology and cognitive decline. Proc Natl Acad Sci USA. 113, E2705–13 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Molofsky AB, Savage AK, Locksley RM, Interleukin-33 in Tissue Homeostasis, Injury, and Inflammation. Immunity. 42, 1005–1019 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molofsky AV et al. , Astrocyte-encoded positional cues maintain sensorimotor circuit integrity. Nature. 509, 189–194 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wicher G, Husic E, Nilsson G, Forsberg-Nilsson K, Developmental expression of IL-33 in the mouse brain. Neurosci Lett. 555, 171–176 (2013). [DOI] [PubMed] [Google Scholar]
- 18.Hooks BM, Chen C, Distinct roles for spontaneous and visual activity in remodeling of the retinogeniculate synapse. Neuron. 52, 281–291 (2006). [DOI] [PubMed] [Google Scholar]
- 19.Pannasch U et al. , Connexin 30 sets synaptic strength by controlling astroglial synapse invasion. Nat Neurosci. 17, 549–58 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Paz JT, Christian CA, Parada I, Prince DA, Huguenard JR, Focal cortical infarcts alter intrinsic excitability and synaptic excitation in the reticular thalamic nucleus. J Neurosci. 30, 5465–5479 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lui H et al. , Progranulin Deficiency Promotes Circuit-Specific Synaptic Pruning by Microglia via Complement Activation. Cell. 165, 921–35 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arber S, Motor Circuits in Action: Specification, Connectivity, and Function. Neuron. 74, 975–989 (2012). [DOI] [PubMed] [Google Scholar]
- 23.Koch M, The neurobiology of startle. Prog Neurobiol. 59, 107–128 (1999). [DOI] [PubMed] [Google Scholar]
- 24.Ruland J, Return to homeostasis: downregulation of NF-κB responses. Nat. Immunol. 12, 709–714 (2011). [DOI] [PubMed] [Google Scholar]
- 25.Haynes SE et al. , The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci. 9, 1512–1519 (2006). [DOI] [PubMed] [Google Scholar]
- 26.Chovatiya R, Medzhitov R, Stress, Inflammation, and Defense of Homeostasis. Molecular Cell. 54, 281–288 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pollen AA et al. , Molecular Identity of Human Outer Radial Glia during Cortical Development. Cell. 163, 55–67 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pichery M et al. , Endogenous IL-33 is highly expressed in mouse epithelial barrier tissues, lymphoid organs, brain, embryos, and inflamed tissues: in situ analysis using a novel Il-33-LacZ gene trap reporter strain. J Immunol. 188, 3488–3495 (2012). [DOI] [PubMed] [Google Scholar]
- 29.Hoshino K et al. , The absence of interleukin 1 receptor-related T1/ST2 does not affect T helper cell type 2 development and its effector function. J. Exp. Med. 190, 1541–1548 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhuo L et al. , hGFAP-cre transgenic mice for manipulation of glial and neuronal function in vivo. Genesis. 31, 85–94 (2001). [DOI] [PubMed] [Google Scholar]
- 31.Yona S et al. , Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 38, 79–91 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen W-Y, Hong J, Gannon J, Kakkar R, Lee RT, Myocardial pressure overload induces systemic inflammation through endothelial cell IL-33. Proc Natl Acad Sci USA. 112, 7249–7254 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gong S et al. , A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 425, 917–925 (2003). [DOI] [PubMed] [Google Scholar]
- 34.Jung S et al. , Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 20, 4106–4114 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galatro TF, Vainchtein ID, Brouwer N, Boddeke EWGM, Eggen BJL, Isolation of Microglia and Immune Infiltrates from Mouse and Primate Central Nervous System. Methods Mol. Biol. 1559, 333–342 (2017). [DOI] [PubMed] [Google Scholar]
- 36.Srinivasan K et al. , Untangling the brain’s neuroinflammatory and neurodegenerative transcriptional responses. Nat Commun. 7, 11295 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andrews S, FastQC: a quality control tool for high throughput sequence data. Available online at: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (2010).
- 38.Aken BL et al. , The Ensembl gene annotation system. Database (Oxford). 2016, baw093 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim D et al. , TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 14, R36 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anders S, Pyl PT, Huber W, HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics. 31, 166–169 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Love MI, Huber W, Anders S, Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trapnell C et al. , Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nature biotechnology. 28, 511–515 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dunkley PR, Jarvie PE, Robinson PJ, A rapid Percoll gradient procedure for preparation of synaptosomes. Nat Protoc. 3, 1718–1728 (2008). [DOI] [PubMed] [Google Scholar]
- 44.Liddelow SA et al. , Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 541, 481–487 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Risher WC et al. , Astrocytes refine cortical connectivity at dendritic spines. Elife. 3, 166–24 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh SK et al. , Astrocytes Assemble Thalamocortical Synapses by Bridging NRX1α and NL1 via Hevin. Cell. 164, 183–196 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akil O et al. , Restoration of hearing in the VGLUT3 knockout mouse using virally mediated gene therapy. Neuron. 75, 283–293 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paz JT et al. , Closed-loop optogenetic control of thalamus as a tool for interrupting seizures after cortical injury. Nat Neurosci. 16, 64–70 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sorokin JM et al. , Bidirectional Control of Generalized Epilepsy Networks via Rapid Real-Time Switching of Firing Mode. Neuron. 93, 194–210 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


