Abstract
Background
Advanced glycation end products (AGEs) accumulation, a measure of cumulative metabolic stress, constitute a novel pathogenic mechanism involved in aging, diabetes, cardiovascular (CVD) and chronic kidney disease (CKD). Despite removal of uremic toxins and AGEs after a successful renal transplant (RT), CVD remains the leading cause of mortality. We hypothesized that AGEs measurement by Skin Autofluorescence (SAF) might be useful even after a successful RT and thus reflect the high cardiovascular risk burden of these patients.
Methods
189 stable RT (61% men, aged 56±13.0 years), CKD stages 1–4 and >12 months since RT were enrolled. Variables collected comprised comorbid history, medication use, smoking habit, routine biochemistry, subclinical atheromatosis by ankle-brachial-index (ABI) and allograft resistivity index (RI), 24-h ABPM, anthropometry and handgrip strength. AGEs were measured by SAF and expressed in arbitrary units (AU). Vascular age was estimated by Koetsier´s formula (SAF-0.83/0.024) and expected 10-years cardiovascular death risk was calculated with the REGICOR score.
Results
Mean SAF was 3.00±0.83 AU and estimated vascular age 90±34.7 years (30 years above biological age). SAF was higher among men (3.10±0.91 vs 2.81±0.66), diabetic nephropathy (3.49±0.75 vs 2.96±0.83) and steroid users (3.14±0.86 vs 2.71±0.69). We observed a positive correlation of SAF with night-systolic blood pressure (r = 0.25, p = 0.001), parathormone (r = 0.20, p<0.01), phosphate (r = 0.28, p<0.001) and negative with hemoglobin (r = -0.29, p<0.001), CKD-EPI (r = -0.32, p<0.001), albumin (r = -0.17, p<0.05), and dynamometry (r = -0.20, p<0.01). Subclinical vascular atheromatosis (ABI and RI) as well as the REGICOR scale (r = 0.35 p<0.001) were also correlated with SAF. In multivariable analysis age, gender, steroid use, serum phosphate and handgrip strength remained independently associated with SAF.
Conclusions
SAF levels are elevated in RT patients and correlate with CVD risk. Besides age and male sex, our results suggest that phosphate overload, steroid use and nutritional status are important factors linking to AGEs accumulation.
Introduction
Advanced glycation end products (AGEs) are a variety of compounds produced by the nonenzymatic Maillard reaction that binds reducing sugars with the free amino groups in proteins, lipids or nucleic acids [1]. AGEs can be degraded by enzymes, such as glyoxalase (I and II), and they can also be modified by innate defense machineries, such as lysozyme, which sequesters AGEs and accelerates their renal excretion [2]. AGEs receptor 1 (AGER1) binds AGEs, leading to their sequestration and detoxification, resulting in antioxidant properties. AGE–specific receptor (RAGE) is a multi-ligand transmembrane receptor that binds many ligands, including AGEs, triggering inflammatory and oxidative injury. A misbalance between RAGE and AGER1 levels result in suppression of the antioxidant defense system and increased levels of pro-oxidant mechanisms [2]. Accumulation of AGEs has been proposed to be a pathogenic mechanism of oxidative stress, inflammation and structural tissue damage leading to chronic vascular complications in different disorders including aging, diabetes and chronic renal disease (CKD) [3, 4]. Diabetic nephropathy illustrates a classical model for AGEs pathogenic injury [5]. More recently, it has been suggested that AGEs accumulation may be involved in the progression of CKD and cardiovascular disease (CVD) both in diabetic and in non-diabetic patients [6,7].
Levels of plasma or urine AGEs have been linked with the severity and prognosis of diabetes, renal and CVD. However, these samples frequently fail to reproduce tissue levels, which more precisely reflect long-standing negative interactions of AGEs [8, 9]. Whereas direct determination of tissue AGEs is an invasive and expensive procedure, skin autofluorescence (SAF), a noninvasive automated measure of tissue AGEs accumulation has recently become an easy and validated method for clinical use. SAF has been shown to be highly correlated with serum AGEs concentrations and more importantly reflects long-standing AGEs accumulation that is associated with tissue and end organ damage [10,11]. Several reports have shown SAF to be independently associated with aging and the development of long-term complications of diabetes, CKD progression and CVD mortality [11–16].
In CKD, accumulation of AGEs is attributed to impaired renal clearance, increased endogenous formation and excess dietary intake [17, 18]. Renal transplantation (RT) is the treatment of choice for end stage renal disease. Despite conferring maximal kidney function and survival benefits, postransplant cardiovascular risk remains high even with a healthy graft [19]. Although plasmatic clearance of AGEs dramatically improves after RT, its removal from slow-turnover tissues might be difficult to achieve and may contribute to this high CVD burden [8, 20]. Some studies have linked tissue levels of AGEs, estimated by SAF, with both chronic allograft dysfunction and a poorer CVD outcome [21, 22]. However, the magnitude of AGE contribution to the important cardiovascular risk of these patients remains to be clarified. Moreover, biochemical, nutritional and subclinical atherosclerotic markers related with AGE accumulation after renal transplantation have not been studied in depth.
Therefore, we hypothesized that AGEs measurement by Skin Autofluorescence might be useful to screen cardiovascular risk even after long-term successful renal grafting. For this aim, we assess SAF in stable RT, with well-characterized clinical and biochemical parameters, in order to investigate factors linked with tissue accumulation of AGEs and analyze its potential significance as a marker of recipient’s cardiovascular risk.
Materials and methods
Study population
A total of 189 stable renal transplant patients followed at the nephrology outpatient clinic (EOXI Lugo-Cervo-Monforte, Lugo-Spain) were asked to participate in this observational cross-sectional study and written informed consent was obtained from all subjects. Recruitment was performed between May 2016 to June 2017. All procedures were in accordance with the ethical standards for research involving human subjects in the Helsinki Declaration and were approved by the local ethics committee (Comité Autonómico de Ética da Investigación de Galicia. Dictamen del Comité Santiago-Lugo; code number 2017/236). Only Caucasian patients with stable renal function for the previous three months were included. Subjects under 18 years old, pregnant women, those with acute inflammatory or infectious disease, or those hospitalized in the previous 3 months were excluded. Patients with a double kidney-pancreas graft, type 1 diabetes mellitus or those with uncontrolled malignancy on chemotherapy were not included. As AGEs levels may improve after a renal graft, only those patients followed for more than 12 months after transplantation were screened. To minimize confounding that would be introduced by poor allograft function; participants with estimated glomerular filtration rate (eGFR) of less than 15 mL/min per 1.73 m2 were also excluded. Immunosuppressive therapy generally consisted of prednisone with a calcineurin inhibitor (mainly tacrolimus) or m-TOR inhibitor, combined with mycophenolate.
Skin autofluorescence measurement (SAF)
SAF was assessed by a validated reader (Diagnoptics Technologies BV, Groningen, the Netherlands) as previously described in detail [10]. The autofluorescence reader (AFR) is a desk-top device based on the characteristic fluorescent properties of certain AGEs to quantify their level of accumulation in the skin. It illuminates a skin surface of 1 cm2, guarded against surrounding light, with an excitation light source between 300 and 420 nm (peak excitation approximately 370 nm). Only light from the skin is measured with a spectrometer in the 300–600 nm range, using a 200 mm glass fiber. SAF is calculated as the ratio between the emission light and reflected excitation light, multiplied by 100 and expressed in arbitrary units (AU). Reproducibility of the device has been tested in different study populations and showed a mean relative error in skin AF of ∼5% [10]. Mean age-corrected skin AF per intraindividual seasonal variance or even per AFR system did not differ significantly as well.
Measurements were performed at room temperature with the patient in a seated position, at the ventral side of the lower arm approximately 10–15 cm below the elbow fold. Care was taken to perform the measurements at normal skin site, that is, without visible dermatosis, bruising or pigmentation disorders, malignancies or other skin abnormalities including arteriovenous fistula. Autofluorescence was calculated by automated analysis and was therefore observer-independent. Using skin AF result we calculated “vascular age” by the relation previously described by Koetsier et al [23], for age and SAF in a healthy Caucasian population [vascular age = (skin AF– 0.83) / 0.024)]
Clinical, laboratory and other parameters
History of cardiovascular disease: ischemic heart disease (coronary syndrome or revascularization), cerebrovascular and peripheral artery disease (amputation or revascularization) were extracted from medical records. In addition, peripheral artery disease (PAD) was also screened by means of the ankle-brachial index (ABI). A pathologic ABI was defined as a value ≤0.9 or ≥1.4. Subclinical atheromatosis was assessed by means of the the intrarenal resistivity index (RI) of the graft. RI, measured by doppler-ultrasound examination at three different places of the graft, was calculated as [1 − (Vmin ÷ Vmax)] where Vmax is the peak of systolic velocity and Vmin the minimal diastolic velocity. Results are expressed as the average of all these measures. A pathologic RI was defined as a value ≥ 0.8. Blood pressure was monitored by 24 h-ambulatory blood pressure monitoring (24h-ABPM). Concomitant medications including antihypertensive treatment, immunosuppression (IS) and statins were also recorded.
The REGICOR (REgistre GIroní del COR) formula was used to estimate the 10-year risk of mortality from cardiac disease. This is a modified Framingham Risk Equation based on age, gender, systolic and diastolic blood pressure, smoking status, diabetes, total cholesterol and high-density lipoprotein cholesterol that has been validated for the Spanish population [24].
Anthropometric measurements, including mid-upper arm circumference (MUAC), height, waist and weight were performed during the clinical examination. Body mass index (BMI) was calculated (kg/m2) and handgrip muscle strength measured in the non-dominant arm using the Baseline Hydraulic Hand Dynamometer (NexGen Ergonomics Inc, Quebec. Canada).
Blood and urine laboratory measurements (Cobas system, Roche diagnostics, Basel, Switzerland) included serum creatinine, uric acid, proteinuria (albumin to creatinine ratio), bicarbonate, calcium, phosphate, parathyroid hormone (PTH), vitamin D, C-reactive protein, ferritin, hemoglobin, glycated hemoglobin (HbA1c) and lipid profile. Albumin and transferrin concentrations were used as biochemical markers of nutritional status. All measurements were part of our routine patient visits and analyzed centrally at the hospital laboratory. eGFR expressed as mL/min per 1.73m2 was estimated using the Chronic Kidney Disease Epidemiology Collaboration equation (CKD-EPI) [25]. Phosphate tubular reabsorption rate was calculated as: [1- ((urine phosphate x serum creatinine) / (urine creatinine x serum phosphate)) x 100].
Statistical analyses
Statistical analyses were performed using SPSS version 21 (SPSS Inc, Chicago, IL). The primary goal was to evaluate SAF (AU) relationship with clinical, biochemical, nutritional and surrogate CVD markers. Normal distribution of the variables was evaluated using the Kolmogorov-Smirnov test. Accordingly, both parametric and non-parametric tests were used as appropriate. Differences in continuous variables for important selected strata were tested using the t-student or Mann-Whitney U-test. All results were expressed as means and standard deviation unless otherwise stated. Pearson´s or Spearman´s correlation coefficient, as appropriate, was used to evaluate bivariate relationships between continuous variables. The χ2 test or Fisher exact test, if applicable, were used to compare categorical variables by cross tabulation. Multivariate linear regression analysis using the stepwise backward-selection method was also performed to identify the independent predictors of SAF (measured as a continuous variable) after adjusting for the significant univariate variables. Unadjusted P-values less than 0.05 were considered significant.
Results
Patient´s characteristics
Results, for the total 189 enrolled patients (116 males and 73 females), are presented in Fig 1. Mean age of the group was 56 ± 13.0 years. Time from transplantation ranged from 12 to 468 (median 118) months. Diabetes was present in 34% of cases (65 patients) whereas diabetic nephropathy accounted only in 17 cases (9%). Major vascular events, namely coronary heart disease, cerebrovascular and peripheral artery disease were present in 29 patients (15%). Actual immunosuppressive medications included steroids (128 patients), tacrolimus (130 patients), cyclosporine A (24 patients), m-TOR inhibitors (32 patients) and mycophenolate (158 patients). 22 patients (12%) had a living related donor. Patients were on a mean of 1.8 ± 1.26 antihypertensive medications. 72 patients were receiving renin-angiotensin-aldosterone system (RAAS) inhibitors and 76% were on statins. 21 patients (11%) were currently smokers. Pretransplant dialysis technique was hemodialysis (137 patients) and peritoneal dialysis (40 patients).
Fig 1. Patient’s characteristics.
Data is expressed as means ± standard deviation. Range is in brackets when appropriate. HD = Hemodialysis, PD = Peritoneal Dialysis, ABI = Ankle-Brachial Index, BP = Blood Pressure, RAAS = Renin-Angiotensin-Aldosterone System, eGFR = Estimated Glomerular Filtration Rate, CKD = Chronic Kidney Disease, BMI = Body Mass Index, MUAC = Mid-upper arm circumference.
Graft function estimated by the CKD-EPI was 49.6 ± 20.01 ml/min/1.73 m2. According to CKD stages (KDOQI classification), 5 patients (3%) were on stage 1, 50 (26%) on stage 2, 101 (53%) on stage 3 and 33 (18%) on stage 4. Whereas microalbuminuria was present in 44% of cases, 16% showed overt proteinuria. Nutritional parameters, namely mean BMI and handgrip strength, were 28.9 ± 5.45 kg/m2 and 30 ± 10.4 Kg respectively.
Correlations of skin AF with demographic, clinical and biological parameters
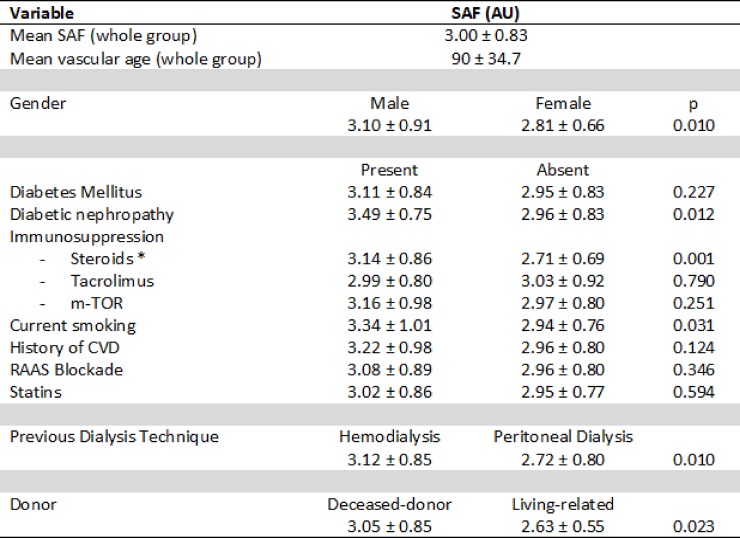
Mean skin autofluorescence was 3.00 ± 0.83 (1.5–6.4) AU. Estimated vascular age for the whole group was 90 ± 34.7 years (>30 years older than chronological age). Comparison of potentially relevant categorical variables (gender, diabetes type of previous dialysis, donor, immunosuppression, etc.) are showed in Fig 2. SAF levels were significantly higher in men (3.10 ± 0.91 vs 2.81 ± 0.66 AU) and in those with diabetic nephropathy. Interestingly, RT recipients with a history of peritoneal dialysis (PD) showed SAF values significantly lower than those with a history of hemodialysis (HD). Though not specifically analyzed, a shorter dialysis vintage for PD, age or a better-preserved residual renal function prior to transplant in PD patients might be responsible of this finding. Likewise, transplants from a living-related donor also showed less SAF levels (2.63 ± 0.55 vs 3.05 ± 0.85 AU). Regarding immunosuppression regimen, patients currently on steroids had higher SAF intensity than those without (3.14 ± 0.86 vs 2.71 ± 0.69). Subsequently, vascular age was significantly higher among steroid treated patients (96 ± 36.0 years vs 78 ± 28.6 years). This difference was not attributable either to diabetes or chronological age since patients with and without steroids had comparable ages (57 ± 11.8 vs 55 ± 15.2 years, respectively) and a lower diabetes frequency (31 vs 42%, respectively). No other differences were found regarding immunosuppression therapy, time since transplantation, statin or RAAS inhibitors use. Despite active smokers were younger than non-smokers (45 ± 15.1 vs 58 ± 12.6 years), they statistically showed higher SAF-values than non-smokers.
Fig 2. SAF values in selected categorical variables.
Data is expressed as means ± standard deviation. SAF = Skin Autofluorescence, AU = Arbitrary Units, CVD = Cardiovascular Disease, RAAS = Renin-Angiotensin-Aldosterone System. *Steroids refers as steroid use at the time of SAF determination (present or absent).
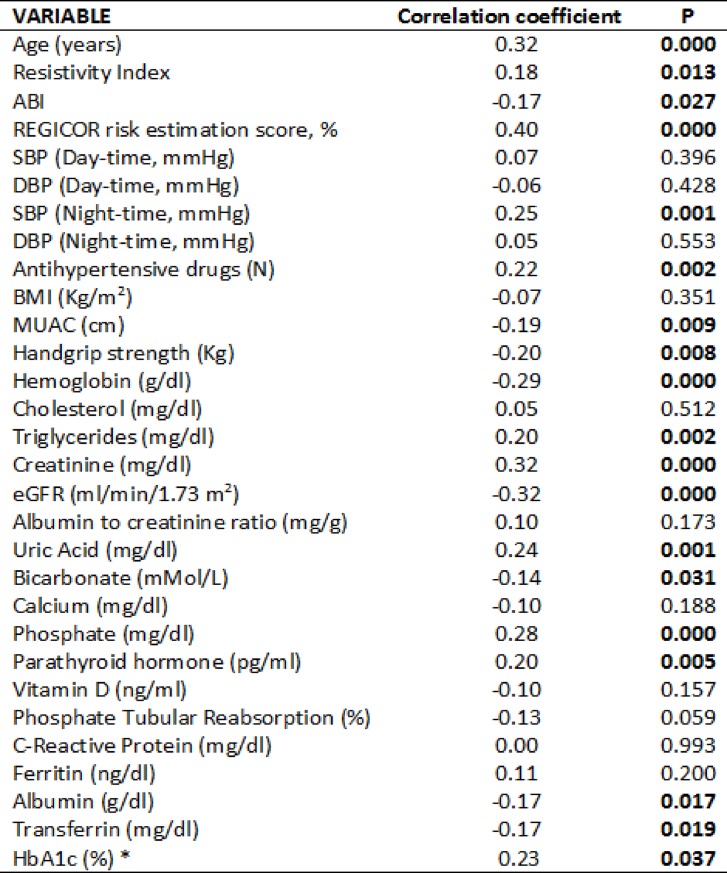
Univariate correlates of SAF are displayed in Fig 3. As expected, SAF showed a positive correlation with age (r = 0.32). SAF intensity was also positively associated with nighttime systolic blood pressure, the number of antihypertensive drugs, triglycerides, uric acid, PTH and phosphate concentration. Phosphate tubular reabsorption rate showed a negative correlation (r = -0.13, p = 0.05). Renal function (eGFR), bicarbonate and hemoglobin were also negatively correlated with SAF. Nutritional status-related variables, including serum albumin, transferrin, MUAC and handgrip strength also showed a negative correlation with SAF. No significant association was observed with BMI, waist, reactive protein C, ferritin, albuminuria, vitamin D, calcium and total cholesterol or its fractions. In diabetics, glycated hemoglobin (%) was positively correlated with SAF (r 0.23; p = 0.037).
Fig 3. Univariable correlations between SAF and continuous variables.
ABI = Ankle-Brachial Index, SBP = Systolic Blood Pressure, DBP = Diastolic Blood Pressure, BMI = Body Mass Index, MUAC = Mid-upper arm circumference, eGFR = Estimated Glomerular Filtration Rate, HbA1c = Glycated hemoglobin, *Diabetic patients only, Significant variables are shown in negrita.
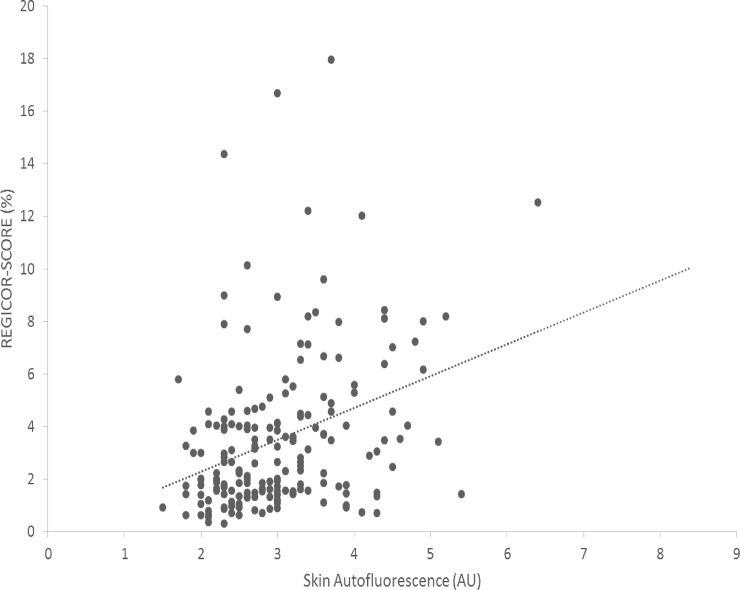
SAF was associated with atherosclerosis damage estimated by ABI (r = -0.17) and intragraft resistivity index (r = 0.18). Of note, as shown in Fig 2, SAF values were not statistically associated with a history of cardiovascular event (probably due to the reduced number of patients with this condition in the series). Nonetheless, application of the REGICOR scale showed a very close correlation with SAF (Fig 4).
Fig 4. Correlation between SAF and the REGICOR-risk score.
r = 0.35, p < 0.001; AU = Arbitrary Units.
Linear regression analysis
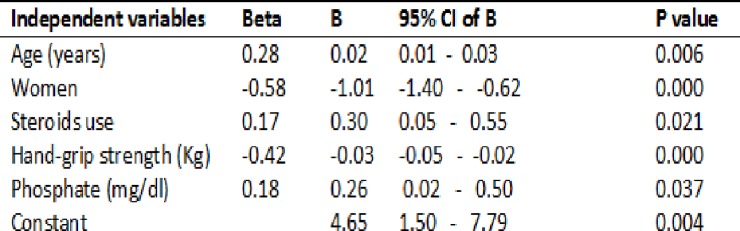
Stepwise linear regression analysis was performed to determine the parameters that independently associated to SAF. In a final model which explained 53% of the variance of skin-AF, age, male gender, steroids use, serum phosphate and handgrip strength emerged as independent contributors, as shown in Fig 5. Renal function (CKD-EPI), uric acid, systolic blood pressure (night-time), number of antihypertensives, hemoglobin, albumin, bicarbonate, parathormone, triglycerides and transferrin did not significantly influence SAF in the model. Addition of REGICOR risk score, Resistivity index, ABI, replacement of diabetes with diabetic nephropathy, type of donor or previous dialysis technique (HD or PD) in the model did not change the results.
Fig 5. Stepwise multivariable linear regression model (SAF = dependent variable).
R2 = 0.535, p < 0.001. Variables stepwise excluded: Diabetes Mellitus, renal function (eGFR), uric acid, bicarbonate, systolic blood pressure (night-time), number of antihypertensives, hemoglobin, albumin, parathyroid hormone, triglycerides, transferrin, REGICOR risk score, Resistivity index and ABI.
Discussion
SAF is a marker of AGEs accumulation and predictor of CVD mortality in aging, diabetes mellitus and CKD [11–16]. We performed a cross-sectional study to find out clinical determinants for SAF among renal transplant patients. SAF levels in our study were comparable with previous reports pinpointing RT as an incomplete solution for AGE accumulation in uremia [21, 22]. Several vascular and transplant-specific factors may explain this AGEs excess after RT and are also strengthened by our results. AGEs are considered to affect the biomechanical properties of matrix proteins, thereby contributing to the physiological process of chronological aging [7, 26]. Using a validated equation based on reference SAF values [23], we observed a marked increase in the vascular age of our patients (more than 30 years higher than chronological age). This disparity has also been documented in a recent research of Sanchez et al within CKD population with subclinical atherosclerosis [27].
We also found an association between SAF intensity and male sex that has not been consistently seen in previous studies also carried out after RT [21, 22]. However, sex-differences in SAF values may not be fully attributed to sex per se but to underlying comorbidities and conditions like diabetes, CVD, CKD, smoking and dietetic habits among others, that may prevail in one sex over the other [11, 28]. Ovarian dysfunction common in uremia and the decrease in skin collagen after menopause may also account for these diverse associations [29–30].
Glycation adducts in tobacco and smoke can form cross-links with proteins [31]. Despite the low percentage of smokers included (11%) and their younger age, our results support the harmful association of smoking in AGEs accumulation after RT. In contrast, our data did not observe other previously reported conditions linked with a favorable AGEs´ balance like RAAS blockade or statins [32]. However, the cross-sectional design of our investigation is not suitable to study the potential anti-AGE benefit of these treatments after renal transplantation.
As expected, we have found a relation with diabetic nephropathy but it did not stand multivariable adjustment. Moreover, postransplant diabetes was also not statistically associated with SAF. Diabetes might be a sufficient condition but it is not essential for AGE cardiovascular damage [5]. We speculate that other conditions beyond diabetes (such as chronic inflammation and nutritional status) may interact with the unfavorable post-transplant metabolic milieu of dyslipemia, insulin resistance and reduced antioxidant defenses. Collectively, this may enhance both lipid and protein glycosylation contributing to elevated SAF values [20, 33, 34]. Moreover, in the presence of diabetes, our results also show a positive correlation between SAF and HbA1c.
Interestingly, a novel finding in our study was the independent association between use of steroids and SAF intensity, something that would need confirmation in subsequent studies. The wide range of post-transplant period (12–468 months) that may include different immunosuppressive protocols (including steroid use and withdrawal strategies) and the cross-sectional design of the study (comparing patients with and without steroids at that moment), may interfere with this observation. Moreover, as only patients from a single center were enrolled, these results are not to be generalized without further considerations.
It has been proposed that in the presence of certain risk factors, circulating AGEs may deposit in blood vessels including graft microvasculature and tubular cells, contributing to renal function decline [12,35]. In chronic allograft nephropathy, Raj et al [34], showed that AGEs might even increase independently of renal function itself reinforcing this vicious cycle. In our study, renal function but not proteinuria, was significantly correlated with SAF. However, multivariable analysis of data failed to support graft function as an independent marker of SAF. Because age and sex play an important role in the calculation of eGFR, the observed association perhaps represents a residual confounding [36]. Serum AGEs are linked to high turnover proteins that may rapidly improve with a healthy graft [19, 33]. In the long run after transplantation, formation, accumulation, breakdown and clearance of tissue AGEs on long-stable tissue proteins is a slow process that reflects accumulative metabolic stress [37]. This idea is also supported by our study by the finding that PD and living donor recipients, with a shorter dialysis vintage or a better preserved residual renal function prior to transplantation, had lower levels of SAF in our analysis. Crowley and colleagues, reported a relationship between SAF and time since transplantation in a previous work [38]. Unexpectedly, our results failed to prove this relation. The wide range of post-transplant period (12–468 months) and the exclusion of patients with less than one year after transplant (who could dramatically improve relatively soon in the short-term after RT) might explain this result.
In contrast to other studies [21,22], we did not find an association between SAF and inflammation (estimated by either ferritin or C-reactive protein). The wide range of post-transplant observation period and the exclusion criteria applied in our study may explain this result. On the other hand, an interesting and previously unknown observation from of our study is the consistent association between SAF intensity both nutritional biochemical markers (i.e. albumin, transferrin) and muscle-related parameters (MUAC and handgrip strength). Sarcopenia has been recently linked with AGEs accumulation and SAF in Japanese population [39]. Furthermore, oxidant stress, inflammation and malnutrition characterize a novel unifying model for CVD risk in uremia [40]. Protein energy wasting (PEW) is an independent factor associated with morbi-mortality in both CKD and renal transplantation with a major impact at dialysis stage [41]. Anemia, another associate of SAF in our study, has been also significantly linked with PEW in renal transplants [42]. Though RT may share similar scores for malnutrition-inflammation with eGFR-paired CKD patients [41], not all these correlates have been studied in depth [43].
Uremic mineral-metabolism disturbances (CKD-MBD) are largely restored after successful renal transplantation. For instance, fibroblast growth factor (FGF-23) returns to normal values one year after RT though hyperparatiroidism and relatively low phosphate levels may persist in the long-term [19]. However, even average values of serum phosphate (> 3.5 mg/dL), are predictors of all-cause mortality after 4 years of RT independently of allograft function [44]. We found an interesting correlation between serum phosphate, phosphate excretion, parathyroid hormone and SAF. Unfortunately we did not analyze other components of the CKD-MBD axis like klotho or FGF-23 levels. However, if phosphate control or AGE restriction may influence RT outcome requires prospective studies.
Beyond phosphate influence, AGEs deposit in the vessel wall and atherosclerotic plaques have been postulated to contribute to the progression of CVD in diabetes or CKD and to associate with the risk of mortality [12, 45, 46]. Limited by the reduced number of patients with cardiovascular events in our series, we failed to find a significant association with skin AF and major CVD. Instead, we used surrogate markers for subclinical atherosclerosis like ABI, RI and nocturnal BP (a marker of arterial stiffness) and observed univariate associations. Our findings might be also in agreement with previous reports that argue in favor of graft RI as a correlate of recipient´s hemodynamic and vascular characteristics rather than with graft function itself [47, 48]. Finally, our results show an interesting correlation between SAF and the REGICOR-calibrated Framingham´s cardiac risk equation. The multimorbidity nature of our patients may explain this association, while contrast with a previous report observing no-link between cardiovascular risk SCORE and SAF in non-CKD, non-diabetic participants [49].
Conclusions
As hypothesized, skin AF may represent a clinically useful, non-invasive method, to assess cardiovascular risk in RT. Limitations of our analysis are, however, its cross-sectional nature that precludes any causation in the associations observed, and the lack of circulating or direct tissue AGEs assessment. Besides age and male gender, our results indicate that serum phosphate, steroid use and nutritional status are the major determinants of SAF after long-term RT. If AGE deposition is the corner stone linking PEW, glucocorticoids and CVD after RT as well as their role as a new therapeutic target requires further prospective investigations.
Acknowledgments
Fondo de Investigaciones Sanitarias grant PI16/01298, Madrid Society of Nephrology and REDinREN support Secundino Cigarran and Juan Latorre.
The authors would like to thank to Jaime Uribarri, professor in Medicine, Nephrology, The Mount Sinai Hospital, NY, USA, by his assistance in review the manuscript and useful comments.
Data Availability
Confidentiality of data is required according to ethical approval (Comité Autonómico de Ética da Investigación de Galicia. Resolution of the Comité Santiago-Lugo; code number 2017/236). Patient´s informed consent guaranteed confidentiality of personal data. For interested researchers, data access may be requested to: Fundación Instituto de Investigación Sanitaria de Santiago de Compostela (FIDIS). Hospital Universitario Lucus Augusti. Unidad de Investigación. 1ª Planta. C/ Ulises Romero. Nº 1. 27003. Lugo gestionlugo.fidis@sergas.es.
Funding Statement
The authors received no specific funding for this work. Fondo de Investigaciones Sanitarias grant PI16/01298, Madrid Society of Nephrology and REDinREN support Secundino Cigarran and Juan Latorre.
References
- 1.Luevano-Contreras C, Chapman-Novakofski K. Dietary advanced glycation end products and aging. Nutrients 2010; 2:1247–65. 10.3390/nu2121247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stinghen AE, Massy ZA, Vlassara H, Striker GE, Boullier A. Uremic Toxicity of Advanced Glycation End Products in CKD. J Am Soc Nephrol 2016; 27:354–70. 10.1681/ASN.2014101047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brownlee M. Advanced protein glycosylation in diabetes and aging. Annu Rev Med 1995; 46:223–34. 10.1146/annurev.med.46.1.223 [DOI] [PubMed] [Google Scholar]
- 4.Miyata T, van Ypersele de Strihou C, Kurokawa K, Baynes JW. Alterations in nonenzymatic biochemistry in uremia: origin and significance of “carbonyl stress” in long-term uremic complications. Kidney Int 1999; 55:389–99. 10.1046/j.1523-1755.1999.00302.x [DOI] [PubMed] [Google Scholar]
- 5.Makita Z, Radoff S, Rayfield EJ, Yang Z, Skolnik E, Delaney V, et al. Advanced glycosylation end products in patients with diabetic nephropathy. N Engl J Med 1991; 325:836–42. 10.1056/NEJM199109193251202 [DOI] [PubMed] [Google Scholar]
- 6.Arsov S, Graaff R, van Oeveren W, Stegmayr B, Sikole A, Rakhorst G, et al. Advanced glycation end-products and skin autofluorescence in end-stage renal disease: a review. Clin Chem Lab Med 2014; 52: 11–20 10.1515/cclm-2012-0832 [DOI] [PubMed] [Google Scholar]
- 7.Semba RD, Ferrucci L, Fink JC, Sun K, Beck J, Dalal M, et al. Advanced glycation end products and their circulating receptors and level of kidney function in older community-dwelling women. Am J Kidney Dis 2009; 53: 51–8. 10.1053/j.ajkd.2008.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartog JW, Smit AJ, van Son WJ, Navis G, Gans RO, Wolffenbuttel BH, et al. Advanced glycation end products in kidney transplant patients: A putative role in the development of allograft dysfunction. Am J Kidney Dis 2004; 43: 966–75. [DOI] [PubMed] [Google Scholar]
- 9.Busch M, Franke S, Ruster C, Wolf G. Advanced glycation endproducts and the kidney. Eur J Clin Invest 2010; 40: 742–55 10.1111/j.1365-2362.2010.02317.x [DOI] [PubMed] [Google Scholar]
- 10.Meerwaldt R, Graaff R, Oomen PH, Links TP, Jager JJ, Alderson NL, et al. Simple non-invasive assessment of advanced glycation endproduct accumulation. Diabetologia 2004; 47:1324–30 10.1007/s00125-004-1451-2 [DOI] [PubMed] [Google Scholar]
- 11.McIntyre NJ, Fluck RJ, McIntyre CW, Taal MW. Skin autofluorescence and the association with renal and cardiovascular risk factors in chronic kidney disiease stage 3. Clin J Am Soc Nephrol 2011; 6:2356–63. 10.2215/CJN.02420311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smit AJ, Gerrits EG. Skin autofluorescence as a measure of advanced glycation endproduct deposition: a novel risk marker in chronic kidney disease. Curr Opin Nephrol Hypertens 2010; 19:527–33. 10.1097/MNH.0b013e32833e9259 [DOI] [PubMed] [Google Scholar]
- 13.Hartog JW, de Vries AP, Lutgers HL, Meerwaldt R, Huisman RM, van Son WJ, et al. Accumulation of advanced glycation end products, measured as skin autofluorescence, in renal disease. Ann N Y Acad Sci 2005; 1043:299–307. 10.1196/annals.1333.037 [DOI] [PubMed] [Google Scholar]
- 14.Meerwaldt R, Lutgers HL, Links TP, Graaff R, Baynes JW, Gans RO, et al. Skin autofluorescence is a strong predictor of cardiac mortality in diabetes. Diabetes Care 2007; 30:107–12. 10.2337/dc06-1391 [DOI] [PubMed] [Google Scholar]
- 15.Corstjens H, Dicanio D, Muizzuddin N, Neven A, Sparacio R, Declercq L, et al. Glycation associated skin autofluorescence and skin elasticity are related to chronological age and body mass index of healthy subjects. Exp Gerontol 2008; 43: 663–7 10.1016/j.exger.2008.01.012 [DOI] [PubMed] [Google Scholar]
- 16.Tanaka K, Nakayama M, Kanno M, Kimura H, Watanabe K, Tani Y, et al. Skin Autofluorescence is associated with the progression of Chronic Kidney Disease: A Prospective Observational Study. PLoS One 2013; 8(12):e83799 10.1371/journal.pone.0083799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agalou S, Ahmed N, Babaei-Jadidi R, Dawnay A, Thornalley PJ. Profound mishandling of protein glycation degradation products in uremia and dialysis. J Am Soc Nephrol 2005;16: 1471–85 10.1681/ASN.2004080635 [DOI] [PubMed] [Google Scholar]
- 18.Uribarri J, Peppa M, Cai W, Goldberg T, Lu M, Baliga S, et al. Dietary glycotoxins correlate with circulating advanced glycation end product levels in renal failure patients. Am J Kidney Dis 2003; 42:532–8. [DOI] [PubMed] [Google Scholar]
- 19.Wolf M, Weir MR, Kopyt N, Mannon RB, Von Visger J, Deng H, et al. A prospective cohort study of mineral metabolism after kidney transplantation. Transplantation 2016; 100:184–93 10.1097/TP.0000000000000823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galli F. Protein damage and inflammation in uraemia and dialysis patients. Nephrol Dial Transplant 2007; 22 (Suppl 5): 20–36 [DOI] [PubMed] [Google Scholar]
- 21.Hartog JW, de Vries AP, Bakker SJ, Graaff R, van Son WJ, van der Heide JJ, et al. Risk factors for chronic transplant dysfunction and cardiovascular disease are related to accumulation of advanced glycation end-products in renal transplant recipients. Nephrol Dial Transplant 2006; 21:2263–9. 10.1093/ndt/gfl132 [DOI] [PubMed] [Google Scholar]
- 22.Hartog JW, Gross S, Oterdoom LH, van Ree RM, de Vries AP, Smit AJ, et al. Skin-autofluorescence is an independent predictor of graft loss in renal transplant recipients. Transplantation 2009; 87:1069–77. 10.1097/TP.0b013e31819d3173 [DOI] [PubMed] [Google Scholar]
- 23.Koetsier M, Lutgers HL, de Jonge C, Links TP, Smit AJ, Graaff R. Reference values of skin autofluorescence. Diabetes Technol Ther 2010; 12: 399–403. 10.1089/dia.2009.0113 [DOI] [PubMed] [Google Scholar]
- 24.Marrugat J, Subirana I, Comín E, Cabezas C, Vila J, Elosua R, et al. ; VERIFICA Investigators. Validity of an adaptation of the Framingham cardiovascular risk function: the VERIFICA Study. J Epidemiol Community Health 2007; 61:40–7. 10.1136/jech.2005.038505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A New Equation to Estimate Glomerular Filtration Rate. Ann Intern Med 2009; 150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Semba RD, Nicklett EJ, Ferrucci L. Does accumulation of advanced glycation end products contribute to the aging phenotype? J Gerontol A Biol Sci Med Sci 2010; 65:963–75. 10.1093/gerona/glq074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanchez E A, Arroyo D, López C, Hernandez M, Rius F, Fernandez E, Lecube A. Skin Autofluorescence and Subclinical Atherosclerosis in Mild to Moderate Chronic Kidney Disease: A Case-Control Study. PLoS One. 2017; 12: e 0170778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ueno H, Koyama H, Tanaka S, Fukumoto S, Shinohara K, Shoji T, et al. Skin autofluorescence, a marker for advanced glycation end product accumulation, is associated with arterial stiffness in patients with end-stage renal disease. Metabolism 2008; 57:1452–7. 10.1016/j.metabol.2008.05.016 [DOI] [PubMed] [Google Scholar]
- 29.Pitha J, Teplan V, Kalousova M, Racek J, Stollova M, Mareckova O, et al. Gender-related determinants of advanced subclinical atherosclerosis in patients undergoing kidney transplantation. Kidney Blood Press Res 2010; 33:227–34. 10.1159/000316709 [DOI] [PubMed] [Google Scholar]
- 30.Affinito P, Palomba S, Sorrentino C, Di Carlo C, Bifulco G, Arienzo MP, et al. Effects of postmenopausal hypoestrogenism on skin collagen. Maturitas 1999; 33:239–47. [DOI] [PubMed] [Google Scholar]
- 31.Cerami C, Founds H, Nicholl I, Mitsuhashi T, Giordano D, Vanpatten S, et al. Tobacco smoke is a source of toxic reactive glycation products. Proc Natl Acad Sci USA 1997; 94: 13915–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nenna A, Nappi F, Avtaar Singh SS, Sutherland FW, Di Domenico F, Chello M, et al. Pharmacologic approaches against advanced glycation end products (AGEs) in diabetic cardiovascular disease. Res Cardiovasc Med 2015; 4:e26949 10.5812/cardiovascmed.4(2)2015.26949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bucala R, Makita Z, Koschinsky T, Cerami A, Vlassara H. Lipid advanced glycosylation: pathway for lipid oxidation in vivo. Proc Natl Acad Sci USA 1993; 90:6434–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raj DS, Lim G, Levi M, Qualls C, Jain SK. Advanced glycation end products and oxidative stress are increased in chronic allograft nephropathy. Am J Kidney Dis 2004; 43:154–60. [DOI] [PubMed] [Google Scholar]
- 35.Yoshida K, Yoneda T, Fujimoto K, Hirao Y, Konishi N. Pentosidine and its deposition in renal tissue in renal transplantation. Transplant Proc 2005; 37:4266–72. 10.1016/j.transproceed.2005.11.018 [DOI] [PubMed] [Google Scholar]
- 36.Gerrits EG, Smit AJ, Bilo HJ. AGEs, autofluorescence and renal function. Nephrol Dial Transplant 2009; 24:710–3 10.1093/ndt/gfn634 [DOI] [PubMed] [Google Scholar]
- 37.Baynes JW. From life to death the struggle between chemistry and biology during aging: the Maillard reaction as an amplifier of genomic damage. Biogerontology 2000; 1:235–46 [DOI] [PubMed] [Google Scholar]
- 38.Crowley LE, Johnson CP, McIntyre N, Fluck RJ, McIntyre CW, Taal MW, et al. Tissue advanced glycation end product deposition after kidney transplantation. Nephron Clin Pract 2013;124:54–9. 10.1159/000355692 [DOI] [PubMed] [Google Scholar]
- 39.Kato M, Kubo A, Sugioka Y, Mitsui R, Fukuhara N, Nihei F, et al. Relationship between advanced glycation end-product accumulation and low skeletal muscle mass in Japanese men and women. Geriatr Gerontol Int 2017; 17:785–90. 10.1111/ggi.12787 [DOI] [PubMed] [Google Scholar]
- 40.Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM. The elephant in uremia: Oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int 2002; 62:1524–38 10.1046/j.1523-1755.2002.00600.x [DOI] [PubMed] [Google Scholar]
- 41.Molnar MZ, Carrero JJ, Mucsi I, Remport A, Rhee CM, Kalantar-Zadeh K, et al. Comparison of the malnutrition-inflammation score in chronic kidney disease patients and kidney transplant recipients. Int Urol Nephrol 2015; 47:1025–33. 10.1007/s11255-015-0984-2 [DOI] [PubMed] [Google Scholar]
- 42.Molnar MZ, Czira ME, Rudas A, Ujszaszi A, Haromszeki B, Kosa JP, et al. Association between the malnutrition-inflammation score and post-transplant anemia. Nephrol Dial Transplant 2011; 26:2000–6. 10.1093/ndt/gfq690 [DOI] [PubMed] [Google Scholar]
- 43.Ter Wee PM. Protein energy wasting and transplantation. J Ren Nutr 2013; 23:246–9. 10.1053/j.jrn.2013.02.004 [DOI] [PubMed] [Google Scholar]
- 44.Connolly GM, Cunningham R, McNamee PT, Young IS, Maxwell AP. Elevated serum phosphate predicts mortality in renal transplant recipients. Transplantation 2009; 87: 1040–4. 10.1097/TP.0b013e31819cd122 [DOI] [PubMed] [Google Scholar]
- 45.Sakata N, Imanaga Y, Meng J, Tachikawa Y, Takebayashi S, Nagai R, et al. Increased advanced glycation endproducts in atherosclerotic lesions of patients with end-stage renal disease. Atherosclerosis 1999; 142:67–77. [DOI] [PubMed] [Google Scholar]
- 46.Stitt AW, He C, Friedman S, Scher L, Rossi P, Ong L, et al. Elevated AGE-modified ApoB in sera of euglycemic, normolipidemic patients with atherosclerosis: relationship to tissue AGEs. Mol Med 1997; 3:617–27 [PMC free article] [PubMed] [Google Scholar]
- 47.Heine GH, Gerhart MK, Ulrich C, Köhler H, Girndt M. Renal Doppler resistance indices are associated with systemic atherosclerosis in kidney transplant recipients. Kidney Int 2005; 68: 878–85. 10.1111/j.1523-1755.2005.00470.x [DOI] [PubMed] [Google Scholar]
- 48.Naesens M, Heylen L, Lerut E, Claes K, De Wever L, Claus F, et al. Intrarenal resistive index after renal transplantation. N Engl J Med 2013; 369:1797–806. 10.1056/NEJMoa1301064 [DOI] [PubMed] [Google Scholar]
- 49.Tiessen AH, Jager W, ter Bogt NC, Beltman FW, van der Meer K, Broer J, et al. Skin autofluorescence as proxy of tissue AGE accumulation is dissociated from SCORE cardiovascular risk score, and remains so after 3 years. Clin Chem Lab Med 2014;52: 121–7. 10.1515/cclm-2012-0825 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Confidentiality of data is required according to ethical approval (Comité Autonómico de Ética da Investigación de Galicia. Resolution of the Comité Santiago-Lugo; code number 2017/236). Patient´s informed consent guaranteed confidentiality of personal data. For interested researchers, data access may be requested to: Fundación Instituto de Investigación Sanitaria de Santiago de Compostela (FIDIS). Hospital Universitario Lucus Augusti. Unidad de Investigación. 1ª Planta. C/ Ulises Romero. Nº 1. 27003. Lugo gestionlugo.fidis@sergas.es.