Abstract
Background/Aim: Triple-negative breast cancer (TNBC) is characterized by the absence of hormone receptors (estrogen, progesterone and human epidermal growth factor receptor-2) and a relatively poor prognosis due to inefficacy of hormone receptor-based chemotherapies. It is imperative that we continue to explore natural products with potential to impede growth and metastasis of TNBC. In this study, we screened over 1,000 natural products for capacity to induce cell death in TNBC (MDA-MB -231) cells. Materials and Methods: Frankincense (Boswellia serrata extract (BSE)) and 3-O-Acetyl-β-boswellic acid (3-OAβBA) were relatively potent, findings that corroborate the body of existing literature. The effects of BSE and 3-OAβBA on genetic parameters in MDA-MB-231 cells were evaluated by examining whole-transcriptomic influence on mRNAs, long intergenic non-coding RNA transcripts (lincRNA) and non-coding miRNAs. Results: Bio-statistical analysis demarcates the primary effect of both BSE/3-OAβBA on the up-regulation of PERK (protein kinase RNA-like endoplasmic reticulum kinase)– endoplasmic reticulum (ER)/unfolded protein response (UPR) pathways that are closely tied to activated programmed cell death (APCD). Global profiling confirms concomitant effects of BSE/3-OAβBA on upwardly expressed ER/URP APCD key components PERK (EIF2AK3), XBP1, C/EBP homologous protein transcription factor (CHOP), ATF3 and DDIT3,4/DNA-damage-inducible transcript 3,4 (GADD34). Further, BSE and/or 3-OAβBA significantly down-regulated oncogenes (OG) which, heretofore, lack functional pathway mapping, but are capable of driving epithelial–mesenchymal transition (EMT), cell survival, proliferation, metastasis and drug resistance. Among these are cell migration-inducing protein hyaluronan binding (CEMIP) [–7.22]; transglutaminase 2 [–4.96], SRY box 9 (SOX9) [–4.09], inhibitor of DNA binding 1, dominant negative helix-loop-helix protein (ID1) [–6.56]; and endothelin 1 (EDN1, [-5.06]). Likewise, in the opposite manner, BSE and/or 3-OAβBA induced the robust overexpression of tumor suppressor genes (TSGs), including: glutathione-depleting ChaC glutathione-specific gamma-glutamylcyclotransferase 1 (CHAC1) [+21.67]; the mTOR inhibitors - sestrin 2 (SESN2) [+16.4] Tribbles homolog 3 (TRIB3) [+6.2], homocysteine-inducible, endoplasmic reticulum stress-inducible, ubiquitin-like domain member 1 (HERPUD1) [+12.01]; and cystathionine gamma-lyase (CTH) [+11.12]. Conclusion: The anti-cancer effects of the historically used frankincense sap (BSE) appear to involve major impact on the ER/UPR response, concomitant to effecting multiple targets counter to the growth, proliferation and metastasis of TNBC cancer cells. The microarray data are available at Expression Omnibus GEO Series accession number GSE102891.
Keywords: Boswellic acid, Boswellia, apoptosis, Frankincense, cancer, microarray, endoplasmic reticulum, UPR, CHOP, ATF, ER, PERK, CHAC1, SESN2, TG2, sestrin, dual specificity phosphatase, histone cluster, epigenetics
Frankincense has been used as a valuable multi-purpose natural product for over 5,000 years, where its medicinal form is derived from the tree sap resin of diverse species from the genus Boswellia/family Burseraceae. Its extended historical use reflects valuable insight about its properties from our ancestors who had a greater dependency on natural medicines. In the past century, with the rapid development of synthetic medicines, botanical therapeutics are perceived as menial compared to that of current medical treatment. Yet, at the same time, scientific literature continues to report Boswellia and its active component: boswellic acid can exert diverse antitumor properties (1) with the capacity to attenuate proliferation, angiogenesis, invasion and metastasis in established models (2-7).
With the availability of current biotechnologies, it is evident that Boswellia can mediate anti-cancer effects through direct reduction of pro-oncogenic proteins and transcription factors that otherwise drive aggressive malignancies. Just for a few examples, Boswellia and its constituents suppress NF-ĸB, Bcl-2, bcl-xL, Mcl-1, IAP-1, BIRC5, VEGF (2,8,9) mPGES-1, MMP-2,7,9, PGE2 (5) cyclin D1, PCNA, c-Myc (10), cyclin E, CDK 2 and 4 and retinoblastoma (Rb) (11). Central to these effects are control over STAT3 phosphorylation of Jak 2/Src or Akt/GSK3β signaling tantamount to triggering apoptotic pathways through caspase-9, caspase-3, and cleaved PARP (12,13). Other reported anti-cancer attributes of Boswellia include its potential to block the development of chemically induced cancers such as that by azomethane (14), prevent multidrug resistance (15) and act as a chemo-sensitizing agent (4,16). These effects are consistently observed both in in vitro and in vivo (10). With regards to triple negative breast cancer (TNBC), Boswellia serrata extract (BSE) and 3-O-Acetyl-β-boswellic acid (3-OAβBA) are equally effective against its growth and that of other malignant breast tumor cell lines (8,17,18).
Here, we further investigate precipitating transcriptome changes induced by Boswellia serrata extract and 3-OAβBA, in order to determine the major cause of cell death in TNBC breast cancer cells. These findings can serve as a general directive in future studies investigating the anti-cancer properties of frankincense.
Materials and Methods
Hanks Balanced Salt Solution, (4-(2-hydroxyethyl)-1-piperazine-ethanesulfonic acid) (HEPES), absolute ethanol ≥99.8%, 96 well plates, pipette tips, Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), penicillin/streptomycin general reagents and supplies were all purchased from Sigma-Aldrich Co. (St. Louis, MO, USA) and VWR International (Radnor, PA, USA). Triple-negative human breast tumor (MDA-MB-231) cells were obtained from the American Type Culture Collection (Rockville, MD, USA). Boswellia serrata was obtained from Starwest Botanicals (Sacramento, CA, USA) and 3-O-Acetyl-β-boswellic acid was purchased from Cayman Chemical (Ann Arbor, MI, USA). All microarray equipment, reagents and materials were purchased from Affymetrix/ Thermo Fisher (Waltham, MA, USA).
All natural chemicals, reference drugs and (3-OAβBA) were dissolved in DMSO [5-20 mg/mL], where the crude herbs including Boswellia serrata were prepared in absolute ethanol [50 mg/mL] after being diced, macerated and powdered prior to being stored at –20˚C. All dilutions were prepared in sterile HBSS + 5 mM HEPES, adjusted to a pH of 7.4, ensuring solvent concentration of DMSO or absolute ethanol at less than 0.5%.
Cell culture. MDA-MB-231 cells were cultured in 175 cm2 flasks containing DMEM supplemented with 10% FBS and 100 U/ml penicillin G sodium/100 μg/ml streptomycin sulfate. Cells were grown at 37˚C in 5% CO2/atmosphere and sub-cultured every three to five days.
Cell viability assay. Alamar Blue cell viability assay was used to determine cytotoxicity. Viable cells are capable of reducing resazurin to resorufin (a detectable fluoroprobe). Briefly, 96-well plates were seeded with MDA-MB-231 cells at a density of 5×106 cells/ml. Cells were treated with HBSS (control) and various concentrations of Boswellia serrata extract or 3-O-Acetyl-β-boswellic acid for 24 h at 37˚C, 5% CO2 in atmosphere. Alamar blue (0.1 mg/ml in HBSS) was added at 15% v/v to each well, and the plates were incubated for 6-8 h. Quantitative analysis of dye conversion was measured on a Synergy™ HTX Multi-Mode microplate reader (BioTek, Winooski, VT, USA), 550nm /580nm (excitation/emission). The data were expressed as a percentage of untreated controls.
Fluorescence microscopy. Live cell imaging was conducted using Fluorescein diacetate (FDA), which is a cell-permeable esterase substrate. The fluorescein molecule accumulates in cells that possess intact membranes, serving as a marker of cell viability. Briefly, FDA was dissolved in ethanol 4.2 mg/ml and subsequently prepared at 20 μM in HBSS. After 30 min of incubation, samples were analyzed photographically using a fluorescent /inverted microscope, CCD camera and data acquisition by ToupTek View (ToupTek Photonics Co, Zhejiang, P.R. China).
Microarray WT 2.1 human datasets. After treatment, cells were washed three times in HBSS, rapidly frozen and stored at –80˚C. Total RNA was isolated/ purified using the TRIzol/chloroform method, quality was assessed and concentration was equalized to 82 ng/μl in nuclease free water. Whole transcriptome analysis was conducted according to the GeneChip™ WT PLUS Reagent Manual for Whole Transcript (WT) Expression Arrays. Briefly, RNA was synthesized to first strand cDNA, second-strand cDNA, followed by transcription to cRNA. cRNA was purified and assessed for yield, prior to 2nd cycle single stranded cDNA synthesis, hydrolysis of RNA and purification of 2nd cycle single stranded cDNA. cDNA was then quantified for yield and equalized to 176 ng/ml. Subsequently, cDNA was fragmented, labeled and hybridized on to the arrays prior to being subjected to fluidics and imaging using the Gene Atlas (Affymetrix, ThermoFisher Scientific, Waltham, MA, USA).
The array data quality control and initial processing from CEL to CHP files were conducted using expression console, prior to data evaluation using the Affymetrix transcriptome analysis console. Supportive analysis was accomplished using geneontology.org (19) and DAVID Bioinformatics Resources 6.8 National Institute of Allergy and Infectious Diseases (NIAID), NIH (20).
Microarray miRNA 4.1 human datasets. miRNA was isolated using the QIAzol reagent and miRNeasy Mini Kit (Qiagen, Germantown, MD). Briefly, after RNA purification, samples were labeled with a POLY A tail, and ligated using a flash tag ligation mix from the FlashTag™ Biotin HSR RNA kit (Affymetrix). Subsequently, biotin labeled RNA was detected using streptavidin – EP and hybridized onto a Genechip miRNA 4.1 human array, prior to fluidics and imaging by the Gene Atlas. The array data quality control and initial processing from CEL to CHP files were conducted using expression console, prior to data evaluation using the transcriptome analysis console provided by Affymetrix. Supportive analysis was accomplished using geneontology.org (19) and DIANA miRPath tools (21,22).
Data analysis. Statistical analysis was performed using Graph Pad Prism (version 3.0; Graph Pad Software Inc. San Diego, CA, USA) with significance of difference between the groups assessed using a one-way ANOVA, then followed by a Tukey post hoc means comparison test, or a Student’s t-test. LC50s were determined by regression analysis using Origin Software (Origin Lab, Northampton, MA, USA).
Results
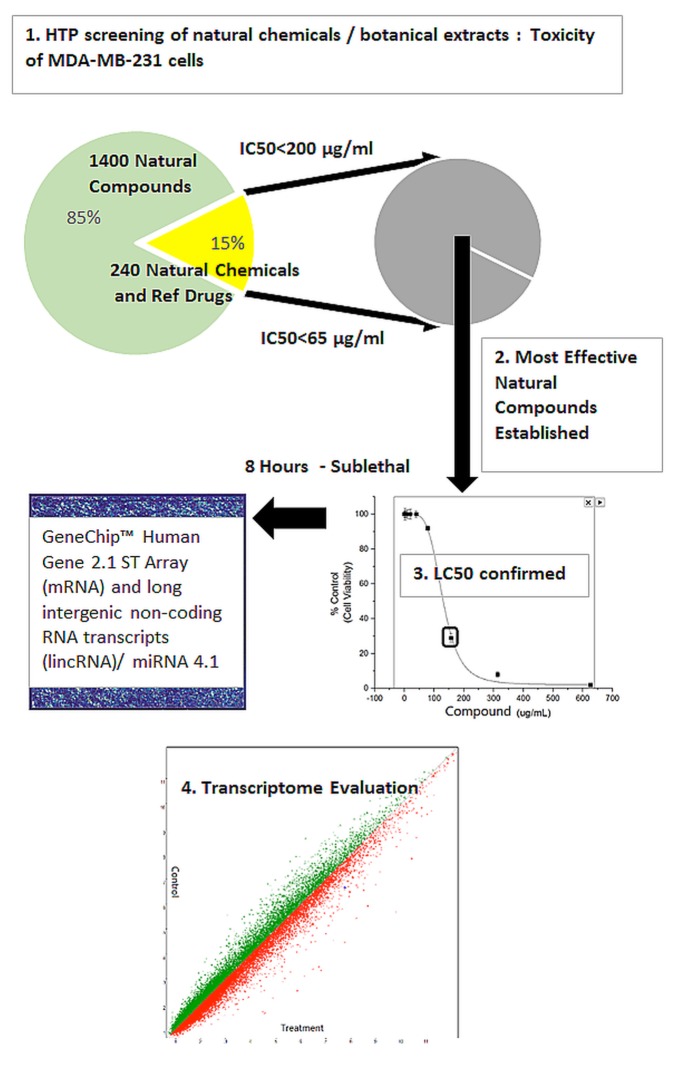
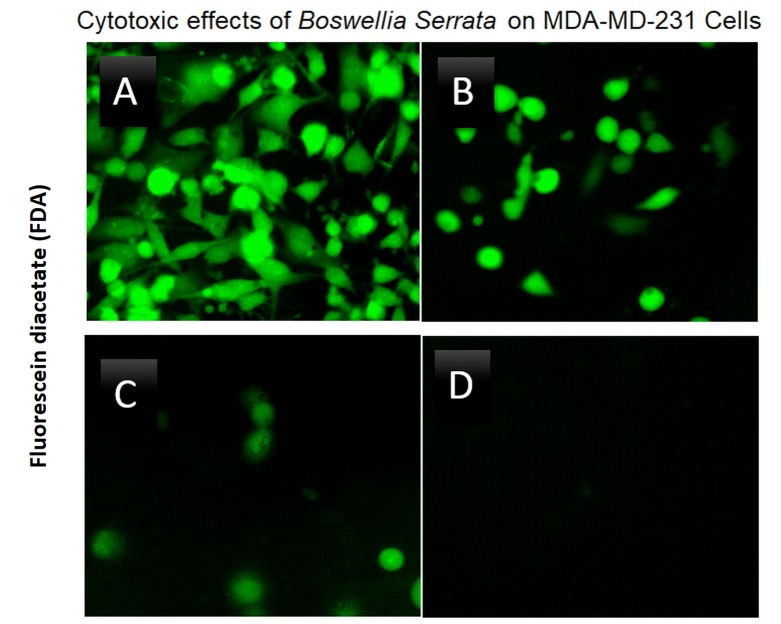
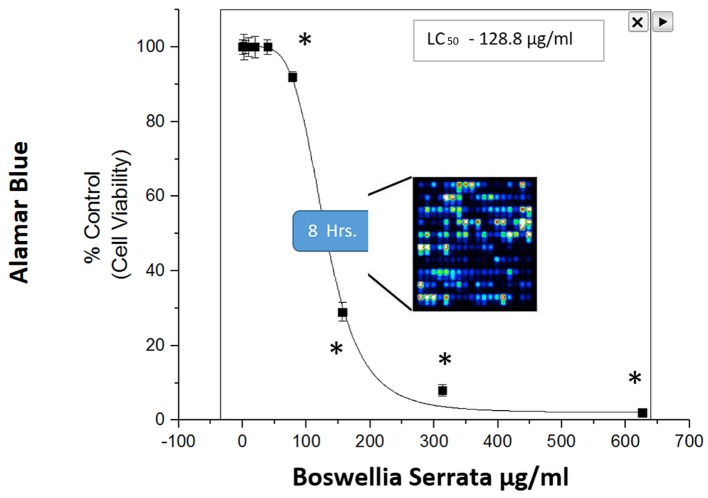
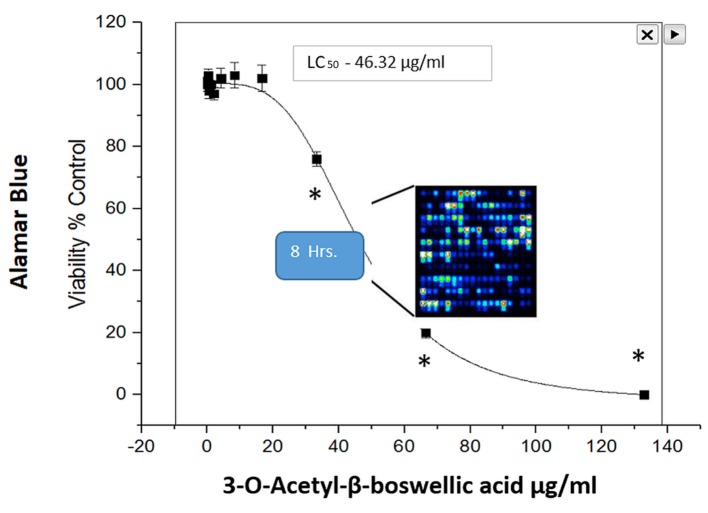
A high throughput (HTP) screening module is routinely used in our facility to enable the preliminary evaluation of thousands of herbs and plant chemicals on selective targets, and in this case for relative capacity to induce cell death in MDA-MB-231 cells (Figure 1). Briefly, LC50s were established, natural products were ranked for potency and lead compounds identified. Here we focus on the natural herb: Boswellia serrata (BSE), where we present fluorescence FDA staining showing a loss of viability over concentration (Figure 2) and corresponding cytotoxicity as determined by Alamar Blue (Figure 3). The LC50s were determined (128.8 μg/ml) for BSE and its active component 3-OAβBA (46.32 μg/ml) (Figure 4).
Figure 1. Natural compound screening procedure: A total of 240 in -house natural plant-derived chemicals (<65 μg/ml) and 1,400 botanical herbs (<200 μg/ml) were tested for capacity to induce cytotoxicity on MDA-MB-231 cells, relative to chemotherapy drugs. Lead compounds Frankincense (Boswellia serrata extract (BSE)) and 3-O-Acetyl-β-boswellic acid (3-OAβBA) were then cultured for 8 hours at the LC50, prior to cell death – and immediately frozen at –80˚C. Microarray analysis was performed to identify biological influence on the entire transcriptome.
Figure 2. Cytotoxic effects of Boswellia serrata (BSE) on MDA-MB-231 cells at 24 h of incubation at 37˚C, 5% CO2/Atm. The data reflect loss of cell viability using FDA which is cleaved only by viable cells. (A) Untreated Control (B) 156 μg/ml (C) 313 μg/ml (D) 626 μg/ml.
Figure 3. Cytotoxicity of BSE on MDA-MB-231 cells at 24 h of incubation at 37˚C, 5% CO2/Atm. The data represent loss of viability as % control values as determined with Alamar Blue assay. The data are presented as the Mean±S.E. M, n=4, and significant difference from the controls were determined by a one-way ANOVA followed by a Tukey post hoc comparison test, *p<0.05.
Figure 4. Cytotoxicity of 3-OAβBA on MDA-MB-231 cells at 24 h of incubation at 37˚C, 5%CO2/Atm. The data represent loss of viability as % Control values as determined with Alamar Blue assay. The data are presented as the mean±S.E. M, n=4, and significant differences from the controls were determined by a one-way ANOVA followed by a Tukey post-hoc comparison test, *p<0.05.
For whole transcriptome microarray studies, the LC50s of BSE and 3-OAβBA were applied to fully viable cells at Time 0 (zero minutes), and morphological changes were monitored every hour, to ensure no cell death was evident. At 6-8 h, the cells retained morphological shape, flask attachment and had no obvious signs of cell death. At this point, cells were rapidly washed in HBSS 3x, spun and frozen at –80˚C. This time of acquisition was ascertained as appropriate to ensure capture of information on pivotal events elicited/precipitating cell death.
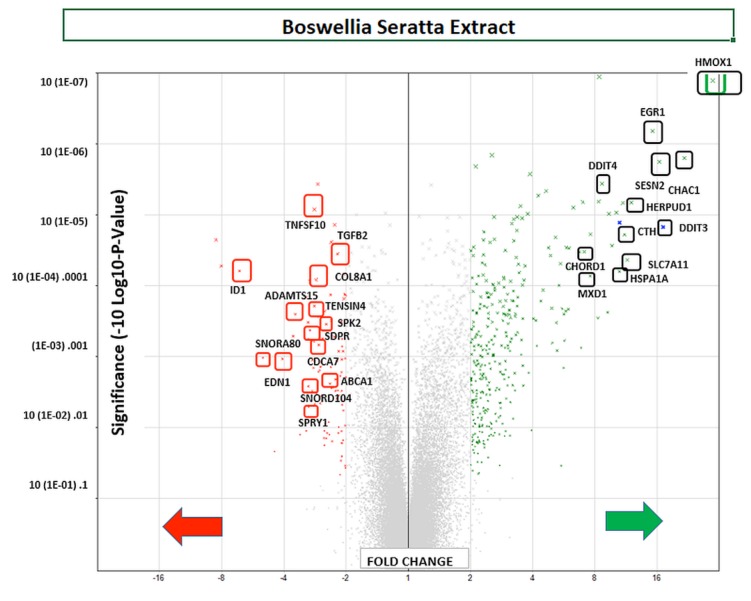
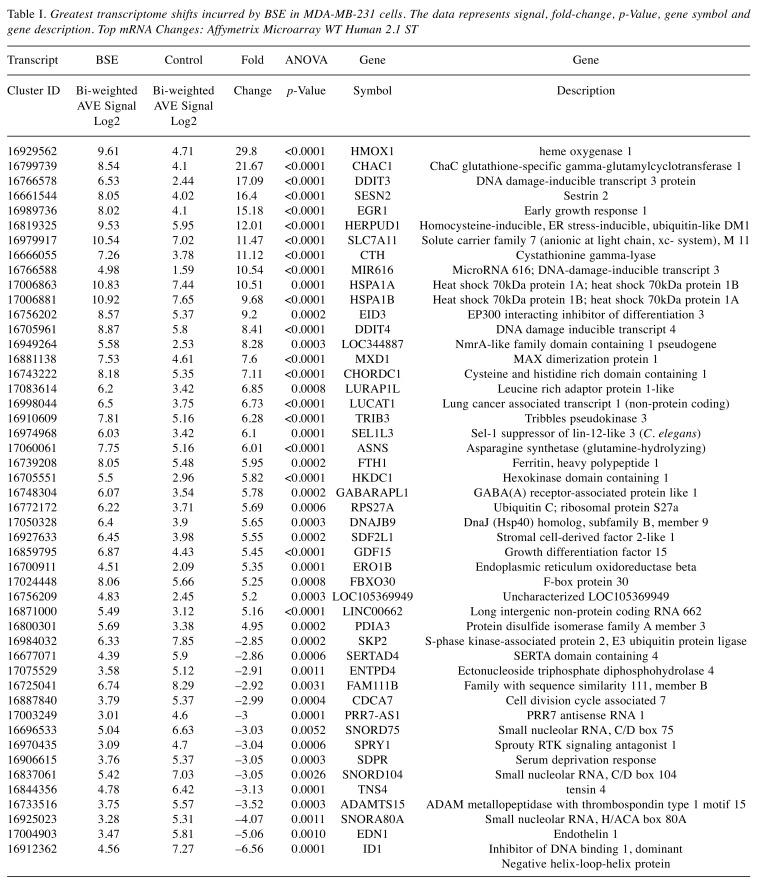
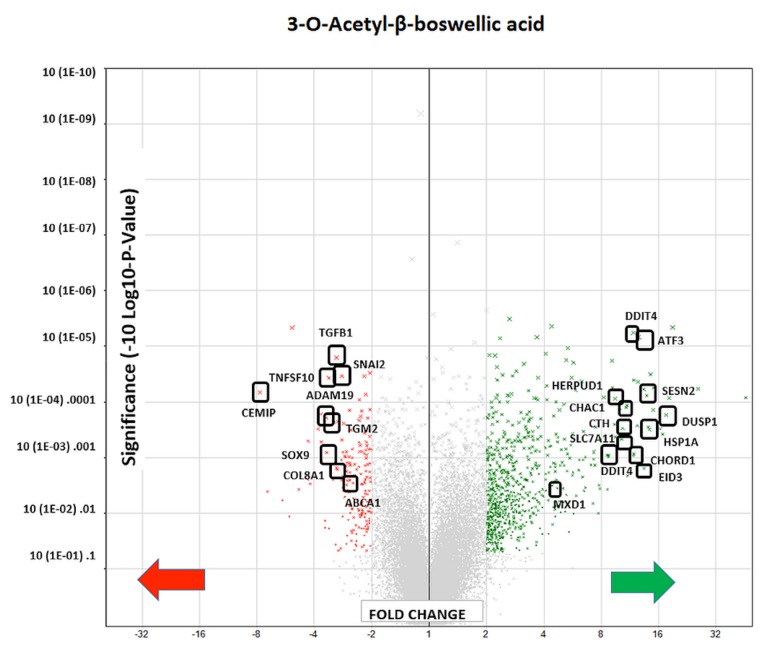
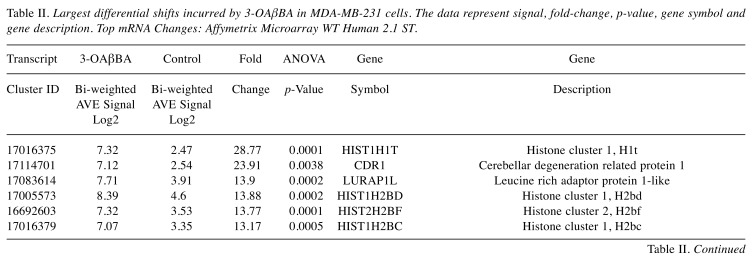
Using affymetrix human whole transcriptome arrays [GeneChip Human Gene Array 2.1], the data showed that of the 48226 transcripts tested, there were 300 differentially expressed genes (DEGs) for BSE (265 up-regulated/65 down-regulated) and for 3-OAβBA: 931 DEGs (391 up-regulated/540 down-regulated). An overview of the transcriptome data for BSE treatment are presented by a volcano plot (Figure 5) showing fold change (FC) vs. significance – then cross referenced to Table I, which presents the largest differentially expressed changes. An overview of microarray data for 3-OAβBA treatments are presented by a similar volcano plot (Figure 6) also cross referenced to Table II, showing the largest differentially expressed changes. The data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus and are accessible through GEO Series accession number GSE102891 located at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE102891.
Figure 5. WT changes in BSE treated cells using GeneChip™ Human Gene 2.1 ST Array. 48226 transcripts were tested, 300 differentially expressed genes (DEGs) were identified for (265 up-regulated/65 down-regulated). The data are presented by a volcano plot (fold-change by significance) for whole transriptome changes in BSE treated MDA-MB-231 cells vs. controls, n=3. The left panel shows down-regulated genes (red)/right panel (green) shows up-regulated genes: highlighting some of the highest differential changes, also listed in Table Ι.
Table I. Greatest transcriptome shifts incurred by BSE in MDA-MB-231 cells. The data represents signal, fold-change, p-Value, gene symbol and gene description. Top mRNA Changes: Affymetrix Microarray WT Human 2.1 ST.
Figure 6. WT changes in 3-OAβBA treated cells using GeneChip™ Human Gene 2.1 ST Array. 48226 transcripts tested: 931 DEGs were identified (391 up-regulated/540 down-regulated). The data are presented as a volcano Plot (fold change by significance) for whole transcriptome changes in 3-OAβBA treated MDA-MB-231 cells vs. controls, n=3. The left panel shows down-regulated genes (red)/right panel shows up-regulated genes (green): highlighting some of the top changes, also listed in Table ΙΙ.
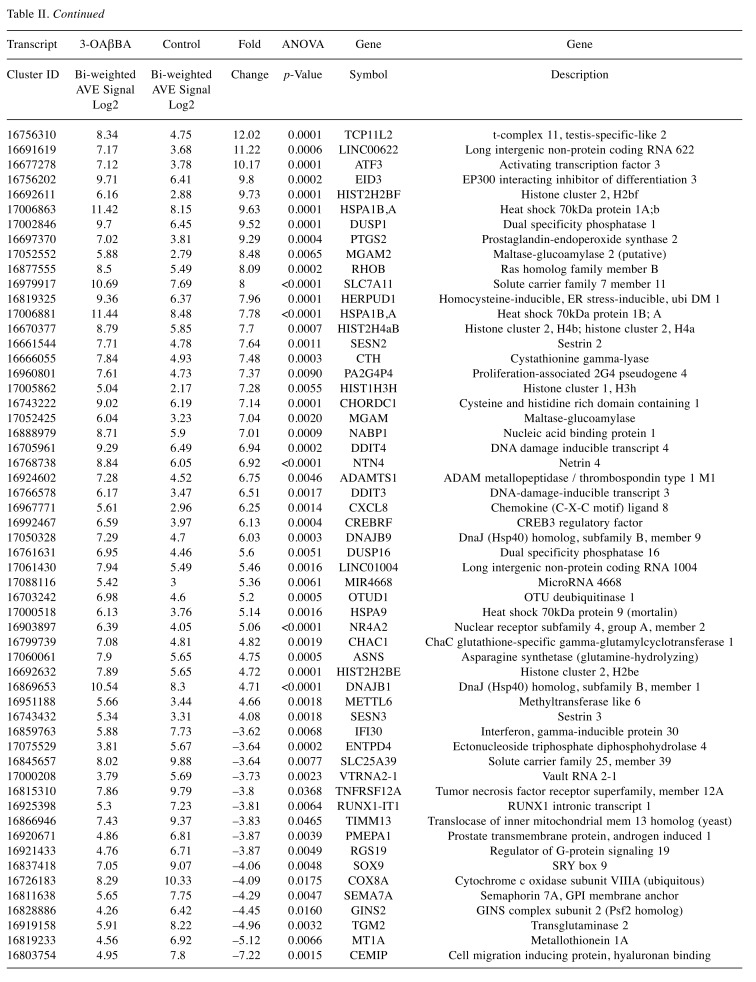
Table II. Largest differential shifts incurred by 3-OAβBA in MDA-MB-231 cells. The data represent signal, fold-change, p-value, gene symbol and gene description. Top mRNA Changes: Affymetrix Microarray WT Human 2.1 ST.
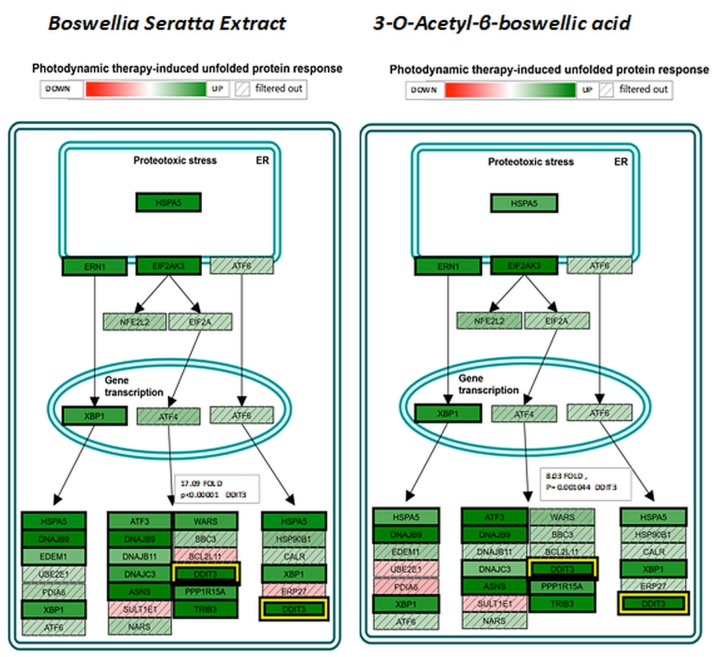
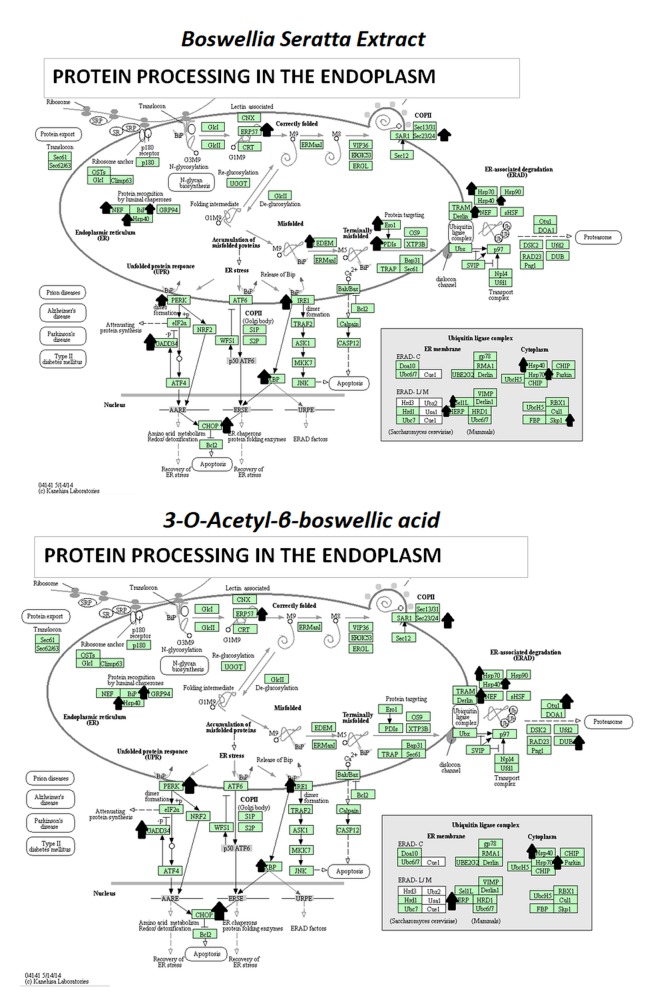
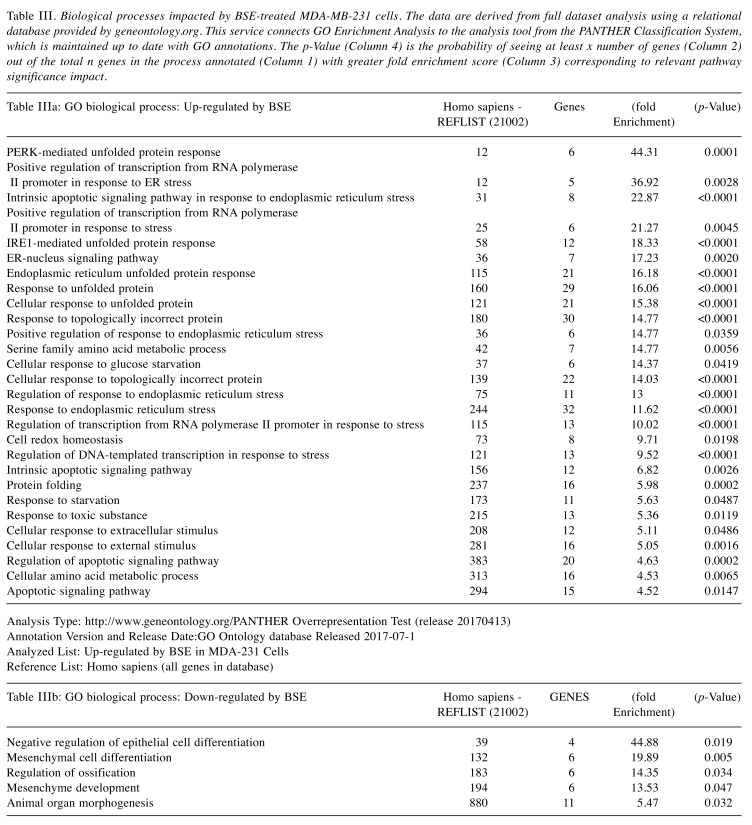
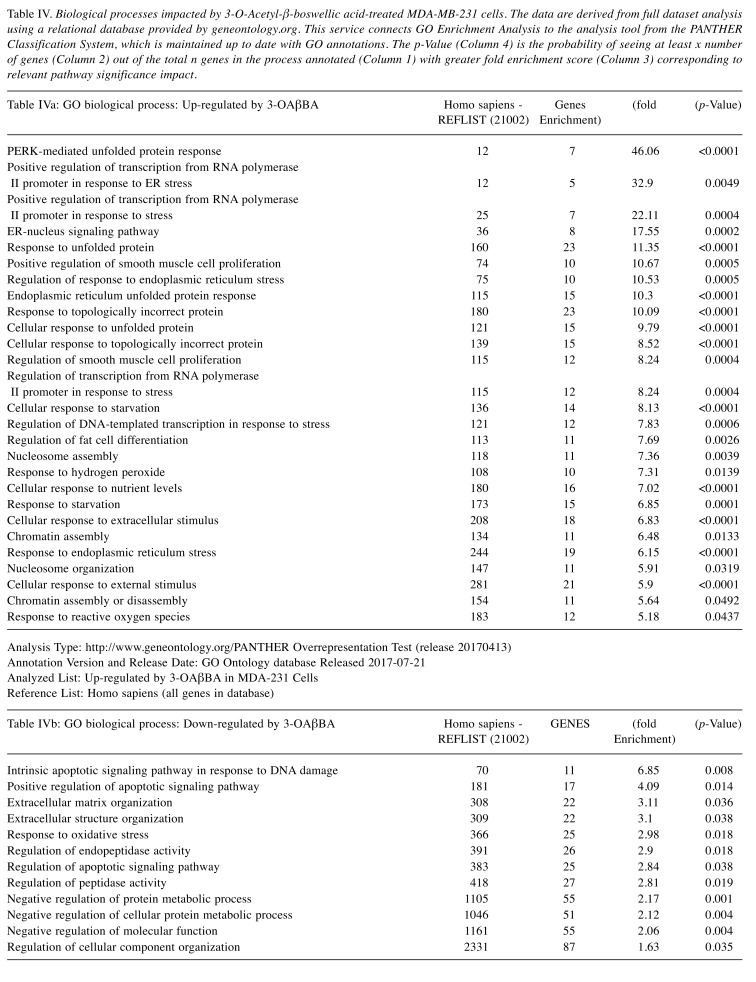
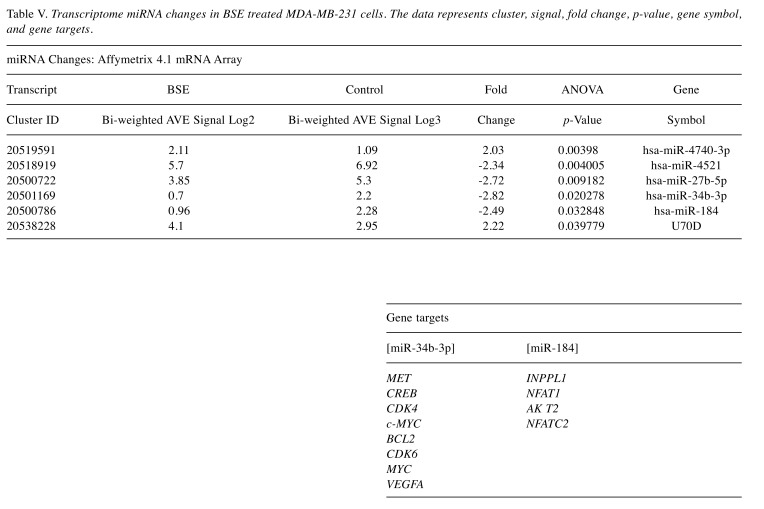
The pathway of greatest impact for both BSE and 3-OAβBA elucidated by the Affymetrix transcriptome analysis console, sorted by greatest relevance was up up-regulation of the photodynamic therapy unfolded protein response (Figure 7). Using DAVID Functional Annotation Bioinformatics Microarray Analysis (20), we also found that the endoplasm reticulum was largely affected – up-regulated genes are shown on a KEGG overlay pathway map (Figure 8). Again, there was a very close overlay between BSE and 3-OAβBA to elicit these ER mediated responses. In terms of dataset analysis for functional biological relevance, we used geneontology.org enrichment analysis tool, which also confirms the findings from Affymetrix and David bio analytic tools, corroborating uniform up-regulation on the ER stress, unfolded protein responses as well as glucose depletion/starvation (Table III and Table IV). Interestingly, very few changes were reported for the miRNAs, with reported pathways for hsa-miR-34b-3p (target of 14 genes) and hsa-miR-184 (target of 6 genes), as presented in Table V. These findings provide an overview of Boswellia seratta and its pharmacologically active compound, boswellic acid on the transcriptome of TNBCs.
Figure 7. Affymetrix Transcription Analysis Console/Wikipaths Correlation by Significance shows impact on Photodynamic therapy unfolded protein response by BSE and 3-3-OAβBA in MDA-MB-231 cells, with a high degree of overlap. The data represents relative fold change by intensity (green) up-regulation, (red) down-regulation with (/////) filtered out as non-significant directional changes. Highlighted in yellow is the shift in DDIT3, with values presented.
Figure 8. DAVID Functional Annotation Bioinformatics Microarray Analysis. DAVID Bioinformatics Resources 6.8. KEGG Diagram Overlap of up-regulated transcripts in response by BSE and 3-O-Acetyl-β-boswellic acid in MDA-MB-231 cells, with a high degree of overlap. The data represents Protein Processing in the ER and up-regulated transcripts noted by an arrow.
Table III. Biological processes impacted by BSE-treated MDA-MB-231 cells. The data are derived from full dataset analysis using a relational database provided by geneontology.org. This service connects GO Enrichment Analysis to the analysis tool from the PANTHER Classification System, which is maintained up to date with GO annotations. The p-Value (Column 4) is the probability of seeing at least x number of genes (Column 2) out of the total n genes in the process annotated (Column 1) with greater fold enrichment score (Column 3) corresponding to relevant pathway significance impact.
Table IV. Biological processes impacted by 3-O-Acetyl-β-boswellic acid-treated MDA-MB-231 cells. The data are derived from full dataset analysis using a relational database provided by geneontology.org. This service connects GO Enrichment Analysis to the analysis tool from the PANTHER Classification System, which is maintained up to date with GO annotations. The p-Value (Column 4) is the probability of seeing at least x number of genes (Column 2) out of the total n genes in the process annotated (Column 1) with greater fold enrichment score (Column 3) corresponding to relevant pathway significance impact.
Table V. Transcriptome miRNA changes in BSE treated MDA-MB-231 cells. The data represents cluster, signal, fold change, p-value, gene symbol, and gene targets.
Discussion
The data in this study suggest a primary mode of cell death by 3-OAβBA and BSE to involve ER stress leading to a UPR (unfolded protein response), this commonly associated with activated cell death. There has been a recent surge in research describing the importance of the ER/UPR in a variety of human pathologies, many of these relevant to cancer (23-25).
A literature review of the ER/UPR involvement in cancer unveils a scientific uncertainty and need for answers as to why activation of ER/UPR creates a double-edged sword. On the one hand, ER stress inducers (e.g. hypoxia, glucose, nutrient deprivation (26,27) activate the ER/UPR which leads to tumor adaptation (a persistent elevation of pro-survival proteins, a resistance to chemotherapy, greater tumor progression, angiogenesis, invasion and thriving of dormant stem cells (28-30). Yet at the same time, activation of main pathways in the ER/UPR an also trigger programmed cell death (PCD) evidenced by many natural anti-cancer agents (31-41) alkylating/ platinum based drugs and anti-cancer steroids (42-47). Several articles have expressed the need for further understanding of the pro-survival/pro-death ER/UPR processes and the relevance timing on cancer initiation, progression and treatment (48). It is believed that if we can further understand control of ER stress regulators on cancer growth, we can successfully use this information to overcome acquired resistance to (49) and augment existing cancer therapies (50). The data from this study show BSE and 3-OAβBA to impact several processes within the ER/UPR.
ER/UPR. If we take a look at the normal function of the ER under non-stress conditions, its main purpose is in the post-translational modification and folding of mature proteins using chaperones and foldases, which are then trafficked to the Golgi. Anything that impairs this system elicits ER stress and a UPR. This later response (ER/UPR) serves a primary means to reduce protein load by decreasing translation, and removing mis-folded proteins. This is accomplished by increasing the folding capability of the ER, and the degradation rate of damaged proteins through binding to glucose-regulated protein 78 (Bip/GRP78) (a pivotal event) in preparation for disposal through an endoplasmic reticulum-associated degradation pathway (ERAD) by the ubiquitin/proteasome pathway or alternatively, an autophagic/lysosomal pathway (51,52).
Briefly, the ER/UPR main branches can all initiate pro-apoptotic events. These include:
[Pathway 1] protein kinase RNA-like endoplasmic reticulum kinase (PERK),
[Pathway 2] inositol-requiring enzyme-1 (IRE1), or
[Pathway 3] activating transcription factor-6 (ATF-6) (53,54).
The effects of 3-OAβBA and BSE on the transcriptome suggests extensive up-regulation on many of these processes.
Pathway 1/PERK: Briefly, when proteins are misfolded in the ER, they bind to BiP/Grp78 which triggers X-box-binding protein 1 (XBP1) splicing, which then initiates PERK to phosphorylate +P (eIF2α). We found evidence of BSE not only up-regulating XBP1 +3.31, p=0.0003 (3-OAβBA ), +3.04, p=0.0003 (BSE) but also PERK (EIF2AK3) +4.6 p=0.0003 (3-OAβBA) and +3.67, p<0.0003 (BSE). This active +PeIF2 α, is central to the control of downstream events which halting protein synthesis, cell cycle arrest in addition to activating ATF4, which in turn elevates ATG12, TRB3 (AKT/mTOR inhibitor), triggering autophagy required for removal of unfolded proteins. These events are often simultaneous with the rise in C/EBP homologous protein transcription factor (CHOP)/DNA damage-inducible transcript 3, 4 or GADD153,GADD34, and ATF3 (triggering cell death) (55,56). The data in this study show mediated effects for TRB3 [+6.3 fold, p<0.0001 BSE/+3.68, p<0.0001 3-OAβBA] ATF3 [+12.61 fold, p<0.0001 BSE/+2.9, p<0.0001 3-OAβBA], DDIT3 [+17.09 fold, p<0.0001 BSE/+8.03, p<0.0001 3-OAβBA] and DDIT4 [+8.41 fold, p<0.0001 BSE/+11.77, p<0.0001 3-OAβBA]. If CHOP driven ER stress mediated apoptosis prevails, this would drive up-regulation of death molecules (BIM, BAX, PUMA), death receptors (Tnfrsf10b/Dr5) juxtaposed to a reduction of BLC2 (anti-apoptotic molecules) (55,57), activation of JNK and p38MAPK, rise in immediate early response genes (EGR-1), and ASK1 recruitment to IRE1-TRAF2, linking pathway 1 to the next ER/UPR stress pathway Pathway 2/ IRE1α; ERN1. The data in this study again, show consistent trends in downstream events including elevated levels of EGR-1, [+ 15.18 fold, p<0.0001 BSE/+2.48, p<0.0001 3-OAβBA], TRB3 [+6.3 fold, p<0.0001 BSE/+3.68, p<0.0001 3-OAβBA] and ATF3 [+12.61 fold, p<0.0001 BSE/+2.9, p<0.0001 3-OAβBA].
Pathway 2/ IRE1α; ERN1: In response to unfolded proteins, IRE1α; ERN1 is cleaved by endoribonuclease activity at the 26bp intron of XBP1 (involved with pathway 1 above), which then facilitates the formation of transcription factor XBP1 mRNA, where IRE1-XBP1 can trigger recruitment of TRAF2 to the ER membrane (+ASK1 recruitment). TRAF2 is an activator of apoptosis signal-regulating kinase 1 (ASK1), which can lead to JNK mediated apoptosis. Also, this pathway can trigger ERO1α to activate the ER calcium channel inositol-1,4,5-trisphosphate receptor 1 (IP3R1) enabling activate cAMP response elements (CREs).
Pathway 3/ATF 6: Upon ER stress, ATF6 dissociates from GRP78/BiP – leaving it free to translocate to the Golgi, where it is cleaved by S1P and S2P, and its fragment released to the cytosol. ATF6 fragments can include the active 50kDa transcription factor (ATF6 p50) which translocate to the nucleus. There, ATF6 p50 and XBP1 bind ERSE promoters and up-regulate chaperones that are involved with unfolded protein response including GRP78.
ER/UPR stress mediated apoptosis and cancer drugs. Many natural products are being reported to impact the aforementioned, including a spiked rise in Grp78, CHOP with activated ER/UPR – PCD occurring through PERK, IRE1alpha and ATF6 pathways as in the case of cryptotanshinone (32) 2-(3,4-dihydroxyphenyl)ethanol (olive oil) (58) selenium (59) methylseleninic acid, sodium selenite (33) xanthohumol (hops), docosahexaenoic acid (34,35) isochaihulactone (Nan-Chai-Hu) (36) Shikonin (Lithospermum erythrorhizon) (37) chrysin (31) curcumin (40) silibinin (41) or whole herbs such as the Chinese herbal medicine Tu Bei Mu (39). A number of drugs also mediate similar effects, such as steroids, platins, taxol, alkylating agents, or cancer chemicals which on the one hand block the growth of diverse cancers, and on the other hand elevate ER/UPR – PCD, associated with up-regulation of GRP78, CHOP and three UPR-associated pathways, PERK, IRE1alpha, and ATF6 (42-44,46,60). It is also believed that hydrogen peroxide tumor mediated cell death also corresponds to up-regulation of the PERK branch evident by +P eIF2α and the mRNA levels of activating transcription factor 4 (ATF4), C/EBP homologous (CHOP) and tribbles homolog 3 (TRB3)(61). The findings in this work, place 3-OAβBA and BSE in this category of anti-cancer agents.
While discussing all the changes in the transcriptome initiated by 3-OAβBA and BSE are beyond the scope of this paper, noteworthy is the rise in CHAC1, which is involved in the degradation of glutathione (62,63) reported to occur in parallel to rise of ATF4-ATF3-CHOP PERK and the phosphorylation of EIF2α, where its rise creates vulnerability of cancer cells to the losses of glutathione associated with radiation and oxidative insult (64,65) also rendering losses on glutathione detoxification systems (66).
In conclusion, we provide whole transcriptome data analysis of RNA from TNBC cells treated with 3-OAβBA and BSE. The data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus and are accessible through GEO Series accession number GSE102891 located at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE10 2891.The findings reflect a high probability of ER/UPR involvement through PERK phosphorylation of eIF2α, leading to up-regulation of ATF3, 4, TRB3, DNA damage-inducible transcript 3, 4 (CHOP) and rise in immediate early response genes. Future research will be required to determine the unique controlling factors in common between natural products and the ER/ UPR programmed death events in tumor cells.
Conflicts of Interest
The Authors wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Acknowledgements
This project was supported by the National Institutes of Health, National Institute on Minority Health and Health Disparities, RCMI grant (8G12MD007582-28.) and COE grant (P20 MD006738).
References
- 1.Estrada AC, Syrovets T, Pitterle K, Lunov O, Buchele B, Schimana-Pfeifer J, Schmidt T, Morad SA, Simmet T. Tirucallic acids are novel pleckstrin homology domain-dependent Akt inhibitors inducing apoptosis in prostate cancer cells. Mol Pharmacol. 2010;77:378–387. doi: 10.1124/mol.109.060475. [DOI] [PubMed] [Google Scholar]
- 2.Yadav VR, Prasad S, Sung B, Gelovani JG, Guha S, Krishnan S, Aggarwal BB. Boswellic acid inhibits growth and metastasis of human colorectal cancer in orthotopic mouse model by down-regulating inflammatory, proliferative, invasive and angiogenic biomarkers. Int J Cancer. 2012;130:2176–2184. doi: 10.1002/ijc.26251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frank MB, Yang Q, Osban J, Azzarello JT, Saban MR, Saban R, Ashley RA, Welter JC, Fung KM, Lin HK. Frankincense oil derived from Boswellia carteri induces tumor cell specific cytotoxicity. BMC Complement Altern Med. 2009;9:6. doi: 10.1186/1472-6882-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park B, Prasad S, Yadav V, Sung B, Aggarwal BB. Boswellic acid suppresses growth and metastasis of human pancreatic tumors in an orthotopic nude mouse model through modulation of multiple targets. PLoS One. 2011;6:e26943. doi: 10.1371/journal.pone.0026943. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Ranjbarnejad T, Saidijam M, Moradkhani S, Najafi R. Methanolic extract of Boswellia serrata exhibits anti-cancer activities by targeting microsomal prostaglandin E synthase-1 in human colon cancer cells. Prostaglandins Other Lipid Mediat. 2017;131:1–8. doi: 10.1016/j.prostaglandins.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Dozmorov MG, Yang Q, Wu W, Wren J, Suhail MM, Woolley CL, Young DG, Fung KM, Lin HK. Differential effects of selective frankincense (Ru Xiang) essential oil versus non-selective sandalwood (Tan Xiang) essential oil on cultured bladder cancer cells: a microarray and bioinformatics study. Chin Med. 2014;9:18. doi: 10.1186/1749-8546-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee DH, Kim SS, Seong S, Woo CR, Han JB. A case of metastatic bladder cancer in both lungs treated with korean medicine therapy alone. Case Rep Oncol. 2014;7:534–540. doi: 10.1159/000365884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thummuri D, Jeengar MK, Shrivastava S, Areti A, Yerra VG, Yamjala S, Komirishetty P, Naidu VG, Kumar A, Sistla R. Boswellia ovalifoliolata abrogates ROS mediated NF-kappaB activation, causes apoptosis and chemosensitization in Triple Negative Breast Cancer cells. Environ Toxicol Pharmacol. 2014;38:58–70. doi: 10.1016/j.etap.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Takada Y, Ichikawa H, Badmaev V, Aggarwal BB. Acetyl-11-keto-beta-boswellic acid potentiates apoptosis, inhibits invasion, and abolishes osteoclastogenesis by suppressing NF-kappa B and NF-kappa B-regulated gene expression. J Immunol. 2006;176:3127–3140. doi: 10.4049/jimmunol.176.5.3127. [DOI] [PubMed] [Google Scholar]
- 10.Zhang YS, Xie JZ, Zhong JL, Li YY, Wang RQ, Qin YZ, Lou HX, Gao ZH, Qu XJ. Acetyl-11-keto-beta-boswellic acid (AKBA) inhibits human gastric carcinoma growth through modulation of the Wnt/beta-catenin signaling pathway. Biochim Biophys Acta. 2013;1830:3604–3615. doi: 10.1016/j.bbagen.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Liu JJ, Huang B, Hooi SC. Acetyl-keto-beta-boswellic acid inhibits cellular proliferation through a p21-dependent pathway in colon cancer cells. Br J Pharmacol. 2006;148:1099–1107. doi: 10.1038/sj.bjp.0706817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qurishi Y, Hamid A, Sharma PR, Wani ZA, Mondhe DM, Singh SK, Zargar MA, Andotra SS, Shah BA, Taneja SC, Saxena AK. NF-kappaB down-regulation and PARP cleavage by novel 3-alpha-butyryloxy-beta-boswellic acid results in cancer cell specific apoptosis and in vivo tumor regression. Anticancer Agents Med Chem. 2013;13:777–790. doi: 10.2174/1871520611313050012. [DOI] [PubMed] [Google Scholar]
- 13.Kunnumakkara AB, Nair AS, Sung B, Pandey MK, Aggarwal BB. Boswellic acid blocks signal transducers and activators of transcription 3 signaling, proliferation, and survival of multiple myeloma via the protein tyrosine phosphatase SHP-1. Mol Cancer Res. 2009;7:118–128. doi: 10.1158/1541-7786.MCR-08-0154. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Chou YC, Suh JH, Wang Y, Pahwa M, Badmaev V, Ho CT, Pan MH. Boswellia serrata resin extract alleviates azoxymethane (AOM)/dextran sodium sulfate (DSS)-induced colon tumorigenesis. Mol Nutr Food Res. 2017 doi: 10.1002/mnfr.201600984. [DOI] [PubMed] [Google Scholar]
- 15.Xue X, Chen F, Liu A, Sun D, Wu J, Kong F, Luan Y, Qu X, Wang R. Reversal of the multidrug resistance of human ileocecal adenocarcinoma cells by acetyl-11-keto-beta-boswellic acid via down-regulation of P-glycoprotein signals. Biosci Trends. 2016;10:392–399. doi: 10.5582/bst.2016.01115. [DOI] [PubMed] [Google Scholar]
- 16.Buchele B, Zugmaier W, Estrada A, Genze F, Syrovets T, Paetz C, Schneider B, Simmet T. Characterization of 3alpha-acetyl-11-keto-alpha-boswellic acid, a pentacyclic triterpenoid inducing apoptosis in vitro and in vivo. Planta Med. 2006;72:1285–1289. doi: 10.1055/s-2006-951680. [DOI] [PubMed] [Google Scholar]
- 17.Mazzio EA, Soliman KF. In vitro screening for the tumoricidal properties of international medicinal herbs. Phytother Res. 2009;23:385–398. doi: 10.1002/ptr.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yazdanpanahi N, Behbahani M, Yektaeian A. Effect of boswellia thurifera gum methanol extract on cytotoxicity and p53 gene expression in human breast cancer cell line. Iran J Pharm Res. 2014;13:719–724. [PMC free article] [PubMed] [Google Scholar]
- 19.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 21.Vlachos IS, Zagganas K, Paraskevopoulou MD, Georgakilas G, Karagkouni D, Vergoulis T, Dalamagas T, Hatzigeorgiou AG. DIANA-miRPath v3.0: deciphering microRNA function with experimental support. Nucleic Acids Res. 2015;43:W460–466. doi: 10.1093/nar/gkv403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papadopoulos GL, Alexiou P, Maragkakis M, Reczko M, Hatzigeorgiou AG. DIANA-mirPath: Integrating human and mouse microRNAs in pathways. Bioinformatics. 2009;25:1991–1993. doi: 10.1093/bioinformatics/btp299. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Qu X, Jiang L. An oasis in the desert of cancer chemotherapeutic resistance: The enlightenment from reciprocal crosstalk between signaling pathways of UPR and autophagy in cancers. Biomed Pharmacother. 2017;92:972–981. doi: 10.1016/j.biopha.2017.05.132. [DOI] [PubMed] [Google Scholar]
- 24.Papaioannou A, Chevet E. Driving cancer tumorigenesis andmetastasis through UPR signaling. Curr Top Microbiol Immunol. 2017 doi: 10.1007/82_2017_36. doi: 10.1007/82_2017_36. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Obacz J, Avril T, Le Reste PJ, Urra H, Quillien V, Hetz C, Chevet E. Endoplasmic reticulum proteostasis in glioblastoma-From molecular mechanisms to therapeutic perspectives. Sci Signal : 2017;10:pii: eaal2323. doi: 10.1126/scisignal.aal2323. [DOI] [PubMed] [Google Scholar]
- 26.Koong AC, Chauhan V, Romero-Ramirez L. Targeting XBP-1 as a novel anti-cancer strategy. Cancer Biol Ther. 2006;5:756–759. doi: 10.4161/cbt.5.7.2973. [DOI] [PubMed] [Google Scholar]
- 27.Le Mercier M, Lefranc F, Mijatovic T, Debeir O, Haibe-Kains B, Bontempi G, Decaestecker C, Kiss R, Mathieu V. Evidence of galectin-1 involvement in glioma chemoresistance. Toxicol Appl Pharmacol. 2008;229:172–183. doi: 10.1016/j.taap.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 28.Hsiao JR, Chang KC, Chen CW, Wu SY, Su IJ, Hsu MC, Jin YT, Tsai ST, Takada K, Chang Y. Endoplasmic reticulum stress triggers XBP-1-mediated up-regulation of an EBV oncoprotein in nasopharyngeal carcinoma. Cancer Res. 2009;69:4461–4467. doi: 10.1158/0008-5472.CAN-09-0277. [DOI] [PubMed] [Google Scholar]
- 29.Salaroglio IC, Panada E, Moiso E, Buondonno I, Provero P, Rubinstein M, Kopecka J, Riganti C. PERK induces resistance to cell death elicited by endoplasmic reticulum stress and chemotherapy. Mol Cancer. 2017;16:91. doi: 10.1186/s12943-017-0657-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corazzari M, Gagliardi M, Fimia GM, Piacentini M. Endoplasmic Reticulum Stress, Unfolded Protein Response, and Cancer Cell Fate. Front Oncol. 2017;7:78. doi: 10.3389/fonc.2017.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryu S, Lim W, Bazer FW, Song G. Chrysin induces death of prostate cancer cells by inducing ROS and ER stress. J Cell Physiol. 2017;232:3786–3797. doi: 10.1002/jcp.25861. [DOI] [PubMed] [Google Scholar]
- 32.Wu CF, Seo EJ, Klauck SM, Efferth T. Cryptotanshinone deregulates unfolded protein response and eukaryotic initiation factor signaling in acute lymphoblastic leukemia cells. Phytomedicine. 2016;23:174–180. doi: 10.1016/j.phymed.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 33.Shigemi Z, Manabe K, Hara N, Baba Y, Hosokawa K, Kagawa H, Watanabe T, Fujimuro M. Methylseleninic acid and sodium selenite induce severe ER stress and subsequent apoptosis through UPR activation in PEL cells. Chem Biol Interact. 2017;266:28–37. doi: 10.1016/j.cbi.2017.01.027. [DOI] [PubMed] [Google Scholar]
- 34.Jakobsen CH, Storvold GL, Bremseth H, Follestad T, Sand K, Mack M, Olsen KS, Lundemo AG, Iversen JG, Krokan HE, Schonberg SA. DHA induces ER stress and growth arrest in human colon cancer cells: associations with cholesterol and calcium homeostasis. J Lipid Res. 2008;49:2089–2100. doi: 10.1194/jlr.M700389-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Slagsvold JE, Pettersen CH, Follestad T, Krokan HE, Schonberg SA. The antiproliferative effect of EPA in HL60 cells is mediated by alterations in calcium homeostasis. Lipids. 2009;44:103–113. doi: 10.1007/s11745-008-3263-5. [DOI] [PubMed] [Google Scholar]
- 36.Tsai SF, Tao M, Ho LI, Chiou TW, Lin SZ, Su HL, Harn HJ. Isochaihulactone-induced DDIT3 causes ER stress-PERK independent apoptosis in glioblastoma multiforme cells. Oncotarget. 2017;8:4051–4061. doi: 10.18632/oncotarget.13266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piao JL, Cui ZG, Furusawa Y, Ahmed K, Rehman MU, Tabuchi Y, Kadowaki M, Kondo T. The molecular mechanisms and gene expression profiling for shikonin-induced apoptotic and necroptotic cell death in U937 cells. Chem Biol Interact. 2013;205:119–127. doi: 10.1016/j.cbi.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 38.Zhang B, Han H, Fu S, Yang P, Gu Z, Zhou Q, Cao Z. Dehydroeffusol inhibits gastric cancer cell growth and tumorigenicity by selectively inducing tumor-suppressive endoplasmic reticulum stress and a moderate apoptosis. Biochem Pharmacol. 2016;104:8–18. doi: 10.1016/j.bcp.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 39.Xu Y, Chiu JF, He QY, Chen F. Tubeimoside-1 exerts cytotoxicity in HeLa cells through mitochondrial dysfunction and endoplasmic reticulum stress pathways. J Proteome Res. 2009;8:1585–1593. doi: 10.1021/pr801001j. [DOI] [PubMed] [Google Scholar]
- 40.Rivera M, Ramos Y, Rodriguez-Valentin M, Lopez-Acevedo S, Cubano LA, Zou J, Zhang Q, Wang G, Boukli NM. Targeting multiple pro-apoptotic signaling pathways with curcumin in prostate cancer cells. PLoS One. 2017;12:e0179587. doi: 10.1371/journal.pone.0179587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ham J, Lim W, Bazer FW, Song G. Silibinin stimluates apoptosis by inducing generation of ROS and ER stress in human choriocarcinoma cells. J Cell Physiol. 2017 doi: 10.1002/jcp.26069. doi: 10.1002/jcp.26069. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 42.Zhang L, Hapon MB, Goyeneche AA, Srinivasan R, Gamarra-Luques CD, Callegari EA, Drappeau DD, Terpstra EJ, Pan B, Knapp JR, Chien J, Wang X, Eyster KM, Telleria CM. Mifepristone increases mRNA translation rate, triggers the unfolded protein response, increases autophagic flux, and kills ovarian cancer cells in combination with proteasome or lysosome inhibitors. Mol Oncol. 2016;10:1099–1117. doi: 10.1016/j.molonc.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boelens J, Lust S, Offner F, Bracke ME, Vanhoecke BW. Review. The endoplasmic reticulum: a target for new anticancer drugs. In Vivo. 2007;21:215–226. [PubMed] [Google Scholar]
- 44.Zanotto-Filho A, Dashnamoorthy R, Loranc E, de Souza LH, Moreira JC, Suresh U, Chen Y, Bishop AJ. Combined Gene Expression and RNAi Screening to Identify Alkylation Damage Survival Pathways from Fly to Human. PLoS One. 2016;11:e0153970. doi: 10.1371/journal.pone.0153970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gifford JB, Hill R. GRP78 influences chemoresistance and prognosis in cancer. Curr Drug Targets. 2017 doi: 10.2174/1389450118666170615100918. doi: 10.2174/1389450118666170615100918. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 46.Holtrup F, Bauer A, Fellenberg K, Hilger RA, Wink M, Hoheisel JD. Microarray analysis of nemorosone-induced cytotoxic effects on pancreatic cancer cells reveals activation of the unfolded protein response (UPR) Br J Pharmacol. 2011;162:1045–1059. doi: 10.1111/j.1476-5381.2010.01125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nawrocki ST, Carew JS, Pino MS, Highshaw RA, Dunner K Jr., Huang P, Abbruzzese JL, McConkey DJ. Bortezomib sensitizes pancreatic cancer cells to endoplasmic reticulum stress-mediated apoptosis. Cancer Res. 2005;65:11658–11666. doi: 10.1158/0008-5472.CAN-05-2370. [DOI] [PubMed] [Google Scholar]
- 48.Vanacker H, Vetters J, Moudombi L, Caux C, Janssens S, Michallet MC. Emerging Role of the Unfolded Protein Response in Tumor Immunosurveillance. Trends Cancer. 2017;3:491–505. doi: 10.1016/j.trecan.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 49.Ojha R, Amaravadi RK. Targeting the unfolded protein response in cancer. Pharmacol Res. 2017;120:258–266. doi: 10.1016/j.phrs.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cubillos-Ruiz JR, Bettigole SE, Glimcher LH. Tumorigenic and Immunosuppressive Effects of Endoplasmic Reticulum Stress in Cancer. Cell. 2017;168:692–706. doi: 10.1016/j.cell.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cerezo M, Rocchi S. New anti-cancer molecules targeting HSPA5/BIP to induce endoplasmic reticulum stress, autophagy and apoptosis. Autophagy. 2017;13:216–217. doi: 10.1080/15548627.2016.1246107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang J, Lee J, Liem D, Ping P. HSPA5 Gene encoding Hsp70 chaperone BiP in the endoplasmic reticulum. Gene. 2017;618:14–23. doi: 10.1016/j.gene.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.So AY, de la Fuente E, Walter P, Shuman M, Bernales S. The unfolded protein response during prostate cancer development. Cancer Metastasis Rev. 2009;28:219–223. doi: 10.1007/s10555-008-9180-5. [DOI] [PubMed] [Google Scholar]
- 54.Mohamed E, Cao Y, Rodriguez PC. Endoplasmic reticulum stress regulates tumor growth and anti-tumor immunity: a promising opportunity for cancer immunotherapy. Cancer Immunol Immunother. 2017;66:1069–1078. doi: 10.1007/s00262-017-2019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rozpedek W, Pytel D, Mucha B, Leszczynska H, Diehl JA, Majsterek I. The Role of the PERK/eIF2alpha/ATF4/CHOP Signaling Pathway in Tumor Progression During Endoplasmic Reticulum Stress. Curr Mol Med. 2016;16:533–544. doi: 10.2174/1566524016666160523143937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cho YM, Jang YS, Jang YM, Chung SM, Kim HS, Lee JH, Jeong SW, Kim IK, Kim JJ, Kim KS, Kwon OJ. Induction of unfolded protein response during neuronal induction of rat bone marrow stromal cells and mouse embryonic stem cells. Exp Mol Med. 2009;41:440–452. doi: 10.3858/emm.2009.41.6.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Farooqi AA, Li KT, Fayyaz S, Chang YT, Ismail M, Liaw CC, Yuan SS, Tang JY, Chang HW. Anticancer drugs for the modulation of endoplasmic reticulum stress and oxidative stress. Tumour Biol. 2015;36:5743–5752. doi: 10.1007/s13277-015-3797-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guichard C, Pedruzzi E, Fay M, Marie JC, Braut-Boucher F, Daniel F, Grodet A, Gougerot-Pocidalo MA, Chastre E, Kotelevets L, Lizard G, Vandewalle A, Driss F, Ogier-Denis E. Dihydroxyphenylethanol induces apoptosis by activating serine/threonine protein phosphatase PP2A and promotes the endoplasmic reticulum stress response in human colon carcinoma cells. Carcinogenesis. 2006;27:1812–1827. doi: 10.1093/carcin/bgl009. [DOI] [PubMed] [Google Scholar]
- 59.Zu K, Bihani T, Lin A, Park YM, Mori K, Ip C. Enhanced selenium effect on growth arrest by BiP/GRP78 knockdown in p53-null human prostate cancer cells. Oncogene. 2006;25:546–554. doi: 10.1038/sj.onc.1209071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Notte A, Rebucci M, Fransolet M, Roegiers E, Genin M, Tellier C, Watillon K, Fattaccioli A, Arnould T, Michiels C. Taxol-induced unfolded protein response activation in breast cancer cells exposed to hypoxia: ATF4 activation regulates autophagy and inhibits apoptosis. Int J Biochem Cell Biol. 2015;62:1–14. doi: 10.1016/j.biocel.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 61.Pierre N, Barbe C, Gilson H, Deldicque L, Raymackers JM, Francaux M. Activation of ER stress by hydrogen peroxide in C2C12 myotubes. Biochem Biophys Res Commun. 2014;450:459–463. doi: 10.1016/j.bbrc.2014.05.143. [DOI] [PubMed] [Google Scholar]
- 62.Bachhawat AK, Kaur A. Glutathione Degradation. Antioxid Redox Signal. 2017 doi: 10.1089/ars.2017.7136. doi: 10.1089/ars.2017.7136. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 63.Kaur A, Gautam R, Srivastava R, Chandel A, Kumar A, Karthikeyan S, Bachhawat AK. ChaC2, an enzyme for slow turnover of cytosolic glutathione. J Biol Chem. 2017;292:638–651. doi: 10.1074/jbc.M116.727479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crawford RR, Prescott ET, Sylvester CF, Higdon AN, Shan J, Kilberg MS, Mungrue IN. Human CHAC1 Protein degrades glutathione, and mRNA induction is regulated by the transcription factors ATF4 and ATF3 and a bipartite ATF/CRE regulatory element. J Biol Chem. 2015;290:15878–15891. doi: 10.1074/jbc.M114.635144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mungrue IN, Pagnon J, Kohannim O, Gargalovic PS, Lusis AJ. CHAC1/MGC4504 is a novel proapoptotic component of the unfolded protein response, downstream of the ATF4-ATF3-CHOP cascade. J Immunol. 2009;182:466–476. doi: 10.4049/jimmunol.182.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu Y, Hyde AS, Simpson MA, Barycki JJ. Emerging regulatory paradigms in glutathione metabolism. Adv Cancer Res. 2014;122:69–101. doi: 10.1016/B978-0-12-420117-0.00002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]