Abstract
Purpose
To investigate the surgical outcome of patients with osteophyte-associated dysphagia (OAD) using the functional outcome swallowing scale (FOSS).
Methods
A retrospective chart review of 10 surgical cases of OAD (9 male and 1 female patient; mean age of 65 years) from 1982 to 2017 was performed, and radiographic evaluations were conducted by video fluoroscopic swallow study (VFSS) and conventional radiography. All OAD cases were treated at a single institution, and osteophytes were surgically resected by the anterior approach under gentle retraction of the affected esophagus. FOSS (0 for normal, 5 for worst) was used for clinical evaluations, and surgical complications were recorded.
Results
VFSS evaluation of OAD showed that the affected osteophyte was located at C4/5 in four patients, followed by C3/4 in three patients. The mean FOSS showed significant improvement from 2.5 preoperatively to 0.3 postoperatively, and no major surgical complications were recorded. Comorbidities were diabetes mellitus in four patients, ossification of the posterior longitudinal ligament in three patients, and lumbar spinal stenosis (LSS) in three patients.
Conclusion
Surgical treatment of OAD was promising, and all patients showed clinical recovery. Evaluation of dysphagia using FOSS was easy and reliable for OAD management, and FOSS 2 might be a good indication for surgical intervention.
Introduction
Anterior cervical osteophytes are a common finding in degenerative spinal conditions, especially among the elderly population, with a reported 30% incidence of osteophytosis and with diffuse idiopathic skeletal hyperostosis (DISH) or Forestier disease [1–5].
Clinical studies have showed that up to 6% of patients with DISH manifested symptoms of osteophyte-associated dysphagia (OAD) [5–6]. However, clinical assessment of dysphagia has been heterogeneous among spine surgeons. Recent studies used the Functional Outcome Swallowing Scale (FOSS) (0 for normal, 1 for episodic dysphagia, 2 for dysphagia with no body weight (BW) change, 3 for BW loss <10%, 4 for BW loss >10%, and 5 for non-oral feeding) and reported reliable evaluation of dysphagia among patients with OAD (Table 1) [7]. The purpose of this study was to investigate the clinical features and surgical outcomes of patients with OAD.
Table 1. Functional outcome swallowing scale.
| Stage | Symptoms |
|---|---|
| 0 | Normal function and asymptomatic |
| 1 | Normal function with episodic or daily symptoms of dysphagia |
| 2 | Compensated abnormal function, prolonged mealtime, no body weight loss |
| 3 | Decompensated abnormal function, body weight loss<10% over 6 months |
| 4 | Severely decompensated abnormal function, body weight loss>10% over 6 months |
| 5 | Non-oral feeding for all nutrition |
Methods
This study was a retrospective chart review of 10 consecutive surgical cases of OAD (male, 9; female, 1; mean age, 65.0 (55–78) years) treated at our institution between 1982 and 2017. The mean follow-up period was 4 years.
Radiographic evaluations were done using video fluoroscopic swallow study (VFSS) and conventional radiography. VFSS was performed at designated division of our hospital using continuous recording of esophageal movement while swallowing contrast medium.
Patients’ demographics, comorbidity, surgical complications were investigated, and clinical evaluation of dysphagia was performed using FOSS.
All patients gave written informed consent, and the study was approved by the institutional review board at our university.
Surgical procedure
Under general anesthesia, the anterior Smith-Robinson type approach, usually from the left side, was used, and osteophytes were exposed with careful retraction of the affected esophagus. A high-speed diamond-tipped burr was used, and the affected osteophyte was thinned off to the original depth of the anterior vertebral cortex and width of Luschka’s joints. Intraoperative radiography was performed to confirm that the affected osteophytes were resected, and surfaces were smoothed as planned. Bone wax was applied to the surface of the resected area for hemostasis, although no additional procedures or spinal fusions were applied in all cases. The wound was cleansed and closed with soft drainage tube placed at the resected surface, mostly until next day postoperatively.
Statistical analysis
We used t-tests to compare preoperative and postoperative FOSS in the same group. p-value of less than 0.05 was considered statistically significant. All statistical analyses were carried out using the StatView software (Abacus Concepts, Inc., Berkley, CA).
Results
Radiographic analysis showed that maximal osteophyte formation was at C4/5 in four patients, C3/4 in three patients, C5/6 in two patients, and C6/7 in one patient.
VFSS analysis showed that the contrast medium was obstructed at the maximal osteophyte level in all patients.
The preoperative mean FOSS score was 2.5, and postoperative mean FOSS score was 0.3. Improvement of FOSS after surgery was significant in all patients (p<0.05).
Complications included transient recurrent nerve paralysis in one patient immediately postoperative, which recovered fully during follow-up. No major complication, such as laryngeal or esophageal perforation or vascular injury, was observed in our series. Recurrence of OAD was found in one patient after 9 years.
Patients’ comorbidities at enrollment included diabetes mellitus (DM, n = 4), ossification of the posterior longitudinal ligament (OPLL, n = 3), and lumbar spinal stenosis (LSS, n = 3) (Table 2).
Table 2. Clinical and demographic data and preoperative/postoperative FOSS scores.
| No | Age | Sex | Comorbidity | Maximal neophyte level | Preop FOSS |
Postop FOSS | P-value |
|---|---|---|---|---|---|---|---|
| 1 | 55 | M | LSS | C4/5 | 2 | 0 | |
| 2 | 68 | F | C3/4 | 2 | 0 | ||
| 3 | 61 | M | C4/5 | 2 | 0 | ||
| 4 | 56 | M | OPLL LSS | C5/6 | 3 | 1 | |
| 5 | 71 | M | LSS DM | C3/4 | 2 | 1 | |
| 6 | 57 | M | C3/4 | 4 | 0 | ||
| 7 | 67 | M | OPLL DM | C4/5 | 2 | 1 | |
| 8 | 73 | M | DM | C4/5 | 3 | 0 | |
| 9 | 59 | M | OPLL DM | C5/6 | 2 | 0 | |
| 10 | 78 | M | M | C6/7 | 3 | 0 | |
| Average | 2.5 | 0.3 | < .001 | ||||
DM, diabetes mellitus; FOSS, functional outcome swallowing scale; LSS, lumbar spinal stenosis; OPLL, ossification of the posterior longitudinal ligament
Case presentation (patient no. 4)
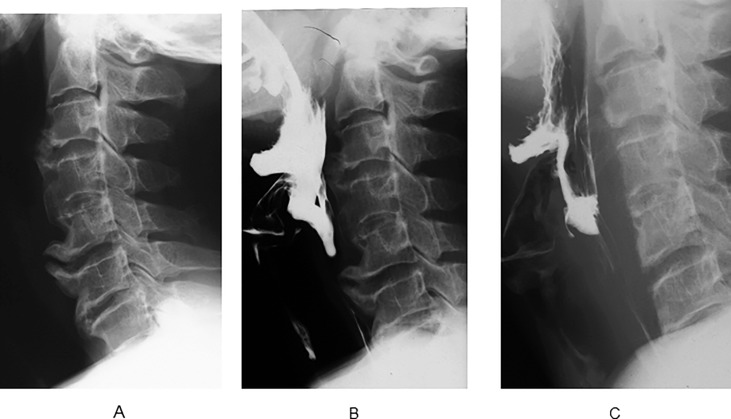
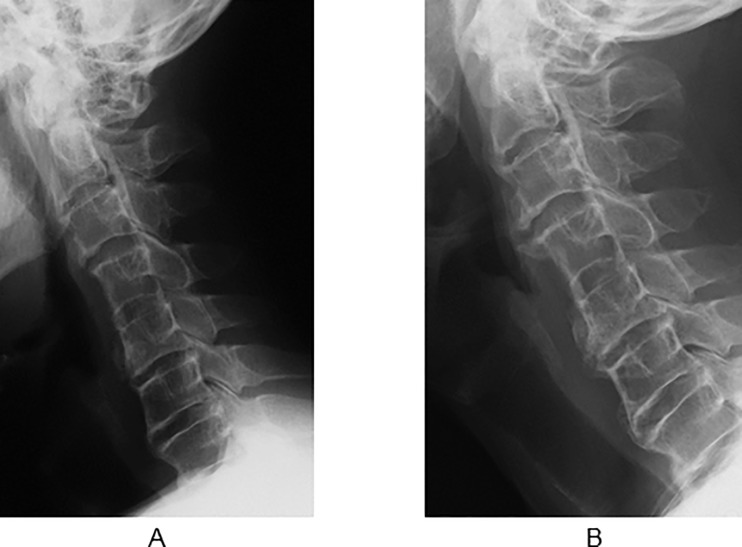
This case involved a 59-year-old male patient with OAD that started 6 years ago. The patient also showed DISH and OPLL with tendency of diffuse ligament ossification. Conservative treatments were unsuccessful, and surgical treatment was recommended. Preoperative VFSS revealed obstruction at the C5/6 osteophyte level. Postoperative VFSS showed normal movement of the esophagus, and reduced symptoms of dysphagia (FOSS 3 to 1) (Fig 1). Radiographic follow-up showed osteophytes at the C3/4 and C5/6 levels that demonstrated gradual regrowth at 9 and 17 years postoperatively (Fig 2).
Fig 1. Preoperative and postoperative lateral radiographs of patient no. 4.
(A) Cervical osteophytes at C5/6 and OPLL at C2/3. (B) Preoperative VFSS showed that the contrast medium was obstructed at C5/6 level and the esophagus was compressed. (C) Postoperative VFSS contrast medium was not obstructed, and the osteophyte at C5/6 was excised completely.
Fig 2. Lateral radiographs of patient no. 4 obtained 9 and 17 years after surgery (long-term follow-up).
(A) Nine years after surgery, recurrent anterior osteophytes were observed at the C3/4 and C5/6 levels. (B) Seventeen years after surgery, the osteophytes were gradually enlarged at the C3/4 and C5/6 levels.
Discussion
This study showed that surgical treatment for OAD is promising and that all patients showed clinical recovery according to the FOSS scores.
In previous studies, patients with OAD had a higher prevalence of DM and OPLL [8,9]. Our series also confirmed the tendency for DM in 40% and OPLL in 30% of the cases. Denko reported that type 2 DM was a risk factor for DISH, and several growth factors and inflammatory mediators lead to proliferation of osteoblast [10,11].
In general, ossification of the anterior longitudinal ligament (OALL) is frequently associated with OPLL, and Song reported that OPLL and OALL coexisted in 11 of 17 patients [12].
We did not have information on the association with LSS. Interestingly, the scar tissue was changed to ossification after decompression surgery for LSS, and recurrence of stenosis induced reoperation surgery [13].
The affected site of the osteophyte was mainly at C3/5 according to a systematic review by Verlaan [8]. Their results coincided with our results. Degenerative changes are most frequent at these cervical levels, which might have been related to anterior osteophytosis and OAD [12].
Initial treatment strategies for OAD should be conservative, including diet modifications, postural changes, muscle relaxants, anti-reflux medications, and steroids. If conservative treatment is not effective, surgical treatment should be indicated for improving the patient’s quality of life (QOL) [2–5,9].
Oppenlander reported clinical improvement of dysphagia after surgical resection of the osteophyte in nine OAD cases [14].
Urrutia and Bono reported that dysphagia resolved within 2 weeks postoperatively, with no recurrence at approximately 5 years of follow-up of five OAD cases [15].
The present study found that the surgical result was satisfactory, but osteophyte regrowth at the site of resection was observed in one patient after 9 years of follow-up.
Miyamoto reported a long-term follow-up of seven patients with OAD and reported that two patients had recurrence of OAD symptom after 10 and 11 years, respectively, with one patient requiring reoperation. They found osteophyte regrowth in all patients, with an average of 1 mm per year, and recommended more than 10 years of follow-up for OAD [16].
Several assessment tools for dysphagia have been reported, including the Bazas dysphagia scoring system [17], the swallowing quality of life, and the dysphagia disability index [18]. Recently, the FOSS was reported by Salassa, which should be suitable for patients with OAD [3,4,7,19]. Salassa and Ozgursoy reported 13 cases of OAD evaluation using FOSS, and the preoperative FOSS was 2.4 on average, which improved to 1.0 postoperatively [19]. Their results showed that most surgical cases acquired normal function at 6-month follow-up. Erdur reported on a series of eight patients with OAD who recovered, with an improvement in the FOSS score from 2.6 preoperatively to 0.4 postoperatively [3]. Similarly, Vodičar reported nine OAD cases managed by surgical resection of cervical osteophytes, and the FOSS of these patient improved from 3.4 to 0.8 [4] (Table 3).
Table 3. Comparison of functional outcome swallowing scale (FOSS) scores between previous studies and our study.
| Study | Cases (n) | Preop FOSS | Postop FOSS | Change in FOSS |
|---|---|---|---|---|
| Our study | 10 | 2.5 [2–4] | 0.3 [0–1] | 2.2 [1–4] |
| Ozgursoy (2010) | 13 | 2.4 [2–4] | 1.0 [0–3] | 1.4 [1–2] |
| Vodičar (2016) | 8 | 3.4 [2–5] | 0.8 [0–3] | 2.6 [0–4] |
| Erdur (2017) | 8 | 2.6 [2–3] | 0.4 [0–1] | 2.2 [1–3] |
Our study and three previous reports showed almost the same degree of improvement in FOSS scores, and the surgical indication has been suggested according to these results. An FOSS score of 2, which shows compensated abnormal function manifested by significant dietary modifications or prolonged meal time, might be a good indication for surgical intervention, yielding good surgical results for OAD.
This study has some limitations. The incidence of OAD is low, as there were only 10 cases identified in 35 years at our university and 13 cases in 10 years at the Mayo Clinic. Therefore, these case reports provide no firm evidence, and further studies could provide sound guidelines for OAD treatment. FOSS is a useful tool. However, it is a physician-based evaluation and lacks patients’ perspectives of the quality of life or psychosocial aspects.
In conclusion, clinical and radiological outcomes of 10 OAD cases were studied. All patients recovered normal function. Considering other studies, surgery for OAD was found to be promising when indicated on the basis of an FOSS score of 2. Furthermore, long-term follow-up is important for recurrent dysphagia because of osteophyte regrowth.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Resnick D, Shaul SR, Robins JM. Diffuse idiopathic skeletal hyperostosis (DISH):Forestier’s disease with extraspinal manifestations. Radiology. 1975;115: 513–524. 10.1148/15.3.513 [DOI] [PubMed] [Google Scholar]
- 2.Parker MD. Dysphagia due to cervical osteophytes: a controversial entity revisited. Dysphagia. 1989; 3: 157–160. [DOI] [PubMed] [Google Scholar]
- 3.Erdur Ö, Taşli H, Polat B, Sofiyev F, Tosun F, Çolpan B, et al. Surgical management of dysphagia due to anterior cervical osteophytes. J Craniofacial Surg. 2017;28: e80–e84. [DOI] [PubMed] [Google Scholar]
- 4.Vodičar M, Košak R, Vengust R. Long-term results of surgical treatment for symptomatic anterior cervical osteophytes a case series with review of the literature. Clin Spine Surg. 2016;29: E482–E487. 10.1097/BSD.0b013e31829046af [DOI] [PubMed] [Google Scholar]
- 5.Carlson ML, Archibald DJ, Graner DE, Kasperbauer JL. Surgical management of dysphagia and airway obstruction in patients with prominent ventral cervical osteophytes. Dysphagia. 2011;26: 34–40. 10.1007/s00455-009-9264-6 [DOI] [PubMed] [Google Scholar]
- 6.Kmucha ST, Cravens RB Jr. DISH syndrome and its role in dysphagia. Otolaryngol Head Neck Surg. 1994;110: 431–436. 10.1177/019459989411000414 [DOI] [PubMed] [Google Scholar]
- 7.Salassa JR. A functional outcome swallowing scale for staging oropharyngeal dysphagia. Dig Dis. 1999;17: 230–234. 10.1159/000016941 [DOI] [PubMed] [Google Scholar]
- 8.Verlaan J-J, Boswijk PFE, de Ru JA, Dhert WJ, Oner FC. Diffuse idiopathic skeletal hyperostosis of the cervical spine: an underestimated cause of dysphagia and airway obstruction. Spine J. 2011;11: 1058–1067. 10.1016/j.spinee.2011.09.014 [DOI] [PubMed] [Google Scholar]
- 9.Von-der-Hoeh NH, Voelker A, Jarvers JS, Gulow J, Heyde CE. Results after the surgical treatment of anterior cervical hyperostosis causing dysphagia. Eur spine J. 2015;24(suppl 4): s489–s493. [DOI] [PubMed] [Google Scholar]
- 10.Denko CW, Malemud CJ. Body mass index and blood glucose: correlations with serum insulin, growth hormone, and insulin-like growth factor-1 levels in patients with diffuse idiopathic skeletal hyperostosis(DISH). Rheumatol Int. 2006;26: 292–297. 10.1007/s00296-005-0588-8 [DOI] [PubMed] [Google Scholar]
- 11.Egerter AC, Kim ES, Lee DJ, Liu JJ, Cadena G, Panchal RR, et al. Dysphagia secondary to anterior osteophytes of the cervical spine. Global Spine J. 2015;5: e78–83. 10.1055/s-0035-1546954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song J, Mizuno J, Nakagawa H. Clinical and radiological analysis of ossification of the anterior longitudinal ligament causing dysphagia and hoarseness. Neurosurgery. 2006;58: 913–919. 10.1227/01.NEU.0000209936.46946.99 [DOI] [PubMed] [Google Scholar]
- 13.McCafferty RR, Harrison MJ, Tamas LB, Larkins MV. Ossification of the anterior longitudinal ligament and Forestiers disease: an analysis of seven cases. J Neurosurg. 1995;83: 13–17. 10.3171/jns.1995.83.1.0013 [DOI] [PubMed] [Google Scholar]
- 14.Oppenlander ME, Orringer DA, La Marca F, McGillicuddy JE, Sullivan SE, Chandler WF, et al. Dysphagia due to anterior cervical hyperosteophytosis. Surg Neurol. 2009;72: 266–271. 10.1016/j.surneu.2008.08.081 [DOI] [PubMed] [Google Scholar]
- 15.Urrutia J, Bono CM. Long-term results of surgical treatment of dysphagia secondary to cervical diffuse idiopathic skeletal hyperostosis. Spine J. 2009;9: e13–e17. [DOI] [PubMed] [Google Scholar]
- 16.Miyamoto K, Sugiyama S, Hosoe H, Iinuma N, Suzuki Y, Shimizu K. Postsurgical recurrence of osteophytes causing dysphagia in patients with diffuse idiopathic skeletal hyperostosis. Eur Spine J. 2009;18: 1652–1658. 10.1007/s00586-009-1133-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bazas R, Lee MJ, Yoo JU. Incidence of dysphagia after anterior cervical spine surgery: a prospective study. Spine (Phila Pa 1976). 2002;27: 2453–2458. [DOI] [PubMed] [Google Scholar]
- 18.Cho SK, Lu Y, Lee DH. Dysphagia following anterior cervical spinal surgery. Bone Joint J. 2013; 95-B: 868–873. 10.1302/0301-620X.95B7.31029 [DOI] [PubMed] [Google Scholar]
- 19.Ozgursoy OB, Salassa JR, Reimer R, Wharen RE, Deen HG. Anterior cervical osteophyte dysphagia: manofluorographic and functional outcomes after surgery. Head Neck. 2010;32: 588–593. 10.1002/hed.21226 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.