Abstract
Some amphibians use chemical signals in addition to optical and acoustical signals to transmit information. Males of mantellid frogs from Madagascar and hyperoliid frogs from Africa emit complex, species- and sex-specific bouquets of volatiles from their femoral or gular glands. We report here on the identification, synthesis, and determination of the absolute configuration of a macrocyclic lactone occurring in several species of both families, (S)-3,7,11-dodec-6,10-dien-12-olide (S-14, frogolide). Macrolides are a preferred compound class of frog volatiles. Nevertheless, frogolide is the first macrocyclic lactone obviously derived from the terpene pathway, in contrast to known frog macrolides that are usually formed via the fatty acid biosynthetic pathway.
Keywords: Natural products, Terpenoids, Pheromones, Macrocycles, Lactones
Introduction
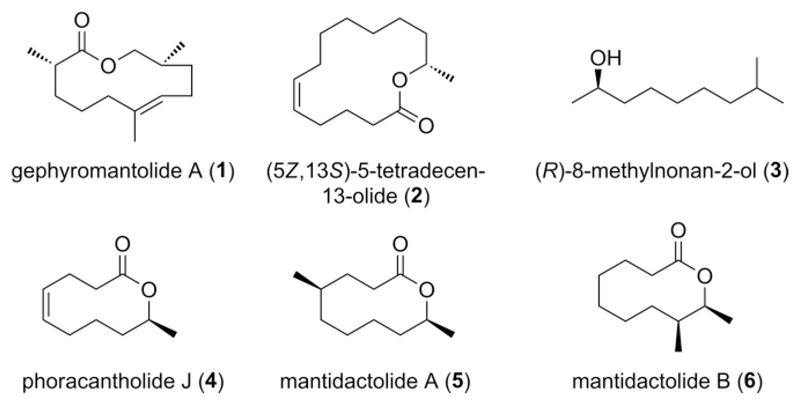
Anuran amphibians (frogs and toads) communicate via acoustic, visual, and tactile signals. In addition to these traits, some representatives of anuran families such as the Hyperoliidae and Mantellidae use the chemical communication channel to transmit information via volatile pheromones (Figure 1). The first volatile pheromone compounds identified from frogs were (R)-8-methylnonan-2-ol (3), and the macrolide phoracantholide J (4). Both compounds induce directional movement in both females and males of Mantidactylus multiplicatus, although the exact biological function of the pheromone is still open to discussion.[1] M. multiplicatus belongs to the family Mantellidae, endemic to Madagascar. Males of many mantellids possess femoral glands on the ventral sides of their shanks that disseminate volatile compounds. The volatiles are mostly alcohols and macrocyclic lactones, e.g. phoracantholide J (4),[1,2] gephyromantolide A (1),[1] mantidactolides (5, 6),[3] or other macrolides with even larger rings (2).[4,5] Further studies have provided evidence that macrolactones can actually be perceived by the olfactory system of mantellids[6] and that these frogs contain species-specific mixtures of volatiles despite occasional large variations within species.[2,6]
Figure 1.
Compounds produced in scent glands of anuran amphibians. For compounds 3 and 4, a species- and sex-specific, biological function has been experimentally tested.
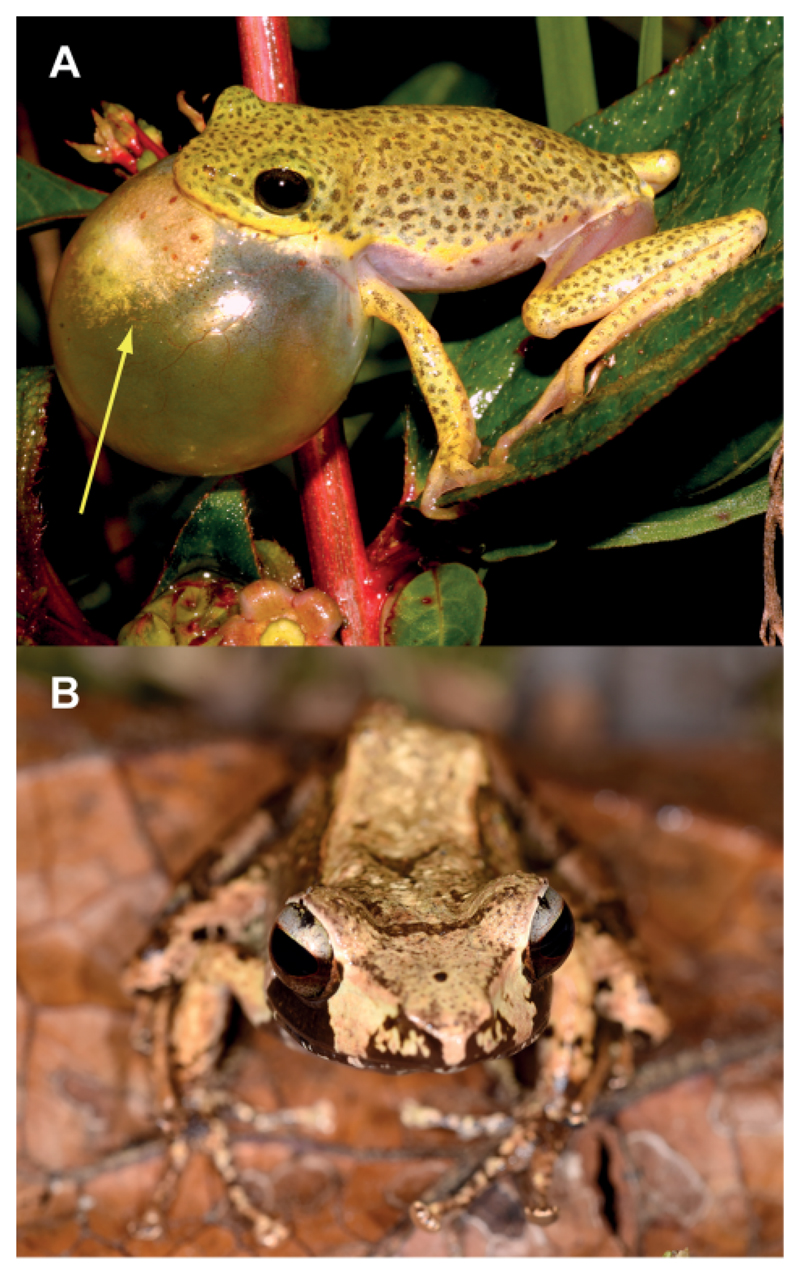
Another family obviously using volatile signals are the African reed frogs of the family Hyperoliidae. They emit acoustic and visual signals to attract females by inflation of a vocal sac that is innervated during the mating season. On these sacs a colorful gular gland is visible (Figure 2A) that releases a complex mixture of volatile compounds during calling. An initial screening study on several species revealed that these glands contain some volatiles similar to those of the Mantellidae, but additionally often sesquiterpenes, many of which not readily identifiable using mass spectroscopic databases.[7]
Figure 2.
Frog species with male scent glands. (A) Hyperolius viridiflavus during calling. The yellow gular gland is clearly visible (arrow) on the vocal sac. (B) Male of Gephyromantis leucomaculatus. The femoral glands on the hindlegs are not visible.
Currently we work on the identification of these sesquiterpenes in the Hyperoliidae that occur in complex species-specific blends. Due to the small amounts and the complex composition of the gland contents, the isolation and structural identification of unknown constituents is challenging. Therefore, microderivatization and mass spectral information are used to develop structural proposals. Finally, structure verification has to be performed by synthesis.[8]
Here we report on the identification of a novel unique sesquiterpene lactone, frogolide, by the approach described. This compound was of particular importance, because it occurs in several frog species of both mantellids and hyperoliids. Frogolide is again a macrolactone, but originates from the terpene pathway in contrast to other macrolactones produced by these frogs.
Results and Discussion
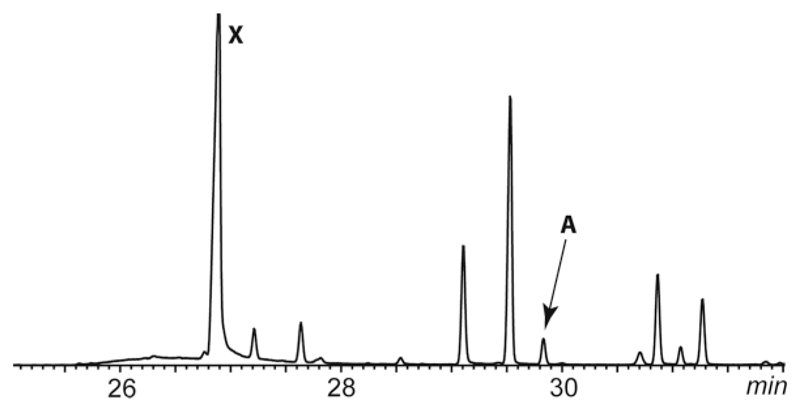
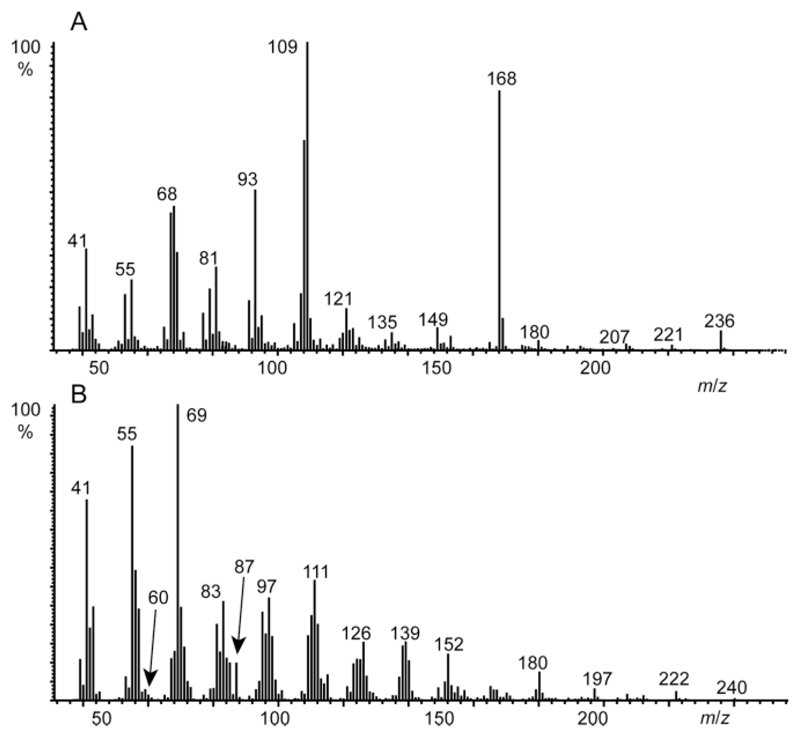
GC/MS analysis of an extract of the gular gland of Hyperolius viridiflavus (Figure 2A) revealed the presence of an unknown compound A with a gas chromatographic retention index I 1710 that occurred in several other frog species like e.g. Gephyromantis leucomaculatus (Figure 2B) as well. Figure 3 shows the total ion chromatogram of the gular gland extract of H. viridiflavus. The mass spectrum of compound A is shown in Figure 4A.
Figure 3.
Total ion chromatogram of a gular gland extract of H. viridiflavus. The title compound A is indicated. X indicates a contaminant. All other peaks are oxidized sesquiterpenes of unknown structure.
Figure 4.
Mass spectra of (A) natural compound A, and (B) hydrogenated natural compound A. The shift of the M+-ion by 4 amu indicates the presence of two C–C double bonds in A.
HR-MS delivered a molecular mass of m/z 236.1753 (calcd. 236.1776) consistent with the composition C15H24O2 with four double-bond equivalents. Hydrogenation of the natural sample furnished the mass spectrum shown in Figure 4B, most likely derived from A. A mass of 240 amu was obtained, hinting at two C–C double bonds in the molecule. We recently published a ruleset for obtaining structural information from the mass spectra of macrolactones.[8] A consecutive loss of two H2O units together with the M-60 ion indicate a macrolide. The hydrogenation product showed small ions at m/z 60 and 87, typical for 3-methyl-branched macrocyclic lactones. These ions arise by McLafferty-type rearrangement followed by a second McLafferty rearrangement or a β-cleavage, respectively.[8] The mass spectrum of A showed a strong ion m/z 168, a loss of 68 amu from M+. This fragment is typical for the loss of an isoprene unit, suggesting the unknown compound to be of terpenoid origin.[9] In addition, a double bond near the alcohol end of A becomes likely.
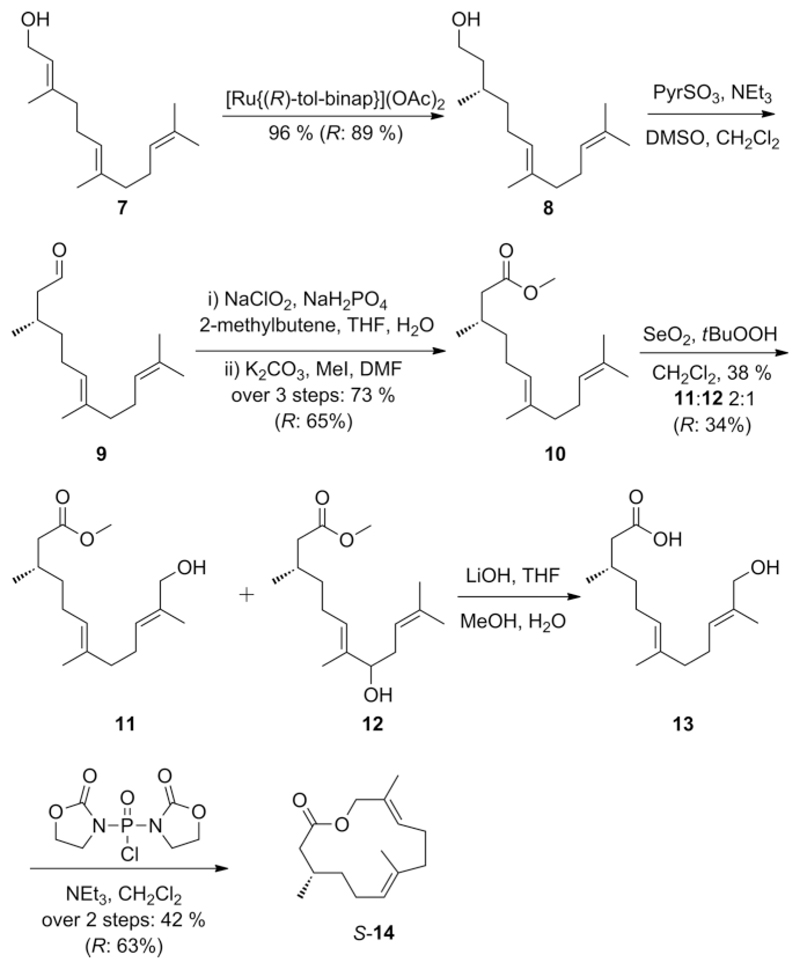
With these data in hand, we proposed A to be a macrolide derived from partly hydrogenated, terminally oxidized farnesoic acid. Often the 2,3-double-bond is hydrogenated in volatile farnesoic acid derived signaling compounds.[10] Therefore, 3,7,11-dodec-6,10-dien-12-olide (14) was chosen as target for our synthesis as being the most likely structure of A. The synthesis was performed according to Scheme 1.
Scheme 1.
Synthesis of frogolide (S-14). The synthesis was also performed with [Ru{(S)-tol-binap}](OAc)2 as catalyst, giving access to R-14.
Enantio- and regioselective hydrogenation of stereochemical pure (E,E)-farnesol (7) according to Pfaltz et al. using commercially available [Ru{(R)-tol-binap}](OAc)2 under 35 bar H2-atmosphere gave alcohol S-8 in 96 % yield.[11] The ee was 90 %, calculated back from final product 14. Subsequent stepwise oxidation using Parikh–Doering and Pinnick–Lindgren oxidation[12] followed by methylation[13] led to methyl ester 10 in 73 % yield over three steps. This stepwise procedure proved to be more effective than direct oxidation of alcohol 8 to the corresponding acid with 2-azaadamantane N-oxyl (AZADO) and PhI(OAc)2[13] that gave lower yields. Also oxidation of alcohol 8 using IBX led to acid catalyzed cyclization forming undesired byproducts and thus reducing the yield significantly.[14] The following Riley-Oxidation of methyl ester 10 gave two major products, alcohols 11 and 12 in a 2:1 ratio.[13] These alcohols were separable by column chromatography. Saponification of isolated 11 using LiOH furnished acid 13 that was directly lactonized with bis(2-oxo-3-oxazolidinyl)phosphinic chloride (BOP-Cl) and triethylamine according to Corey et al.[15] Target compound S-14 was obtained as clean product by simple filtration of the catalyst and removal of the solvent.
Gratifyingly, comparison of mass spectral and gas chromatographic data proved the identity of natural compound A and 14. The synthetic sequence was then performed again using [Ru{(S)-tol-binap}](OAc)2 as hydrogenation catalyst to obtain R-14 as well.
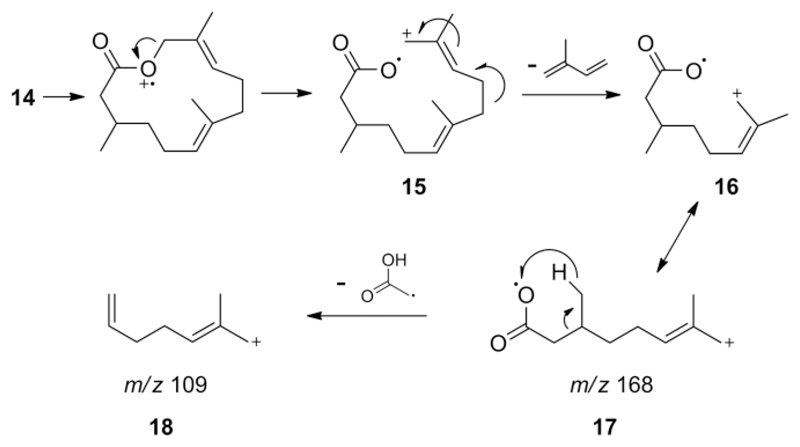
The formation of the characteristic peaks m/z 109 and 168 in the mass spectrum of 14 can be explained as shown in Scheme 2. Initial cleavage led to ion 16 that loses a stable isoprene molecule, the 68 amu fragment mentioned above. The distonic species 17 is formed with high intensity, stabilized by the allylic cation. An acetyl radical loss leads to the base peak m/z 109 (18) after H-transfer and double bond formation. The loss of isoprene can also be observed in mass spectra of other macrolides with the typical 1,5-dimethyl-1,5-diene structural motif such as brassicalactone[9] and niaviolide.[16]
Scheme 2.
Proposed fragmentation of frogolide (14) leading to the characteristic ions m/z 168 (17) and m/z 109 (18) of the mass spectrum (Figure 4A).
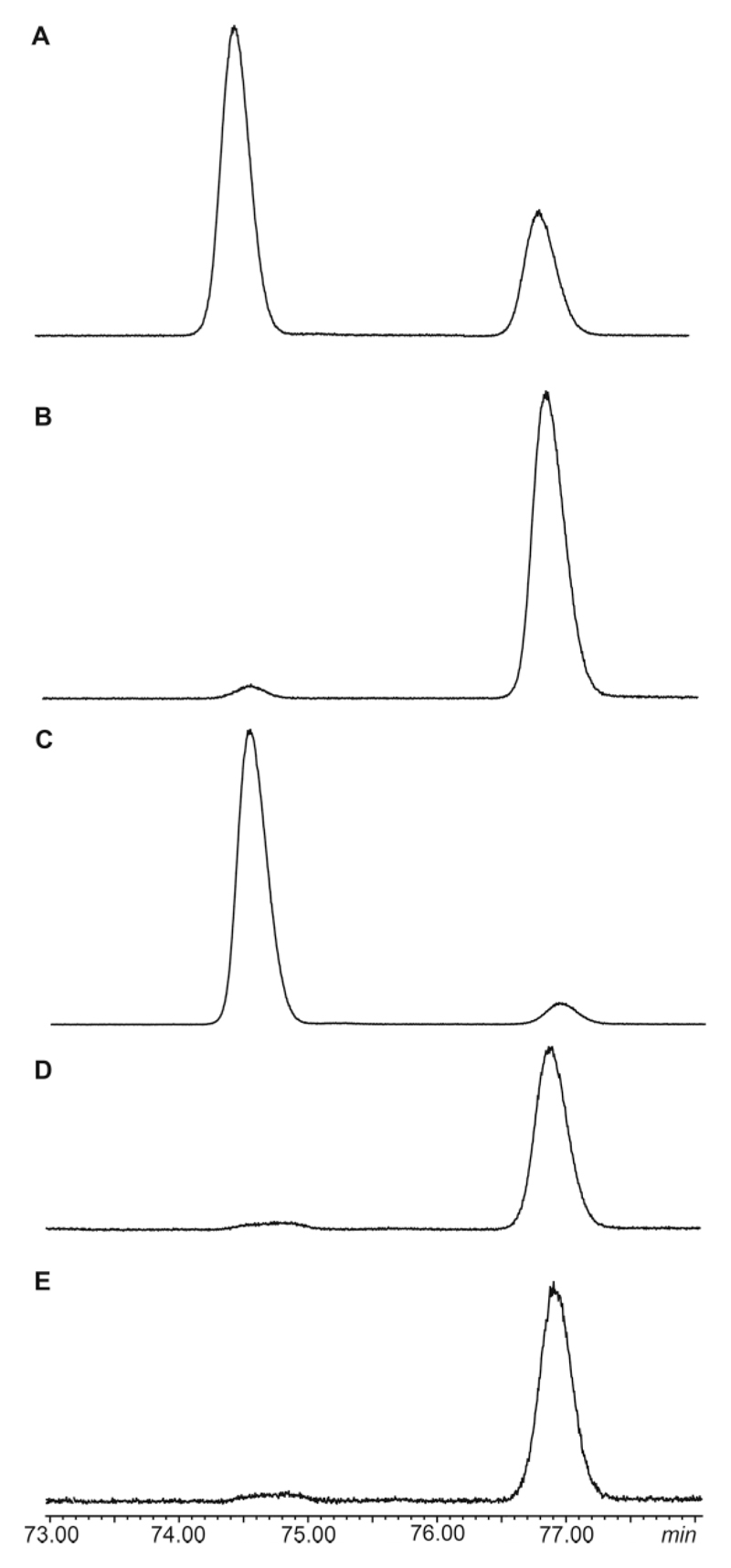
With the two enantiomers in hand, the absolute configuration of the natural compound was determined. Both enantiomers were separated by GC on a chiral β-TBDMS-hydrodex phase as shown in Figure 5. Coinjection of the racemic mixture with the (S)-enantiomer proved the later eluting peak to be the (S)-enantiomer. Synthetic S-14 had an ee of 90 % while synthetic R-14 showed an ee of 85 %. Natural 14 was a 98:2 S/R mixture.
Figure 5.
Total ion chromatogram of the gas chromatographic enantiomer separation of 14 on a chiral β-TBDMS-hydrodex phase. Temperature program: isothermal for 60 min at 110 °C, then with 2 °C/min to 160 °C, followed by a sharp ramp of 25 °C/min to 220 °C. (A) racemic mixture; (B) S-14; (C) R-14; (D) natural extract of a gland extract of H. cinnamomeoventris; (E) coinjection of the natural extract and S-14. Peak identities were confirmed by GC/MS.
Compound A was not only found in extracts of H. viridiflavus, but also in other species, both hyperoliids and mantellids (Table 1). These include H. cinnamomeoventris and several Gephyromantis, Guibemantis, Mantella, and Spinomantis species. In several of them only minor amounts occur, but extracts of H. viridiflavus, Spinomantis aglavei and Guibemantis liber were analyzed by chiral GC as well [see Supporting Information]. While H. viridiflavus contained a 98.5:1.5 S/R mixture, G. liber produced only S-14. S. aglavei also contained S-14, but its low abundance prohibited accurate ee determination.
Table 1.
Frog species containing frogolide (14) in their femoral or gular gland extracts obtained from males.
| Species | Frog family |
|---|---|
| Hyperolius cinnamomeoventris | Hyperoliidae |
| Hyperolius viridiflavus | Hyperoliidae |
| Gephyromantis granulatus | Mantellidae |
| Gephyromantis leucomaculatus | Mantellidae |
| Gephyromantis luteus | Mantellidae |
| Guibemantis liber | Mantellidae |
| Guibemantis pulcher | Mantellidae |
| Guibemantis tornieri | Mantellidae |
| Mantella aurantiaca | Mantellidae |
| Spinomantis aglavei | Mantellidae |
| Spinomantis taravatra | Mantellidae |
Because of its abundance across species, we propose the name frogolide for this new natural compound. Surprisingly, the prevalent macrolactone pattern in frog volatiles is also followed in the structure of this sesquiterpene. Macrolactones identified earlier, e.g. 2 and 4–6 are most likely derived from the fatty acid pathway.[17] In contrast, 14 is the first sesquiterpene macrolactone identified in frogs. The compound is closely related to niaviolide that additionally contains a C-2–C-3 doublebond. Niaviolide was isolated from the androconial organs of the African butterfly Amauris niavius.[16] Other sesquiterpenoid macrolactones used in chemical communication are the tris(norsesquiterpene)s cucujolide I [ferrulactone I, (4E,8E)-4,8-dimethyldeca-4,8-dien-10-olide], employed as pheromone in both cucujolid beetles[18] and Pieris butterflies,[9] and its isomer suspensolide (3E,8E)-4,8-dimethyldeca-3,8-dien-10-olide.[19]
Frogolide is another example of the lactone ring as a preferred structural motif of volatile signals.[17] Some macrolides are used by both insects and frogs, e.g. 2 and 4.[1,4,20] This might implicate that frogs potentially obtain the macrolides from their insect diet, which synthesize macrolides de novo[18,21] but can also take them up from plants.[22] Experiments with a laboratory colony of Mantidactylus betsileanus exclusively fed with fruit flies showed de novo synthesis of 4, which was absent in the food (unpublished). It seems likely that also the frogs investigated can synthesize 14 de novo, effectively using the terpene biosynthetic pathway besides fatty acid metabolism to synthesize macrolides.
Conclusions
We herein report a novel, unprecedented sesquiterpene macrolide, a rare class of natural products. The structure of frogolide was determined to be (3S,6E,10E)-3,7,11-trimethyldodeca-6,10-dien-12-olide (14). This compound is found in gular glands of male African hyperoliid frogs as well as femoral glands of male mantellid frogs endemic to Madagascar. We hypothesize that frogolide is involved in chemical communication of these frogs, although its exact function is yet unknown.
Experimental Section
General Remarks
Chemicals were obtained from commercial suppliers and used without further purification unless otherwise noted. Reactions were carried out in flame-dried vessels under a nitrogen atmosphere. Conventionally dried solvents were distilled before use. Purification of synthetic compounds was performed by column chromatography with silica (silica gel 60, particle size 0.040–0.063 mm, mesh 230–440 ASTM, Fluka) using ethyl acetate, pentane, and diethyl ether as solvents. Thin layer chromatography was performed using silica coated plates Polygram SIL G/UV254 (Macherey & Nagel) with molybdatophosphoric acid (10 % in ethanol) for detection. 1H NMR- and 13C NMR spectra were acquired with the following instruments (Bruker): AV II-300 (300 MHz for 1H and 75 MHz for 13C), DRX-400 (400 MHz for 1H and 100 MHz for 13C), AV III-400 (400 MHz for 1H and 100 MHz for 13C) and AV II-600 (600 MHz for 1H and 150 MHz for 13C). Tetramethylsilane was used as an internal standard (TMS, δ = 0 ppm). Multiplicities of the protons are described as singlets (s), doublets (d), triplets (t), quartets (q), quintets (quint), sextets (sext), septets (sept), or multiplets (m). The connectivities of the carbon atoms are described as primary (CH3), secondary (CH2), tertiary (CH), or quaternary (Cq). GC/MS analyses of synthetic products were performed with a GC HP6890/MSD HP5973 combination (Hewlett Packard) and natural samples were analyzed with a GC 7890A/MSD 5975C combination (Agilent Technologies). Mass spectrometry was performed in electron ionization mode (EI) with 70 eV. Fused-silica capillary columns HP-5MS (Agilent Technologies, 30 m, 0.25 mm i.d., 0.25 μm film thickness) were used with helium as the carrier gas. High resolution mass spectrometry data were obtained with a GC 6890 gas chromatograph (Agilent) equipped with a Phenomenex ZB5-MS column (30 m, 0.25 mm i.d., 0.25 μm film thickness) coupled with a time-of-flight mass spectrometer JMS-T100GC, GCAccuTOF (JEOL, Japan), operated in EI mode (70 eV). JEOL MassCenter Workstation Software was used. The instrument was calibrated with PFK to reach a resolution of 5000 (fwhm) at m/z 292.9824. Chiral gas chromatography was performed with a Hydrodex-β-6-TBDMS-column (25 m, 0.25 mm i.d., Macherey–Nagel). IR spectra were acquired with a Tensor 27 (Bruker) by using the diamond-ATR-technique, and GC/IR analysis was performed using a GC 7890B (Agilent Technologies) gas chromatograph coupled to a DiscovIR instrument (Dani Instruments). The samples eluting from the GC column were deposited on a cooled ZnSe disc at – 40 °C using a disc speed of 4 mm/min. The gas chromatograph was equipped with an Agilent HP-5 column (30 m, 0.25 mm i.d., 0.25 μm film thickness) with helium as the carrier gas. The resulting infrared spectra had a resolution of 4 wavenumbers and were normalized and processed using GRAMS/AI 9.2 software by Thermo Fisher Scientific Inc. modified with workbooks provided by Dani Instruments. The peaks are listed with wave numbers in cm–1. Intensities are described with s (strong), m (medium), w (weak) and br (broad). Optical rotation was measured with a Dr. Kernchen Propol Digital Automatic polarimeter with 1 cm cuvettes at a wavelength of 589 nm. Free-living adult male specimens of the target amphibian species were caught in the field at night in 2013 and 2014, during the respective breeding season; males emitting advertisement calls and thus being in the breeding condition were specifically selected. The frogs were sedated by the application of a small quantity of benzocaine, which is absorbed through the frog's skin, and euthanized by an overdose of the same substance in the field laboratory. Tissue from the vocal sac (hyperoliids) or the femoral gland (mantellids) was removed with sterilized scissors and tweezers, and immediately fixed in dichloromethane for chemical analysis. Samples were stored in 1 mL GC vials sealed with Teflon-lined caps to prevent modification or evaporation of compounds.
(S,E)-2,3-Dihydrofarnesol (8)
The catalyst [Ru{(R)-tol-binap}](OAc)2 (324 mg, 0.36 mmol, 0.02 equiv.) was added to a solution of (E,E)-farnesol (7, 4.00 g, 17.99 mmol, 1.0 equiv.) in degassed MeOH (35 mL) in a 40 mL cylindrical glass vial equipped with a magnetic stirrer bar that was transferred into a Teflon-lined high-pressure hydrogenation autoclave. The autoclave was purged with nitrogen, filled with hydrogen gas (35 bar), and the solution was stirred for 5 h at room temperature. The crude reaction mixture was then stirred for 10 min with added silica (3.24 g, 1000 wt.-% of catalyst) and then filtered. The filtrate was stirred again with activated charcoal (16 g, 400 wt.-% of starting material) for 15 min and filtered through a short silica plug. (S,E)-2,3-dihydrofarnesol (8) was obtained as a light yellow liquid ready to use without further purification in the next step (yield: 3.89 g, 17.34 mmol, 96 %). (R,E)-2,3-dihydrofarnesol was obtained in 89 % yield by using [Ru{(S)-tol-binap}](OAc)2 as the catalyst.[11] 1H NMR (400 MHz, CDCl3): δ = 0.91 [d, 3J(H,H) = 6.6 Hz, 3 H], 1.14–1.43 (m, 5 H), 1.60 (s, 6 H), 1.68 (s, 3 H, CH3), 1.96–2.09 (m, 6 H), 3.63–3.74 (m, 2 H), 5.07–5.13 (m, 2 H) ppm. 13C NMR (100 MHz, CDCl3): δ = 15.9, 17.7, 19.5, 25.4, 25.7, 26.7, 29.2, 37.2, 39.7, 39.9, 61.2, 124.3, 124.6, 131.3, 134.9 ppm. EI-MS (70 eV): m/z (%) 224 (1) [M]+, 209 (1), 181 (33), 163 (19), 149 (2), 137 (5), 123 (54), 109 (21), 95 (47), 81 (71), 69 (100), 55 (26), 41 (56). IR (GC/IR): [cm–1] 3274 (br.s), 2965 (s), 2926 (s), 2863 (s), 2853 (s), 1676 (w), 1451 (s), 1377 (s), 1107 (m), 1060 (s), 1013 (m). (S)-8: = +24.2 (c = 0.96 in CH2Cl2). (R)-8: = –26.4 (c = 1.10 in CH2Cl2).
Methyl (S,E)-2,3-Dihydrofarnesenoate (10)
A solution of pyridine·SO3 (2.12 g, 13.32 mmol, 3.0 equiv.) in DMSO (50 mL) was stirred for 15 min at room temperature. This mixture was added to a solution of (S)-8 (1.00 g, 4.45 mmol, 1.0 equiv.) and NEt3 (3.08 mL, 44.50 mmol, 10.0 equiv.) in CH2Cl2 (150 mL) at 0 °C. After stirring for 3 h at room temperature water was added (100 mL). The aqueous phase was extracted three times with CH2Cl2 (3 × 50 mL), and the combined organic phases were dried with MgSO4. The crude aldehyde 9 was used directly in the next reaction step after removal of the solvents.[12]
Aldehyde 9 (989 mg, 4.45 mmol, 1.0 equiv.) was added to a solution of NaH2PO4 (5.34 g, 44.5 mmol, 10.0 equiv.), 2-methyl-2-butene (20 mL), tert-butanol (15 mL) in THF (50 mL) and water (50 mL). Sodium chlorite (2.01 g, 22.25 mmol, 5.0 equiv.) was added partially in three equal portions every 45 min. After addition of the last portion, the mixture was stirred for an additional hour. Water was added (50 mL), and the aqueous phase was extracted three times with ethyl acetate (3 × 75 mL). The organic phase was washed with brine (50 mL), dried with MgSO4, and the solvents were removed. The residue was taken up in DMF (15 mL) and methyl iodide (1.264 g, 8.9 mmol, 2.0 equiv.) and K2CO3 (0.922 g, 6.68 mmol, 1.5 equiv.) were added. After stirring for 30 min, sat. NaHCO3 solution (30 mL) was added. The aqueous phase was extracted with ethyl acetate (3 × 75 mL). The organic phase was washed with brine (50 mL) and dried with MgSO4. After removal of the solvents, column chromatography (pentane/Et2O, 20:1) yielded the desired methyl ester 10 in 73 % yield over three steps (819 mg, 3.25 mmol). The (R)-enantiomer was obtained using (R)-dihydrofarnesol in 65 % yield over 3 steps under identical conditions. TLC (pentane/Et2O, 20:1): Rf 0.43. 1H NMR (300 MHz, CDCl3): δ = 0.94 (d, 3JH,H = 6.6 Hz, 3 H, CH3), 1.26–1.42 (m, 2 H, CH2), 1.60 (s, 6 H, 2 × CH3), 1.68 (d, 3JH,H = 1.1 Hz, 3 H, CH3), 1.90–2.07 (m, 7 H, 3 × CH2, CH), 2.08–2.36 (m, 2 H, CH2), 3.67 (s, 3 H, CH3), 5.07–5.13 (m, 2 H, 2 × CH) ppm. 13C NMR (75 MHz, CDCl3): δ = 15.9, 17.7, 19.6, 25.3, 25.7, 26.7, 30.0, 36.7, 39.7, 41.6, 51.3, 124.1, 124.3, 131.3, 135.7, 179.7 ppm. EI-MS (70 eV): m/z (%) 252 (2) [M]+, 237 (2), 209 (55), 177 (18), 163 (3), 151 (6), 135 (7), 123 (41), 109 (100), 95 (26), 85 (13), 81 (24), 73 (8), 69 (99), 59 (15), 55 (18), 41 (51). IR (GC-IR): = 2961 (s), 2921 (s), 2853 (s), 2729 (w), 1738 (s), 1702 (w), 1672 (w), 1437 (s), 1377 (s), 1289 (s), 1261 (m), 1197 (s), 1153 (s), 1108 (m), 1009 (m), 838 (m) cm–1. (S)-10: = –4.6 (c = 1.01 in CH2Cl2). (R)-10: = +4.1 (c = 0.78 in CH2Cl2).
Methyl (3S,6E,10E)-12-Hydroxy-2,3-dihydrofarnesenoate (11)
A solution of SeO2 (44 mg, 0.396 mmol, 0.2 equiv.) and tBuOOH (3.96 mmol, 2.0 equiv., 0.72 mL of a 5.5 m solution in nonane) in CH2Cl2 (10 mL) was stirred for 30 min at room temperature.[13] Ester 10 (0.5 g, 1.98 mmol, 1 equiv.) was added at 0 °C. After stirring for 5 h at room temperature Na2SO3 solution (10 mL) was added, and the aqueous phase was extracted three times with ethyl acetate (3 × 15 mL). The combined organic phases were washed with brine (10 mL), dried with MgSO4, and the solvents were removed. Column chromatography (pentane/ethyl acetate, 2:1) yielded 25 % of the desired product (133 mg, 0.495 mmol) 11 and 13 % of its regioisomer 12 (69 mg, 0.257 mmol). The (R)-enantiomer of 11 was obtained similarly in 22 % yield. TLC (pentane/EtOAc, 2:1): Rf 0.54. 1H NMR (400 MHz, CDCl3): δ = 0.94 (d, 3JH,H = 6.6 Hz, 3 H, CH3), 1.18–1.40 (m, 2 H, CH2), 1.60 (s, 3 H, CH3), 1.66 (s, 3 H, CH3), 1.94–2.34 (m, 9 H, 4 × CH2, CH), 3.67 (s, 3 H, CH3), 3.98 (s, 2 H, CH2), 5.08–5.12 (m, 1 H, CH), 5.36–5.40 (m, 1 H, CH) ppm. 13C NMR (100 MHz, CDCl3): δ = 13.6, 15.9, 19.5, 25.2, 25.9, 29.8, 36.6, 39.2, 41.5, 51.3, 68.7, 124.4, 125.6, 134.7, 134.8, 173.8 ppm. EI-MS (70 eV): m/z (%) 250 (14) [M – H2O]+, 235 (2), 219 (1), 207 (3), 199 (51), 194 (4), 181 (3), 175 (6), 167 (100), 161 (4), 149 (16), 139 (28), 121 (47), 107 (36), 93 (30), 81 (22), 77 (8), 69 (43), 65 (4), 59 (17), 55 (29), 41 (36). IR (GC-IR): = 3279 (br.s), 2956 (s), 2918 (s), 2853 (s), 1736 (s), 1672 (w), 1438 (s), 1377 (s), 1290 (s), 1198 (s), 1154 (s), 1105 (m), 1072 (s), 1011 (s), 958 (w), 879 (w), 844 (m) cm–1. S-11: = –2.4 (c = 0.98 in CH2Cl2). R-11: = +3.4 (c = 1.80 in CH2Cl2).
(3S,6E,10E)-3,7,11-Dodec-6,10-dien-12-olide, Frogolide (14)
Lithium hydroxide (97 mg, 4.06 mmol, 20 equiv.) was added to the ester 11 (55 mg, 0.203 mmol, 1 equiv.) dissolved in a mixture of THF (2 mL), methanol (2 mL), and water (1 mL).[15] The resulting mixture was heated to reflux at 80 °C for 14 h. After addition of sat. NH4Cl solution (3 mL), the mixture was acidified using 2 m HCl (ca. 1 mL), and the aqueous phase was extracted three times with ethyl acetate (3 × 5 mL). After removal of the solvent, the resulting crude acid 13 was used directly in the next step without further purification.
Triethylamine (144 mg, 200 μL, 1.42 mmol, 7 equiv.) and bis(2-oxo-3-oxazolidinyl)phosphinic chloride (BOP-Cl, 155 mg, 0.609 mmol, 3 equiv.) were added to a solution of the hydroxyacid 13 (52 mg, 0.203 mmol, 1 equiv.) in CH2Cl2 (5 mL). The resulting mixture was stirred for 6 h at room temperature, and sat. NH4Cl solution (5 mL) was added. The aqueous phase was extracted three times with CH2Cl2 (3 × 5 mL), and the combined organic phases were dried with MgSO4. After removal of the solvents, a short silica filter column using pentane/diethyl ether (20:1) yielded frogolide (14) in 42 % yield over two steps (20 mg, 0.085 mmol). The (R)-enantiomer was obtained in 63 % yield with upscaling by the factor of two. TLC (pentane/Et2O, 20:1): Rf 0.62. 1H NMR (400 MHz, CDCl3): δ = 1.01 (d, 3JH,H = 6.0 Hz, 3 H, CH3), 1.18–1.43 (m, 2 H, CH2), 1.53, (s, 3 H, CH3), 1.64 (s, 3 H, CH3), 1.86–2.35 (m, 9 H, 4 × CH2, CH), 4.07 (d, 2JH,H = 12.7 Hz, 1 H, CH2) 4.88 (d, 2JH,H = 12.7 Hz, 1 H, CH2), 5.14 (t, 3JH,H = 7.9 Hz, 1 H, CH), 5.31–5.34 (m, 1 H, CH) ppm. 13C NMR (100 MHz, CDCl3): δ = 14.7, 14.9, 20.8, 23.7, 24.4, 29.3, 36.0, 38.9, 43.6, 67.1, 126.4, 126.7, 130.4, 133.4, 172.4 ppm. EI-MS (70 eV): m/z (%) 236 (7) [M]+, 221 (1), 208 (1), 175 (1), 168 (88), 153 (5), 135 (6), 121 (12), 109 (100), 105 (4), 93 (44), 81 (24), 77 (8), 67 (45), 55 (20), 41 (32). IR (GC-IR): = 2957 (s), 2929 (s), 2862 (s), 1739 (s), 1690 (w), 1444 (s), 1378 (m), 1362 (m), 1304 (m), 1288 (s), 1256 (m), 1236 (m), 1221 (s), 1181 (w), 1071 (s), 1028 (m), 984 (w), 857 (m) cm–1. S-14: = –46.3 (c = 0.99 in CH2Cl2) R-14: = +36.1 (c = 0.61 in CH2Cl2).
Supplementary Material
Supporting information and ORCID(s) from the author(s) for this article are available on the WWW under https://doi.org/10.1002/ejoc.201800199.
Acknowledgments
We thank the Deutsche Forschungsgemeinschaft through research grant SCHU 984/10-1 and the Austrian Science Fund (FWF): P25612 for financial support. Work in Madagascar was carried out in the framework of a collaboration accord between TU Braunschweig and the Université d'Antananarivo, Département de Biologie Animale. We are grateful to the Malagasy authorities for research and export permits. Specimen collection in Rwanda was conducted within a collaboration between the University of Vienna and the University of Koblenz-Landau, Germany. We would like to particularly thank M. Dehling and P. M. Maier for their support. Specimen collection and export was authorized by the Rwanda Development Board.
References
- [1].Poth D, Wollenberg KC, Vences M, Schulz S. Angew Chem Int Ed. 2012;51:2187–2190. doi: 10.1002/anie.201106592. [DOI] [PubMed] [Google Scholar]; Angew Chem. 2012;124:2229. [Google Scholar]
- [2].Poth D, Peram PS, Vences M, Schulz S. J Nat Prod. 2013;76:1548–1558. doi: 10.1021/np400131q. [DOI] [PubMed] [Google Scholar]
- [3].Vences M, Wahl-Boos G, Hoegg S, Glaw F, Spinelli Oliveira E, Meyer A, Perry S, Vences M. Biol J Linn Soc. 2007;92:529–539. [Google Scholar]
- [4].Menke M, Peram PS, Starnberger I, Hödl W, Jongsma GFM, Blackburn DC, Rödel M-O, Vences M, Schulz S. Beilstein J Org Chem. 2016;12:2731–2738. doi: 10.3762/bjoc.12.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].a) Hötling S, Haberlag B, Tamm M, Collatz J, Mack P, Steidle JLM, Vences M, Schulz S. Chem Eur J. 2014;20:3183–3191. doi: 10.1002/chem.201304414. [DOI] [PubMed] [Google Scholar]; b) Peram PS, Vences M, Schulz S. Org Biomol Chem. 2017;15:6967–6977. doi: 10.1039/c7ob00849j. [DOI] [PubMed] [Google Scholar]
- [6].Nowack C, Peram PS, Wenzel S, Rakotoarison A, Glaw F, Poth D, Schulz S, Vences M. J Zool. 2017;303:72–81. [Google Scholar]
- [7].Starnberger I, Poth D, Peram PS, Schulz S, Vences M, Knudsen J, Barej MF, Rödel M-O, Walzl M, Hödl W. Biol J Linn Soc. 2013;110:828–838. doi: 10.1111/bij.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Schulz S, Peram PS, Menke M, Hötling S, Röpke R, Melnik K, Poth D, Mann F, Henrichsen S, Dreyer K. J Nat Prod. 2017;80:2572–2582. doi: 10.1021/acs.jnatprod.7b00366. [DOI] [PubMed] [Google Scholar]
- [9].Yildizhan S, van Loon J, Sramkova A, Ayasse M, Arsene C, ten Broeke C, Schulz S. ChemBioChem. 2009;10:1666–1677. doi: 10.1002/cbic.200900183. [DOI] [PubMed] [Google Scholar]
- [10].a) Luxová A, Urbanová K, Valterová I, Terzo M, Borg-Karlson A-K. Chirality. 2004;16:228–233. doi: 10.1002/chir.20017. [DOI] [PubMed] [Google Scholar]; b) Goodwin TE, Rasmussen EL, Guinn AC, McKelvey SS, Gunawardena R, Riddle SW, Riddle HS. J Nat Prod. 1999;62:1570–1572. doi: 10.1021/np990191n. [DOI] [PubMed] [Google Scholar]; c) Goodwin TE, Brown FD, Counts RW, Dowdy NC, Fraley PL, Hughes RA, Liu DZ, Mashburn CD, Rankin JD, Roberson RS, Wooley KD, et al. J Nat Prod. 2002;65:1319–1322. doi: 10.1021/np010647c. [DOI] [PubMed] [Google Scholar]
- [11].Wang A, Wüstenberg B, Pfaltz A. Angew Chem Int Ed. 2008;47:2298–2300. doi: 10.1002/anie.200705521. [DOI] [PubMed] [Google Scholar]; Angew Chem. 2008;120:2330. [Google Scholar]
- [12].Govek SP, Overman LE. Tetrahedron. 2007;63:8499–8513. [Google Scholar]
- [13].Sun Y, Chen P, Zhang D, Baunach M, Hertweck C, Li A. Angew Chem Int Ed. 2014;53:9012–9016. doi: 10.1002/anie.201404191. [DOI] [PubMed] [Google Scholar]; Angew Chem. 2014;126:9158. [Google Scholar]
- [14].Bartlett SL, Beaudry CM. J Org Chem. 2011;76:9852–9855. doi: 10.1021/jo201810c. [DOI] [PubMed] [Google Scholar]
- [15].Corey EJ, Hua DH, Pan BC, Seitz SP. J Am Chem Soc. 1982;104:6818–6820. [Google Scholar]
- [16].Stritzke K, Schulz S, Boppré M. Eur J Org Chem. 2003:1337–1342. [Google Scholar]
- [17].Schulz S, Hötling S. Nat Prod Rep. 2015;32:1042–1066. doi: 10.1039/c5np00006h. [DOI] [PubMed] [Google Scholar]
- [18].Vanderwel D, Johnston B, Oehlschlager AC. Insect Biochem Mol Biol. 1992;22:875–883. [Google Scholar]
- [19].Chuman T, Sivinski J, Heath RR, Calkins CO, Tumlinson JH, Battiste MA, Wydra RL, Strekowski L, Nation JL. Tetrahedron Lett. 1988;29:6561–6563. [Google Scholar]
- [20].a) Oehlschlager AC, King GGS, Pierce HD, Jr, Pierce AM, Slessor KN, Millar JG, Borden HJ. J Chem Ecol. 1987;13:1543–1554. doi: 10.1007/BF01012296. [DOI] [PubMed] [Google Scholar]; b) Moore BP, Brown WV. Aust J Chem. 1976;29:1365–1374. [Google Scholar]
- [21].Vanderwel D, Pierce HD, Jr, Oehlschlager AC, Borden JH, Pierce AM. Insect Biochem. 1990;20:567–572. [Google Scholar]
- [22].Schulz S. Eur J Org Chem. 1998:13–20. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.