Abstract
Therapeutic ultrasound is an established technique for biomodulation used by physical therapists. Typically it is used to deliver energy locally for the purpose of altering tissue plasticity and increasing local circulation. Access to ultrasound therapy has been limited by equipment and logistic requirements, which has reduced the overall efficacy of the therapy. Ultrasound miniaturization allows for development of portable, wearable, self-applied ultrasound devices that sidestep these limitations. Additionally, research has shown that the timescale of acoustic stimulation matters, and directly affects the quality of result. This paper describes a novel, long duration approach to therapeutic ultrasound and reviews the current data available for a variety of musculoskeletal conditions.
Keywords: Therapeutic ultrasound, regeneration, biomodulation, SAM
1. INTRODUCTION
Ultrasound, or mechanical pressure waves with frequencies above 20 kHz, has been studied for over 70 years. Ultrasound waves are used for a diverse range of biomedical applications including imaging, tissue ablation, and exerting forces on objects in the body such as kidney stones1, 2. In each case, the energy transferred by the wave is utilized in the body to mediate some beneficial medical effect. Another technique is the use of ultrasound as a direct therapy, so the acoustic waves transfer energy into tissue to mediate beneficial effects3, 4. This is similar to therapies utilizing light, however, the longer wavelength associated with acoustic waves means they are attenuated to a lesser degree, enabling the waves to penetrate much deeper into the body than optical frequency electromagnetic waves of similar intensity. This therapy is used clinically to apply heat to deep tissue, in order to modulate cellular metabolism and tissue properties, and to apply mechanical stimulation, which causes increased nutrient transfer out of the blood stream and to the surrounding tissue5, 6.
Therapeutic ultrasound is used in the treatment of muscle spasms, joint contractures, and to accelerate healing. Typical application of ultrasound therapy is performed in a clinical setting6. This is primarily due to the potential for thermal injury if traditional ultrasound therapy is provided improperly1. Ultrasound causes heating of tissue, and improper treatment can cause tissue damage. As a secondary cause, ultrasound equipment costs thousands of dollars, and typically is large enough to require dedicated space for treatment. An ultrasound transducer, typically engineered into a hand-held wand, is applied to the body by a trained medical practitioner and activated for 5–20 minutes.
In studies designed to elucidate the mechanism of action exploited by the clinical applications of therapeutic ultrasound, the direct biological effects of the mechanical wave have been studied. There are a number of different means for mechanical energy to influence biological systems. On the molecular level, ultrasound provides mechanical agitation, which is converted to heat as it is absorbed5, 6. Heat increases the Brownian motion of molecules in the blood stream, interstitial fluid, and cytosol7. The elevated temperature increases the rate of biochemical reactions8. The mechanical waves of ultrasound directionally accelerate transport kinetics, providing a force that drives convective mass transport, which occurs significantly faster than diffusion9. On the cellular level, ultrasound causes cells and tissue structures to experience a cycle of compression and rarefaction. This phenomenon increases the exchange rate on the interfaces between the cytosol, interstitial fluid, and bloodstream10. Cellular processes can be activated through mechanosensing proteins on the cell membrane, which modulate cell behavior11. These changes in cell behavior can lead to increased cellular migration, proliferation, or protein synthesis, depending on the cell type and the signaling proteins expressed on the cell surface11, 12. The cellular level is also modulated by the changes on the molecular level, because the increased transport kinetics will modulate the concentration and gradient of soluble cell signaling factors13. On the tissue level, ultrasound provides mechanical cues that can align collagen fibers or other extracellular matrix structures14, 15. The heat deposited in the area and the mechanical waves lead to increased local circulation, enhancing the nutrient supply to an area that is primed to distribute those nutrients from the blood stream to the cells16. The rate of waste removal is also increased, further improving the local cellular environment. Ultrasound also increases the elasticity of the overall tissue and increases the lubricity of the synovial fluid in the joint space17.
2. CLINICAL BENEFITS AND LIMITATIONS OF ULTRASOUND
The biological effects of ultrasound have been successfully evaluated in both human trials of clinical benefit and animal studies of mechanisms of action. A profile of randomized clinical trials for shoulder ailments found that the most benefits were seen with ultrasound when at least 4,000 J, on average, of acoustic energy was applied per treatment18–20. Ultrasound was also typically applied multiple times per week (3–5 in these studies). When used to treat tendinitis in the elbow and the knee, daily therapeutic ultrasound over 12 weeks resulted in significant reduction in pain and improvement in strength21, 22. In addition to these standard musculoskeletal ailments, therapeutic ultrasound has also been used to treat osteoarthritis. A pilot test of daily ultrasound for pain therapy found a clinically significant 2 point decrease in pain on a 10 point visual-analog scale23. The scientific community has built a clinical consensus on the benefit of daily treatment with more than 4,000 J of acoustic energy depositived per treatment. Multiple animal models have demonstrated that daily ultrasound therapy on models of osteoarthritis has resulted in improved gross appearance of the joint, delayed the progression of the disease, and improved histology scores in terms of cellular morphology at the bone interface24–28. Tendons treated with daily or multiple daily sessions of ultrasound therapy for 5–20 minutes possessed greater tensile strength and demonstrated increased collagen synthesis when compared with untreated tendons.
Therapeutic ultrasound is a widespread technique, with over 80% of physiotherapists using it in their practice29–31. However, ultrasound is often not applied the same way or with the same protocol in practice as it is during clinical research. Therapeutic ultrasound has been limited in its application by the requirement that a trained clinician administers the treatment. Due to the logistical difficulties associated with the therapy, the majority of patients who receive therapeutic ultrasound get therapy once or twice a week. The problem with this treatment paradigm is that the dosing provided is sub-optimal compared to what clinical research has shown is possible with optimal dosing.
3. SUSTAINED ACOUSTIC MEDICINE: A NOVEL TREATMENT CONCEPT TO IMPROVE CLINICAL PRACTICE
In order to facilitate the translation of clinical research to clinical practice, it is clear that the therapy must be adapted for self-administration. It is not reasonable to expect patients to access their healthcare practitioners on a daily basis. As mentioned in the introduction, therapeutic ultrasound is applied by practitioners because it can pose a safety risk if it is not applied properly or when effective acoustic coupling is not maintained for the entire treatment. Reducing the power output of the device mitigates some of the risk associated with thermal injury. Research has shown that ultrasound intensity under 150 mW/cm2 can be applied to the body for multiple hours without damaging tissue32–34. This type of therapy, called Low Intensity Therapeutic Ultrasound, or LITUS, has been studied for its ability to mediate significant results in recovery. The second obstacle to self-application requires innovative device engineering to overcome the challenges associated with proper treatment placement and protocol. Finally, in order to optimize the acoustic dose given the reduced intensity and to make sure sufficient energy is delivered to trigger a robust biological response, the treatment time could be extended to guarantee maximum delivery of acoustic energy.
Combining those features into a low intensity ultrasound device that can be safely applied by a user for multiple hours would enable a new type of ultrasound therapy, designed around the premise of prolonging the mechanical stimulus of the tissue to provide a robust biological response, and matching the time scale of the therapy to the timescale of the cell and tissue processes which it is accelerating during healing and recovery. This represents a new paradigm in therapeutic ultrasound, where duration is considered to be as important as frequency and intensity. Because this concept provides sustained mechanical stimulation of tissue, this treatment regimen is called sustained acoustic medicine (SAM). SAM conveys that the prolonged ultrasound therapy is having distinct, duration dependent effects, exponentially increasing the biological benefits of traditional Low Intensity Therapeutic Ultrasound (LITUS). SAM simultaneously increases the total dose of acoustic energy and the effectiveness of that dose, optimizing the strength of the therapy.
4. TECHNOLOGY FACILITATING THE SAM APPROACH TO ULTRASOUND THERAPY
4.1. Ultrasound Circuit Miniaturization
Novel circuit design and construction has dramatically increased the achievable efficiency with ultrasound, facilitating a transformation in equipment size from the bench scale to the handheld scale. This ultrasound device is a diverging-wave system that operates near 3 MHz with 0.132 W/cm2 ultrasound intensity for 4 hrs of treatment. The high efficiency RF ultrasound driver35–38 employs a parallel pin-driver configuration of MOSFET(s) to obtain a non-reactive output impedance of approximately 0.5 Ohm and a frequency bandwidth from DC-40 MHz. The voltage and frequency operating range of the ultrasound driver made it particularly well suited for portable and low-voltage battery powered ultrasound applications. The element is a silver-plated piezocrystal of lead-zirconate-titanate (PZT-4), that is air backed to uniformly channel the ultrasound towards the body.
4.2. Portable, Wearable Device Design
Inspired by low impedance design principles, a system can be effectively designed to be entirely portable and wearable. The first FDA cleared device using the principles of SAM (conveniently named sam®) to enhance ultrasound therapy is currently available for characterization and study. The circuit and the transducer were housed in a custom built plastic shell, sealed to prevent access and tampering, with the air-backed crystal positioned in a 5° divergent lens. The battery, along with timer and control circuitry are located in a separate housing, connected to the transducer driver assembly by a cable and a custom interlock. There is a split-cable adaptor that facilitates the connection of two transducers to one battery pack (Figure 1).
Figure 1:
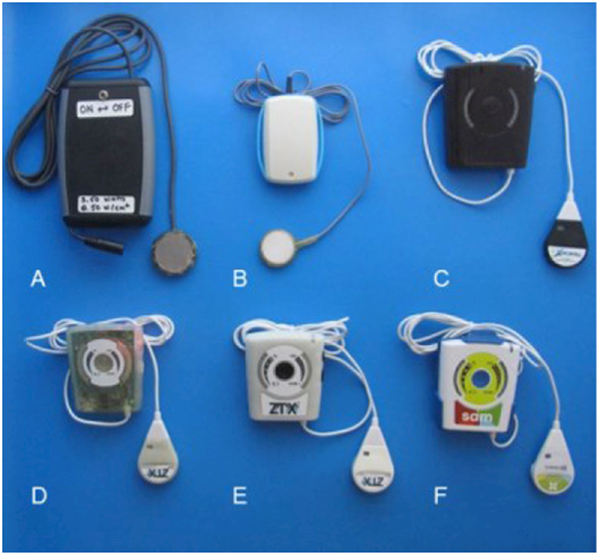
(a-f) Depictions of design iterations of portable, wearable device design, progressing from initial prototype towards final design.
SAM devices have been designed with efficiencies upward of 90% in battery to acoustic energy conversion. The corresponding transducer efficiency was measured to be 96%. When operated continuously from the battery at an acoustic output power of 1.3 W, or 0.132 W/cm2, the system can operate for up to 4 hours, which provides an acoustic dose in excess of 18,000 J. In comparison to the average clinical therapeutic ultrasound systems, the wearable ultrasound device is less than 1/20th of the size, does not require wall power, and provides an increased acoustic dose.
4.3. Long Duration Coupling Reservoir
The bandage is the first FDA-cleared ultrasound coupling system designed for four hours of use (Figure 2). During traditional ultrasound therapy, patients expose the area to be treated, and the area has a small amount of coupling medium applied to the skin, to prevent air from interrupting the wave path. Because of the dramatically different speed of sound through aqueous media and air, air reflects and scatters the acoustic wave, preventing it from arriving at its intended destination. The practitioner will regularly add more coupling medium throughout the treatment as it evaporates. For a self-administered treatment, the coupling reservoir would need to function for up to four hours without any intervention. This was achieved using a bandage which maintains attachment to the body during treatment, firmly secures the transducer in place, and maintains the coupling medium between the lens and the body for four hours. The coupling medium used is a novel, hydrogel based coupling medium that is over 90% water content, giving it the acoustic properties necessary for quality coupling39. The bandage was tested to maintain effective coupling of over 93% of the treatment area between the applicator lens and the body. Throughout the testing, the human factors performance was assessed to guarantee the bandage would perform in a variety of locations on different body types, and a patient could successfully apply it with minimal training and effort.
Figure 2:
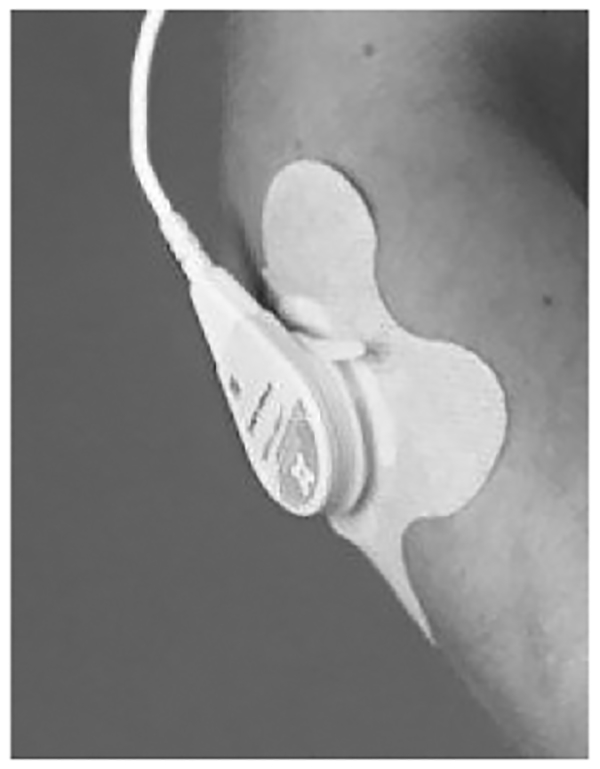
Bandage containing long-duration coupling medium attached to the body.
4.4. Closed Loop Temperature Monitoring
Despite the low intensity of the acoustic energy, there were concerns that the SAM therapy approach could unknowingly generate hazardous transfer of thermal energy to the body. To prevent this, a temperature monitor was added to the piezocrystal, and connected to a switch. This final precaution guaranteed that the system would operate within the validated realm of mechanical and thermal therapy.
5. SUSTAINED ACOUSTIC MEDICINE ENABLES RAPID TRANSLATION OF THERAPEUTIC ULTRASOUND’S BENEFITS TO CLINICAL PRACTICE
With an effective SAM device, there was the need to put the concept of the therapy into practice, to test if a prescription-strength therapy could be successfully applied by a layperson and if the therapy would have the expected beneficial effects. To test the potential power of SAM therapy, pilot clinical trials were run on conditions where ultrasound is either currently used, or where ultrasound therapy is of interest to the research community.
5.1. Condition #1: Trapezius muscle spasm38
Subjects (N=30) diagnosed with chronic trapezius muscle spasm whose pain was not managed with opioid pain medication alone were prescribed a system to be used in conjunction with pain medication. Subjects applied the device at the onset of a muscle spasm for one hour. Subjects successfully recorded their pain and overall therapeutic benefit scores using the (VAS) and global rate of change (GROC) scales, respectively.
On average, placebo users had an 8% pain reduction, while active users experienced a significant 16% reduction in pain (p <0.05). Figure 3 shows the temporal percent change in VAS over a 60-min treatment and 60-min post treatment as recorded in the patients diary averaged across the 10 treatment sessions. Active male and female users respond faster than placebo treatment as shown by the greater slope in the percent pain reduction graphs.
Figure 3:
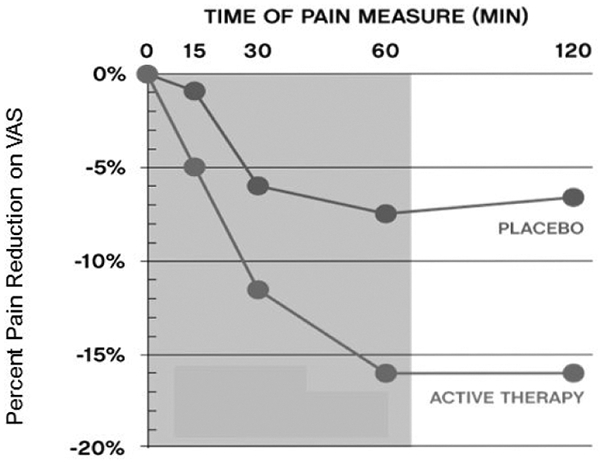
Placebo controlled pain relief for trapezius muscle spasms in a patient population where opioids were not providing adequate pain relief.
5.2. Condition #2: Rotator Cuff Pilot Data40
This pilot study evaluated SAM therapy in the management of shoulder pain from rotator cuff tendinopathy. Four subjects were enrolled and instructed to wear the device for 3–4 hours per day for 12 consecutive treatment sessions. Subjects recorded their daily pain score on the visual analog scale (1 to 10) and global rate of health improvement scale (−7 to 7). Across the 12 treatments, subjects reported a 30% reduction in pain and 52% improvement in health compared to baseline scores (p<0.05). This pilot study demonstrated the ability of patients to self-apply the SAM device and to utilize it to improve pain related to a widespread medical condition.
5.3. Condition #3: Osteoarthritis of the Knee23
Subjects with radiographic mild to moderate clinical knee osteoarthritis (Grade 1–2 on the OARSI scale) in one or both knees, average pain score >4 on a 10 point VAS scale during the week prior to enrollment were accepted into a clinical evaluation of SAM therapy for the relief of osteoarthritis pain. Participants were asked to report their medications but were not asked to modify them. Participants in the sample were taking a median of four prescription medications including Opiod/Narcotic, NSAID, Neurocognitive and Muscle Relaxers. Subjects were trained on the use of a device delivering SAM therapy, and asked to self-treat their affected knee at least four times per week for six weeks, recording their pain before and after treatment. Participants attended bi-weekly visits to the clinical study site to assess compliance.
Forty seven (N=47) subjects completed the study (n=28 active, n=19 placebo). For subjects with moderate to severe starting pain (VAS score ≥5, n=20 active, n=8 placebo), patients with active devices reported a 2.5 point decrease in pain over the six week study which was statistically different from the 1.23 point decrease of the placebo group (p<0.03) (Figure 4). The Initiative on Methods, Measurements, and Pain Assessment in Clinical Trials (IMMPACT) suggests that a decrease of 2/10 points on a VAS scale is clinically meaningful41, and the active device met that threshold.
Figure 4:
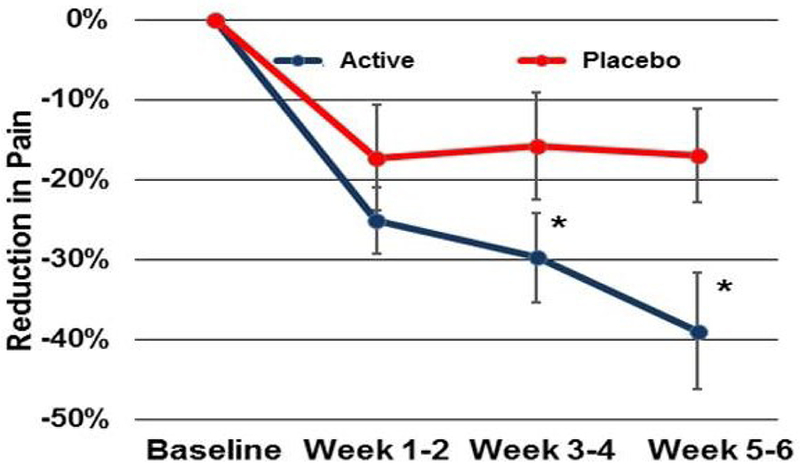
Placebo controlled pain relief for osteoarthritis over six weeks of SAM therapy.
5.4. Tendon Pain Relief and Recovery42
This study evaluated the ability of SAM therapy to relieve pain for patients suffering from tendon problems in the elbow, knee, or ankle. Subjects (N=25) were recruited into this study if they had a tendon issue that had been persistent for at least six weeks, and had been treated previously by a physical therapist without successful resolution. Subjects treated themselves daily with the device for a six week period. All subjects experienced reduced tendon pain with ultrasound therapy. VAS measurements decreased significantly throughout the 6-week study (Figure 5 top). The largest reduction in pain was observed at week 6, with a 4.28 VAS point decrease from baseline, p < 0.001. An overwhelming majority of patients who completed the trial, 93.75%, experienced at least a 50% decrease in pain. Pain also decreased significantly each day from start to end of treatment (0.60 VAS points, p < 0.001, Figure 5 bottom). VAS progressively decreased from pre-treatment (M = 2.67 ± 1.32), 30 minutes (M = 2.44 ± 1.31), 2 hours (M = 2.21 ± 1.29), and 4 hours into treatment (M = 2.06 ± 1.30), F(3,19) = 7.89, p = 0.001. There was a similar reduction in pain of 0.64 VAS points during the first 2 weeks of treatment when pain scores were higher, with a change from 3.35 pre-treatment to 2.71 post 4-hour treatment, F(3,19) = 13.84, p < 0.001.
Figure 5:
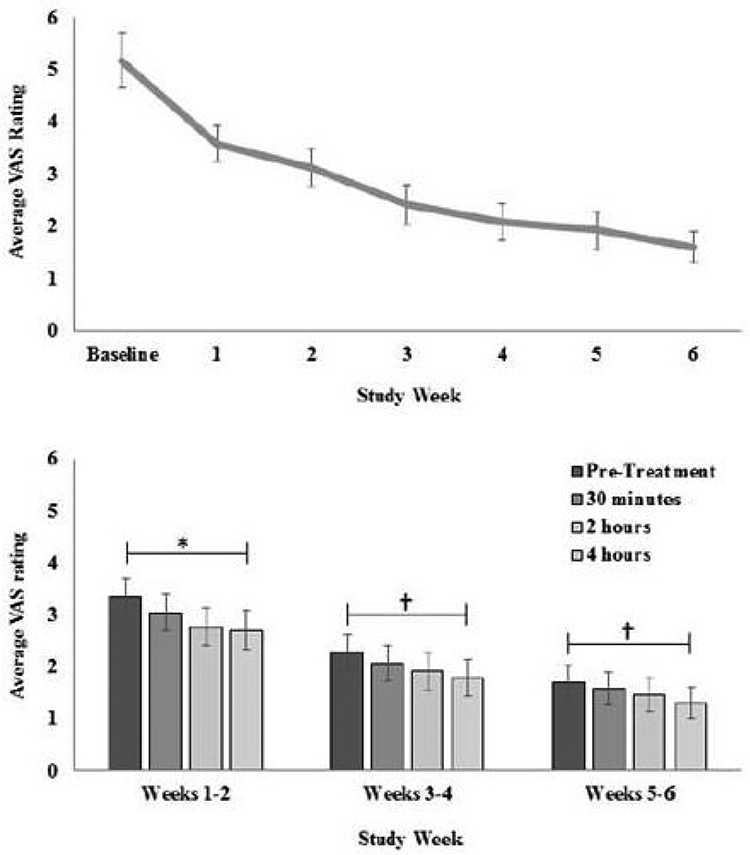
(top) Subject pain over six weeks of SAM therapy. The average pain decrease was 4.28 points on a 10 pt VAS scale (p < 0.001). (bottom) Subject pain decrease across single treatments (averaged over two week data periods). *(p < 0.001), †(p = 0.08)
6. DISCUSSION OF THERAPY, TECHNOLOGY, AND OPPORTUNITIES
SAM therapy is an innovative treatment approach method to promote more rapid ultrasound-mediated biomodulation using long duration exposures to acoustic waves. By prolonging the time in which the cells and tissue are stimulated with mechanical oscillations, SAM therapy seeks to trigger a more robust mechanotransduction response, leading to a greater activation of cells in the form of protein synthesis, cell proliferation, and integration between the cells and the extracellular matrix. The clinical application of SAM is as innovative as its therapeutic benefit. A self-applied treatment, SAM therapy engages patients directly, empowering them to take a direct role in administering their treatment and involving them with care. Patients who are actively involved with their care generally have better outcomes to their treatment, and data from the trials discussed above has suggested that there are patients who began exercise regimens or taking other active steps to ensure their health in addition to applying SAM on a daily basis.
In addition to the therapeutic innovation of the device, it is technically innovative. There were substantial engineering challenges to incorporating prescription strength, non-pharmaceutical therapy into a wearable device that can be easily used by the majority of patients without issue. Additionally, this wearable product actually provides treatment to a patient during normal daily activities. Most wearable wellness technology currently functions to obtain and report data only. This system moves a step beyond that, by providing a medically proven therapy that can accelerate healing and recovery, and gathering local information on temperature to regulate the rate at which that therapy is delivered. To successfully administer this therapy, innovative materials were required to successful couple and transfer the acoustic energy into the body efficiently. As this technology is advanced, further miniaturization may allow for the development of a system that is easily wearable with the entire therapeutic unit in a single shell. The technology will also need to be developed to allow for multiple different frequencies, intensities, and treatment profiles. As SAM is studied more in the field, researchers will begin to describe specific effects of ultrasound for conditions, and begin to optimize acoustic dose, duty cycle, frequency, intensity to maximize the beneficial biological response for individual conditions. As wearable technology and sensors develop further, additional technical features may also for direct observation of the body’s response to therapeutic ultrasound, both physically and biochemically. Additionally, the user interface for the device can be made more accessible to the average user. Particularly, by developing secure algorithms that can work with existing portable technology, software will allow patients to observe and monitor their treatment, and may interact with other wearable technology to assess things like strength, range of motion, or daily activity for the purpose of evaluating the body’s performance and recovery.
SAM has successfully mediated biomodulation in a large number of pre-clinical and clinical trials. SAM therapy has stimulated the deposition of collagen and increased the tensile strength of healing tendons in animal models. This compliments its pain relieving benefits that have been seen in the clinical trials. SAM therapy provides a long duration, robust mechanotransduction signal that is processed repeatedly as cells experience a continuous, oscillating pressure wave. Further research is required to identify the exact mechanisms of action and the basis for the sustained effect. The promise of SAM is a therapeutic technology that could be used by anyone, anywhere to treat soft tissue injuries. SAM may represent the next step in ultrasound therapies, and is the first available technology to bring a prescription strength biomodulation treatment to a wearable form factor.
REFERENCES
- [1].Miller DL, Smith NB, Bailey MR et al. , “Overview of therapeutic ultrasound applications and safety considerations,” J Ultrasound Med, 31(4), 623–34 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Heller M, and Johle D, “Fundamentals of Ultrasonography,” Emergency Medicine Clinics of North America, 15(4), 921–974 (1997). [DOI] [PubMed] [Google Scholar]
- [3].Mitragotri S, “Healing sound: the use of ultrasound in drug delivery and other therapeutic applications,” Nat Rev Drug Discov, 4(3), 255–60 (2005). [DOI] [PubMed] [Google Scholar]
- [4].Baker KG, Robertson VJ, and Duck FA, “A Review of Therapeutic Ultrasound: Biophysical Effects,” Physical Therapy, 81(7), 1351–1358 (2001). [PubMed] [Google Scholar]
- [5].Lehmann J, [Therapeutic Heat and Cold] Williams & Wilkins, Baltimore, MD, 429–530 (1990). [Google Scholar]
- [6].Draper DO, Castel JC, and Castel D, “Rate of temperature increase in human muscle during 1 MHz and 3 MHz continuous ultrasound,” Journal of Orthopaedic & Sports Physical Therapy, 22(4), 142–150 (1995). [DOI] [PubMed] [Google Scholar]
- [7].Le Bihan D, Delannoy J, and Levin RL, “Temperature mapping with MR imaging of molecular diffusion: application to hyperthermia,” Radiology, 171(3), 853–7 (1989). [DOI] [PubMed] [Google Scholar]
- [8].Busija DW, Leffler CW, and Pourcyrous M, “Hyperthermia increases cerebral metabolic rate and blood flow in neonatal pigs,” Am J Physiol, 255(2 Pt 2), H343–6 (1988). [DOI] [PubMed] [Google Scholar]
- [9].Johns LD, “Nonthermal effects of therapeutic ultrasound: the frequency resonance hypothesis,” J Athl Train, 37(3), 293–9 (2002). [PMC free article] [PubMed] [Google Scholar]
- [10].Rawool NM, Goldberg BB, Forsberg F et al. , “Power Doppler assessment of vascular changes during fracture treatment with low-intensity ultrasound,” J Ultrasound Med, 22(2), 145–53 (2003). [DOI] [PubMed] [Google Scholar]
- [11].Ross TD, Coon BG, Yun S et al. , “Integrins in mechanotransduction,” Current Opinion in Cell Biology, 25(5), 613–618 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tabdili H, Langer M, Shi Q et al. , “Cadherin-dependent mechanotransduction depends on ligand identity but not affinity,” Journal of cell science, 125(18), 4362–4371 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pounder NM, and Harrison AJ, “Low intensity pulsed ultrasound for fracture healing: A review of the clinical evidence and the associated biological mechanism of action,” Ultrasonics, 48(4), 330–338 (2008). [DOI] [PubMed] [Google Scholar]
- [14].Jackson BA, Schwane JA, and Starcher BC, “Effect of ultrasound therapy on the repair of Achilles tendon injuries in rats,” Med Sci Sports Exerc, 23(2), 171–6 (1991). [PubMed] [Google Scholar]
- [15].Fu SC, Shum WT, Hung LK et al. , “Low-intensity pulsed ultrasound on tendon healing: a study of the effect of treatment duration and treatment initiation,” Am J Sports Med, 36(9), 1742–9 (2008). [DOI] [PubMed] [Google Scholar]
- [16].Mundi R, Petis S, Kaloty R et al. , “Low-intensity pulsed ultrasound: Fracture healing,” Indian J Orthop, 43(2), 132–40 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Knight K, and Draper DO, [Therapeutic modalities: the art and science] Lippincot Williams & Wilkins, Baltimore, MD, 252–281 (2013). [Google Scholar]
- [18].Alexander LD, Gilman DRD, Brown DR et al. , “Exposure to Low Amounts of Ultrasound Energy Does Not Improve Soft Tissue Shoulder Pathology: A Systematic Review,” Physical Therapy, 90(1), 14–25 (2010). [DOI] [PubMed] [Google Scholar]
- [19].Ebenbichler GR, Erdogmus CB, Resch KL et al. , “Ultrasound Therapy for Calcific Tendinitis of the Shoulder,” New England Journal of Medicine, 340(20), 1533–1538 (1999). [DOI] [PubMed] [Google Scholar]
- [20].Shomoto K, Takatori K, Morishita S et al. , “Effects of Ultrasound Therapy on Calcificated Tendinitis of the Shoulder,” Journal of the Japanese Physical Therapy Association, 5(1), 7–11 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].D’Vaz AP, Ostor AJ, Speed CA et al. , “Pulsed low-intensity ultrasound therapy for chronic lateral epicondylitis: a randomized controlled trial,” Rheumatology (Oxford), 45(5), 566–70 (2006). [DOI] [PubMed] [Google Scholar]
- [22].Warden SJ, Metcalf BR, Kiss ZS et al. , “Low-intensity pulsed ultrasound for chronic patellar tendinopathy: a randomized, double-blind, placebo-controlled trial,” Rheumatology, 47(4), 467–471 (2008). [DOI] [PubMed] [Google Scholar]
- [23].Langer M, Taggart R, Ortiz R et al. , “Sustained Acoustic Medicine for the Treatment of Osteoarthritis of the Knee: A Randomized, Placebo Controlled Clinical Study.” [Google Scholar]
- [24].Cook SD, Salkeld SL, Patron LP et al. , “The effect of low-intensity pulsed ultrasound on autologous osteochondral plugs in a canine model,” Am J Sports Med, 36(9), 1733–41 (2008). [DOI] [PubMed] [Google Scholar]
- [25].Gurkan I, Ranganathan A, Yang X et al. , “Modification of osteoarthritis in the guinea pig with pulsed low-intensity ultrasound treatment,” Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society, 18(5), 724–733 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Jia XL, Chen WZ, Zhou K et al. , “Effects of low-intensity pulsed ultrasound in repairing injured articular cartilage,” Chin J Traumatol, 8(3), 175–8 (2005). [PubMed] [Google Scholar]
- [27].Lu H, Qin L, Fok P et al. , “Low-Intensity Pulsed Ultrasound Accelerates Bone-Tendon Junction Healing: A Partial Patellectomy Model in Rabbits,” The American Journal of Sports Medicine, 34(8), 1287–1296 (2006). [DOI] [PubMed] [Google Scholar]
- [28].Naito K, Watari Τ, Muta Τ et al. , “Low-intensity pulsed ultrasound (LIPUS) increases the articular cartilage type II collagen in a rat osteoarthritis model,” J Orthop Res, 28(3), 361–9 (2010). [DOI] [PubMed] [Google Scholar]
- [29].Robertson VJ, and Spurritt D, “Electrophysical Agents: Implications of their Availability and Use in Undergraduate Clinical Placements,” Physiotherapy, 84(7), 335–344 (1998). [Google Scholar]
- [30].Pope GD, Mockett SP, and Wright JP, “A Survey of Electrotherapeutic Modalities: Ownership and Use in the NHS in England,” Physiotherapy, 81(2), 82–91 (1995). [Google Scholar]
- [31].Wong RA, Schumann B, Townsend R et al. , “A Survey of Therapeutic Ultrasound Use by Physical Therapists Who Are Orthopaedic Certified Specialists,” Physical Therapy, 87(8), 986–994 (2007). [DOI] [PubMed] [Google Scholar]
- [32].Qin L, Fok P, Lu H et al. , “Low intensity pulsed ultrasound increases the matrix hardness of the healing tissues at bone–tendon insertion—a partial patellectomy model in rabbits,” Clinical biomechanics (Bristol, Avon), 21(4), 387–394 (2006). [DOI] [PubMed] [Google Scholar]
- [33].Cook S, Salkeld S, Popich-Patron L et al. , “Improved cartilage repair after treatment with low intensity pulsed ultrasound,” Clinical orthopaedics and related research, 391, S231–43 (2001). [DOI] [PubMed] [Google Scholar]
- [34].Huang M-H, Yang R-C, Ding H-J et al. , “Ultrasound effect on level of stress proteins and arthritic histology in experimental arthritis,” Archives of physical medicine and rehabilitation, 80(5), 551–556 (1999). [DOI] [PubMed] [Google Scholar]
- [35].Lewis G, and Olbricht JW, “Development of a portable therapeutic ultrasound system for military, medical and research use,” The Journal of the Acoustical Society of America, 124(4), 2551–2551 (2008). [Google Scholar]
- [36].Lewis GK Jr., and Olbricht WL, “Development of a portable therapeutic and high intensity ultrasound system for military, medical, and research use,” Rev Sci Instrum, 79(11), 114302 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lewis GK Jr., and Olbricht WL, “Design and characterization of a high-power ultrasound driver with ultralow-output impedance,” Rev Sci Instrum, 80(11), 114704 (2009). [DOI] [PubMed] [Google Scholar]
- [38].Lewis GK, Langer MD, Henderson CR et al. , “Design and Evaluation of a Wearable Self-Applied Therapeutic Ultrasound Device for Chronic Myofascial Pain,” Ultrasound in medicine & biology, 39(8), 1429–1439 (2013). [DOI] [PubMed] [Google Scholar]
- [39].Langer M, Guarino S, Fleshman S et al. , “Hydrogels for ultrasound coupling and imaging.” [Google Scholar]
- [40].Lewis G, Hernandez L, Lewis GK Sr. et al. , “Wearable long duration ultrasound therapy pilot study in rotator cuff tendinopathy,” Proceedings of Meetings on Acoustics, 19(1), - (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Dworkin RH, Turk DC, McDermott MP et al. , “Interpreting the clinical importance of group differences in chronic pain clinical trials: IMMPACT recommendations,” Pain, 146(3), 238–44 (2009). [DOI] [PubMed] [Google Scholar]
- [42].Draper DO, Moorman CT, Henderson S et al. , “Sustained Acoustic Medicine: A novel therapeutic modality for sustained deep tissue heating and accelerating tendon recovery.” [Google Scholar]


