Abstract
Background
Non-union affects up to 10% of fractures and is associated with substantial morbidity. There is currently no single effective therapy for the treatment or prevention of non-union. Potential treatments are currently selected for clinical trials based on results from limited animal studies, with no attempt to compare results between therapies to determine which have the greatest potential to treat non-union.
Aim
The aim of this systematic review was to define the range of therapies under investigation at the preclinical stage for the prevention or treatment of fracture non-union. Additionally, through meta-analysis, it aimed to identify the most promising therapies for progression to clinical investigation.
Methods
MEDLINE and Embase were searched from 1St January 2004 to 10th April 2017 for controlled trials evaluating an intervention to prevent or treat fracture non-union. Data regarding the model used, study intervention and outcome measures were extracted, and risk of bias assessed.
Results
Of 5,171 records identified, 197 papers describing 204 therapies were included. Of these, the majority were only evaluated once (179/204, 88%), with chitosan tested most commonly (6/204, 3%). Substantial variation existed in model design, length of survival and duration of treatment, with results poorly reported. These factors, as well as a lack of consistently used objective outcome measures, precluded meta-analysis.
Conclusion
This review highlights the variability and poor methodological reporting of current non-union research. The authors call for a consensus on the standardisation of animal models investigating non-union, and suggest journals apply stringent criteria when considering animal work for publication.
Introduction
Fracture non-union can be defined as occurring when the normal healing processes of bone cease to the extent that solid healing cannot occur without further intervention[1]. The condition is estimated to affect 5–10% of fractures[2, 3], with wide variation depending on anatomical location[4]. The negative effect on quality of life associated with non-union has been demonstrated as being greater than that of diabetes mellitus, stroke and acquired immunodeficiency syndrome[5], with substantial financial consequences[6].
The failure of a fracture to unite is multifactorial and the result of both predisposing and contributing factors[1, 7]. There is no consensus or accepted guidelines for the treatment of non-union, but most current management strategies involve hospital admission and revision surgery, frequently using bone graft or synthetic substitutes, with varied and unpredictable results. In order to either primarily prevent non-union, increase the likelihood of success of revision surgery, or potentially offer an alternative to surgery, researchers continue to evaluation novel therapies in this field.
Preclinical studies are defined as those using animals to determine if a treatment is likely to be effective, before progression to testing in humans [8].
It is currently not clear on what basis researchers select potential therapies for translation into clinical studies. It is likely that positive results from a single, or a small number, of animal studies are used to justify progression to clinical trial. However, it is problematic to rely on the positive effects of a therapy in a single animal study to justify direct translation to clinical testing due to the likely existence of bias and methodological weakness. There is no evidence that researchers in this field have compared different preclinical studies in an attempt to determine which therapies are the most promising and therefore should be prioritised for translation into clinical studies.
Systematic reviews summarise the literature for a defined research question; when combined with a meta-analysis of results they are considered to represent the highest level in the hierarchy of evidence[9]. Despite this, meta-analyses are reliant upon the quality of data in the original studies included, and can risk propagating any errors included in the original research. The methodology for systematic reviews of preclinical research is still evolving, but it is recognised that the technique has the potential to clarify the existing evidence base and potentially increase the precision of effect estimates through meta-analysis[10, 11]. To date there has not been a systematic review or meta-analysis of preclinical studies aiming to prevent or treat fracture non-union.
The aim of this systematic review was firstly to establish the range of therapies under investigation at the preclinical stage for the prevention or treatment of fracture non-union. Secondly, by conducting a meta-analysis of results of methodologically similar studies, it aimed to systematically and objectively identify the most promising therapies for progression to clinical investigation.
Materials and methods
Search strategy and inclusion criteria
Full methodological details can be found in the previously published protocol[12]. The protocol was registered with Collaborative Approach to Meta-Analysis and Review of Animal Data from Experimental Studies (CAMARADES)[13]. A summary of the methods is reported below. Reporting of the full systematic review was in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines[14], (S1 Table).
MEDLINE and Embase were searched via Ovid from 1st January 2004 to 10th April 2017 (see S2 Table for full search strategy). The citation lists of included studies were searched for additional studies. In a deviation from the methodology published in the study protocol, due to the large volume of studies retrieved from the primary searches, no further additional sources were searched.
Two reviewers (PMB/SKS) independently screened titles and abstracts. Where eligibility for inclusion could not be determined from the abstract the full manuscript was obtained and reviewed for clarification. Any disagreements were resolved through discussion with a third reviewer (JPB). Controlled trials evaluating an intervention to prevent or treat non-union and measuring bone formation were eligible for inclusion; the focus of this review was to examine preclinical therapies with clinical potential and so treatments which had already been evaluated in a clinical study were excluded. Full inclusion and exclusion criteria were listed in the previously published protocol and are summarised in Table 1. Relevant preclinical studies evaluating therapies that had subsequently progressed to clinical trial were excluded, unless the therapy was combined with a novel therapy.
Table 1. Summary of study inclusion and exclusion criteria.
| Inclusion Criteria | |
| Types of studies | Controlled trials |
| Unpublished and published works | |
| Types of participants | Mammalian model testing an intervention to treat or prevent fracture non-union |
| Induced co-morbidities | |
| Intervention | Interventions aim to:
|
| Administered after formation of a bony defect | |
| Established interventions in a novel vehicle | |
| Comparator | Control group described receiving:
|
| Outcome measures | Quantifiable measure of bone formation through radiological and/or histological means |
| Exclusion Criteria | |
| Types of studies | Review articles |
| Types of participants | Clinical trials |
| Intervention | Any intervention that has subsequently progressed to clinical trial |
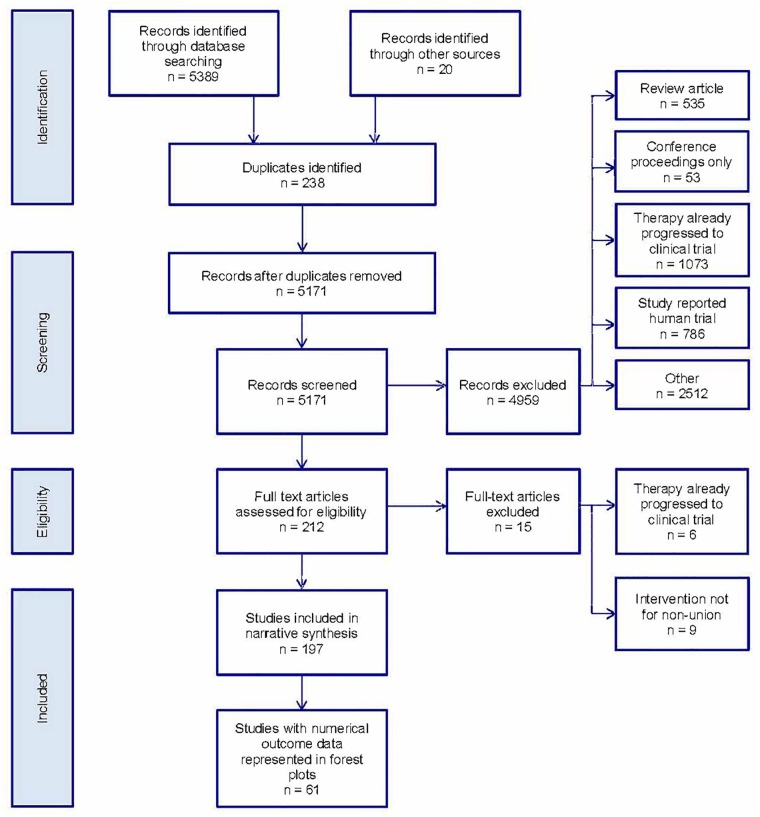
After duplicates were removed, 5,171 records were identified in the literature search as shown in the PRISMA flow diagram (Fig 1). After inclusion/exclusion criteria were applied 197 studies were included in the systematic review. The commonest single reason for study exclusion (1,073 studies, 21%) was that the article described a therapy that had already progressed to clinical trial.
Fig 1. PRISMA flow diagram for study inclusion/exclusion.
Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) flow diagram detailing numbers of studies excluded and reasons at each stage of the review process.
Data extraction and risk of bias assessment
Data relating to the model, defect location and method of creation, length of survival, number of animals included, outcome measures (radiological or histological) were extracted from manuscripts.
Where incomplete data was provided in the manuscript authors were contacted for clarification: of the 64 authors contacted, only 9 replied with the required information (14%). Numerical data extraction from papers presenting results in graphical format only was performed using ImageJ v.2.0 software (National Institute of Health, Bethesda, MD) using a standardised method[15, 16].
The Systematic Review Centre for Laboratory Animal Experimentation’s (SYRCLE) risk of bias tool was used to assess risk of bias across all studies[17]. The SYRCLE tool assesses ten domains across six types of bias: selection bias (sequence generation, baseline characteristics, allocation concealment), performance bias (random housing, blinding), detection bias (random outcome assessment, blinding), attrition bias (incomplete outcome data), reporting bias (selecting outcome reporting) and other sources of bias. Risk of bias assessment was performed by one author (PMB or SKS). Each domain was given a rating of high risk, low risk or unclear where information was incomplete or not reported. These ratings were based on the signalling questions designed to assist judgement, as detailed in the SYRCLE tool[17].
Analysis
Where studies reported sufficient data (numbers in intervention and control group, mean and standard deviation), results for the most consistently reported measures (bone formation (%), bone volume (mm3) or bone density (mg/cm3)) were represented in forest plots for illustrative purposes. Results for the remaining studies were tabulated. Where several time-points were reported, only the longest follow-up was considered.
Therapies were grouped into the following nine categories:
Animal derivatives
Plant extracts
Minerals/elements/chemicals
Pharmaceuticals
Cells/tissues
Vibration/motion
Light/lasers
Gases
Human proteins/hormones
If a therapy related to more than one category, it was included in both it pertained to (e.g. mesenchymal stem cells with insulin-like growth factor-1 was recorded in both the ‘cells/tissues’ and ‘human proteins/hormones’ categories.) Combination therapies using both an established therapy already in clinical trial with a novel preclinical therapy were again recorded in both categories to which they pertained.
Results
The spectrum of potential treatments
The 197 included studies evaluated a total of 204 different interventions (Table 2). The objective of approximately half of all studies was to promote or accelerate healing of a bony defect (103/197, 52%) or treat non-union (93/103, 47%), with further information available in S3 Table. The majority of therapies (179/204 (88%)) were only evaluated once, while five interventions (chitosan [18–23], adipose stromal cells [24–27], erythropoietin [28–31], vascular endothelial growth factor [32–35] and SDF-1 [36–38]) were investigated by multiple studies (Table 3). Chitosan as a single therapy was evaluated by six studies: four of these found significantly greater bone formation in the intervention group compared to control [18, 20–22], with further detail in Table 3.
Table 2. Number of evaluations under investigation by category*.
| Group | Number of evaluations included in tables | Number of evaluations included in forest plots | Total |
|---|---|---|---|
| Animal derivatives | 27 | 5 | 32 |
| Plant extracts | 23 | 13 | 36 |
| Minerals / elements / chemicals | 25 | 7 | 32 |
| Pharmaceuticals | 16 | 13 | 29 |
| Cells / tissues | 32 | 18 | 50 |
| Vibration / motion | 2 | 5 | 7 |
| Light / lasers | 3 | 0 | 3 |
| Gases | 3 | 5 | 8 |
| Human proteins / hormones | 59 | 41 | 100 |
| Total | 190 | 107 | 297 |
*Combination therapies are duplicated in all groups they pertain to, e.g. mesenchymal stem cells + vascular endothelial growth factor will be counted in “cells / tissues” and “human proteins / hormones”.
Single therapies tested in multiple concentrations are counted more than once, e.g. Ngueguim 2012 evaluates two plant based therapies: both therapies are evaluated at three different concentrations, thereby contributing 6 evaluations.
A total of 197 studies were included, investigating a total of 204 distinct therapies.
Total number of studies included in tables = 136, total number of studies included in forest plots = 61.
Table 3. Most frequently evaluated therapies across all studies (n = 197).
| Therapy | Number of studies evaluating therapy | Direction of effect |
|---|---|---|
| Chitosan | 6 | Four studies [18, 20–22] favoured intervention over control. One study [19] favoured control over intervention. One study [23] showed no difference between intervention and control. |
| Adipose stromal cells | 4 | Two studies [25, 27] favoured intervention over control. Two studies [24,26] showed no difference between intervention and control. |
| Erythropoietin | 4 | Four studies [28–31] showed no difference between intervention and control |
| Vascular endothelial growth factor | 4 | Two studies [32, 35] favoured intervention over control. Two studies [33, 34] showed no difference between intervention and control. |
| SDF-1 | 3 | Two studies [36, 38] favoured intervention over control. One study [37] showed no difference between intervention and control. |
| Therapies tested twice | 40 | |
| Therapies tested once | 179 |
Risk of bias
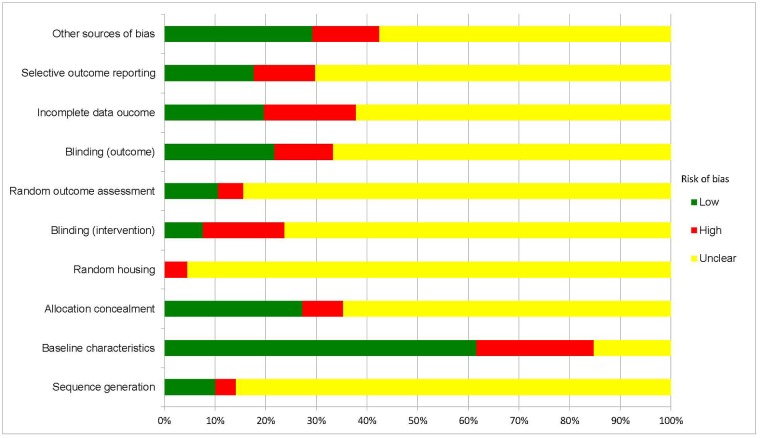
Details necessary to assess risk of bias were vastly underreported, particularly with regard to random housing, random outcome assessment (randomisation), sequence generation, blinding of outcome assessment and selective outcome reporting (Fig 2). Between 4 and 23% of studies were judged to be at high risk of bias for a given criterion. No study reported details for all ten domains of the SYRCLE tool.
Fig 2. Risk of bias analysis.
Bias assessed as per the Systematic Review Centre for Laboratory Animal Experimentation’s (SYRCLE) tool for all 197 studies included.
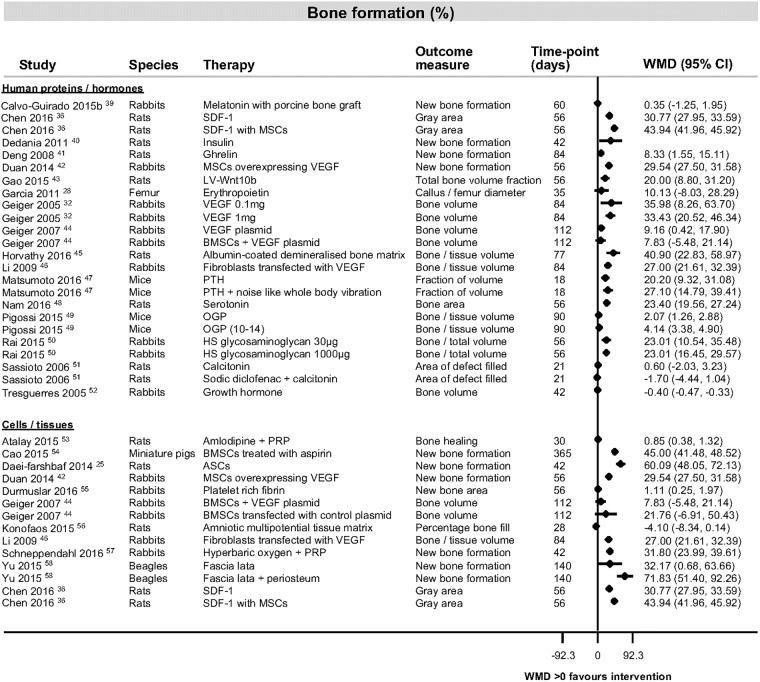
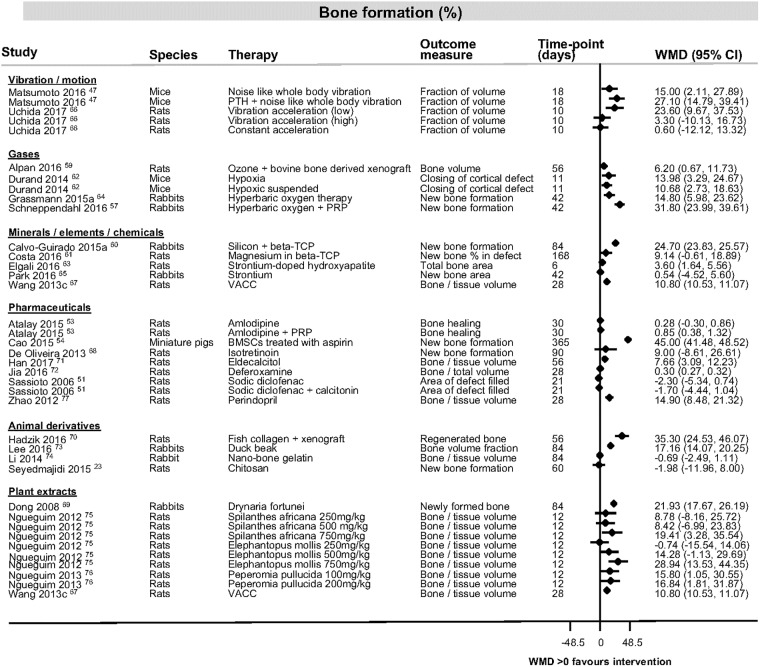
The most consistently reported outcome measure was percentage bone formation in the category of human proteins and hormones (Fig 3 [25, 28, 32, 36, 39–58]). Study findings across all categories for bone formation, bone volume and bone density are shown in Fig 4, [23, 47, 51, 53, 54, 57, 59–77], Fig 5 [29, 37, 38, 78–86] and Fig 6 [87–91]). Table 4 ([92–105]) shows the findings for the pharmaceutical therapies that could not be represented in forest plots, with findings for the remaining categories available as supporting information (S4, S5, S6, S7, S8, S9, S10 and S11 Tables).
Fig 3. Bone formation data for studies looking at interventions of human proteins and hormones or cells and tissues.
Forest plot illustrating mean difference in percentage of bone formation as measured by different histological or radiological measures. Abbreviations: ASCs, adipose tissue stem cells; BMSCs, bone marrow stromal cells; CI, confidence interval; HS, heparan sulphate; LV-Wnt10b, lentivirus vector encoding Wnt10b gene; MSCs, mesenchymal stem cells; OGP, osteogenic growth peptide; PRP, platelet rich plasma; PTH, parathyroid hormone; SDF-1, stromal cell derived factor 1; VEGF, vascular endothelial growth factor; WMD, weighted mean difference.
Fig 4. Bone formation data for studies looking at interventions of vibration and motion, gases, minerals, elements and chemicals, pharmaceuticals, animal derivatives or plant extracts.
Forest plot illustrating mean difference in percentage of bone formation as measured by different histological or radiological measures. Abbreviations: BMSCs, bone marrow stromal cells; CI, confidence interval; PRP, platelet rich plasma; PTH, parathyroid hormone; VACC, vanadium absorbed by Coprinus comatus; WMD, weighted mean difference.
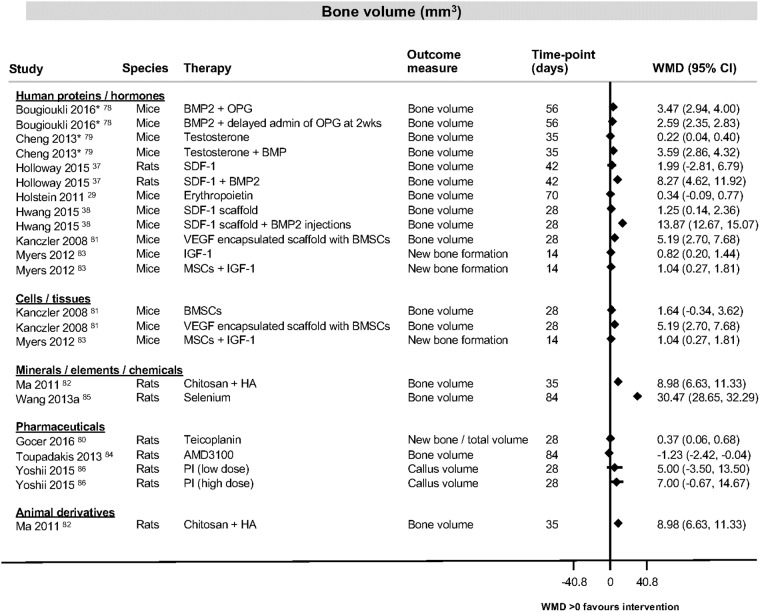
Fig 5. Bone volume data for studies looking at interventions of human proteins and hormones, cells and tissues, minerals, elements and chemicals, pharmaceuticals or animal derivatives.
Forest plot illustrating mean difference in cubic millimetre (mm3) of bone volume as measured by different histological or radiological measures. *Since none of the control groups healed, the increase in bone volume was set as 0 and the standard deviation as 0.0000001 in order to be able to illustrate those results in a forest plot using STATA. Abbreviations: BMP2, bone morphogenetic protein 2; BMSCs, bone marrow stromal cells; CI, confidence interval; HA, hyaluronic acid; IGF-1, insulin growth factor-1; MSCs, mesenchymal stem cells; OPG, osteoprotegerin; PI, proteasome inhibitor; SDF-1, stromal cell derived factor 1; VEGF, vascular endothelial growth factor; wks, weeks; WMD, weighted mean difference.
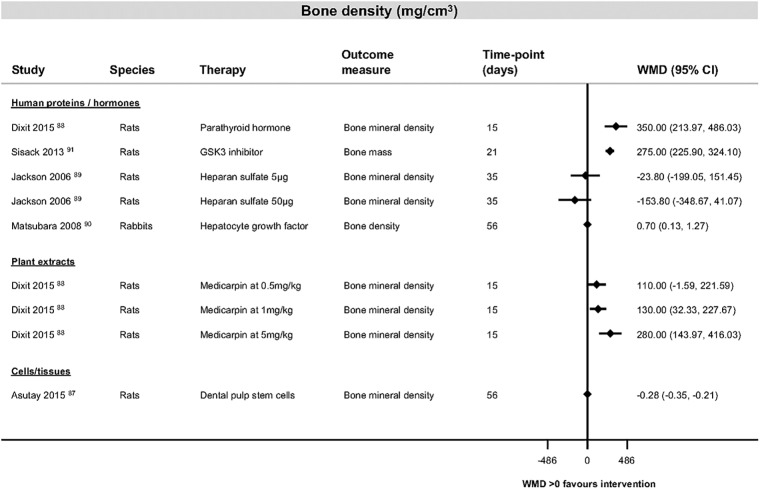
Fig 6. Bone density data for studies looking at interventions of human proteins and hormones, cells and tissues or plant extracts.
Forest plot illustrating mean difference in milligrams per cubic centimetre (mg/cm3) of bone density as measured by different histological or radiological measures. Abbreviations: CI, confidence interval; GSK3, glycogen synthase kinase 3; WMD, weighted mean difference.
Table 4. Defect repair data for studies evaluating therapies based on pharmaceuticals (16 therapies, 14 studies).
| Study | Therapy | Species | Maximum length of survival (days) | Outcome | Overall effect |
|---|---|---|---|---|---|
| Alic 2016 [92] | Cilostazol | Rats | 21 | No difference between groups at end of 21 days | = |
| Baht 2017 [93] | Nefopam | Mice | 21 | Treatment with Nefopam resulted in fracture calluses that contained higher proportions of bone and lower proportions of fibrous tissue | → |
| Bernick 2014 [94] | Lithium | Rats | 28 | Fracture healing was maximised with low dose, later onset and longer treatment duration of lithium, resulting in significantly greater yield torque in the therapeutic group | ↑ |
| Cai 2015 [95] | Lithium | Rabbits | 84 | New bone area for lithium containing mesoporous bioglass markedly higher than that for lithium containing bioglass at 56 and 84 days | → |
| Cakmak 2015 [96] | Pentoxyfylline | Rats | 56 | No bone growth in control or systemic pentoxyfylline only groups | = |
| Cakmak 2015 [96] | Pentoxyfylline + iliac crest autograft | Rats | 56 | Radiological bone union was observed in the iliac crest autograft and systemic pentoxyfylline group compared to no new bone growth in the control group | → |
| Del Rosario 2015 [97] | Simvastatin | Rats | 56 | No significant difference between groups | = |
| Donneys 2013 [98] | Deferoxamine | Rats | 40 | Greater union rate in treatment group than in irradiated group, but both less than control group | ↓ |
| Fan 2017 [99] | Phenamil | Rats | 86 | Incomplete mandibular restoration was observed in the defect treated with phenamil alone | ? |
| Fan 2017 [99] | Phenamil + BMP | Rats | 86 | Addition of BMP to phenamil synergistically augmented bone healing, resulting in almost complete bone healing | → |
| Ishack 2017 [100] | Dipyridamole | Mice | 56 | Significant increase in percentage of bone regenerated in dipyridamole group compared to control group | ↑ |
| Kutan 2016 [101] | Doxycycline | Rats | 28 | Osteogenesis in the test group was significantly higher than that of the control group | ↑ |
| Limirio 2016 [102] | Doxycycline + alendronate | Rats | 15 | Statistically greater bone density in therapeutic group compared to control group at 15 days | ↑ |
| Wada 2013 [103] | Salicylic acid | Rats | 84 | Significantly higher new bone in defect in therapeutic group compared to control group | ↑ |
| Werkman 2006 [104] | Risedronate | Rats | 28 | No significant difference between therapeutic and control groups | = |
| Wixted 2009 [105] | Zileuton | Mice | 28 | Net increase in callus size relative to control | → |
↑ indicates statistically significant effect on bone formation in trial therapy compared to control
→ indicates greater bone formation in trial therapy compared to control, but the effect did not reach statistical significance
= indicates no difference in bone formation rates between the therapeutic or control groups
↓ indicates less effect on bone formation in trial therapy compared to control
? indicates results are unclear, and no effect size could be determined
In total 53 human protein and hormone therapy evaluations (30 in forest plots, 23 in tables, 53/100, 53%) reported statistically significant improvements in bone healing compared to the control groups. Statistically significant improvements for the other categories were 50% animal derivatives (16/32), 53% plant extracts (19/36), 55% minerals/elements/chemicals (18/33), 38% pharmaceuticals (11/29), 54% cells/tissues (26/48), 30% vibration/motion (3/10), 100% light/lasers (3/3) and 75% gases (6/8). In total, 135 separate therapy evaluations (135/204, 66%) showed a significantly greater effect on healing of fracture non-union when compared to the control. Only a minority of interventions (9/204, 4%) resulted in significantly less effect on bone union than the comparator arm.
Meta-analysis
Substantial heterogeneity across studies in terms of type and site of defect, method of defect creation, species, length of follow-up and method of outcome reporting precluded meta-analysis.
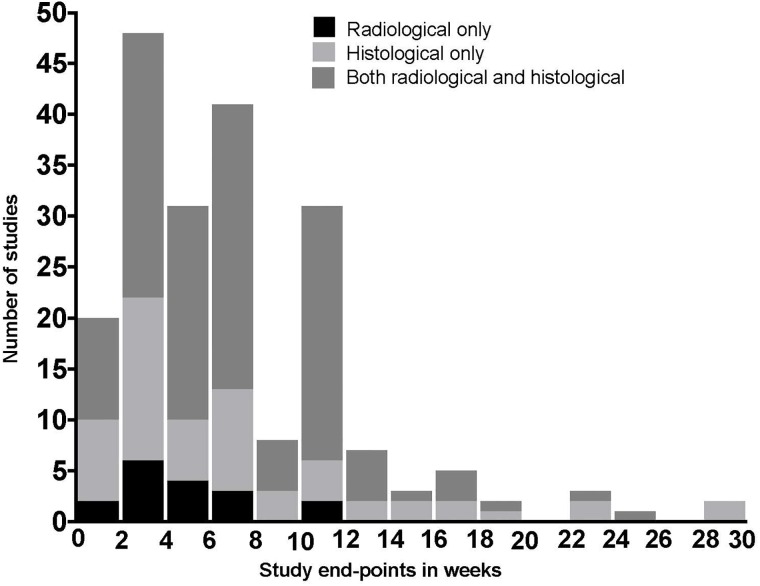
Rats were the most common animal model, used in 105 studies (105/197, 53%), with the calvarium being the commonest site of bony defect (71/197, 36%). Pigs, dogs, goats, rabbits and mice were also used. Further detail on animal and defect location is given in Table 5. It was not possible to determine the total number of animals used in 28 studies (28/197, 14%) with further detail in S2 Table. Studies used both radiological and histological outcome measures, with follow-up times ranging from 1–30 weeks (Fig 7).
Table 5. Model of non-union by species and anatomical location.
| Calvarium | Femur | Humerus | Mandible | Radius | Rib | Tibia | Ulna | Zygomatic arch | Total | |
|---|---|---|---|---|---|---|---|---|---|---|
| Pigs | 2 | 2 | ||||||||
| Dogs | 1 | 1 | 2 | |||||||
| Goats | 1 | 1 | ||||||||
| Rabbits | 17 | 8 | 1 | 2 | 14 | 10 | 4 | 1 | 57 | |
| Rats | 42 | 40 | 6 | 1 | 1 | 14 | 1 | 105 | ||
| Mice | 10 | 12 | 8 | 30 | ||||||
| Total | 71 | 60 | 1 | 8 | 16 | 1 | 34 | 5 | 1 | 197 |
Fig 7. Bar graph demonstrating varied study methodology.
Illustration of study-end point in weeks and outcome measure used by all 197 studies.
Regarding the defect, the majority of studies (75/197, 38%) did not report how the defect was created. A bur was used in 51 studies (51/197, 26%), with other methods including drills (14%), saws (12%), three-point bending (5%), drop weights or pendulums (3%), and being cut with scissors (3%). The defect was explicitly stated as being critical in 75 studies (75/197, 38%) and non-critical in 2 (2/197, 1%), with the remainder of studies (155/197, 79%) not providing this detail. Ten studies (6%) cauterised or stripped the periosteum surrounding the osteotomy.
Only one third of studies (61/197, 31%) included sufficient data to permit illustration in forest plots (without quantitative pooling), due to insufficient reporting of outcome data, or use of less commonly used outcome metrics.
Discussion
Fracture non-union is a common complication of a common condition [1–3]. This systematic review highlights not only the range of research activity in this field but the poor quality of contemporary animal research investigating this condition. Meta-analysis was not possible due to the diverse and non-standardised nature of the preclinical research, range of outcome measures and poor reporting of results. Despite there being a large amount of data– 204 evaluations across 197 studies—it has not been possible to make a valid comparison between any two studies nor draw firm conclusions regarding relative efficacies from different interventions and therefore identify those therapies that should be prioritised in translational research.
When developing preclinical models of fracture non-union various factors need to be considered. Fundamentally these include the species of animal to be used and the anatomical location of the fracture. Additionally, the type of fracture (transverse or segmental), whether it is subsequently stabilised or not and whether or not the periosteum is stripped are all variables that will affect the union rates of the fracture model. Finally, the delivery method of the therapy under investigation, including the use of scaffolds and carriers, must also be considered. The greater the number of differences that exist between model designs, the less reliably any differences in union rates can be attributed to the therapy under investigation alone, as model variations will act as confounders.
In clinical practice the progression of a fracture to established non-union is multi-factorial, with different types of non-union existing. The majority of primary research contained within this systematic review failed to consider this variability during model development: though the stated aim was to test a therapy designed to prevent or treat non-union, very few used proven models of non-union. The poor fidelity to clinical situations further limits the utility of the preclinical findings.
This systematic review used a methodologically rigorous approach to identifying, selecting and appraising primary studies. There were however some deviations from the previously published protocol; the authors chose to use the MEDLINE version of PUBMED to allow easier duplication of the search strategy on OVID. The decision to limit the systematic review to only these two primary databases was made due to the large volume of eligible studies included. The authors judged it unlikely that the inclusion of a small number of additional studies identified through other sources would significantly alter any conclusions, particularly given the variable and methodologically poor reporting of studies identified in the main databases. Additionally, the large number of studies meant that the risk of bias assessment was performed by one reviewer only for each study.
The studies included in this systematic review were limited by inadequate reporting of methodological details and results. Applying the risk of bias tool developed by SYRCLE showed that many risk of bias criteria were not reported and the rating of ‘unclear’ risk of bias was most common. This in turn hampers interpretation of results. It is however in line with the findings of previous studies which found poor reporting of randomisation procedures and blinding of assessors in animal studies[106], despite multiple resources for study design and reporting available to researchers[107–109]. Some omissions were extremely basic, for example 11% of studies had to be excluded from the forest plots for not stating whether their results were reported as mean with standard deviation, or standard error of the mean, with authors failing to provide clarification when contacted. The use of ± in methodological reporting without further explanation has previously been identified as a persistent problem[110, 111].
To address the problems identified by this review, the authors recommend that the orthopaedic trauma community attempt to reach a consensus on preferred animal models of bone healing similar to the standardisation of fracture classification with the OTA/AO/Muller system[112]. Once a consensus on the standardisation of species, defect and outcome measure is achieved, funding could be restricted to researchers using agreed models and study methodology[113], and journals should similarly restrict publication to studies that would allow direct comparison and insist on reporting results in detail. However, even if this were achieved, the translatability of animal research into effective clinical trials remains controversial [114–116], with even highly cited animal studies failing to translate into successful interventions in clinical trials[117].
This systematic review describes the diverse range of treatments currently under investigation at the preclinical stage for the prevention or treatment of fracture non-union. These therapies can be divided into nine broad treatment categories. Approximately 90% of interventions were only evaluated by a single study, and only five were evaluated three or more times. Reliance on a single study is problematic given the methodological limitations of the research and when considered in the context of publication bias.
Publication bias is an established problem of clinical trials, and its prevalence in animal studies is increasingly recognised[115, 118]. Failing to publish non-significant results of preclinical research limits the ability of researchers to interpret the efficacy of a therapy in the context of the wider literature. It is also unethical: subjecting animals to experiments without publishing the results effectively wastes those animals. The majority of studies included in this review (66%) reported significantly greater rates of bone healing in the therapeutic group compared to the control group. While formal assessment of publication bias was not possible it is reasonable to speculate that a bias against publication of negative or non-significant results persists.
The variability across studies meant that no two studies from the 197 included in this review were judged to be sufficiently similar across clinical and methodological parameters to allow pooling of results in a meta-analysis. Only 31% of studies presented their results in sufficient detail to be illustrated graphically in a forest plot. Not only does this preclude a rapid visual comparison of results from different studies, but it is also indicative of a lack of detail in reporting scientific findings.
Heterogeneity is expected in systematic reviews of preclinical research. Indeed, it could be argued that the aim of a systematic review in this field is to explore and demonstrate the breadth of the evidence, the variability between studies and the consistency of any findings. The generation of a precise pooled effect estimate through meta-analysis even where this is deemed feasible may be of limited value given translatability issues. Yet in this review it was mostly not possible to comment on the consistency of benefit of a particular intervention, as they were mostly only explored in one or two studies.
This systematic review has defined the considerable range of therapies currently being investigated at the preclinical phase for the treatment and prevention of fracture non-union. Though some studies report statistically significant results for some therapies, high levels of clinical and methodological heterogeneity and poor methodological quality and reporting severely hamper the ability to prioritise therapies for translation into clinical trials. If the orthopaedic trauma community were to collectively agree on a standardised animal model for investigating this question, and standards for reporting of all results regardless of findings were mandated, improved clinical treatments for fracture non-union will be developed more efficiently.
Supporting information
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist for reporting of study methodology, results and discussion.
(DOC)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We thank Professor Sarah Stapley FRCS for her contribution to the protocol phase of this study.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Costs for publication were met by a grant from UNIFY, Engineering and Physical Sciences Research Council, EP/N027221/1.
References
- 1.Brinker M. Nonunions: Evaluation and Treatment In: Browner BD, Jupiter JB, Levine AM, Trafton PG, editors. Skeletal Trauma Basic Science, Management, and Reconstruction. One. Sixth ed. Philadephia: Saunders; 2003. [Google Scholar]
- 2.Calori G, Mazza E, Colombo M, Ripamonti C, Tagliabue L. Treatment of long bone non-unions with polytherapy: indications and clinical results. Injury. 2011;42(6):587–90. 10.1016/j.injury.2011.03.046 [DOI] [PubMed] [Google Scholar]
- 3.Zura R, Xiong Z, Einhorn T, Watson JT, Ostrum RF, Prayson MJ, et al. Epidemiology of fracture nonunion in 18 human bones. JAMA surgery. 2016;151(11):e162775–e. 10.1001/jamasurg.2016.2775 [DOI] [PubMed] [Google Scholar]
- 4.Tzioupis C, Giannoudis PV. Prevalence of long-bone non-unions. Injury. 2007;38:S3–S9. [DOI] [PubMed] [Google Scholar]
- 5.Schottel PC, O’Connor DP, Brinker MR. Time trade-off as a measure of health-related quality of life: long bone nonunions have a devastating impact. JBJS. 2015;97(17):1406–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hak DJ, Fitzpatrick D, Bishop JA, Marsh JL, Tilp S, Schnettler R, et al. Delayed union and nonunions: epidemiology, clinical issues, and financial aspects. Injury. 2014;45:S3–S7. [DOI] [PubMed] [Google Scholar]
- 7.Calori G, Albisetti W, Agus A, Iori S, Tagliabue L. Risk factors contributing to fracture non-unions. Injury. 2007;38:S11–S8. [DOI] [PubMed] [Google Scholar]
- 8.National Cancer Institute. NCI Dictionary of Cancer Terms https://www.cancer.gov/publications/dictionaries/cancer-terms?cdrid=44517 [cited 2017]. https://www.cancer.gov/publications/dictionaries/cancer-terms?cdrid=44517.
- 9.Cook DJ, Mulrow CD, Haynes RB. Systematic reviews: synthesis of best evidence for clinical decisions. Annals of internal medicine. 1997;126(5):376–80. [DOI] [PubMed] [Google Scholar]
- 10.Pound P, Ebrahim S, Sandercock P, Bracken MB, Roberts I, Group RATS. Where is the evidence that animal research benefits humans? BMJ: British Medical Journal. 2004;328(7438):514 10.1136/bmj.328.7438.514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sandercock P, Roberts I. Systematic reviews of animal experiments. The Lancet. 2002;360(9333):586. [DOI] [PubMed] [Google Scholar]
- 12.Stewart SK, Bennett PM, Stapley SA, Dretzke J, Bem D, Penn-Barwell JG. Pre-clinical evaluation of therapies to prevent or treat bone non-union: a systematic review protocol. Systematic reviews. 2015;4(1):161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collaborative Approach to Meta Analysis and Review of Animal Data from Experimental Studies (CAMARADES). http://www.dcn.ed.ac.uk/camarades/default.htm http://www.dcn.ed.ac.uk/camarades/default.htm [13 August 2017].
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine. 2009;6(7):e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oliveira IR, Santos-Jesus R, Po ALW, Poolsup N. Extracting numerical data from published reports of pharmacokinetics investigations: method description and validation. Fundamental & clinical pharmacology. 2003;17(4):471–2. [DOI] [PubMed] [Google Scholar]
- 16.Schindelin J, Rueden CT, Hiner MC, Eliceiri KW. The ImageJ ecosystem: An open platform for biomedical image analysis. Molecular reproduction and development. 2015;82(7–8):518–29. 10.1002/mrd.22489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE’s risk of bias tool for animal studies. BMC medical research methodology. 2014;14(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azevedo AS, Sa MJ, Fook MV, Neto PI, Sousa OB, Azevedo SS, et al. Use of chitosan and beta-tricalcium phosphate, alone and in combination, for bone healing in rabbits. Journal of Materials Science-Materials in Medicine. 2014;25(2):481–6. 10.1007/s10856-013-5091-2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canter HI, Vargel I, Korkusuz P, Oner F, Gungorduk DB, Cil B, et al. Effect of use of slow release of bone morphogenetic protein-2 and transforming growth factor-Beta-2 in a chitosan gel matrix on cranial bone graft survival in experimental cranial critical size defect model. Annals of Plastic Surgery. 2010;64(3):342–50. 10.1097/SAP.0b013e3181a73045 . [DOI] [PubMed] [Google Scholar]
- 20.Cui X, Zhao D, Zhang B, Gao Y. Osteogenesis mechanism of chitosan-coated calcium sulfate pellets on the restoration of segmental bone defects. Journal of Craniofacial Surgery. 2009;20(5):1445–50. 10.1097/SCS.0b013e3181af1529 . [DOI] [PubMed] [Google Scholar]
- 21.Ezoddini-Ardakani F, Navabazam A, Fatehi F, Danesh-Ardekani M, Khadem S, Rouhi G. Histologic evaluation of chitosan as an accelerator of bone regeneration in microdrilled rat tibias. Dental Research Journal. 2012;9(6):694–9. . [PMC free article] [PubMed] [Google Scholar]
- 22.Park SS, Kim SG, Lim SC, Ong JL. Osteogenic activity of the mixture of chitosan and particulate dentin. Journal of Biomedical Materials Research Part A. 2008; 87(3):618–23. 10.1002/jbm.a.31812 . [DOI] [PubMed] [Google Scholar]
- 23.Seyedmajidi M, Rabiee S, Haghanifar S, Seyedmajidi S, Jorsaraei SGA, Alaghehmand H, et al. Histopathological, histomorphometrical, and radiographical evaluation of injectable glass-ceramic-chitosan nanocomposite in bone reconstruction of rat. International Journal of Biomaterials 2015, 2015 Article Number: 719574 Date of Publication: 2015. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheung WK, Working DM, Galuppo LD, Leach JK. Osteogenic comparison of expanded and uncultured adipose stromal cells. Cytotherapy. 2010;12(4):554–62. 10.3109/14653241003709694 . [DOI] [PubMed] [Google Scholar]
- 25.Daei-farshbaf N, Ardeshirylajimi A, Seyedjafari E, Piryaei A, Fadaei Fathabady F, Hedayati M, et al. Bioceramic-collagen scaffolds loaded with human adipose-tissue derived stem cells for bone tissue engineering. Molecular Biology Reports 41 (2) (pp 741–749), 2014. Date of Publication: February 2014. 2014. 10.1007/s11033-013-2913-8 . [DOI] [PubMed] [Google Scholar]
- 26.Heo SC, Shin WC, Lee MJ, Kim BR, Jang IH, Choi EJ, et al. Periostin accelerates bone healing mediated by human mesenchymal stem cell-embedded hydroxyapatite/tricalcium phosphate scaffold. PLoS ONE 10 (3) (no pagination), 2015. Article Number: e0116698 Date of Publication: 16 Mar 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin Y, Wang T, Wu L, Jing W, Chen X, Li Z, et al. Ectopic and in situ bone formation of adipose tissue-derived stromal cells in biphasic calcium phosphate nanocomposite. Journal of Biomedical Materials Research Part A. 2007; 81(4):900–10. 10.1002/jbm.a.31149 . [DOI] [PubMed] [Google Scholar]
- 28.Garcia P, Speidel V, Scheuer C, Laschke MW, Holstein JH, Histing T, et al. Low dose erythropoietin stimulates bone healing in mice. Journal of Orthopaedic Research 29 (2) (pp 165–172), 2011. Date of Publication: February 2011. 2011. 10.1002/jor.21219 . [DOI] [PubMed] [Google Scholar]
- 29.Holstein JH, Orth M, Scheuer C, Tami A, Becker SC, Garcia P, et al. Erythropoietin stimulates bone formation, cell proliferation, and angiogenesis in a femoral segmental defect model in mice. Bone. 2011;49(5):1037–45. 10.1016/j.bone.2011.08.004 . [DOI] [PubMed] [Google Scholar]
- 30.Omlor GW, Kleinschmidt K, Gantz S, Speicher A, Guehring T, Richter W. Increased bone formation in a rabbit long-bone defect model after single local and single systemic application of erythropoietin. Acta Orthopaedica 87 (4) (pp 425–431), 2016. Date of Publication: 03 Jul 2016. 10.1080/17453674.2016.1198200 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wan L, Zhang F, He Q, Tsang WP, Lu L, Li Q, et al. EPO promotes bone repair through enhanced cartilaginous callus formation and angiogenesis.[Erratum appears in PLoS One. 2014;9(10):e111830]. PLoS ONE [Electronic Resource]. 2014;9(7):e102010 10.1371/journal.pone.0102010 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geiger F, Bertram H, Berger I, Lorenz H, Wall O, Eckhardt C, et al. Vascular endothelial growth factor gene-activated matrix (VEGF 165-GAM) enhances osteogenesis and angiogenesis in large segmental bone defects. Journal of Bone and Mineral Research 20 (11) (pp 2028–2035), 2005. Date of Publication: November 2005. 2005. 10.1359/JBMR.050701 . [DOI] [PubMed] [Google Scholar]
- 33.Ogilvie CM, Lu C, Marcucio R, Lee M, Thompson Z, Hu D, et al. Vascular endothelial growth factor improves bone repair in a murine nonunion model. Iowa Orthopaedic Journal. 2012;32:90–4. . [PMC free article] [PubMed] [Google Scholar]
- 34.Patel ZS, Young S, Tabata Y, Jansen JA, Wong ME, Mikos AG. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone. 2008;43(5):931–40. 10.1016/j.bone.2008.06.019 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cz Xu, Wg Yang, Xf He, Lt Zhou, Xk Han, Xf Xu. Vascular endothelial growth factor and nano-hydroxyapatite/collagen composite in the repair of femoral defect in rats. Journal of Clinical Rehabilitative Tissue Engineering Research 15 (38) (pp 7118–7122), 2011. Date of Publication: 2011. 2011. [Google Scholar]
- 36.Chen G, Fang T, Qi Y, Yin X, Di T, Feng G, et al. Combined use of mesenchymal stromal cell sheet transplantation and local injection of SDF-1 for bone repair in a rat nonunion model. Cell Transplantation 25 (10) (pp 1801–1817), 2016. Date of Publication: 2016. 10.3727/096368916X690980 . [DOI] [PubMed] [Google Scholar]
- 37.Holloway JL, Ma H, Rai R, Hankenson KD, Burdick JA. Synergistic Effects of SDF-1alpha and BMP-2 Delivery from Proteolytically Degradable Hyaluronic Acid Hydrogels for Bone Repair. Macromolecular Bioscience 15 (9) (pp 1218–1223), 2015. Date of Publication: 01 Sep 2015. 10.1002/mabi.201500178 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hwang HD, Lee JT, Koh JT, Jung HM, Lee HJ, Kwon TG. Sequential Treatment with SDF-1 and BMP-2 Potentiates Bone Formation in Calvarial Defects. Tissue engineering Part A 21(13–14):2125–35, 2015. July 10.1089/ten.TEA.2014.0571 . [DOI] [PubMed] [Google Scholar]
- 39.Calvo-Guirado JL, Gomez-Moreno G, Mate-Sanchez JE, Lopez-Mari L, Delgado-Ruiz R, Romanos GE. New bone formation in bone defects after melatonin and porcine bone grafts: experimental study in rabbits. Clinical Oral Implants Research 26(4):399–406, 2015. April 10.1111/clr.12364 . [DOI] [PubMed] [Google Scholar]
- 40.Dedania J, Borzio R, Paglia D, Breitbart EA, Mitchell A, Vaidya S, et al. Role of local insulin augmentation upon allograft incorporation in a rat femoral defect model. Journal of Orthopaedic Research. 2011;29(1):92–9. 10.1002/jor.21205 . [DOI] [PubMed] [Google Scholar]
- 41.Deng F, Ling J, Ma J, Liu C, Zhang W. Stimulation of intramembranous bone repair in rats by ghrelin. Experimental Physiology 93 (7) (pp 872–879), 2008. Date of Publication: July 2008. 2008. 10.1113/expphysiol.2007.041962 . [DOI] [PubMed] [Google Scholar]
- 42.Duan C, Liu J, Yuan Z, Meng G, Yang X, Jia S, et al. Adenovirus-mediated transfer of VEGF into marrow stromal cells combined with PLGA/TCP scaffold increases vascularization and promotes bone repair in vivo. Archives of Medical Science 10 (1) (pp 174–181), 2014. Date of Publication: February 2014. 2014. 10.5114/aoms.2012.30950 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao F, Zhang C, Chai Y, Li X. Lentivirus-mediated Wnt10b overexpression enhances fracture healing in a rat atrophic non-union model. Biotechnology letters 37 (3) (pp 733–739), 2015. Date of Publication: 01 Mar 2015. 10.1007/s10529-014-1703-2 . [DOI] [PubMed] [Google Scholar]
- 44.Geiger F, Lorenz H, Xu W, Szalay K, Kasten P, Claes L, et al. VEGF producing bone marrow stromal cells (BMSC) enhance vascularization and resorption of a natural coral bone substitute. Bone. 2007;41(4):516–22. 10.1016/j.bone.2007.06.018 . [DOI] [PubMed] [Google Scholar]
- 45.Horvathy DB, Vacz G, Szabo T, Szigyarto IC, Toro I, Vamos B, et al. Serum albumin coating of demineralized bone matrix results in stronger new bone formation. Journal of Biomedical Materials Research Part B, Applied Biomaterials 104(1):126–32, 2016. January 10.1002/jbm.b.33359 . [DOI] [PubMed] [Google Scholar]
- 46.Li R, Stewart DJ, Von Schroeder HP, Mackinnon ES, Schemitsch EH. Effect of cell-based VEGF gene therapy on healing of a segmental bone defect. Journal of Orthopaedic Research 27 (1) (pp 8–14), 2009. Date of Publication: January 2009. 2009. 10.1002/jor.20658 . [DOI] [PubMed] [Google Scholar]
- 47.Matsumoto T, Sato D, Hashimoto Y. Individual and combined effects of noise-like whole-body vibration and parathyroid hormone treatment on bone defect repair in ovariectomized mice. Proceedings of the Institution of Mechanical Engineers Part H, Journal of engineering in medicine 230 (1) (pp 30–38), 2016. Date of Publication: 01 Jan 2016. 10.1177/0954411915616987 . [DOI] [PubMed] [Google Scholar]
- 48.Nam SS, Lee JC, Kim HJ, Park JW, Lee JM, Suh JY, et al. Serotonin Inhibits Osteoblast Differentiation and Bone Regeneration in Rats. Journal of Periodontology 87(4):461–9, 2016. April 10.1902/jop.2015.150302 . [DOI] [PubMed] [Google Scholar]
- 49.Pigossi SC, de Oliveira GJ, Finoti LS, Nepomuceno R, Spolidorio LC, Rossa C Jr., et al. Bacterial cellulose-hydroxyapatite composites with osteogenic growth peptide (OGP) or pentapeptide OGP on bone regeneration in critical-size calvarial defect model. Journal of Biomedical Materials Research Part A 103(10):3397–406, 2015. October 10.1002/jbm.a.35472 . [DOI] [PubMed] [Google Scholar]
- 50.Rai B, Chatterjea A, Lim ZXH, Tan TC, Sawyer AA, Hosaka YZ, et al. Repair of segmental ulna defects using a beta-TCP implant in combination with a heparan sulfate glycosaminoglycan variant. Acta Biomaterialia 28 (pp 193–204), 2015. Date of Publication: December 2015. 10.1016/j.actbio.2015.09.008 . [DOI] [PubMed] [Google Scholar]
- 51.Sassioto MC, Inouye CM, Aydos RD, Figueiredo AS. Bone repair in rats treated with sodic diclofenac and calcitonin. Acta Cirurgica Brasileira. 2006;21 Suppl 4:40–4. . [DOI] [PubMed] [Google Scholar]
- 52.Tresguerres IF, Alobera MA, Baca R, Tresguerres JA. Histologic, morphometric, and densitometric study of peri-implant bone in rabbits with local administration of growth hormone. International Journal of Oral & Maxillofacial Implants. 2005;20(2):193–202. . [PubMed] [Google Scholar]
- 53.Atalay Y, Bozkurt MF, Gonul Y, Cakmak O, Agacayak KS, Kose I, et al. The effects of amlodipine and platelet rich plasma on bone healing in rats. Drug Des Devel Ther. 2015;9:1973–81. 10.2147/DDDT.S80778 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cao Y, Xiong J, Mei S, Wang F, Zhao Z, Wang S, et al. Aspirin promotes bone marrow mesenchymal stem cell-based calvarial bone regeneration in mini swine. Stem Cell Research and Therapy 6 (1) (no pagination), 2015. Article Number: 210 Date of Publication: 31 Oct 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Durmuslar MC, Balli U, Ongoz Dede F, Bozkurt Dogan S, Misir AF, Baris E, et al. Evaluation of the effects of platelet-rich fibrin on bone regeneration in diabetic rabbits. Journal of Cranio-Maxillo-Facial Surgery 44(2):126–33, 2016. February 10.1016/j.jcms.2015.11.009 . [DOI] [PubMed] [Google Scholar]
- 56.Konofaos P, Petersen D, Jennings JA, Smith RA, Doty H, Reves BT, et al. Evaluation of Amniotic Multipotential Tissue Matrix to Augment Healing of Demineralized Bone Matrix in an Animal Calvarial Model. Journal of Craniofacial Surgery 26(4):1408–12, 2015. June 10.1097/SCS.0000000000001741 . [DOI] [PubMed] [Google Scholar]
- 57.Schneppendahl J, Jungbluth P, Sager M, Benga L, Herten M, Scholz A, et al. Synergistic effects of HBO and PRP improve bone regeneration with autologous bone grafting. Injury 47 (12) (pp 2718–2725), 2016. Date of Publication: 01 Dec 2016. 10.1016/j.injury.2016.09.039 . [DOI] [PubMed] [Google Scholar]
- 58.Yu Z, Geng J, Gao H, Zhao X, Chen J. Evaluations of guided bone regeneration in canine radius segmental defects using autologous periosteum combined with fascia lata under stable external fixation. Journal of Orthopaedics and Traumatology 16 (2) (pp 133–140), 2015. Article Number: 321 Date of Publication: 12 Oct 2015. 10.1007/s10195-014-0321-z . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alpan AL, Toker H, Ozer H. Ozone Therapy Enhances Osseous Healing in Rats With Diabetes With Calvarial Defects: A Morphometric and Immunohistochemical Study. Journal of Periodontology 87(8):982–9, 2016. August 10.1902/jop.2016.160009 . [DOI] [PubMed] [Google Scholar]
- 60.Calvo-Guirado JL, Garces M, Delgado-Ruiz RA, Ramirez Fernandez MP, Ferres-Amat E, Romanos GE. Biphasic beta-TCP mixed with silicon increases bone formation in critical site defects in rabbit calvaria. Clinical Oral Implants Research 26(8):891–7, 2015. August 10.1111/clr.12413 . [DOI] [PubMed] [Google Scholar]
- 61.Costa NM, Yassuda DH, Sader MS, Fernandes GV, Soares GD, Granjeiro JM. Osteogenic effect of tricalcium phosphate substituted by magnesium associated with Genderm membrane in rat calvarial defect model. Materials Science & Engineering C, Materials for Biological Applications 61:63–71, 2016. April 01 10.1016/j.msec.2015.12.003 . [DOI] [PubMed] [Google Scholar]
- 62.Durand M, Collombet JM, Frasca S, Begot L, Lataillade JJ, Le Bousse-Kerdiles MC, et al. In vivo hypobaric hypoxia performed during the remodeling process accelerates bone healing in mice. Stem Cells Translational Medicine. 2014;3(8):958–68. 10.5966/sctm.2013-0209 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Elgali I, Turri A, Xia W, Norlindh B, Johansson A, Dahlin C, et al. Guided bone regeneration using resorbable membrane and different bone substitutes: Early histological and molecular events. Acta Biomaterialia 29:409–23, 2016. January 10.1016/j.actbio.2015.10.005 . [DOI] [PubMed] [Google Scholar]
- 64.Grassmann JP, Schneppendahl J, Hakimi AR, Herten M, Betsch M, Logters TT, et al. Hyperbaric oxygen therapy improves angiogenesis and bone formation in critical sized diaphyseal defects. Journal of Orthopaedic Research. 2015;33(4):513–20. 10.1002/jor.22805 . [DOI] [PubMed] [Google Scholar]
- 65.Park JW, Kang DG, Hanawa T. New bone formation induced by surface strontium-modified ceramic bone graft substitute. Oral Diseases 22(1):53–61, 2016. January 10.1111/odi.12381 . [DOI] [PubMed] [Google Scholar]
- 66.Uchida R, Nakata K, Kawano F, Yonetani Y, Ogasawara I, Nakai N, et al. Vibration acceleration promotes bone formation in rodent models. PLoS ONE 12 (3) (no pagination), 2017. Article Number: e0172614 Date of Publication: March 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang G, Wang J, Fu Y, Bai L, He M, Li B, et al. Systemic treatment with vanadium absorbed by Coprinus comatus promotes femoral fracture healing in streptozotocin-diabetic rats. Biological Trace Element Research. 2013;151(3):424–33. 10.1007/s12011-012-9584-5 . [DOI] [PubMed] [Google Scholar]
- 68.de Oliveira HT, Bergoli RD, Hirsch WD, Chagas OL Jr., Heitz C, Silva DN. Isotretinoin effect on the repair of bone defects—a study in rat calvaria. Journal of Cranio-Maxillo-Facial Surgery. 2013;41(7):581–5. 10.1016/j.jcms.2012.11.030 . [DOI] [PubMed] [Google Scholar]
- 69.Dong GC, Chen HM, Yao CH. A novel bone substitute composite composed of tricalcium phosphate, gelatin and drynaria fortunei herbal extract. Journal of Biomedical Materials Research Part A. 2008; 84(1):167–77. 10.1002/jbm.a.31261 . [DOI] [PubMed] [Google Scholar]
- 70.Hadzik J, Kubasiewicz-Ross P, Kunert-Keil C, Jurczyszyn K, Nawrot-Hadzik I, Dominiak M, et al. A silver carp skin derived collagen in bone defect treatment-A histological study in a rat model. Annals of Anatomy 208:123–128, 2016. November 10.1016/j.aanat.2016.07.009 . [DOI] [PubMed] [Google Scholar]
- 71.Han X, Du J, Liu D, Liu H, Amizuka N, Li M. Histochemical examination of systemic administration of eldecalcitol combined with guided bone regeneration for bone defect restoration in rats. Journal of Molecular Histology 48(1):41–51, 2017. February 10.1007/s10735-016-9705-0 . [DOI] [PubMed] [Google Scholar]
- 72.Jia P, Chen H, Kang H, Qi J, Zhao P, Jiang M, et al. Deferoxamine released from poly(lactic-co-glycolic acid) promotes healing of osteoporotic bone defect via enhanced angiogenesis and osteogenesis. Journal of Biomedical Materials Research Part A 104(10):2515–27, 2016. October 10.1002/jbm.a.35793 . [DOI] [PubMed] [Google Scholar]
- 73.Lee JY, Son SJ, Son JS, Kang SS, Choi SH. Bone-healing capacity of PCL/PLGA/duck beak scaffold in critical bone defects in a rabbit model. BioMed Research International 2016. (no pagination), 2016 Article Number: 2136215 Date of Publication: 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li W, Zhao Z, Xiong J, Zeng Y. The modification experimental study in vivo of nano-bone gelatin. Artificial Cells, Nanomedicine, & Biotechnology. 2014;42(5):309–15. 10.3109/21691401.2013.821411 . [DOI] [PubMed] [Google Scholar]
- 75.Ngueguim FT, Khan MP, Donfack JH, Siddiqui JA, Tewari D, Nagar GK, et al. Evaluation of Cameroonian plants towards experimental bone regeneration. Journal of Ethnopharmacology. 2012;141(1):331–7. 10.1016/j.jep.2012.02.041 . [DOI] [PubMed] [Google Scholar]
- 76.Ngueguim FT, Khan MP, Donfack JH, Tewari D, Dimo T, Kamtchouing P, et al. Ethanol extract of Peperomia pellucida (Piperaceae) promotes fracture healing by an anabolic effect on osteoblasts. Journal of Ethnopharmacology. 2013;148(1):62–8. 10.1016/j.jep.2013.03.063 . [DOI] [PubMed] [Google Scholar]
- 77.Zhao X, Wu ZX, Zhang Y, Gao MX, Yan YB, Cao PC, et al. Locally administrated perindopril improves healing in an ovariectomized rat tibial osteotomy model. PLoS ONE [Electronic Resource]. 2012;7(3):e33228 10.1371/journal.pone.0033228 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bougioukli S, Jain A, Sugiyama O, Tinsley BA, Tang AH, Tan MH, et al. Combination therapy with BMP-2 and a systemic RANKL inhibitor enhances bone healing in a mouse critical-sized femoral defect. Bone. 2016;84 (pp 93–103), 2016. Date of Publication:March 01. 10.1016/j.bone.2015.12.052 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cheng BH, Chu TMG, Chang C, Kang HY, Huang KE. Testosterone Delivered with a Scaffold Is as Effective as Bone Morphologic Protein-2 in Promoting the Repair of Critical-Size Segmental Defect of Femoral Bone in Mice. PLoS ONE 8 (8), 2013. Article Number: e70234 Date of Publication: 05 Aug 2013. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gocer H, Onger ME, Kuyubasi N, Cirakli A, Kir MC. The effect of teicoplanin on fracture healing: an experimental study. Eklem hastaliklari ve cerrahisi = Joint Diseases & Related Surgery 27(1):16–21, 2016. . [DOI] [PubMed] [Google Scholar]
- 81.Kanczler JM, Ginty PJ, Barry JJA, Clarke NMP, Howdle SM, Shakesheff KM, et al. The effect of mesenchymal populations and vascular endothelial growth factor delivered from biodegradable polymer scaffolds on bone formation. Biomaterials 29 (12) (pp 1892–1900), 2008. Date of Publication: April 2008. 2008. 10.1016/j.biomaterials.2007.12.031 . [DOI] [PubMed] [Google Scholar]
- 82.Ma X, Wang Y, Guo H, Wang J. Nano-hydroxyapatite/chitosan sponge-like biocomposite for repairing of rat calvarial critical-sized bone defect. Journal of Bioactive and Compatible Polymers 26 (4) (pp 335–346), 2011. Date of Publication: July 2011. 2011. [Google Scholar]
- 83.Myers TJ, Yan Y, Granero-Molto F, Weis JA, Longobardi L, Li T, et al. Systemically delivered insulin-like growth factor-I enhances mesenchymal stem cell-dependent fracture healing. Growth Factors 30 (4) (pp 230–241), 2012. Date of Publication: August 2012. 2012. 10.3109/08977194.2012.683188 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Toupadakis CA, Granick JL, Sagy M, Wong A, Ghassemi E, Chung DJ, et al. Mobilization of endogenous stem cell populations enhances fracture healing in a murine femoral fracture model. Cytotherapy. 2013;15(9):1136–47. 10.1016/j.jcyt.2013.05.004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang Y, Lv P, Ma Z, Zhang J. Enhanced healing of rat calvarial critical size defect with selenium-doped lamellar biocomposites. Biological Trace Element Research 155 (1) (pp 72–81), 2013. Date of Publication: October 2013. 2013. 10.1007/s12011-013-9763-z . [DOI] [PubMed] [Google Scholar]
- 86.Yoshii T, Nyman JS, Yuasa M, Esparza JM, Okawa A, Gutierrez GE. Local application of a proteasome inhibitor enhances fracture healing in rats. Journal of Orthopaedic Research. 2015;33(8):1197–204. 10.1002/jor.22849 . [DOI] [PubMed] [Google Scholar]
- 87.Asutay F, Polat S, Gul M, Subasi C, Kahraman SA, Karaoz E. The effects of dental pulp stem cells on bone regeneration in rat calvarial defect model: micro-computed tomography and histomorphometric analysis. Arch Oral Biol. 2015;60(12):1729–35. 10.1016/j.archoralbio.2015.09.002 . [DOI] [PubMed] [Google Scholar]
- 88.Dixit M, Raghuvanshi A, Gupta CP, Kureel J, Mansoori MN, Shukla P, et al. Medicarpin, a Natural Pterocarpan, Heals Cortical Bone Defect by Activation of Notch and Wnt Canonical Signaling Pathways. PLoS ONE 10 (12) (no pagination), 2015. Article Number: e0144541 Date of Publication: 01 Dec 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jackson RA, McDonald MM, Nurcombe V, Little DG, Cool SM. The use of heparan sulfate to augment fracture repair in a rat fracture model. Journal of Orthopaedic Research. 2006;24(4):636–44. 10.1002/jor.20103 . [DOI] [PubMed] [Google Scholar]
- 90.Matsubara H, Tsuchiya H, Watanabe K, Takeuchi A, Tomita K. Percutaneous nonviral delivery of hepatocyte growth factor in an osteotomy gap promotes bone repair in rabbits: A preliminary study. Clinical Orthopaedics and Related Research 466 (12) (pp 2962–2972), 2008. Date of Publication: December 2008. 2008. 10.1007/s11999-008-0493-z . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sisask G, Marsell R, Sundgren-Andersson A, Larsson S, Nilsson O, Ljunggren O, et al. Rats treated with AZD2858, a GSK3 inhibitor, heal fractures rapidly without endochondral bone formation. Bone 54 (1) (pp 126–132), 2013. Date of Publication: May 2013. 2013. 10.1016/j.bone.2013.01.019 . [DOI] [PubMed] [Google Scholar]
- 92.Alic T, Cirakli A, Sahin Y, Tomak Y. Effects of cilostazol on fracture healing: an experimental study. Acta Orthop Traumatol Turc. 2016;50(1):103–10. 10.3944/AOTT.2016.15.0211 . [DOI] [PubMed] [Google Scholar]
- 93.Baht GS, Nadesan P, Silkstone D, Alman BA. Pharmacologically targeting beta-catenin for NF1 associated deficiencies in fracture repair. Bone 98 (pp 31–36), 2017. Date of Publication: 01 May 2017. 10.1016/j.bone.2017.02.012 . [DOI] [PubMed] [Google Scholar]
- 94.Bernick J, Wang Y, Sigal IA, Alman BA, Whyne CM, Nam D. Parameters for lithium treatment are critical in its enhancement of fracture-healing in rodents. Journal of Bone & Joint Surgery—American Volume. 2014;96(23):1990–8. 10.2106/JBJS.N.00057 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cai Y, Guo L, Shen H, An X, Jiang H, Ji F, et al. Degradability, bioactivity, and osteogenesis of biocomposite scaffolds of lithium-containing mesoporous bioglass and mPEG-PLGA-b-PLL copolymer. International Journal of Nanomedicine 10:4125–36, 2015. 10.2147/IJN.S82945 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cakmak G, Sahin MS, OzdemIr BH, KaradenIz E. Effect of pentoxifylline on healing of segmental bone defects and angiogenesis. Acta orthopaedica et traumatologica turcica 49 (6) (pp 676–682), 2015. Date of Publication: 2015. 10.3944/AOTT.2015.15.0158 . [DOI] [PubMed] [Google Scholar]
- 97.Del Rosario C, Rodriguez-Evora M, Reyes R, Simoes S, Concheiro A, Evora C, et al. Bone critical defect repair with poloxamine-cyclodextrin supramolecular gels. International Journal of Pharmaceutics 495 (1) (pp 463–473), 2015. Date of Publication: 10 Nov 2015. 10.1016/j.ijpharm.2015.09.003 . [DOI] [PubMed] [Google Scholar]
- 98.Donneys A, Weiss DM, Deshpande SS, Ahsan S, Tchanque-Fossuo CN, Sarhaddi D, et al. Localized deferoxamine injection augments vascularity and improves bony union in pathologic fracture healing after radiotherapy. Bone. 2013;52(1):318–25. 10.1016/j.bone.2012.10.014 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fan J, Guo M, Im CS, Pi-Anfruns J, Cui ZK, Kim S, et al. Enhanced Mandibular Bone Repair by Combined Treatment of Bone Morphogenetic Protein 2 and Small-Molecule Phenamil. Tissue Engineering—Part A 23 (5–6) (pp 195–207), 2017. Date of Publication: March 2017. 10.1089/ten.TEA.2016.0308 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ishack S, Mediero A, Wilder T, Ricci JL, Cronstein BN. Bone regeneration in critical bone defects using three-dimensionally printed beta-tricalcium phosphate/hydroxyapatite scaffolds is enhanced by coating scaffolds with either dipyridamole or BMP-2. Journal of Biomedical Materials Research—Part B Applied Biomaterials 105 (2) (pp 366–375), 2017. Date of Publication: 01 Feb 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kutan E, Duygu-Capar G, Ozcakir-Tomruk C, Dilek OC, Ozen F, Erdogan O, et al. Efficacy of doxycycline release collagen membrane on surgically created and contaminated defects in rat tibiae: A histopathological and microbiological study. Archives of Oral Biology 63:15–21, 2016. March 10.1016/j.archoralbio.2015.11.001 . [DOI] [PubMed] [Google Scholar]
- 102.Limirio PHJO, Rocha FS, Batista JD, Guimaraes-Henriques JC, de Melo GB, Dechichi P. The Effect of Local Delivery Doxycycline and Alendronate on Bone Repair. AAPS PharmSciTech 17 (4) (pp 872–877), 2016. Date of Publication: 01 Aug 2016. 10.1208/s12249-015-0411-0 . [DOI] [PubMed] [Google Scholar]
- 103.Wada K, Yu W, Elazizi M, Barakat S, Ouimet MA, Rosario-Melendez R, et al. Locally delivered salicylic acid from a poly(anhydride-ester): impact on diabetic bone regeneration. Journal of Controlled Release. 2013;171(1):33–7. 10.1016/j.jconrel.2013.06.024 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Werkman C, Senra GS, da Rocha RF, Brandao AA. Comparative therapeutic use of Risedronate and Calcarea phosphorica—allopathy versus homeopathy—in bone repair in castrated rats. Pesquisa Odontologica Brasileira = Brazilian Oral Research. 2006;20(3):196–201. . [DOI] [PubMed] [Google Scholar]
- 105.Wixted JJ, Fanning PJ, Gaur T, O’Connell SL, Silva J, Mason-Savas A, et al. Enhanced fracture repair by leukotriene antagonism is characterized by increased chondrocyte proliferation and early bone formation: a novel role of the cysteinyl LT-1 receptor. Journal of Cellular Physiology. 2009;221(1):31–9. 10.1002/jcp.21809 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kilkenny C, Parsons N, Kadyszewski E, Festing MF, Cuthill IC, Fry D, et al. Survey of the quality of experimental design, statistical analysis and reporting of research using animals. PloS one. 2009;4(11):e7824 10.1371/journal.pone.0007824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Festing MF, Altman DG. Guidelines for the design and statistical analysis of experiments using laboratory animals. ILAR journal. 2002;43(4):244–58. [DOI] [PubMed] [Google Scholar]
- 108.Festing MF, Overend P, Gaines Das R, Cortina-Borja M, Berdoy M. The design of animal experiments: reducing the use of animals in research through better experimental design: The Royal Society of Medicine Press Limited; 2002. [Google Scholar]
- 109.McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. British journal of pharmacology. 2010;160(7):1573–6. 10.1111/j.1476-5381.2010.00873.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Olsen CH. Review of the use of statistics in infection and immunity. Infection and immunity. 2003;71(12):6689–92. 10.1128/IAI.71.12.6689-6692.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Altman DG, Bland JM. Standard deviations and standard errors. Bmj. 2005;331(7521):903 10.1136/bmj.331.7521.903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Marsh J, Slongo TF, Agel J, Broderick JS, Creevey W, DeCoster TA, et al. Fracture and dislocation classification compendium-2007: Orthopaedic Trauma Association classification, database and outcomes committee. LWW; 2007. [DOI] [PubMed]
- 113.Reichert JC, Saifzadeh S, Wullschleger ME, Epari DR, Schütz MA, Duda GN, et al. The challenge of establishing preclinical models for segmental bone defect research. Biomaterials. 2009;30(12):2149–63. 10.1016/j.biomaterials.2008.12.050 [DOI] [PubMed] [Google Scholar]
- 114.de Vries R, Wever KE, Avey MT, Stephens ML, Sena ES, Leenaars M. The usefulness of systematic reviews of animal experiments for the design of preclinical and clinical studies. ILAR journal. 2014;55(3):427–37. 10.1093/ilar/ilu043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Van der Worp HB, Howells DW, Sena ES, Porritt MJ, Rewell S, O’Collins V, et al. Can animal models of disease reliably inform human studies? PLoS medicine. 2010;7(3):e1000245 10.1371/journal.pmed.1000245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.O’Loughlin PF, Morr S, Bogunovic L, Kim AD, Park B, Lane JM. Selection and development of preclinical models in fracture-healing research. JBJS. 2008;90(Supplement_1):79–84. [DOI] [PubMed] [Google Scholar]
- 117.Hackam DG, Redelmeier DA. Translation of research evidence from animals to humans. Jama. 2006;296(14):1727–32. [DOI] [PubMed] [Google Scholar]
- 118.ter Riet G, Korevaar DA, Leenaars M, Sterk PJ, Van Noorden CJ, Bouter LM, et al. Publication bias in laboratory animal research: a survey on magnitude, drivers, consequences and potential solutions. PLoS One. 2012;7(9):e43404 10.1371/journal.pone.0043404 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist for reporting of study methodology, results and discussion.
(DOC)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.