Abstract
Background
Symptom burden, medical comorbidities, and functional well-being of patients with chronic hepatitis C virus (HCV) initiating direct acting antiviral (DAA) therapy in real-world clinical settings are not known. We characterized these patient-reported outcomes (PROs) among HCV-infected patients and explored associations with sociodemographic, liver disease, and psychiatric/substance abuse variables.
Methods and findings
PROP UP is a large US multicenter observational study that enrolled 1,600 patients with chronic HCV in 2016–2017. Data collected prior to initiating DAA therapy assessed the following PROs: number of medical comorbidities; neuropsychiatric, somatic, gastrointestinal symptoms (PROMIS surveys); overall symptom burden (Memorial Symptom Assessment Scale); and functional well-being (HCV-PRO). Candidate predictors included liver disease markers and patient-reported sociodemographic, psychiatric, and alcohol/drug use features. Predictive models were explored using a random selection of 700 participants; models were then validated with data from the remaining 900 participants. The cohort was 55% male, 39% non-white, 48% had cirrhosis (12% with advanced cirrhosis); 52% were disabled or unemployed; 63% were on public health insurance or uninsured; and over 40% had markers of psychiatric illness. The median number of medical comorbidities was 4 (range: 0–15), with sleep disorders, chronic pain, diabetes, joint pain and muscle aches being present in 20–50%. Fatigue, sleep disturbance, pain and neuropsychiatric symptoms were present in over 60% and gastrointestinal symptoms in 40–50%. In multivariable validation models, the strongest and most frequent predictors of worse PROs were disability, unemployment, and use of psychiatric medications, while liver markers generally were not.
Conclusions
This large multi-center cohort study provides a comprehensive and contemporary assessment of the symptom burden and comorbid medical conditions in patients with HCV treated in real world settings. Pain, fatigue, and sleep disturbance were common and often severe. Sociodemographic and psychiatric markers were the most robust predictors of PROs. Future research that includes a rapidly changing population of HCV-infected individuals needs to evaluate how DAA therapy affects PROs and elucidate which symptoms resolve with viral eradication.
Trial registration
(Clinicaltrial.gov: NCT02601820).
Introduction
Chronic hepatitis C virus (HCV) infection affects over 3 million Americans and is a leading cause of liver failure, cirrhosis, and liver cancer[1, 2]. Though primarily associated with liver disease, recent evidence suggests that HCV is a multi-faceted systemic condition that may be linked to many extra-hepatic disorders (EHDs) including arthritic-like pain, endocrine, metabolic, kidney, neuropsychiatric, and cardiovascular conditions [3–5]. Studies conducted during the interferon (IFN) treatment era found associations between HCV and neuropsychiatric, somatic and gastrointestinal (GI) symptom clusters, most commonly fatigue, sleep disturbance, irritability, depression, and pain[6–11]. Symptoms may be attributable to liver disease or to underlying inflammatory processes that mediate the relationship between HCV infection and EHDs [4, 12]. Beyond disease-related factors, psychosocial factors may also contribute to symptom burden. Psychiatric and substance use disorders, salient risk factors for contracting HCV, are highly prevalent among HCV patients and may be directly associated with poor health outcomes, irrespective of HCV infection or liver disease[9, 10, 13–15]. Indeed, neuropsychiatric symptoms are reported in up to 50% of HCV patients, independent of liver disease severity[16]. Additionally, the HCV population is further disadvantaged by social determinants of poor health (SDoH), including economic instability, low income, unemployment and lack of health insurance[17–19]. Finally, the psychological sequelae of harboring a transmittable disease, social stigma, and anxiety related to deteriorating liver health may also contribute to symptom burden and poor health outcomes[20–24]. Fortunately, the impact of these factors on health outcomes may be mitigated by public awareness that a short course of well tolerated direct acting antiviral (DAA) therapy has over 95% efficacy in achieving virologic cure.
Much of the data on HCV-associated symptoms, health-related quality of life (HRQOL), and treatment side effects, comes from the IFN treatment era [6–8, 25]. Qualitative studies elucidated patients’ experiences of symptoms and HRQOL[7, 26–28], but far fewer quantitative studies provided any in-depth analysis of HCV symptoms, especially among patients not on IFN therapy[6, 7]. During the IFN era, researchers identified 22 key patient-reported outcome (PRO) concepts from qualitative studies as important to the HCV population[7]. The majority of these PRO concepts received inadequate attention then and have received virtually no attention during the current DAA era. Several recent studies of HCV-infected patients treated with DAAs have investigated a few PRO concepts, such as HRQOL, fatigue, and work productivity[29, 30]. However, these data were derived from drug registration trials that enrolled highly selected patients with limited participation of under-represented racial minorities and subgroups with decompensated liver disease, EHDs, and medical, psychiatric and addiction comorbidities.
A comprehensive understanding of baseline symptom burden in patients with HCV is necessary to lay the groundwork for subsequent real-world investigations of potential changes in symptoms during DAA therapy and after virologic cure. We aimed to characterize patient-reported symptoms, medical conditions, and functional well-being in a large multi-center US cohort who initiated DAA therapy in clinical practices in 2016-2017. Our secondary aim was to evaluate sociodemographic/SDoH, liver-related, and other clinical features associated with these health outcomes.
Methods
Study design
The Patient-Reported Outcomes Project of HCV-TARGET (PROP UP) study is funded by the Patient-Centered Outcomes Research Institute (PCORI) and is a unique HCV study developed with engagement of patients affected by HCV and patient advocates. PROP UP is a multi-center, prospective, observational study that enrolled 1,600 patients across the U.S. to characterize patients’ experiences associated with HCV, DAA treatments, and virologic cure. The rationale and study protocol for PROP UP has been previously published[31]. Site recruitment began in January 2016 and ended in October 2017 at 11 U.S. medical centers (9 academic hepatology and 2 private gastroenterology). All sites were under the jurisdiction of their local Institutional Review Board (IRB) and obtained approval prior to study initiation (S1 Table). Inclusion criteria were made purposely broad to capture real world clinical experiences, and mainly required completion of baseline PROs and initiation of one of five DAA regimens. The current analysis used cross-sectional data collected at baseline prior to patients starting DAA therapy.
Measures
Brief details of the measures are provided below, as extensive details are provided elsewhere[31].
Number of medical comorbidities
Participants responded to a list of 30 chronic medical conditions regarding whether they (a) never had the condition; (b) have experienced the condition in the past; or (c) have experienced the condition in the last year (current condition). For these analyses we focused on predictors of current medical comorbidities.
Specific symptoms
The National Institutes of Health PROMIS instruments were used to capture three symptom clusters associated with HCV in the literature: neuropsychiatric (depression, anxiety, anger, cognitive concerns); somatic (pain interference, fatigue, sleep disturbance); and GI (abdominal pain, nausea/vomiting, diarrhea) clusters[32–34]. Psychometric testing of these PROMIS instruments in patients with HCV demonstrated satisfactory reliability and validity[35, 36]. Higher PROMIS T-scores reflect worse symptoms.
Overall symptom burden
A comprehensive list of 32 symptoms common to many medical conditions were assessed using the Memorial Symptom Assessment Scale (MSAS)[37, 38]. Participants reported the presence or absence of symptoms, and if present, its severity, frequency and level of distress. A total symptom burden (TMSAS) score was calculated. A higher TMSAS score reflects higher symptom burden.
Functional well-being
The HCV-PRO is a newly developed HCV-specific survey designed to evaluate the functional well-being of patients with HCV[39, 40]. The scale includes 16 items that measure various aspects of physical and emotional functioning, productivity, intimacy, and perceived quality of life related to having HCV. The total score ranges from 0 to 100; higher scores indicate better functional well-being.
Sociodemographics
Participants reported age, sex, racial background, educational attainment, annual household income, marital status, employment status, and health insurance status were explored as potential predictors of PROs.
Liver-related clinical and laboratory markers
The following data were extracted from participants’ medical records by trained site coordinators: HCV genotype, HCV RNA level, aspartate aminotransferase test (AST), alanine aminotransferase test (ALT), albumin, total bilirubin, platelets, hemoglobin, creatinine, and international normalized ratio (INR). Based on these laboratory data, we calculated various measures of advanced fibrosis/cirrhosis: (a) the AST to platelet ratio index (APRI); (b) the Fibrosis-4 Index for Liver Fibrosis (FIB-4), and (c) the Model for End-Stage Liver Disease (MELD), where FIB-4 >3.25 indicated advanced fibrosis; APRI >2.0 and MELD >6 indicated cirrhosis, and MELD ≥12 indicated advanced cirrhosis[41–43]. Site coordinators were also trained to review multiple sources of evidence in patient medical records, such as biopsy results, ultrasounds, MRIs, transient elastography scores, serum biomarker scores, and clinical notes for evidence of cirrhosis /stage 4 fibrosis. Based on the evidence, the trained site coordinators categorized patients as cirrhotic or noncirrhotic (Yes/No). All cross-referenced information in the dataset (e.g., labs, APRI, FIB-4, MELD, treatment type, duration, use of ribavirin) was reviewed to validate the accuracy of the cirrhosis categorization. When needed, sites were queried for additional information to support cirrhosis categorization (e.g., transient elastography scores). Adjudication of cases with inconsistent data was made by an experienced hepatologist (M.W.F.) who reviewed all available cross-referenced information or in less than .05% of cases, final adjudication was conducted by the site investigator who had access to the comprehensive medical record. These laboratory biomarkers and clinical data were explored as potential predictor variables.
Mental health and substance abuse history
Participants responded to five questions related to psychiatric history, two questions from the AUDIT[44] related to frequency and quantity of alcohol consumption, and two questions from the Substance Abuse Mental Illness Symptoms Screener (SAMISS) queried frequency of drug abuse in the past year, including use of nonprescription street drugs and prescription drugs [45]. Psychiatric questions queried (Yes/No) past and current psychiatric medication use, psychiatric diagnoses, psychiatric treatment, and inpatient psychiatric hospitalization.
Statistical analysis strategy
Descriptive statistics
To characterize the study cohort, graphical and tabular descriptive statistical methods (means, standard deviations (SD), range) were used to visualize the data and describe the empirical distributions of the four PRO constructs: (1) Number of medical comorbidities; (2) Specific Symptom Clusters (PROMIS instruments); (3) Overall Symptom Burden (TMSAS); and (4) Functional Well-Being (HCV-PRO), as well as participant characteristics (sociodemographics, liver-related features, psychiatric and substance abuse history).
Bivariate associations
To help characterize relationships between each patient characteristic and each PRO, unadjusted point and interval estimates of correlation coefficients were tabulated along with unadjusted estimates of subgroup PRO means/medians. Pearson correlation coefficients were used for continuous-vs-continuous pairs of variables; point-biserial correlation coefficients were used for binary (e.g. gender, cirrhosis)-vs-continuous pairs; Spearman correlation coefficients were used for ordinal (e.g., cirrhosis status combined with MELD score)-vs-continuous pairs; and for continuous PRO measures and multi-level nominal categorical variables (e.g., race, employment), we used the square root of the unadjusted R2 for the general linear model of the PRO measure conditional on the categorical variable as a predictor. The square root of R2 is a non-directional absolute value (r).
Multivariable models of association
In the inferential investigation of patient characteristics that may be predictive of PROs, we implemented a cross-validation strategy. Participants were assigned by randomization to two groups: sample 1 or sample 2. Sample 1 (n = 700) was used for exploratory model-building analyses to generate a set of candidate predictor variables that might be associated with each outcome. Sample 2 (n = 900) was used to test and potentially validate the candidate models. Essentially, two identical studies were performed in which the second was used to validate predictor models generated by the first.
Based on documented and informal information from the data collection process, missing values of the outcome variables and the candidate predictor variables are presumed missing at random or missing completely at random. Complete-case analyses (omitting patients with missing data) suffers from selection bias and loss of precision. Therefore, missing values for the predictor variables were addressed via multiple imputation. Patients having a missing value for an outcome variable were omitted from that regression analysis. A multivariate multiple imputation algorithm (SAS procedure MI) was used to generate 40 completed copies of the dataset for the multivariable analyses. Each statistical regression model of interest was fitted to all 40 datasets. The 40 sets of results were combined (SAS procedure MIANALYZE) to produce the final results shown for each multivariable regression model.
The candidate predictor variables for model-building based in Sample 1 are found in Table 1 and include: (1) sociodemographics; (2) liver-related clinical and laboratory markers; and (3) mental health and substance abuse variables. Some of the laboratory markers were transformed to log10 scale. In using Sample 1 to select candidate multivariable models for each PRO, we first examined models accounting for sociodemographics, then we looked at the additional contribution of liver lab and clinical variables, and finally psychiatric and substance abuse variables were included. Model-building with Sample 1 relied on stepwise variable selection algorithms or use LASSO (least absolute shrinkage and selection operator) variable-selection algorithms. Having completed all exploratory analyses and model development with Sample 1 (n = 700), we then used the data from Sample 2 (n = 900) for validation. Candidate predictor variables in models based on Sample 2 were considered “validated” if their regression coefficients were statistically significant at level α = 0.01.
Table 1. Patients characteristics (n = 1600).
| Characteristic | n (%) unless specified |
|---|---|
| Sociodemographics | |
| Age, years (mean (SD), range) | 57 (11), 22-85 |
| Sex | |
| Male | 886 (55) |
| Female | 714 (45) |
| Race | |
| Black | 519 (33) |
| White | 974 (61) |
| Other | 101 (6) |
| Ethnicity | |
| Non-White Hispanic | 66 (4) |
| Marital Status | |
| Single | 570 (36) |
| Divorced, Separated, Widowed | 442 (28) |
| Married or Domestic Partner | 573 (36) |
| Education Level | |
| ≤ High school diploma or equivalent | 863 (55) |
| > High school | 720 (45) |
| Annual Household Income | |
| ≤ $40K | 1164 (75) |
| > $41K | 398 (25) |
| Employment | |
| Working full or part time | 549 (36) |
| Receiving or applying for disability | 696 (45) |
| Unemployed | 110 (7) |
| Retired/homemaker/student | 191 (12) |
| Insurance status | |
| Private insurance | 542 (37) |
| Public insurance Medicaid/Medicare | 750 (51) |
| Uninsured or hospital assistance | 177 (12) |
| Liver Lab and Clinical Markers | Mean (SD), range |
| HCV RNA level (log10IU/ml) | 6 (1), 1-7 |
| AST (IU/L) | 70 (63), 10-1324 |
| ALT (IU/L) | 78 (69), 6-682 |
| Albumin (g/dL) | 4 (0.5), 1.6-5.1 |
| Total Bilirubin (mg/dL) | 1 (1.1), 0.4-21 |
| Platelets (103/μL) | 197 (79), 25-610 |
| Hemoglobin (g/dL) | 14 (2), 6-19 |
| Creatinine (mg/dL) | 1 (1) 0.6-14.9 |
| INR | 1 (0.2), 1-3.9 |
| n (%) | |
| Genotype | |
| Genotype 1 | 1285 (81) |
| Genotype 2-6 | 293 (19) |
| Cirrhosis status | |
| Cirrhotic | 764 (48) |
| Noncirrhotic | 825 (52) |
| MELD status in cirrhotics | |
| MELD 6-11 | 543 (85) |
| MELD ≥ 12 | 94 (15) |
| AST to Platelet Ratio Index (APRI) | |
| APRI ≤ 2.0 | 1313 (86) |
| APRI > 2.0 | 210 (14) |
| Fibrosis-4 (Fib-4) | |
| FIB-4 ≤ 3.25 | 1088 (71) |
| FIB-4 > 3.25 | 435 (29) |
| Psychiatric and Substance Use Markers | n (%) |
| History of psychiatric medication use | 803 (50) |
| If yes, currently taking psychiatric medications | 487 (30) |
| History of mental health diagnosis | 701 (44) |
| History of mental health treatment | 654 (41) |
| History of psychiatric hospitalization | 287 (18) |
| Frequency of current alcohol consumption | |
| Never | 1020 (64) |
| Monthly | 384 (24) |
| Weekly | 195 (12) |
| Number of alcoholic drinks on a drinking day | |
| None | 1001 (63) |
| 1 or 2 | 385 (24) |
| 3 to 6 | 177 (11) |
| 7 or more | 31 (2) |
| Use of nonprescription street drugs in last year | |
| Never | 1277 (80) |
| Less than monthly | 113 (7) |
| Monthly | 57 (4) |
| Weekly | 56 (3) |
| Daily / Almost daily | 90 (6) |
| Use of prescription drugs to get high or change the way you feel in last year | |
| Never | 1453 (91) |
| Less than monthly | 61 (4) |
| Monthly | 23 (1) |
| Weekly | 19 (1) |
| Daily / Almost daily | 39 (3) |
Model-based analyses for the patient-reported ‘Number of Medical Comorbidities’ relied on generalized log-linear models representing a mean count of comorbid conditions as a function of patient characteristics. Similarly, multivariable analyses for TMSAS and HCV-PRO measures relied on generalized linear models. The PROMIS Fatigue and PROMIS Sleep Disturbance T-scores also were studied using generalized linear models because they exhibited symmetric discrete distributions.
In contrast, the other eight symptom-specific PROMIS T-scores exhibited semi-continuous empirical distributions; that is, the scores follow a discrete distribution with a clumping of values at the floor of each scale (patients who reported no symptom). Therefore, the analysis of each of these 8 PROMIS measures relied on a generalized linear model for zero-inflated Poisson distributions. To represent both the probability of having the symptom and the conditional mean score as functions of patient characteristics, the zero-inflated Poisson model specifies two simultaneous regression equations for the modified T-score: one for the distribution of non-zero values (patients who have the symptom) and one for the clump of values at zero (for patients without the symptom). The dependent variable was computed as (T-score − K) with K being the observed floor of the scale. The floor values (K) were: Depression score > 42; Anger score > 35; Anxiety score > 45; Cognitive Concerns > 25; Pain Interference > 45; Belly Pain > 30; Diarrhea > 32; Nausea/Vomiting > 37 (these floor values were also used for other analyses of dichotomized T-scores). In these analyses, we focused on identifying patient characteristics associated with the non-zero values (having the symptom) to identify patients at risk for high symptom burden.
For all the PROs, sensitivity analyses included variations on the type of model fitted, variations on the engine used for exploratory hypothesis generation (e.g. stepwise, LASSO, least-angle regression, model averaging) and variations on the assumptions and criteria used (e.g. link function, α-levels for entry and exit). For the PROMIS T-scores, sensitivity analyses included logistic regression for the dichotomized T-scores, cumulative logit model analysis for categorized T-scores (low, medium, high), and zero-inflated negative binomial model analysis. All statistical estimates were computed along with corresponding 95% confidence intervals. Statistical computations were performed using SAS System software version 9.4 (SAS Institute, Cary, NC). PROMIS T-scores were computed using R software, version 3.1.2 (2014 The R Foundation for Statistical Computing), and RStudio software, version 1.0.136 (RStudio Inc.).
Results
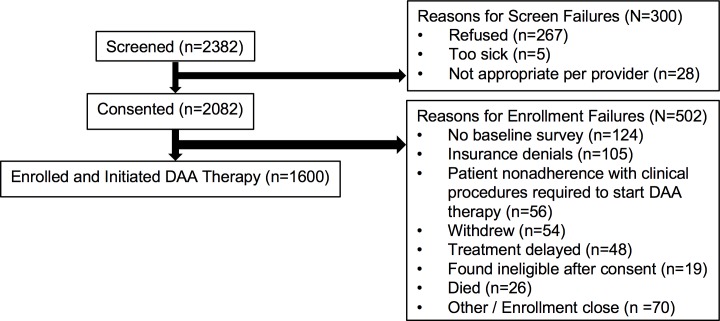
The study flowchart, which includes the number of patients screened, consented, and enrolled in PROP UP, is displayed in Fig 1. Of the nearly 2,400 patients screened, 87% consented to participate in the study. Of the 2082 consented patients, the main reasons patients were not enrolled included incomplete baseline surveys at the time that treatment was initiated (6%); insurance payer denials of DAA therapy (5%); patient lack of follow through with clinical requirements to start DAA therapy (e.g., completion of paperwork, urine toxicology screens) (3%); and verbal withdrawal from the study after consent (3%).
Fig 1. Study flowchart.
To explore the potential for selection bias that could affect generalizability of the study results, we compared the 1600 enrolled patients with 757 patients who were screened or consented, but not enrolled, on age, sex, and race. Compared to the 1600 patients enrolled, the 757 screen/enrollment failures were similar in age (55 years versus 57 years), sex (61% male versus 55%), and race (59% White versus 61%; 29% Black versus 33%).
Patient characteristics
Baseline patient characteristics are detailed in Table 1. Notably, 39% of patients self-identified as Black/African American (n = 519) or Other Race (n = 101), which was comprised of ‘other’ (n = 41), bi-racial/multi-racial (n = 32), American Indian (n = 17), Asian (n = 6), ‘not reported’ (n = 6) and native Hawaiian/Pacific Islander (n = 5). Annual household income was low (less or equal to $40,000) in 75% of patients. Around 45% of patients reported being recipients of disability benefits or in the process of applying for disability benefits, 36% were working a part-time or full-time job, and 7% were unemployed, out of work or looking for work. Fifty-one percent of patients had public health insurance (Medicare or Medicaid), 12% were uninsured, and 37% had private insurance or private plus Medicare.
Number of medical comorbidities and association with patient characteristics
In addition to liver-related problems, patients reported the presence of current and past comorbid conditions, 97% of these conditions was endorsed by at least one participant. The 10 most frequently reported comorbidities are listed in Table 2. Patients endorsed a mean of four comorbidities (range: 0–15) (S2 Table). Less than 10% (n = 154) reported no medical comorbidities, thus the majority had at least one medical condition other than chronic HCV. Notably, many of these comorbid conditions could represent EHDs.
Table 2. Top 10 patient reported medical comorbidities.
| Medical Comorbidities | Current Symptoms n (%) | Past Symptoms n (%) | Combined Symptomsa n (%) |
|---|---|---|---|
| Joint Pain | 804 (50) | 92 (6) | 863 (55) |
| High Blood Pressure | 790 (49) | 122 (8) | 887 (56) |
| Muscle Aches | 686 (43) | 78 (5) | 740 (47) |
| Vision Loss or Problems | 509 (32) | 73 (5) | 560 (36) |
| Sleep Disorder/Insomnia | 494 (31) | 63 (4) | 535 (34) |
| Chronic Pain Disorder/Orthopedic | 473 (30) | 75 (5) | 517 (33) |
| Diabetes/High Sugar Levels | 327 (20) | 46 (3) | 369 (23) |
| Asthma/COPD | 282 (18) | 61 (4) | 330 (21) |
| Hearing Loss or Problems | 267 (17) | 34 (2) | 290 (18) |
| High Cholesterol | 254 (16) | 89 (6) | 333 (21) |
a Each participant only counted once in combined column.
As shown in Table 3, a greater number of medical conditions correlated with sociodemographics (.20 ≤ r ≤ .40; being older, low income, public health insurance, disability), liver-related markers (.17 ≤ r ≤ .18; MELD ≥ 12, low albumin, low hemoglobin, high creatinine), and mental health markers (15 ≤ r ≤ .24; psychiatric medication use, diagnosis, treatment, inpatient hospitalization). Correlations with alcohol and substance abuse were lower (0 ≤ r ≤ .09).
Table 3. Patient characteristic subgroup means and correlations with PRO measures.
| PATIENT CHARACTERISTICS | Number of Comorbid Conditions | TMSAS | Depression | Anger | Anxiety | Cognitive Concerns | Pain Interference | Fatigue | Sleep Disturbance | Belly Pain | Diarrhea | Nausea/ Vomiting | HCV-PRO |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Mean | Mean | Mean | Mean | Mean | Mean | Mean | Mean | Mean | Mean | Mean | Mean | |
| r | r | r | r | r | r | r | r | r | r | r | r | r | |
| SOCIODEMOGRAPHICS | |||||||||||||
| Age (Mean = 57 years old) | 0.23 | -0.05 | -0.13 | -0.19 | -0.16 | -0.13 | 0.00 | -0.13 | -0.09 | -0.09 | 0.03 | -0.10 | 0.12 |
| Sex | 0.07 | 0.15 | 0.10 | 0.06 | 0.06 | 0.08 | 0.04 | 0.15 | 0.05 | 0.08 | 0.05 | 0.06 | 0.05 |
| Male | 3.8 | 0.5 | 48.4 | 48.3 | 49.8 | 32.3 | 52.6 | 50.3 | 51.2 | 35.1 | 35.2 | 41.2 | 72.6 |
| Female | 4.2 | 0.7 | 50.5 | 49.7 | 51.1 | 33.8 | 53.5 | 53.6 | 52.5 | 37.3 | 36.1 | 42.2 | 70.5 |
| Race | 0.06 | 0.12 | 0.13 | 0.15 | 0.13 | 0.13 | 0.04 | 0.18 | 0.13 | 0.10 | 0.03 | 0.11 | 0.14 |
| Black | 4.1 | 0.5 | 47.4 | 46.4 | 48.6 | 31.2 | 52.3 | 49.0 | 49.7 | 34.3 | 35.3 | 40.5 | 76.0 |
| White | 3.9 | 0.6 | 50.2 | 50.1 | 51.1 | 33.8 | 53.2 | 53.0 | 52.7 | 36.8 | 35.7 | 42.0 | 69.9 |
| Other | 4.5 | 0.7 | 51.3 | 50.6 | 53.1 | 33.8 | 53.7 | 53.9 | 53.2 | 38.5 | 36.3 | 43.8 | 66.8 |
| Marital Status | 0.11 | 0.13 | 0.13 | 0.03 | 0.12 | 0.07 | 0.14 | 0.10 | 0.07 | 0.10 | 0.03 | 0.10 | 0.13 |
| Single | 4.0 | 0.6 | 49.5 | 48.7 | 50.6 | 33.1 | 53.0 | 51.0 | 51.6 | 36.1 | 35.6 | 41.6 | 71.2 |
| Divorced, Separated, Widowed | 4.5 | 0.7 | 51.1 | 49.4 | 52.0 | 33.8 | 55.1 | 53.5 | 53.0 | 38.0 | 36.0 | 42.9 | 67.8 |
| Married, domestic partner | 3.6 | 0.5 | 47.8 | 48.6 | 48.9 | 32.3 | 51.3 | 51.2 | 51.0 | 34.7 | 35.3 | 40.7 | 75.0 |
| Education | 0.10 | 0.11 | 0.08 | 0.08 | 0.08 | 0.08 | 0.11 | 0.01 | 0.09 | 0.08 | 0.05 | 0.11 | 0.08 |
| ≤ High school diploma | 4.3 | 0.6 | 50.0 | 49.6 | 51.1 | 33.7 | 54.0 | 51.9 | 52.7 | 37.1 | 36.0 | 42.5 | 70.1 |
| > High school diploma | 3.7 | 0.5 | 48.4 | 47.9 | 49.5 | 32.2 | 51.7 | 51.6 | 50.6 | 34.9 | 35.1 | 40.7 | 73.6 |
| Annual Income | 0.20 | 0.20 | 0.18 | 0.09 | 0.15 | 0.13 | 0.25 | 0.13 | 0.13 | 0.16 | 0.07 | 0.15 | 0.22 |
| ≤ $40K | 4.3 | 0.6 | 50.4 | 49.4 | 51.3 | 33.7 | 54.6 | 52.6 | 52.6 | 37.3 | 35.9 | 42.4 | 68.8 |
| > $41K | 3.0 | 0.4 | 46.1 | 47.2 | 47.7 | 30.9 | 48.2 | 49.3 | 49.1 | 32.5 | 34.5 | 39.5 | 80.3 |
| Employment | 0.40 | 0.36 | 0.28 | 0.19 | 0.27 | 0.27 | 0.42 | 0.24 | 0.26 | 0.27 | 0.17 | 0.26 | 0.39 |
| Working full or part time | 2.7 | 0.4 | 46.0 | 47.1 | 47.7 | 30.5 | 48.1 | 49.2 | 48.7 | 32.3 | 33.8 | 39.3 | 80.4 |
| Receiving or applying for disability | 5.2 | 0.8 | 52.2 | 51.0 | 53.1 | 35.5 | 57.9 | 54.3 | 54.7 | 39.6 | 37.0 | 44.0 | 62.6 |
| Unemployed | 3.3 | 0.6 | 51.6 | 50.0 | 52.7 | 33.9 | 52.8 | 53.4 | 53.7 | 38.3 | 36.4 | 42.1 | 69.0 |
| Retired/homemaker/student | 3.7 | 0.4 | 46.7 | 45.8 | 46.8 | 30.2 | 49.0 | 48.6 | 48.5 | 33.3 | 34.8 | 39.7 | 81.0 |
| Health insurance | 0.32 | 0.24 | 0.19 | 0.13 | 0.18 | 0.17 | 0.31 | 0.17 | 0.20 | 0.20 | 0.11 | 0.22 | 0.27 |
| Private insurance | 2.8 | 0.4 | 46.7 | 47.1 | 48.0 | 31.0 | 48.5 | 49.4 | 48.9 | 32.8 | 34.3 | 39.3 | 79.6 |
| Public insurance Medicaid/Medicare | 4.9 | 0.7 | 50.5 | 49.5 | 51.5 | 34.2 | 55.7 | 53.3 | 53.3 | 38.2 | 36.3 | 43.0 | 66.7 |
| Uninsured or hospital assistance | 3.9 | 0.6 | 51.7 | 51.5 | 53.0 | 34.3 | 54.8 | 52.7 | 53.8 | 38.0 | 36.2 | 43.4 | 68.1 |
| LIVER LAB & CLINICAL MARKERS | |||||||||||||
| HCV RNA viral load (log10IU/ml) (Mean = 6) | 0.03 | 0.04 | 0.04 | 0.02 | 0.02 | 0.03 | 0.05 | 0.03 | 0.01 | 0.02 | 0.04 | 0.01 | -0.04 |
| AST (IU/L) (Mean = 70) | 0.00 | 0.06 | 0.03 | 0.04 | -0.02 | 0.02 | -0.01 | 0.03 | 0.08 | 0.04 | 0.03 | 0.01 | -0.05 |
| ALT (IU/L) (Mean = 78) | -0.07 | 0.00 | 0.02 | 0.08 | 0.00 | 0.01 | -0.04 | 0.02 | 0.06 | -0.01 | -0.03 | -0.03 | -0.05 |
| Albumin (g/dL) (Mean = 4) | -0.18 | -0.16 | -0.05 | -0.02 | -0.01 | -0.03 | -0.11 | -0.09 | -0.08 | -0.12 | -0.14 | -0.08 | 0.09 |
| Total Bilirubin (mg/dL (Mean = 1) | 0.01 | 0.04 | -0.01 | -0.03 | -0.03 | -0.02 | -0.02 | -0.01 | 0.05 | 0.01 | 0.02 | 0.03 | -0.01 |
| Platelets (103/μL) (Mean = 197) | -0.05 | -0.06 | -0.04 | -0.06 | 0.00 | -0.03 | 0.01 | -0.02 | -0.05 | -0.04 | -0.07 | 0.00 | 0.06 |
| Hemoglobin (g/dL) (Mean = 14) | -0.18 | -0.12 | 0.00 | 0.01 | 0.02 | -0.01 | -0.07 | -0.07 | -0.01 | -0.07 | -0.11 | -0.06 | 0.00 |
| Creatinine (mg/dL) (Mean = 1) | 0.13 | 0.02 | 0.00 | 0.00 | -0.02 | -0.02 | 0.05 | 0.02 | 0.03 | 0.00 | 0.04 | 0.06 | -0.01 |
| INR (Mean = 1) | 0.10 | 0.07 | 0.02 | 0.02 | 0.01 | 0.03 | 0.04 | 0.04 | 0.06 | 0.10 | 0.11 | 0.06 | -0.06 |
| Cirrhosis status | 0.07 | 0.05 | 0.03 | 0.01 | 0.02 | 0.05 | 0.02 | 0.03 | 0.05 | 0.05 | 0.03 | 0.02 | 0.05 |
| No | 3.8 | 0.5 | 48.9 | 49.0 | 50.6 | 32.7 | 52.7 | 51.4 | 51.3 | 35.4 | 35.3 | 41.5 | 72.8 |
| Yes | 4.2 | 0.6 | 49.7 | 48.7 | 50.2 | 33.3 | 53.2 | 52.1 | 52.3 | 36.7 | 35.9 | 41.9 | 70.6 |
| If cirrhotic, MELD score | 0.09 | 0.09 | 0.05 | 0.00 | 0.02 | 0.05 | 0.04 | 0.05 | 0.06 | 0.06 | 0.05 | 0.06 | 0.07 |
| MELD 6–11 | 4.0 | 0.6 | 49.4 | 48.3 | 49.8 | 33.1 | 53.3 | 51.9 | 51.9 | 36.3 | 35.1 | 41.5 | 71.4 |
| MELD ≥ 12 | 5.3 | 0.8 | 51.4 | 50.8 | 51.9 | 34.8 | 55.1 | 54.9 | 55.2 | 40.1 | 40.2 | 44.9 | 64.5 |
| AST to Platelet Ratio Index (APRI) | 0.02 | 0.09 | 0.07 | 0.06 | 0.01 | 0.06 | 0.00 | 0.08 | 0.10 | 0.07 | 0.11 | 0.05 | 0.09 |
| APRI ≤ 2.0 | 4.0 | 0.6 | 49.0 | 38.6 | 50.3 | 32.7 | 53.0 | 51.4 | 51.3 | 35.7 | 35.2 | 41.5 | 72.6 |
| APRI > 2.0 | 4.2 | 0.7 | 51.0 | 50.6 | 50.8 | 34.4 | 53.1 | 54.1 | 54.8 | 38.3 | 38.0 | 42.8 | 66.5 |
| Fibrosis-4 | 0.06 | 0.07 | 0.02 | 0.00 | 0.02 | 0.01 | 0.01 | 0.02 | 0.03 | 0.04 | 0.07 | 0.03 | 0.03 |
| FIB-4 ≤ 3.25 | 3.9 | 0.5 | 49.1 | 48.9 | 50.5 | 32.9 | 52.9 | 51.6 | 51.6 | 35.7 | 35.2 | 41.5 | 72.2 |
| FIB-4 > 3.25 | 4.3 | 0.6 | 49.7 | 48.8 | 50.1 | 33.1 | 53.3 | 52.2 | 52.4 | 36.9 | 36.5 | 42.1 | 70.7 |
| PSYCHIATRIC AND SUBSTANCE USE MARKERS | |||||||||||||
| History of psychiatric medication use | 0.21 | 0.35 | 0.38 | 0.30 | 0.36 | 0.33 | 0.27 | 0.30 | 0.24 | 0.20 | 0.10 | 0.19 | 0.38 |
| No | 3.4 | 0.4 | 45.3 | 45.5 | 46.7 | 30.0 | 50.0 | 48.5 | 49.0 | 33.5 | 34.7 | 40.0 | 80.0 |
| Yes | 4.6 | 0.7 | 53.2 | 52.2 | 54.0 | 36.0 | 55.9 | 55.0 | 54.5 | 38.6 | 36.4 | 43.2 | 63.2 |
| Currently on psychiatric medications | 0.12 | 0.23 | 0.22 | 0.12 | 0.19 | 0.19 | 0.14 | 0.16 | 0.04 | 0.15 | 0.10 | 0.13 | 0.17 |
| No | 4.2 | 0.6 | 50.3 | 50.5 | 51.5 | 33.8 | 53.9 | 53.0 | 53.9 | 36.0 | 35.2 | 41.8 | 67.9 |
| Yes | 4.9 | 0.8 | 55.1 | 53.3 | 55.6 | 37.4 | 57.1 | 56.3 | 54.8 | 40.3 | 37.1 | 44.2 | 60.0 |
| History of mental health diagnosis | 0.24 | 0.33 | 0.36 | 0.28 | 0.35 | 0.33 | 0.27 | 0.29 | 0.20 | 0.17 | 0.11 | 0.20 | 0.37 |
| No | 3.4 | 0.4 | 46.0 | 46.0 | 47.3 | 30.3 | 50.3 | 49.0 | 49.7 | 34.1 | 34.7 | 40.1 | 79.0 |
| Yes | 4.8 | 0.8 | 53.6 | 52.5 | 54.4 | 36.4 | 56.3 | 55.3 | 54.3 | 38.6 | 36.7 | 43.6 | 62.1 |
| History of mental health treatment or services | 0.16 | 0.27 | 0.30 | 0.25 | 0.30 | 0.29 | 0.17 | 0.24 | 0.15 | 0.14 | 0.09 | 0.18 | 0.29 |
| No | 3.6 | 0.5 | 46.7 | 46.6 | 47.8 | 30.8 | 51.4 | 49.5 | 50.3 | 34.6 | 34.9 | 40.3 | 77.2 |
| Yes | 4.5 | 0.7 | 53.0 | 52.2 | 54.0 | 36.1 | 55.1 | 54.9 | 53.7 | 38.2 | 36.5 | 43.4 | 64.0 |
| History of psychiatric hospitalization | 0.15 | 0.24 | 0.24 | 0.21 | 0.26 | 0.25 | 0.14 | 0.19 | 0.11 | 0.15 | 0.10 | 0.18 | 0.24 |
| No | 3.8 | 0.5 | 48.1 | 47.8 | 49.2 | 31.9 | 52.2 | 50.8 | 51.1 | 35.2 | 35.2 | 40.9 | 74.2 |
| Yes | 4.9 | 0.8 | 54.7 | 53.9 | 55.9 | 37.9 | 56.4 | 56.2 | 54.5 | 40.1 | 37.5 | 44.8 | 60.0 |
| Frequency of current alcohol consumption | 0.08 | 0.10 | 0.03 | 0.03 | 0.09 | 0.09 | 0.09 | 0.06 | 0.05 | 0.11 | 0.02 | 0.04 | 0.09 |
| Never | 4.2 | 0.6 | 49.6 | 49.2 | 51.1 | 33.6 | 53.7 | 52.3 | 52.2 | 37.1 | 35.7 | 41.9 | 70.2 |
| Weekly to monthly consumption | 3.7 | 0.5 | 48.9 | 48.4 | 49.2 | 31.8 | 51.6 | 50.9 | 51.1 | 34.3 | 35.4 | 41.2 | 74.4 |
| Number of alcoholic drinks on a drinking day | 0.09 | 0.10 | 0.03 | 0.03 | 0.09 | 0.09 | 0.10 | 0.06 | 0.05 | 0.12 | 0.02 | 0.04 | 0.09 |
| None | 4.2 | 0.6 | 49.6 | 49.2 | 51.1 | 33.6 | 53.8 | 52.2 | 52.3 | 37.3 | 35.7 | 41.9 | 70.1 |
| 1+ | 3.7 | 0.5 | 48.9 | 48.5 | 49.3 | 31.9 | 51.6 | 51.0 | 51.0 | 34.1 | 35.3 | 41.2 | 74.2 |
| Use of nonprescription street drugs in last year | 0.00 | 0.07 | 0.10 | 0.11 | 0.08 | 0.07 | 0.02 | 0.03 | 0.07 | 0.04 | 0.03 | 0.09 | 0.05 |
| Never | 4.0 | 0.6 | 48.8 | 48.3 | 50.0 | 32.7 | 52.8 | 51.6 | 51.4 | 35.8 | 35.4 | 41.2 | 72.2 |
| Daily to monthly | 4.0 | 0.6 | 51.3 | 51.3 | 52.1 | 34.2 | 53.5 | 52.4 | 53.5 | 37.0 | 36.2 | 43.3 | 69.3. |
| Use of prescription drugs to get high or change the way you feel in last year | 0.05 | 0.12 | 0.14 | 0.15 | 0.13 | 0.14 | 0.07 | 0.11 | 0.11 | 0.08 | 0.07 | 0.15 | 0.12 |
| Never | 4.0 | 0.6 | 48.8 | 48.3 | 50.0 | 32.6 | 52.7 | 51.4 | 51.4 | 35.8 | 35.4 | 41.2 | 72.5 |
| Daily to monthly | 4.5 | 0.8 | 54.2 | 54.4 | 54.5 | 36.9 | 55.6 | 55.7 | 55.8 | 39.2 | 37.4 | 45.8 | 63.1 |
Mean scores for all patient characteristic subgroups by PRO measures and correlation coefficients (r) as degree of relationship between the predictor variable and PRO measure. Correlations range from 0 (no association) to +/- 1.0 (perfect association).
Table 4 shows the results of the confirmatory multivariable analyses (n = 900) predicting Number of Medical Comorbidities and other PROs. The estimates are the predicted percent (%) increase in the mean score attributable to a specific patient characteristic. For example, a higher Number of Medical Comorbidities was associated with being older (2.7% higher for each additional year) and being disabled (53% higher).
Table 4. Validated predictors of PROs.
| Entries are % increase in the mean score with 95% C.I. | Number of Medical Comorbidities | Overall Symptom Burden (TMSAS) | Functional Well-Beinga (HCV-PRO) | PROMIS Fatigue T-score | PROMIS Sleep Disturbance T-score |
|---|---|---|---|---|---|
| Older Age | 2.7 [2.1, 3.4] | ||||
| Female | 13 [5, 22] | 6 [3, 9] | |||
| White race | 9 [6, 13] | ||||
| Other race | 10 [4, 17] | ||||
| Unemployed | 29 [12, 45] | -11 [-17, -4] | 10 [4,16] | ||
| Disabled | 53 [36, 71] | 41 [31, 51] | -15 [-19, -11] | 9 [5, 13] | 10 [6, 13] |
| APRI > 2 | -6 [-11, -2] | ||||
| Currently on Psych Meds | 33 [19, 48] | -11 [-17, -6] | 7 [2, 13] | 8 [3, 13] | |
| History of Psych Meds | 7 [2, 12] |
a For the HCV-PRO, higher scores are better; estimates reflect the % decrease in HCV-PRO score (lower well-being).
Specific symptom clusters and association with patient characteristics
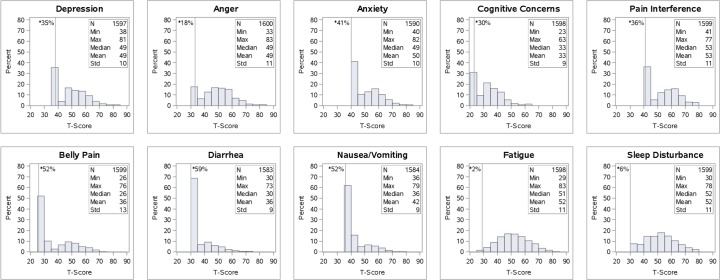
Fig 2 displays histograms for the T-scores for each of the PROMIS symptoms. Eight PROMIS measures showed a bimodal distribution with some patients reporting no symptoms while others reported mild to severe symptoms. T-scores over 55 are 1/2 standard deviation worse than the general US population and considered a clinically meaningful difference in other medical populations[34, 46–48].
Fig 2. Histograms of PROMIS symptom T-scores.
*The vertical line in each histogram shows the proportion of patients reporting no symptoms or responses at the minimum score.
Neuropsychiatric symptom cluster
Approximately 60-80% of patients endorsed symptoms in the neuropsychiatric symptom cluster (Fig 2). Bivariate correlations and unadjusted mean group differences are summarized in Table 3. Of the sociodemographic variables, the largest correlations with this symptom cluster were with employment (.19 ≤ r ≤ .28), health insurance (.13 ≤ r ≤ .19), and income (.09 ≤ r ≤ .18), such that disability, unemployment, low income, and public health insurance were associated with worse symptoms. Correlations between the neuropsychiatric symptom cluster and liver-related markers were quite small (0 ≤ r ≤ .07).
In multivariable models, severity of the neuropsychiatric symptom cluster was frequently associated with being disabled, unemployed, and use of psychiatric medications (Table 5). Patients who were disabled, unemployed, white, single and with past or current psychiatric medication use had 6% to 18% higher depression scores. Anger severity decreased with age and was 4% to 8% higher in patients who were disabled, unemployed, using psychiatric medications and abusing prescription drugs. Anxiety severity decreased with age and was 6% to 11% higher in patients who were disabled, of other race, uninsured, taking psychiatric medications, or had a history of psychiatric diagnosis. Severity in cognitive concerns was 4% to 9% higher in patients who were disabled, unemployed, on psychiatric medications, and history of psychiatric hospitalization.
Table 5. Validated predictors of T-score symptom severity.
| Entries are % increase in the mean scorewith 95% C.I. | Depression | Anger | Anxiety | Cognitive Concerns | Pain | Belly Pain | Diarrhea | Nausea/ Vomiting |
|---|---|---|---|---|---|---|---|---|
| Younger Age | -.3 [-.4, -.2] | -.3 [-.4, -.1] | ||||||
| Other Race | 10 [5, 16] | |||||||
| White Race | 6 [3, 9] | |||||||
| Low Income | 5 [2, 8] | |||||||
| Disabled | 14 [10, 18] | 7 [6, 9] | 11 [7, 16] | 7 [4, 9] | 13 [11, 16] | 18 [14, 21] | 19 [15, 23] | 23 [18, 28] |
| Unemployed | 18 [13, 23] | 5 [2, 8] | 8 [3, 12] | 8 [3, 13] | 13 [9, 18] | 22 [14, 30] | ||
| Retired | -7 [-12, -3] | 11 [3, 19] | ||||||
| Uninsured | 8 [2, 15] | |||||||
| Single | 5 [2, 8] | |||||||
| Currently on Psych Meds | 16 [12, 20] | 8 [5, 10] | 10 [6, 14] | 9 [6, 13] | 5 [1, 8] | 11 [8, 15] | 7 [2, 13] | |
| Hx of Psych Meds | 8 [4, 12] | 4 [2, 6] | 5 [2, 8] | 9 [6, 13] | ||||
| Prescription drug abuse | 5 [3, 7] | 14 [9, 18] | ||||||
| Hx of Psych diagnosis | 6 [2, 10] | -5 [-8, -2] | -8 [-13, 3] | |||||
| Hx of Psych hospitalized | 4 [1, 7] | 7 [3, 12] |
Somatic symptom cluster
Almost all patients endorsed some level of fatigue and sleep disturbance, evidenced by the histograms in Fig 2 and 65% reported pain interference. Bivariate correlations and unadjusted mean differences between the somatic symptom cluster and patient characteristics are summarized in Table 3. In general, the largest correlations with the somatic symptom cluster were the sociodemographic and psychiatric variables. Patients who had lower income, were unemployed or disabled, or on public health insurance had worse somatic symptoms (.13 ≤ r ≤ .42) Patients with psychiatric markers were also observed to experience worse pain, sleep, and fatigue (.11 ≤ r ≤ .29). By contrast, correlations between the somatic symptom cluster and liver-related markers were small (.01 ≤ r ≤ .11).
Multivariable models demonstrated that fatigue severity was worse among patients who were female (6% higher), White or other race (9%-10% higher), disabled (9% higher) and those taking psychiatric medications (7% higher) (Table 4). Sleep disturbance severity was higher in patients who were unemployed (10% higher), disabled (11% higher), using psychiatric medications (7%-8% higher) (Table 4). Patients with lower income (5% higher), disabled (13% higher), unemployed (8% higher), with psychiatric medication use (5% higher) reported more severe pain interference (Table 5). No liver-related markers were identified as validated predictors of the somatic symptom cluster.
Gastrointestinal symptom cluster
Approximately half of the cohort reported symptoms in the GI cluster, including abdominal pain (50%), nausea/vomiting (50%), and diarrhea (40%) (Fig 2). Bivariate correlations between the GI symptom cluster and patient characteristics are summarized in Table 3. Correlations were smaller with the GI cluster than with other symptom clusters. In general, the sociodemographic variables had the largest correlations with the GI cluster (.07 ≤ r ≤ .27), followed by correlations with the psychiatric markers (.10 ≤ r ≤ .20), and liver-related markers (0 ≤ r ≤ .11).
In multivariable models, nausea/vomiting severity was worse in patients who were disabled, unemployed or abusing prescription medications (14% to 23% higher) (Table 5). Abdominal pain severity was worse in patients who were disabled, unemployed, and with psychiatric diagnosis and psychiatric medication use (5% to 18% higher). Diarrhea severity was worse in patients who were disabled, taking psychiatric medications, and with a history of psychiatric diagnosis and hospitalization (7% -19% higher).
Overall symptom burden and associations with patient characteristics
On the MSAS, individual symptoms reported by at least 25% of participants are listed in Table 6 (S3 Table). The median TMSAS score was 0.4 (mean = 0.6, SD = 0.5, range: 0–3.1). The most common symptoms were lack of energy, pain, and difficulty sleeping. These symptoms were also the most severe and frequent.
Table 6. MSAS symptoms reported by at least 25% of patients.
| Symptoms | % Endorsed | % Severitya | % Frequencyb | % Distressingc |
|---|---|---|---|---|
| Lack of energy | 60 | 50 | 39 | 43 |
| Pain | 52 | 46 | 37 | 42 |
| Difficulty sleeping | 48 | 42 | 34 | 35 |
| Worrying | 43 | 33 | 23 | 28 |
| Numbness/tingling in hands/feet | 40 | 31 | 23 | 26 |
| Feeling drowsy | 37 | 28 | 17 | 21 |
| Dry mouth | 34 | 24 | 18 | 17 |
| Feeling irritable | 32 | 22 | 12 | 20 |
| Difficulty concentrating | 31 | 20 | 13 | 20 |
| Feeling nervous | 31 | 24 | 14 | 21 |
| Feeling sad | 29 | 22 | 13 | 20 |
| Cough | 27 | 17 | 12 | 12 |
| Feeling bloated | 27 | 20 | 13 | 17 |
| Shortness of breath | 25 | 19 | 11 | 17 |
| Itching | 25 | 18 | 11 | 15 |
Symptom severity, frequency and distress are reported as percentages of those who endorsed the symptom presence.
a Severity ranges from slight, moderate, severe, very severe; data shown is % reporting moderate, severe, very severe symptoms.
b Frequency ranges from rarely, occasionally, frequently, almost constantly; data shown is % reported frequently or almost constantly.
c Distress or bothersome ranges from not at all, a little bit, somewhat, quite a bit, very much; data shown is % reporting symptoms as somewhat, quite a bit, or very much distressing.
As shown in Table 3, higher overall symptom burden (higher TMSAS scores) was correlated with sociodemographics (.15 ≤ r ≤ .36; being female; lower income; disabled; uninsured; having public health insurance), lab biomarkers (.12 ≤ r ≤ .16; lower albumin, lower hemoglobin) and psychiatric markers (.23 ≤ r ≤ .35). Correlations with alcohol and substance abuse were lower (0 ≤ r ≤ .12). In multivariable regressions, symptom burden was higher in patients who were female (13% higher), disabled (41% higher), unemployed (29% higher), and taking psychiatric medications (33% higher) (Table 4).
HCV-specific functional well-being
The mean HCV-PRO total score was 72 (median = 77, SD = 22; range = 0-100), a score consistent with a previous Phase II clinical trial where patients had a mean baseline HCV-PRO of 78[40]. As shown in Table 3, patients with lower functional well-being tended to be younger; other race; lower income, disabled, unemployed, or on public insurance. Low functional well-being was also correlated with the mental health markers (.24 ≤ r ≤ .38). In contrast, the correlations between HCV-PRO scores and liver-related markers were quite small, with the strongest correlations being with albumin, APRI >2, and MELD ≥12 (.07 ≤ r ≤ .09). In multivariable regression models, functional well-being was lower in patients who had worse liver disease (APRI >2) (6% lower), were unemployed (11% lower), disabled (15% lower), and taking psychiatric medications (11% lower).
Sensitivity analyses
Sensitivity analyses and diagnostics were used to evaluate the robustness of the reported main results in Tables 4 and 5 to reasonable perturbations of the statistical methods and assumptions used. These auxiliary analyses were used to guide our level of trust in the main results. The various sensitivity analyses produced results similar to the main results. For example: (1) For Table 5, similar results were obtained when using a zero-inflated negative binomial (ZINB) model instead of the zero-inflated Poisson (ZIP) model, albeit with wider confidence intervals as expected. The two approaches identified the same predictor variables but ZINB classified fewer as “validated”. In terms of the Akaiki Information Criterion, the ZINB fit was slightly better for some PRO measures. (2) For Table 4, similar results were obtained when using a generalized linear model with identity link function instead of the Poisson model. (3) Results in Tables 4 and 5 were supported when we explored inclusion or exclusion of selected candidate predictor variables. (4) Finally, the results in Tables 4 and 5 relied on multiple imputation (MI) of missing values for patient characteristics using a two-step approach: Markov-chain-Monte-Carlo estimation followed by use of parametric regressions. Closely similar versions of Tables 4 and 5 were obtained when relying on an alternative MI method in which the second step was a nonparametric propensity scores method.
Discussion
With recent advances in the treatment of HCV using highly potent DAAs and resultant changes to demographics of patients initiating DAA treatment, a contemporary and comprehensive assessment of the symptom burden of HCV-infected individuals in a real world setting, outside of carefully selected clinical trials populations, was needed. Towards this end, we conducted an in-depth analysis of patient-reported symptom burden including HCV-associated specific symptoms, medical comorbidities and functional well-being in a large multicenter cohort initiating DAA therapy. We also took the opportunity to thoroughly describe the cohort with regard to several sociodemographics, liver-related features, and mental health and drug and alcohol use parameters of the current treatment population. Through a rigorous analytical approach, we identified and validated key patient characteristics associated with each PRO. Our analysis has three key findings. First, many patients with chronic HCV, although not all, had a large number of medical comorbidities and high symptom burden, with the most common being fatigue, sleep disturbance, pain interference and neuropsychiatric symptoms. Second, most PROs were strongly associated with just a handful of patient characteristics; namely, disability, unemployment and current use of psychiatric medications. Other predictor variables for each PRO were identified, but with lower frequency and strength of association. Third, laboratory biomarkers and clinical markers of liver disease severity were not strong predictors of most PROs. These findings will lay the groundwork for subsequent longitudinal investigations of symptoms and comorbid conditions that may change over the course of DAA therapy and after viral eradication.
This study provides extensive sociodemographic information regarding the chronic HCV population currently being treated in the US. The study cohort was 61% White and 39% non-White (33% identified as Black/African-American, 6% identified as Other Race), in contrast to industry-sponsored clinical trials that under-represent minority populations (i.e., majority are >80% White (range: 66%-97%)[49–51]. The vast majority of patients initiating treatment had low educational attainment (not exceeding high school) and low household income (less or equal to $40,000 per year), well below the average income cited in the 2016 Census survey[52]. Over 50% were recipients of disability benefits, applying for disability benefits, or unemployed and looking for work. The majority was receiving public health insurance or was uninsured. Employment status, including disability and unemployment, as well as low income and lack of insurance were common patient features associated with high symptom burden and comorbidities, consistent with the larger literature on social and economic determinants of poor health in other medical populations). Recognizing the impact of SDoH on the health and treatment outcomes of the HCV population has both clinical and health policy implications. While the emphasis has been on viral eradication to improve liver-related outcomes, our results highlight the need to acknowledge the high burden of concomitant chronic health conditions among the chronic HCV population. In fact, HCV therapy initiation presents a window of opportunity for patients to re-engage with the healthcare system and address other health conditions. Improving the overall health of the HCV population will require improvements and innovation at multiple levels of healthcare including enhanced coordination of care between hepatologists and other subspecialists, multidisciplinary teams, co-location of mental health and addiction specialists, patient navigation, and innovative telehealth models [21, 53–63].
Nearly half of the cohort had cirrhosis and 12% had evidence of advanced cirrhosis (MELD ≥ 12). We suspect that state Medicaid restrictions during the enrollment period partially influenced variability of liver disease severity in this cohort by limiting access to treatment to patients with higher stages of fibrosis and denying treatment to those with minimal fibrosis[64, 65].
Psychiatric disorders were common comorbidities among HCV-infected individuals in the interferon-era[13, 66], and this trend continues. Almost half of the patients reported a psychiatric diagnosis (44%), utilizing mental health treatment or services (41%), or psychiatric medication use (50%), with 30% currently on psychiatric medications. Eighteen percent (n = 287) of the cohort reported a past history of inpatient psychiatric hospitalizations, a proxy for severe psychopathology. The prevalence of these mental health indicators far exceeds the prevalence in the general US population[67]. A total of 36% of patients were still using alcohol at time of treatment initiation and at least 20% had used street drugs and 9% reported using prescription medications in an abusive fashion in the previous year. While these data are alarming, it is likely that patients under-reported these behaviors due to social desirability and fear of treatment being rescinded. In this study, we attempted to mitigate under-reporting by highlighting the confidential nature of data collection and separation of research team and clinical staff. The high prevalence of psychiatric disorders and moderate drug and alcohol use has clinical relevance for all practitioners, as these patients will be at risk for long-term poor health outcomes, beyond liver disease.
The vast majority of patients suffer from multiple chronic health conditions. Patients reported up to 15 comorbid conditions and an average of four. A greater number of comorbidities was found for patients who are disabled and older. Many of the prevalent medical conditions could represent biologically plausible inflammatory conditions, or extrahepatic disorders (EHDs) related to HCV, such as diabetes[68, 69]. Recent literature suggests that EHDs are underestimated because they are non-specific, but may compromise overall health outcomes and result in an estimated economic burden of $1.5 billion per year[70]. The diagnostic guidelines published by the International Study Group of Extrahepatic Manifestations Related to HCV recommend evaluating all patients with HCV for potential EHDs to ensure that the entire spectrum of HCV-related disorders are identified and properly treated[4]. Indeed, patients with all stages of liver disease should be treated for HCV and not delayed until fibrosis advances, as studies indicate that clinical and economic burden of EHDs can be reduced through viral eradication[70, 71].
Like comorbidities, many, but not all patients reported experiencing specific symptoms. For each of the specific neuropsychiatric, somatic and GI symptoms, a proportion of patients did not experience that particular symptom (e.g., 50-60% had no GI symptoms), but the majority reported mild to severe symptoms. For instance, fatigue and sleep disturbance were ubiquitous problems in the entire cohort. With regard to the neuropsychiatric cluster (depression, anxiety, anger, cognitive concerns), about 60-80% endorsed mild to severe symptoms, with severe neuropsychiatric symptoms experienced by patients who were disabled, unemployed, and on psychiatric medications, among other factors. Over 60% of patients endorsed pain interference and 30% endorsed joint/muscle pain, which is consistent with previous reports of pain disorders in patients with chronic HCV[6, 15, 72–74]. Over 90% of patients reporting some level of sleep disturbance; 31% reported a sleep disorder. Estimates from the literature suggest that up to 60% of patients with chronic HCV may have sleep problems, including restless leg syndrome, although confounding effects of other comorbidities has been difficult to tease apart[6, 75]. Disability, unemployment, and psychiatric medication use were the prominent predictors of the pain, sleep interference, and fatigue cluster. Finally, 40-50% of patients endorsed GI symptoms (nausea/vomiting, abdominal pain, diarrhea) and similarly, disability, unemployment and psychiatric markers including psychiatric medication use were associated with more severe GI symptoms.
A comprehensive analysis of symptom prevalence in the chronic HCV population has not been conducted since the work of Lang et al. in 2006[6]; therefore, this study provides a contemporary perspective of the most prevalent, severe, and distressing symptoms in the chronic HCV population. The majority of symptoms endorsed by at least 20% of patients in this cohort overlap considerably with Lang et al.’s findings, validating our results. We also identified new symptoms (e.g., numbness and tingling in hands and feet, dry mouth, cough, feeling bloated, shortness of breath, and lack of sexual interest) that were prevalent and require further investigation. This information can help clinicians identify and better care for these patients and observe changes in symptoms after viral eradication.
The functional well-being of the current cohort was comparable to that obtained from the original HCV-PRO analysis embedded in a Phase II clinical trial[40]. Items endorsed on the HCV-PRO most often included “Having Hepatitis C was very stressful to me”, “I had difficulty sleeping or slept too much”, and “I needed to pace myself to finish what I had planned”. HCV-specific functional well-being was worse in patients with advanced liver disease (APRI > 2), and those disabled, unemployed and on psychiatric medications. The HCV-PRO is a contemporary disease-specific instrument developed with rigorous methods and we would recommend it for use in future HCV investigations; unfortunately, no other studies have been published to date, therefore cross-study comparisons are unable to be conducted.
Not surprisingly, we found that laboratory biomarkers and markers of liver disease severity were not strong predictors of the PROs investigated in this study. We observed differences in the unadjusted means between high and low MELD subgroups by 2 to 7 points across the PROs, reflecting possible clinically significant differences in patients with advanced cirrhosis on symptoms such as sleep disturbance and the GI symptom cluster. However, MELD was not a strong predictor in confirmatory models. There were small differences between patients with high versus low APRI, FIB-4, albumin and creatinine, but only APRI was validated as a predictor of functional well-being in multivariable models. Our findings are consistent with a large body of HrQOL literature from the interferon treatment era that found negligible associations between liver disease parameters and PROs once other variables were accounted for[15, 76–81]. Most of these studies have found psychiatric and/or medical conditions to be the strongest predictors of PROs in multivariate models, especially depression and pain-related conditions[15, 80–82]. Many clinical researchers have speculated that HCV exerts a deleterious effect on PROs through medical and psychiatric comorbidities, which could represent underlying HCV-induced EHDs [15, 80, 81]. These speculations require further investigation.
Limitations of this study should be noted, particularly regarding the generalizability of these results. Our findings may not apply to other subpopulations or clinical settings, including younger people who inject drugs, persons receiving medication-assisted treatment for opioid use disorders with methadone or buprenorphine/naloxone, Veterans, and those incarcerated. These subgroups may represent the primary treatment groups in years to come if the opioid epidemic is not controlled[83]. We found no substantial differences between patients enrolled and not enrolled in this study, therefore we can presume generalizability to similar patients treated in similar settings. Social desirability and response bias issues could have occurred, especially in response to alcohol and drug use questions. However, it’s important to appreciate the necessity of capturing data directly from patients when they are uniquely positioned to assess an outcome (i.e., pain), as research shows that clinicians tend to under-report the frequency and severity of patients’ symptoms[84]. Finally, minimal clinical data were extracted from medical records, thus we are unable to verify patient-reporting of medical comorbidities, psychiatric medications and other clinical information.
Future research should build upon our observations to directly inform clinical knowledge and decision-making for various stakeholders (patients, clinicians, policy makers). There is a critical need to tease apart and elucidate the causal pathways that may influence health outcomes in the HCV population (i.e., psychiatric illness, EHDs, number of medical comorbidities, liver disease, symptoms); this may be achieved through sophisticated path analyses and structural equation modeling[85]. We identified a new predictor of worse PROs, namely employment status, which should be included in future predictive models of HCV-related PROs. Health services research is needed to examine the most effective models to integrate healthcare for liver disease, mental health, and addiction[21, 55–57, 60–63]. More studies derived from real world clinical settings, outside of clinical trials, need to examine changes in overall symptom burden and specific symptom clusters over time, during DAA therapy, after virologic cure, and during long-term follow-up. Comparisons between the DAA regimens and how they affect PROs would provide stakeholders with information to guide treatment decision-making. Importantly, it remains unclear if overall symptom burden lessens after virologic cure or if only specific symptom clusters improve. Therefore, the exploratory work herein to identify and validate the most robust predictors of PROs is fundamental to future PRO research in chronic HCV.
Our findings have a number of strengths and highlight clinical relevance for multiple stakeholders. This study is unique and notably the largest investigation of PROs in the current population of HCV patients seeking DAA therapy outside of clinical trials. We have identified some of the most challenging sociodemographic features, common comorbidities, potentially EHDs, and troublesome symptoms experienced by HCV-infected individuals. More black Americans have been recruited than any previously conducted clinical trial and thus provided an opportunity for a robust evaluation. Interestingly, Black patients reported the lowest levels of symptom burden and neuropsychiatric symptoms. Consistent with many prior studies, we found that laboratory biomarkers and liver disease severity were not helpful in identifying patients with diminished PROs, such as HrQOL, when other variables were accounted for[80–82]. It is also noteworthy that PROP UP is a highly patient-centered study. Patients affected by HCV were engaged since its inception and helped to identify the study outcomes and select PRO measures to ensure the study findings were salient and meaningful to people with HCV. Finally, given the large sample size, we were able to conduct cross-validation analyses to rigorously identify and confirm predictors, essentially conducting two separate identical studies, and then followed up with sensitivity analyses to confirm that perturbations in modeling, missing data, and assumptions did not alter our findings. These methods serve to instill a higher level of trust in the conclusions drawn from this study. Consequently, we hope the comprehensive data provided herein serves as reference guide for future investigations of PROs in the chronic HCV patient population.
Supporting information
(DOCX)
a Each participant only counted once in combined column.
(DOCX)
Symptom severity, frequency and distress are reported as percentages of those who endorsed the symptom presence. a Severity ranges from slight, moderate, severe, very severe; data shown is % reporting moderate, severe, very severe symptoms. b Frequency ranges from rarely, occasionally, frequently, almost constantly; data shown is % reported frequently or almost constantly. c Distress or bothersome ranges from not at all, a little bit, somewhat, quite a bit, very much; data shown is % reporting symptoms as somewhat, quite a bit, or very much distressing.
(DOCX)
Acknowledgments
The authors would like to acknowledge the contributions of the following people: Alan Franciscus of www.HCVadvocate.org, Anquenette Sloan, Summer Wadsworth-Delciotto, Scott Kixmiller, Larry Huston, Finton Brown (UNC Patient Engagement Group); Virginia Sharpless, Ken Berquist, Herleesha Anderson, Courtenay Pierce, Jenn Barr, Bryonna Jackson, Jane Giang, Jama Darling, Paul Hayashi, Steven Zacks, A. Sidney Barritt IV, Scott Elliot, Dawn Harrison, Danielle Cardona (University of North Carolina); Patrick Horne (University of Florida); Kelly Borges, Danielle Ciuffetelli (University of Pennsylvania); Chrissy Ammons, Kathleen Genther, Jessica Mason (Virginia Commonwealth University); Lelani Fetrow, Vicki Shah (Rush University); Theresa Cattoor, Alisha McLendon (Saint Louis University); Mariechristi Candido, Sophia Zaragoza, Sandeep Dhaliwal, Patricia Poole, Rebecca Hluahanich, Kathleen Haight, (University of California, Davis); Andrea Gajos, Elizabeth Wu, Carrie Bergmans (University of Michigan); AnnMarie Liapakis, MD, Kristine Drozd, Carol Eggers, Hong Chau and Claudia Bertuccio (Yale University). William Harlan, Roberta Golden, Kylee Diaz(Asheville Gastroenterology Associates), William King, Megan Marles (Wilmington Gastroenterology Associates).
Abbreviations
- ALT
alanine aminotransferase test
- AST
aspartate aminotransferase tes
- APRI
AST to platelet ratio index
- AUDIT
Alcohol Use Disorders Identification Test
- CI
Confidence interval
- DAA
Direct acting antiviral
- EHDs
extrahepatic disorders
- FIB-4
Fibrosis-4 Index for Liver Fibrosis
- GI
Gastrointestinal
- HCV
hepatitis C virus
- HrQOL
health-related quality of life
- Hx
history
- IFN
Interferon
- INR
international normalized ratio
- IRB
Institutional Review Board
- LASSO
Least absolute shrinkage and selection operator
- MELD
Model for End-Stage Liver Disease
- MSAS
Memorial Symptom Assessment Scale
- PCORI
Patient-Centered Outcomes Research Institute
- PRO
patient-reported outcome
- PROMIS
Patient-Reported Outcome Measurement Information System
- PROP UP
The Patient-Reported Outcomes Project of HCV-TARGET
- RBV
Ribavirin
- SD
Standard deviation
- SDoH
social determinants of health
- TMSAS
Total Memorial Symptom Assessment Scale
- Tx
treatment
Data Availability
The de-identified limited dataset and codebook used for these analyses have been deposited in the University of North Carolina's Carolina Digital Repository (https://cdr.lib.unc.edu/). The link to the dataset in the CDR: https://cdr.lib.unc.edu/record/uuid:f7d43e1e-0adb-4377-afdc-f9905083a1ee. The doi link to the dataset is: https://doi.org/10.17615/C62D42. Please contact the Primary Investigator, Donna Evon, for more details: Donna_Evon@med.unc.edu.
Funding Statement
This study was funded through a Patient Centered Outcomes Research Institute (PCORI) Award to Donna Evon (CER 1408 20660). Additional support for this study (data management) was partially supported by the NIDDK-funded Center for Gastrointestinal Biology and Disease (P30-DK34987; PI: Sandler). Additional support for Dr. Golin’s salary was partially supported by the NIH Eunice Kennedy Shriver National Institute of Child Health and Human Development (K24 HD06920) and by the University of North Carolina Center for AIDS Research (CFAR) (P30 AI 50410). The statements presented in this article are solely the responsibility of the authors and do not necessarily represent the views of PCORI, its Board of Governors or Methodology Committee. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Denniston MM, Jiles RB, Drobeniuc J, Klevens RM, Ward JW, McQuillan GM, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160(5):293–300. 10.7326/M13-1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edlin BR, Eckhardt BJ, Shu MA, Holmberg SD, Swan T. Toward a more accurate estimate of the prevalence of hepatitis C in the United States. Hepatology. 2015;62(5):1353–63. 10.1002/hep.27978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Younossi ZM. Hepatitis C Infection: A Systemic Disease. Clinics in liver disease. 2017;21(3):449–53. 10.1016/j.cld.2017.03.001 [DOI] [PubMed] [Google Scholar]
- 4.Ferri C, Ramos-Casals M, Zignego AL, Arcaini L, Roccatello D, Antonelli A, et al. International diagnostic guidelines for patients with HCV-related extrahepatic manifestations. A multidisciplinary expert statement. Autoimmunity reviews. 2016;15(12):1145–60. 10.1016/j.autrev.2016.09.006 [DOI] [PubMed] [Google Scholar]
- 5.Jacobson IM, Cacoub P, Dal Maso L, Harrison SA, Younossi ZM. Manifestations of chronic hepatitis C virus infection beyond the liver. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2010;8(12):1017–29. [DOI] [PubMed] [Google Scholar]
- 6.Lang CA, Conrad S, Garrett L, Battistutta D, Cooksley WG, Dunne MP, et al. Symptom prevalence and clustering of symptoms in people living with chronic hepatitis C infection. JPain SymptomManage. 2006;31(4):335–44. [DOI] [PubMed] [Google Scholar]
- 7.Kleinman L, Mannix S, Yuan Y, Kummer S, L'Italien G, Revicki D. Review of patient-reported outcome measures in chronic hepatitis C. Health QualLife Outcomes. 2012;10:92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kallman J, O'Neil MM, Larive B, Boparai N, Calabrese L, Younossi ZM. Fatigue and health-related quality of life (HRQL) in chronic hepatitis C virus infection. DigDisSci. 2007;52(10):2531–9. [DOI] [PubMed] [Google Scholar]
- 9.Golden J, O'Dwyer AM, Conroy RM. Depression and anxiety in patients with hepatitis C: prevalence, detection rates and risk factors. GenHospPsychiatry. 2005;27(6):431–8. [DOI] [PubMed] [Google Scholar]
- 10.Dieperink E, Willenbring M, Ho SB. Neuropsychiatric symptoms associated with hepatitis C and interferon alpha: A review. The American journal of psychiatry. 2000;157(6):867–76. 10.1176/appi.ajp.157.6.867 [DOI] [PubMed] [Google Scholar]
- 11.Foster GR. Quality of life considerations for patients with chronic hepatitis C. J Viral Hepat. 2009;16(9):605–11. 10.1111/j.1365-2893.2009.01154.x [DOI] [PubMed] [Google Scholar]
- 12.Grover VP, Pavese N, Koh SB, Wylezinska M, Saxby BK, Gerhard A, et al. Cerebral microglial activation in patients with hepatitis C: in vivo evidence of neuroinflammation. J Viral Hepat. 2012;19(2):e89–96. 10.1111/j.1365-2893.2011.01510.x [DOI] [PubMed] [Google Scholar]
- 13.Fireman M, Indest DW, Blackwell A, Whitehead AJ, Hauser P. Addressing tri-morbidity (hepatitis C, psychiatric disorders, and substance use): the importance of routine mental health screening as a component of a comanagement model of care. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2005;40 Suppl 5:S286–91. [DOI] [PubMed] [Google Scholar]
- 14.Yovtcheva SP, Rifai MA, Moles JK, Van der Linden BJ. Psychiatric comorbidity among hepatitis C-positive patients. Psychosomatics. 2001;42(5):411–5. [DOI] [PubMed] [Google Scholar]
- 15.Hussain KB, Fontana RJ, Moyer CA, Su GL, Sneed-Pee N, Lok AS. Comorbid illness is an important determinant of health-related quality of life in patients with chronic hepatitis C. The American journal of gastroenterology. 2001;96(9):2737–44. 10.1111/j.1572-0241.2001.04133.x [DOI] [PubMed] [Google Scholar]
- 16.Monaco S, Mariotto S, Ferrari S, Calabrese M, Zanusso G, Gajofatto A, et al. Hepatitis C virus-associated neurocognitive and neuropsychiatric disorders: Advances in 2015. World journal of gastroenterology: WJG. 2015;21(42):11974–83. 10.3748/wjg.v21.i42.11974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Establishing a Holistic Framework to Reduce Inequities in HIV, Viral Hepatitis, STDs, Tuberculosis in the United States U.S: Department of Health and Human Services; October 2010. [Google Scholar]
- 18.Ruffin R, Ebneter D, Galanko J, Stephens J, Evon DM. (data unpublished). Psychological Trauma is associated with mental health and substance abuse in patients with chronic hepatitis C being evaluated for antiviral therapy. 2014. [Google Scholar]
- 19.Mason K, Dodd Z, Sockalingam S, Altenberg J, Meaney C, Millson P, et al. Beyond viral response: A prospective evaluation of a community-based, multi-disciplinary, peer-driven model of HCV treatment and support. The International journal on drug policy. 2015;26(10):1007–13. 10.1016/j.drugpo.2015.04.012 [DOI] [PubMed] [Google Scholar]
- 20.Butt G. Stigma in the context of hepatitis C: concept analysis. J Adv Nurs. 2008;62(6):712–24. 10.1111/j.1365-2648.2008.04641.x [DOI] [PubMed] [Google Scholar]
- 21.Evon DM, Golin CE, Fried MW, Keefe FJ. Chronic hepatitis C and antiviral treatment regimens: where can psychology contribute? JConsult ClinPsychol. 2013;81(2):361–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janke EA, McGraw S, Garcia-Tsao G, Fraenkel L. Psychosocial issues in hepatitis C: a qualitative analysis. Psychosomatics. 2008;49(6):494–501. 10.1176/appi.psy.49.6.494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silberbogen AK, Ulloa EW, Janke EA, Mori DL. Psychosocial issues and mental health treatment recommendations for patients with hepatitis C. Psychosomatics. 2009;50(2):114–22. 10.1176/appi.psy.50.2.114 [DOI] [PubMed] [Google Scholar]
- 24.Zickmund S, Ho EY, Masuda M, Ippolito L, LaBrecque DR. 'They Treated Me Like a Leper': Stigmatization and the Qualify of Life of Patients with Hepatitis C. Journal of General Internal Medicine. 2003;18(10):835–44. 10.1046/j.1525-1497.2003.20826.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marcellin F, Roux P, Protopopescu C, Duracinsky M, Spire B, Carrieri MP. Patient-reported outcomes with direct-acting antivirals for the treatment of chronic hepatitis C: current knowledge and outstanding issues. Expert review of gastroenterology & hepatology. 2017;11(3):259–68. [DOI] [PubMed] [Google Scholar]
- 26.Conrad S, Garrett LE, Cooksley WG, Dunne MP, Macdonald GA. Living with chronic hepatitis C means 'you just haven't got a normal life any more'. ChronicIlln. 2006;2(2):121–31. [DOI] [PubMed] [Google Scholar]
- 27.Glacken M, Coates V, Kernohan G, Hegarty J. The experience of fatigue for people living with hepatitis C. JClinNurs. 2003;12(2):244–52. [DOI] [PubMed] [Google Scholar]
- 28.Hill R, Pfeil M, Moore J, Richardson B. Living with hepatitis C: a phenomenological study. Journal of clinical nursing. 2015;24(3–4):428–38. 10.1111/jocn.12620 [DOI] [PubMed] [Google Scholar]
- 29.Younossi ZM, Stepanova M, Afdhal N, Kowdley KV, Zeuzem S, Henry L, et al. Improvement of health-related quality of life and work productivity in chronic hepatitis C patients with early and advanced fibrosis treated with ledipasvir and sofosbuvir. Journal of hepatology. 2015;63(2):337–45. 10.1016/j.jhep.2015.03.014 [DOI] [PubMed] [Google Scholar]
- 30.Younossi ZM, Stepanova M, Zeuzem S, Dusheiko G, Esteban R, Hezode C, et al. Patient-reported outcomes assessment in chronic hepatitis C treated with sofosbuvir and ribavirin: The VALENCE study. Journal of hepatology. 2014;61(2):228–34. 10.1016/j.jhep.2014.04.003 [DOI] [PubMed] [Google Scholar]
- 31.Evon DM, Golin CE, Stewart P, Fried MW, Alston S, Reeve B, et al. Patient engagement and study design of PROP UP: A multi-site patient-centered prospective observational study of patients undergoing hepatitis C treatment. Contemporary clinical trials. 2017;57:58–68. 10.1016/j.cct.2017.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. Journal of clinical epidemiology. 2010;63(11):1179–94. 10.1016/j.jclinepi.2010.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reeve BB, Hays RD, Bjorner JB, Cook KF, Crane PK, Teresi JA, et al. Psychometric evaluation and calibration of health-related quality of life item banks: plans for the Patient-Reported Outcomes Measurement Information System (PROMIS). MedCare. 2007;45(5 Suppl 1):S22–S31. [DOI] [PubMed] [Google Scholar]
- 34.Khanna D, Hays RD, Shreiner AB, Melmed GY, Chang L, Khanna PP, et al. Responsiveness to Change and Minimally Important Differences of the Patient-Reported Outcomes Measurement Information System Gastrointestinal Symptoms Scales. Dig Dis Sci. 2017;62(5):1186–92. 10.1007/s10620-017-4499-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bajaj JS, Thacker LR, Wade JB, Sanyal AJ, Heuman DM, Sterling RK, et al. PROMIS computerised adaptive tests are dynamic instruments to measure health-related quality of life in patients with cirrhosis. Alimentary pharmacology & therapeutics. 2011;34(9):1123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Evon DM, Amador J, Reeve B, Lok AS, Sterling RK, Bisceglie AMD, et al. Psychometric Properties of the PROMIS® Short Form Measures in a Large U.S. Cohort of Patients with Chronic Hepatitis C Prescribed Direct Acting Antiviral Therapy. Contemp Clin Trials. 2017. June;57:58–68. PMCID: PMC5495187. 10.1016/j.cct.2017.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Portenoy RK, Thaler HT, Kornblith AB, Lepore JM, Friedlander-Klar H, Kiyasu E, et al. The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. European journal of cancer (Oxford, England: 1990). 1994;30a(9):1326–36. [DOI] [PubMed] [Google Scholar]
- 38.Chang VT, Hwang SS, Thaler HT, Kasimis BS, Portenoy RK. Memorial symptom assessment scale. Expert review of pharmacoeconomics & outcomes research. 2004;4(2):171–8. [DOI] [PubMed] [Google Scholar]
- 39.Anderson RT, Baran RW, Dietz B, Kallwitz E, Erickson P, Revicki DA. Development and initial psychometric evaluation of the hepatitis C virus-patient-reported outcomes (HCV-PRO) instrument. Quality of life research: an international journal of quality of life aspects of treatment, care and rehabilitation. 2014;23(2):561–70. [DOI] [PubMed] [Google Scholar]
- 40.Anderson RT, Baran RW, Erickson P, Revicki DA, Dietz B, Gooch K. Psychometric evaluation of the hepatitis C virus patient-reported outcomes (HCV-PRO) instrument: validity, responsiveness, and identification of the minimally important difference in a phase 2 clinical trial. Quality of life research: an international journal of quality of life aspects of treatment, care and rehabilitation. 2014;23(3):877–86. [DOI] [PubMed] [Google Scholar]
- 41.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38(2):518–26. 10.1053/jhep.2003.50346 [DOI] [PubMed] [Google Scholar]
- 42.Sterling RK, Kuo A, Rustgi VK, Sulkowski MS, Stewart TG, Fenkel JM, et al. Virological outcomes and treatment algorithms utilisation in observational study of patients with chronic hepatitis C treated with boceprevir or telaprevir. Alimentary pharmacology & therapeutics. 2015;41(7):671–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.UNOS MELD/PELD Calculator Documentation 2009 (accessed July 5, 2017).
- 44.Saunders JB, Aasland OG, Babor TF, de lF Jr., Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption—II. Addiction. 1993;88(6):791–804. [DOI] [PubMed] [Google Scholar]
- 45.Pence BW, Gaynes BN, Whetten K, Eron JJ Jr., Ryder RW, Miller WC. Validation of a brief screening instrument for substance abuse and mental illness in HIV-positive patients. JAcquirImmuneDeficSyndr. 2005;40(4):434–44. [DOI] [PubMed] [Google Scholar]
- 46.Amtmann D, Kim J, Chung H, Askew RL, Park R, Cook KF. Minimally important differences for Patient Reported Outcomes Measurement Information System pain interference for individuals with back pain. Journal of pain research. 2016;9:251–5. 10.2147/JPR.S93391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hays RD, Spritzer KL, Fries JF, Krishnan E. Responsiveness and minimally important difference for the patient-reported outcomes measurement information system (PROMIS) 20-item physical functioning short form in a prospective observational study of rheumatoid arthritis. Annals of the rheumatic diseases. 2015;74(1):104–7. 10.1136/annrheumdis-2013-204053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee AC, Driban JB, Price LL, Harvey WF, Rodday AM, Wang C. Responsiveness and Minimally Important Differences for 4 Patient-Reported Outcomes Measurement Information System Short Forms: Physical Function, Pain Interference, Depression, and Anxiety in Knee Osteoarthritis. The journal of pain: official journal of the American Pain Society. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zepatier Prescribing Information http://www.merck.com/product/usa/pi_circulars/z/zepatier/zepatier_pi.pdf (accessed November 13, 2017).
- 50.Epclusa Prescribing Information http://www.gilead.com/~/media/Files/pdfs/medicines/liver-disease/epclusa/epclusa_pi.pdf (accesssed November 13, 2017).
- 51.Harvoni Prescribing Information https://www.gilead.com/~/media/Files/pdfs/medicines/liver-disease/harvoni/harvoni_pi.pdf (accesssed November 13, 2017).
- 52.U.S Census Bureau https://www.census.gov/data/tables/2017/demo/income-poverty/p60-259.html. (accessed October 20, 2017)
- 53.Arora S, Kalishman S, Thornton K, Dion D, Murata G, Deming P, et al. Expanding access to hepatitis C virus treatment-Extension for Community Healthcare Outcomes (ECHO) project: Disruptive innovation in specialty care. Hepatology. 2010. September;52(3):1124–33. PMCID: PMC3795614. 10.1002/hep.23802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bonner JE, Barritt AS, Fried MW, Evon DM. Time to rethink antiviral treatment for hepatitis C in patients with coexisting mental health/substance abuse issues. DigDisSci. 2012;57(6):1469–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bruggmann P, Litwin AH. Models of care for the management of hepatitis C virus among people who inject drugs: one size does not fit all. ClinInfectDis. 2013;57 Suppl 2:S56–S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brunner N, Senn O, Rosemann T, Falcato L, Bruggmann P. Hepatitis C treatment for multimorbid patients with substance use disorder in a primary care-based integrated treatment centre: a retrospective analysis. European journal of gastroenterology & hepatology. 2013;25(11):1300–7. [DOI] [PubMed] [Google Scholar]
- 57.Evon DM, Simpson K, Kixmiller S, Galanko J, Dougherty K, Golin C, et al. A Randomized Controlled Trial of an Integrated Care Intervention to Increase Eligibility for Chronic Hepatitis C Treatment. AmJGastroenterol. 2011;106(10):1777–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Groessl EJ, Sklar M, Cheung RC, Brau N, Ho SB. Increasing antiviral treatment through integrated hepatitis C care: a randomized multicenter trial. ContempClinTrials. 2013;35(2):97–107. [DOI] [PubMed] [Google Scholar]
- 59.Ho SB, Groessl E, Dollarhide A, Robinson S, Kravetz D, Dieperink E. Management of chronic hepatitis C in veterans: the potential of integrated care models. AmJGastroenterol. 2008;103(7):1810–23. [DOI] [PubMed] [Google Scholar]
- 60.Martinez AD, Dimova R, Marks KM, Beeder AB, Zeremski M, Kreek MJ, et al. Integrated internist—addiction medicine—hepatology model for hepatitis C management for individuals on methadone maintenance. JViral Hepat. 2012;19(1):47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rongey C, Asch S, Knight SJ. Access to care for vulnerable veterans with hepatitis C: a hybrid conceptual framework and a case study to guide translation. Translational behavioral medicine. 2011;1(4):644–51. 10.1007/s13142-011-0098-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Willenbring ML. Integrating care for patients with infectious, psychiatric, and substance use disorders: concepts and approaches. AIDS. 2005;19 Suppl 3:S227–S37. [DOI] [PubMed] [Google Scholar]
- 63.Zaller N, Gillani FS, Rich JD. A model of integrated primary care for HIV-positive patients with underlying substance use and mental illness. AIDS Care. 2007;19(9):1128–33. 10.1080/09540120701335196 [DOI] [PubMed] [Google Scholar]
- 64.Barua S, Greenwald R, Grebely J, Dore GJ, Swan T, Taylor LE. Restrictions for Medicaid Reimbursement of Sofosbuvir for the Treatment of Hepatitis C Virus Infection in the United States. Ann Intern Med. 2015;163(3):215–23. 10.7326/M15-0406 [DOI] [PubMed] [Google Scholar]
- 65.Canary LA, Klevens RM, Holmberg SD. Limited Access to New Hepatitis C Virus Treatment Under State Medicaid Programs. Ann Intern Med. 2015;163(3):226–8. 10.7326/M15-0320 [DOI] [PubMed] [Google Scholar]
- 66.Evon DM, Verma A, Dougherty KA, Batey B, Russo M, Zacks S, et al. High deferral rates and poorer treatment outcomes for HCV patients with psychiatric and substance use comorbidities. Digest Dis Sci. 2007;52(11):3251–8. 10.1007/s10620-006-9669-0 [DOI] [PubMed] [Google Scholar]
- 67.National Institute of Mental Health https://www.nimh.nih.gov/health/statistics/prevalence/any-mental-illness-ami-among-us-adults.shtml (accessed November 3, 2017).
- 68.Kaddai V, Negro F. Current understanding of insulin resistance in hepatitis C. ExpertRevGastroenterolHepatol. 2011;5(4):503–16. [DOI] [PubMed] [Google Scholar]
- 69.White DL, Ratziu V, El-Serag HB. Hepatitis C infection and risk of diabetes: a systematic review and meta-analysis. JHepatol. 2008;49(5):831–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Younossi Z, Park H, Henry L, Adeyemi A, Stepanova M. Extrahepatic Manifestations of Hepatitis C: A Meta-analysis of Prevalence, Quality of Life, and Economic Burden. Gastroenterology. 2016;150(7):1599–608. 10.1053/j.gastro.2016.02.039 [DOI] [PubMed] [Google Scholar]
- 71.Reau N, Vekeman F, Wu E, Bao Y. Prevalence and economic burden of extrahepatic manifestations of hepatitis C virus are underestimated but can be improved with therapy. Hepatology Communications. 2017;1(5):439–52 10.1002/hep4.1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morasco BJ, Huckans M, Loftis JM, Woodhouse J, Seelye A, Turk DC, et al. Predictors of pain intensity and pain functioning in patients with the hepatitis C virus. GenHospPsychiatry. 2010;32(4):413–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rivera J, de Diego A, Trinchet M, Garcia Monforte A. Fibromyalgia-associated hepatitis C virus infection. British journal of rheumatology. 1997;36(9):981–5. [DOI] [PubMed] [Google Scholar]
- 74.Barkhuizen A, Rosen HR, Wolf S, Flora K, Benner K, Bennett RM. Musculoskeletal pain and fatigue are associated with chronic hepatitis C: a report of 239 hepatology clinic patients. The American journal of gastroenterology. 1999;94(5):1355–60. 10.1111/j.1572-0241.1999.01087.x [DOI] [PubMed] [Google Scholar]
- 75.Sockalingam S, Abbey SE, Alosaimi F, Novak M. A review of sleep disturbance in hepatitis C. JClinGastroenterol. 2010;44(1):38–45. [DOI] [PubMed] [Google Scholar]
- 76.Hoofnagle JH. Hepatitis C: the clinical spectrum of disease. Hepatology. 1997;26(3 Suppl 1):15s–20s. [DOI] [PubMed] [Google Scholar]
- 77.Foster GR, Goldin RD, Thomas HC. Chronic hepatitis C virus infection causes a significant reduction in quality of life in the absence of cirrhosis. Hepatology. 1998;27(1):209–12. 10.1002/hep.510270132 [DOI] [PubMed] [Google Scholar]
- 78.Hauser W, Zimmer C, Schiedermaier P, Grandt D. Biopsychosocial predictors of health-related quality of life in patients with chronic hepatitis C. Psychosomatic medicine. 2004;66(6):954–8. 10.1097/01.psy.0000145824.82125.a8 [DOI] [PubMed] [Google Scholar]
- 79.Rowan PJ, Al-Jurdi R, Tavakoli-Tabasi S, Kunik ME, Satrom SL, El-Serag HB. Physical and psychosocial contributors to quality of life in veterans with hepatitis C not on antiviral therapy. Journal of clinical gastroenterology. 2005;39(8):731–6. [DOI] [PubMed] [Google Scholar]
- 80.Spiegel BM, Younossi ZM, Hays RD, Revicki D, Robbins S, Kanwal F. Impact of hepatitis C on health related quality of life: a systematic review and quantitative assessment. Hepatology. 2005;41(4):790–800. 10.1002/hep.20659 [DOI] [PubMed] [Google Scholar]
- 81.Fontana RJ, Moyer CA, Sonnad S, Lok ASF, Sneed-Pee N, Walsh J, et al. Comorbidities and quality of life in patients with interferon-refractory chronic hepatitis C. The American journal of gastroenterology. 2001;96(1):170–8. 10.1111/j.1572-0241.2001.03473.x [DOI] [PubMed] [Google Scholar]
- 82.Lim JK, Cronkite R, Goldstein MK, Cheung RC. The impact of chronic hepatitis C and comorbid psychiatric illnesses on health-related quality of life. Journal of clinical gastroenterology. 2006;40(6):528–34. [DOI] [PubMed] [Google Scholar]
- 83.Grebely J, Robaeys G, Bruggmann P, Aghemo A, Backmund M, Bruneau J, et al. Recommendations for the management of hepatitis C virus infection among people who inject drugs. The International journal on drug policy. 2015;26(10):1028–38. 10.1016/j.drugpo.2015.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Basch E. The missing voice of patients in drug-safety reporting. N Engl J Med. 2010;362(10):865–9. 10.1056/NEJMp0911494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lackner JM, Jaccard J, Krasner SS, Katz LA, Gudleski GD, Blanchard EB. How does cognitive behavior therapy for irritable bowel syndrome work? A mediational analysis of a randomized clinical trial. Gastroenterology. 2007;133(2):433–44. 10.1053/j.gastro.2007.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
a Each participant only counted once in combined column.
(DOCX)
Symptom severity, frequency and distress are reported as percentages of those who endorsed the symptom presence. a Severity ranges from slight, moderate, severe, very severe; data shown is % reporting moderate, severe, very severe symptoms. b Frequency ranges from rarely, occasionally, frequently, almost constantly; data shown is % reported frequently or almost constantly. c Distress or bothersome ranges from not at all, a little bit, somewhat, quite a bit, very much; data shown is % reporting symptoms as somewhat, quite a bit, or very much distressing.
(DOCX)
Data Availability Statement
The de-identified limited dataset and codebook used for these analyses have been deposited in the University of North Carolina's Carolina Digital Repository (https://cdr.lib.unc.edu/). The link to the dataset in the CDR: https://cdr.lib.unc.edu/record/uuid:f7d43e1e-0adb-4377-afdc-f9905083a1ee. The doi link to the dataset is: https://doi.org/10.17615/C62D42. Please contact the Primary Investigator, Donna Evon, for more details: Donna_Evon@med.unc.edu.