Abstract
During the past decade, H5N1 highly pathogenic avian influenza (HPAI) viruses have diversified genetically and antigenically, suggesting the need for multiple H5N1 vaccines. However, preparation of multiple vaccines from live H5N1 HPAI viruses is difficult and economically not feasible representing a challenge for pandemic preparedness. Here we evaluated a novel multi-clade recombinant H5N1 VLP design, in which H5 hemagglutinins (HA) and N1 neuraminidase (NA) derived from four distinct clades of H5N1 virus were co-localized within the VLP structure. The multi-clade H5N1 VLPs were prepared by using a recombinant baculovirus expression system and evaluated for functional hemagglutination and neuraminidase enzyme activities, particle size and morphology, as well as for the presence of baculovirus in the purified VLP preparations. To remove residual baculovirus, VLP preparations were treated with beta-propiolactone (BPL). Immunogenicity and efficacy of multi-clade H5N1 VLPs were determined in an experimental ferret H5N1 HPAI challenge model, to ascertain the effect of BPL on immunogenicity and protective efficacy against lethal challenge. Although treatment with BPL reduced immunogenicity of VLPs, all vaccinated ferrets were protected from lethal challenge with influenza A/VietNam/1203/2004 (H5N1) HPAI virus, indicating that multi-clade VLP preparations treated with BPL represent a potential approach for pandemic preparedness vaccines.
Keywords: Influenza, vaccine, H5N1, VLP, propiolactone
1. Introduction
Avian strains of influenza virus represent a threat to the U.S. and global public health. Highly pathogenic avian influenza (HPAI) viruses continue to circulate in birds and other animals worldwide. Continuing human infections with H5N1 HPAI viruses with high mortality rates suggest that the virus represents a pandemic threat [1,2]. The development of vaccines is an essential part of the global strategy for pandemic preparedness. However, multiple strains and frequent genetic changes represent challenges for the development of H5N1 vaccines [3]. H5N1 viruses have diversified genetically and antigenically since their re-emergence in 2003, leading to the need for multiple vaccines [3,4]. Prediction of pandemic virus strains is currently not possible; therefore, safe and effective vaccines for multiple H5N1 virus strains are needed. Candidate vaccine viruses for several clades of H5N1 have been identified by the WHO [5]. Monovalent vaccines have been approved for use in the US for clade 1 and clade 2.1.3.2 viruses [6,7]. However, preparation of several monovalent H5N1 vaccines is costly and technically challenging. For emergency preparedness, a broadly protective vaccine that provides immunity to several potentially pandemic H5N1 viruses would be advantageous over a monovalent vaccine because of a higher probability of antigenic match with an emerging pandemic virus. Improved antigenic match would result in a potentially better immune coverage in the case of an outbreak of novel influenza, which could save lives until generation of a homologous vaccine [8,9]. Universal experimental vaccines for influenza are being developed [10,11]. Potentially, trivalent or quadrivalent H5N1 vaccines can be prepared using current influenza vaccine technologies. A seasonal trivalent or quadrivalent inactivated vaccine represents a blend of inactivated H1N1, H3N2, and influenza B viruses [12–14]. Each virus is grown separately in eggs, inactivated, and then combined with two other strains to make a trivalent formulation [12,13]. Live attenuated influenza vaccines are also manufactured individually in eggs and then blended to produce a tri- or quadrivalent vaccine [6,15]. However, the need for individual preparations of each vaccine increases vaccine cost. In addition, large-scale propagation of live H5N1 HPAI viruses for vaccine purposes is difficult, expensive, and can raise safety concerns, thus hampering pandemic preparedness strategies. The use of eggs in vaccine production is an additional difficulty for avian influenza viruses due to difficulty of virus propagation, as well as adaptive mutations that lead to structural and antigenic changes [16].
VLPs prepared using recombinant baculovirus (rBV) have been shown to be safe and effective experimental vaccines for influenza viruses [1,17,18]. A multi-subtype VLP design, in which distinct subtypes of hemagglutinin (HA) are co-localized in the envelope of a VLP, was found to induce broad, multi-subtypic immune responses [19–22]. Beyond the HA, a protective role for neuraminidase (NA) has also been identified against H5N1 influenza viruses [23]. Here, we initiated studies employing VLPs that co-localize H5 HA proteins derived from three clades of H5N1 recommended by the WHO for H5N1 vaccine development. We also included N1 NA from a fourth clade of H5N1 virus into VLPs. Such multi-clade vaccine can potentially replace three distinct monovalent H5N1 vaccines thus improving the cost and logistics of pandemic preparedness. Multi-clade H5N1 VLPs were prepared using a rBV expression system and purified from Sf9 cell culture supernatant. VLPs were characterized for purity, antigenicity, functional activity, morphology, and presence of infectious rBV. Difficulty in purifying VLPs from rBV can result in the potential for vaccine products to contain residual amounts of live rBV, limiting the use of this application [24,25]. To remove residual rBV infectivity, an aliquot of VLPs was treated with beta propiolactone (BPL) and the effects of BPL on VLP antigenicity and functional activity were determined. Finally, immunogenicity and protective efficacy of multi- clade H5 VLPs including the effects of BPL treatment were evaluated in the experimental ferret H5N1 HPAI challenge model using two routes of vaccination.
2. Materials and Methods
2.1. Genes and recombinant plasmids
The Influenza A/VietNam/1203/2004 (A/VN, clade 1) virus H5 gene was described in previous studies [18,19,26,27]. The H5 genes from A/Egypt/3300-NAMRU3/2008 (A/Egypt, clade 2.2.1.1) and A/Hubei/1/2010 (A/Hubei, clade 2.3.2.1) viruses were codon optimized and synthesized biochemically (GenScript Corp., Piscataway, NJ) by using sequences of respective H5 proteins available from GenBank (accession #FJ226061.1 and #CY098758.1, respectively). The N1 gene was derived from A/Indonesia/5/2005 (H5N1) (A/Indonesia, clade 2.1.3.2) virus. The bovine immunodeficiency virus (BIV) retrovirus group antigen (Bgag) gene was described elsewhere [20,22]. Genes were initially cloned between BamHI-HindIII sites in the individual pFastBac1 baculovirus transfer plasmids (Thermo, Carlsbad, CA) downstream of the AcMNPV polyhedrin promoter [18]. The expression cassettes for the H5 genes, N1 gene, and Bgag gene were combined in a tandem fashion within a single pFastBac1-based transfer plasmid (Fig. 1a).
Fig. 1.
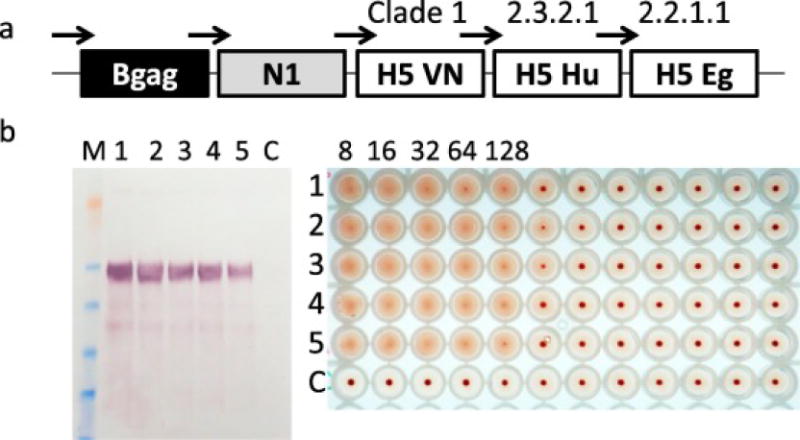
Preparation and characterization of recombinant baculovirus (rBV) for expression of multi-clade H5N1 VLPs. (a) Location and order of Bgag, N1 and H5 genes in the rBV. Polyhedrin promoters are schematically indicated with arrows. Clades of H5 genes are also shown. (b) Detection of H5 in the VLPs prepared by using passages 1-5 of rBV. Passages of rBV used for expression of VLPs are indicated by numbers 1 to 5. VLPs produced by rBV were harvested from Sf9 growth medium and probed by western blot (left panel) and HA assay with 0.5% turkey RBCs (right panel). M, protein molecular weight marker SeeBlue Plus 2 (Thermo). Western blot was done using H5-specific mouse IgG2a monoclonal antibody (H5N1) IT-003-005M6. HA titers prepared using 2-fold dilutions of VLPs are also indicated.
2.2. Preparation of recombinant baculovirus
The rBV expressing three H5, N1 and Bgag genes were generated using a Bac-to-Bac baculovirus expression system (Thermo). Briefly, rBV bacmid DNA was made by site-specific homologous recombination following transformation of pFastBac1-based transfer plasmid containing four influenza genes (clade 1 H5, clade 2.2.1.1 H5, clade 2.3.2.1 H5, N1 from H5N1 clade 2.1.3.2) and Bgag into E. coli DH10Bac competent cells, which contain the Autographa californica Multicapsid Nucleopolyhedrovirus (AcMNPV) baculovirus genome. The recombinant bacmid DNA was extracted from E. coli cells and transfected into Spodoptera frugiperda Sf9 cells (Thermo) using FuGENE 6 reagent (Promega Corp., Madison, WI). Transfected Sf9 insect cells were maintained for 72 h as adherent cultures in Sf900-II serum free medium (Thermo) at 26-28°C. The rBVs were recovered, plaque-purified, and amplified by incubating Sf9 cells with plaque eluate for 72 h to generate rBV passage 1. Titers of rBV stocks were determined by plaque assay using Sf9 cell monolayers and were expressed as plaque-forming units (PFU) per milliliter (PFU/ml).
Stability of the resulting rBV was evaluated by five 72-hr passages in Sf9 cells at a multiplicity of infection (MOI) of 0.01. After each passage, rBV samples was taken and stored at 2-8°C. The collected rBV samples from passages 1 to 5 were used to infect fresh Sf9 cells in order to express VLPs, and the expression of VLPs was evaluated as described below.
2.3. Expression of multi-clade H5N1 VLPs and analytical assays
In order to express multi-clade H5N1 VLPs, Sf9 cells at 2 × 106 cells/ml were infected at 2.L scale with rBV (passage 2) at MOI of 0.1 PFU per cell for 72 hr with rBV encoding three influenza H5 genes, as well as N1 and Bgag proteins. Influenza H5N1 VLPs were harvested from the growth medium, clarified by centrifugation followed by filtration using 0.2 micron filtration, and then purified and concentrated using combination of tangential flow filtration (TFF), ion exchange chromatography and ultracentrifugation at 100 000g. Aliquot of VLPs was treated with 0.1% BPL for 3 h on ice to remove residual live baculovirus, as described elsewhere [28,29]. BPL was removed by incubation of the VLPs at 36-38°C for 2 h.
SDS-PAGE was performed using 4-12% gradient polyacrylamide gels (Invitrogen), stained with GelCode Blue stain, and quantitated by densitometry. Western blot was carried out using monoclonal antibody anti-H5 (H5N1) IT-003-005M6 mouse IgG2a MAb, clone 268D8 (Immune Technology Corp., New York, NY).
For hemagglutination assay, serial dilutions of triple-H5 VLPs in 50 μl volumes were prepared in 96-well microtiter plates, followed by addition of 0.5% turkey erythrocytes (Lampire Biologicals, Pipersville, PA) in phosphate buffered saline (PBS) [19,30]. Plates with erythrocytes and VLPs were mixed by agitation, covered, and erythrocytes were allowed to settle for 30-60 min at 20-25°C. The HA titer was determined by visual inspection. Negative hemagglutination results appeared as compact dots in the center of the 96-well plate, and the titer was calculated as the highest dilution factor that produced a positive reading.
Neuraminidase enzyme activity was determined by using a fluorescence-based NA-Fluor assay (Thermo) in duplicates using methyl umbelliferone N-acetylneuraminic acid as a substrate according to manufacturer’s instructions. Diluent (PBS or saline) was used as a negative control. A standard curve to identify a relative fluorescence unit (RFU) value within the linear range of fluorescence detection was generated using 4-methyl umbelliferone sodium salt (Sigma, St. Louis, MO).
Negative-staining transmission electron microscopy was carried out to evaluate the size and morphology of VLPs. Briefly, VLP samples were adsorbed onto freshly discharged 400 mesh carbon parlodion-coated copper grids (Poly-Sciences, Warrington, PA). The grids were rinsed with buffer containing 20 mM Tris, pH 7.4, and 120 mM KCl and negatively stained with 1% phosphotungstic acid, then dried by aspiration. VLPs were visualized on a Hitachi H-7600 transmission electron microscope (Hitachi High Technologies America, Schaumburg, IL) operating at 80 kV and digitally captured with a CCD camera at 1K×1K resolution (Advanced Microscopy Techniques Corp., Danvers, MA).
2.4. Vaccination of ferrets and serology
All studies involving the use of animals were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at the CDC in an Association for the Assessment and Accreditation of Laboratory Animal Care accredited facility. Purified VLPs (either untreated or BPL-treated) were formulated and stored at 2–8°C in PBS. Male Fitch ferrets Mustela putorius furo (Triple F Farms, Sayre, PA), 4 to 5 months of age, 4 animals per group, were vaccinated on days 0 and 28 intramuscularly (i.m.) with either untreated or BPL-treated VLPs containing 90 μg of HA (as determined by SDS-PAGE and western blot densitometry). One additional group of 5 animals was vaccinated with BPL-treated VLPs intranasally (i.n.) Control animals (n=5) received PBS as placebo. Prior to vaccination, animals were confirmed by hemagglutination inhibition (HI) assay to be seronegative for circulating influenza A (H1N1 and H3N2) and B viruses. Serum was collected one day prior each vaccination, as well as before challenge.
Serum collected from vaccinated ferrets was tested by HI assay for the presence of influenza-specific humoral responses using recombinant influenza H5 antigens, which represented monovalent A/VN, A/Egypt and A/Hubei VLPs prepared in Sf9 cells by using baculovirus expression. HI assays were performed using 0.5% turkey red blood cells with 4 HA units of appropriate recombinant H5 antigen [27].
2.5. Challenge with H5N1 HPAI virus
For virus challenge, vaccinated and control ferrets were transferred into an ABSL3+ containment facility and challenged i.n. (1ml volume) on day 28 after boost with 106 EID50 of influenza A/VN (clade 1) virus. After challenge, survival (mortality) and weight loss (morbidity) were determined; any ferret that lost >25% of pre-inoculation weight or exhibited neurological dysfunction was euthanized. Viral replication was determined by collection and titration of nasal wash specimens on alternate days post-challenge. Nasal wash specimens were stored at −80C until titration in 10-11 day old embryonated hens eggs, and titers were calculated by the method of Reed and Muench. Statistical significance of virus titers in nasal washes was determined by Student’s t-test.
3. Results
3.1. Preparation of multi-clade H5N1 VLPs
The coding sequences of the full-length H5 genes from clades 1 (A/VN), 2.2.1.1 (A/Egypt) and 2.3.2.1 (A/Hubei), the N1 gene from clade 2.1.3.2 (A/Indonesia) and Bgag gene were assembled within the multi-gene baculovirus transfer vector plasmid propagated in E. coli (Fig. 1a). Optimization of translational codons was performed to improve and equalize expression of distinct H5 proteins in Sf9 cells. The assembled expression cassette containing all five genes was transferred into an rBV genome as described in Materials and Methods. The resulting rBV contained three H5 genes with HPAI genotype with furin cleavage sites between HA1 and HA2, as well as genes for influenza N1 and Bgag. Expression of each gene from its own transcription cassette including identical polyhedrin promoters (Fig. 1a). was designed to achieve equivalent expression of genes [19,20]. Sf9 cells were infected with the resulting rBV in serum-free medium to express H5 VLPs. After 3 days of incubation, supernatants of Sf9 cells were harvested as described in the Materials and Methods and examined for the presence of hemagglutination activity using 0.5% turkey red blood cells (RBC). Turkey RBC are recommended by the WHO for detection of avian influenza A(H5N1) virus [31]. The HA activity was confirmed in the harvested Sf9 growth medium supernatants, with approximately 1:128 titer (Fig. 1b., line 1) demonstrating functional HA activity and suggesting properly assembled tertiary and quaternary structures of the HA.
To evaluate stability of rBV for expression of VLPs, rBV was passed five times to generate rBV passages 1-5. The VLPs were prepared with each passage of rBV and quantitated by western blot and HA assay. Western bot showed that expression of HA had a tendency to reduction with multiple passages. However, HA was detected in VLPs generated by rBV from all passages (Fig. 1b). by both western blot and HA assay suggesting relative stability of VLP expression from rBV.
3.2. Purification and characterization of multi-clade VLPs
The VLPs were concentrated approximately 1,000-fold from Sf9 growth medium by TFF and purified by ion exchange chromatography and ultracentrifugation (Fig. 2). Chromatography resulted in two peaks that were identified as VLP and rBV (Fig. 2b). VLPs were purified in the flow-through fraction. After additional purification and concentration using ultracentrifugation, VLPs were analyzed for the presence of HA, NA and Bgag proteins. HA was confirmed by both HA assay (Fig. 2a)., SDS-PAGE and western blot with H5-specific antibody (Fig. 2c). After separation under reducing conditions, two bands of approximately 64 and 55 kDa were detected by SDS-PAGE. The 64 kDa band corresponds in size to the uncleaved HA0 form of influenza HA, which is consistent with previous observations [32]. Proteolytic cleavage of the translation product HA0 into HA1 and HA2 represents a natural and important event for viral activity [33]. However, this processing step does not occur in Sf9 cells and does not prevent VLP budding or their functional activity [18]. The H5 HA0 proteins from three clades have similar molecular weights and were migrating as a single diffuse band on SDS-PAGE. The HA0 band reacted with H5-specific antibody by western blot (Fig. 2c., lane 2). Quantitation of each clade was not possible as H5 antibody reacted with each of three clades of H5. However, the H5 gene sequences in rBV were confirmed by PCR (data not shown). A band of approximately 55 kDa corresponding to Bgag protein was also observed in stained SDS-PAGE, which was consistent with the predicted molecular weight of Bgag protein (Fig. 2c., lane 1). Additional minor bands of approximately 40-42 kDa were detected, likely resulting from the proteolytic processing of HA0 or indicative of cell- or rBV-derived impurities.
Fig. 2.
Preparation and characterization of multi-clade H5N1 VLPs from 2 L culture of Sf9 cells. (a) HA assay of Sf9 culture supernatant. Dilutions of VLPs are indicated. (b) Ion exchange chromatography profile of purification of VLPs. Location of VLP and rBV peaks are shown. (c) SDS-PAGE staining (left panel, lane 1) and western blot of purified VLPs (right panel, lanes 2-5). Western blot was done using H5-specific mouse IgG2a monoclonal antibody (H5N1) IT-003- 005M6. Lanes 1 and 2, purified VLPs; lanes 3 and 4 negative control; lane 5, protein molecular weight marker SeeBlue Plus 2. Positions of H5 HA0 and of Bgag proteins are indicated.
Expression of N1 was confirmed by functional neuraminidase enzyme assay suggesting correct NA protein folding (Fig. 3a). Furthermore, VLPs were characterized by electron microscopy observation of purified preparations. By transmission negative-staining electron microscopy, the VLPs were identified as largely spherical, influenza-like enveloped particles approximately 120-200 nm in diameter with characteristic HA spikes closely resembling those of the influenza virions protruding from the VLP phospholipid membrane envelope (Fig. 3b., left panel).
Fig. 3.
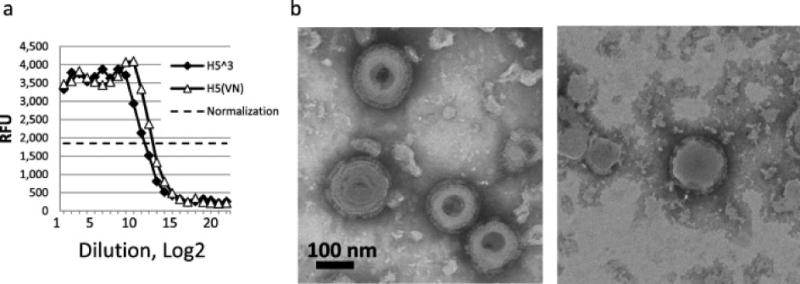
Neuraminidase (NA) enzyme assay (a) and negative stain transmission electron microscopy (b) of purified VLPs. (a) NA enzyme activity was determined in duplicates by using a fluorescence-based NA-Fluor assay (Thermo) with methyl umbelliferone N-acetylneuraminic acid as a substrate. Multi-clade H5N1 VLPs are shown with solid diamonds. As a control, A/VN monovalent VLPs were used (open triangles).Normalization line is indicated with dotted line. (b) For electron microscopy, VLPs were examined untreated (left panel) or treated with 0.1% BPL for 3 h on ice (right panel). VLPs were negatively stained with 1% phosphotungstic acid and visualized on a Hitachi H-7600 transmission electron microscope. Bar, 100 nm.
SDS-PAGE showed no significant presence of rBV proteins suggesting efficient VLP purification procedure (Fig. 2c., lane 1). To detect if purified VLPs contained detectable residual infectious rBV, a plaque assay was performed with purified concentrated VLP. As shown in Table 1. VLP preparations contained considerable amount of residual infectious rBV exceeding 106 PFU/ml (Table 1.), although no considerable amount of rBV proteins was detected by SDS-PAGE.
Table 1.
BPL treatment of H5 VLPs
VLPs were purified from Sf9 culture supernatant using combination of TFF, ion exchange chromatography and ultracentrifugation. HA activity was determined using 0.5% turkey RBCs. Infectivity was determined by plaque assay in Sf9 cell monolayers (10 PFU/ml limit of detection).
Purified VLPs were treated with 0.1% BPL for 3 h on ice, then incubated for 2 h at 37°C to remove BPL.
3.3. Removal of rBV infectivity using BPL
Because residual rBV was detected in the VLP preparation, we investigated if preparations of VLPs containing rBV could be treated with an inactivating agent such as BPL to remove rBV infectivity while maintaining functional HA activity and morphology of VLPs. Treatment with BPL resulted in removal of infectivity, as no detectable plaques were detected in Sf9 cell monolayers after VLP preparations were treated with BPL. However, HA activity with turkey RBCs was reduced only 2-fold after BPL treatment (Table 1). Enveloped VLPs resembling influenza virus were detectable by negative stain electron microscopy (Fig. 3b., right panel). These results indicate that BPL represents an appropriate means to confirm safety of VLP preparations for in vivo use.
3.4. Immunogenicity of multi-clade H5N1 VLPs
Ferret vaccinations were conducted using two doses of 90 μg, similar to the stockpiled clade 1 H5N1 vaccine, except the dose of multi-clade H5 VLPs contained proteins from three clades of H5N1 virus. For vaccinations, purified VLPs were either left untreated or treated with BPL, as BPL treatment can result in alteration of proteins that potentially can affect immunogenicity [34]. Group 1 was vaccinated i.m. with purified untreated H5 VLPs, while groups 2 and 3 received BPL-treated VLPs via i.m. and i.n. routes, respectively (Table 2). Control group 4 received PBS as placebo. Both i.m. and i.n. routes were employed to mimic seasonal influenza virus vaccination with current inactivated (such as Fluzone) or live attenuated (FluMist) preparations, respectively. All animals received prime and boost vaccinations 28 days apart, and were challenged with H5 A/VN HPAI virus 28 days after boost vaccination. No adverse reactions to vaccinations were observed following vaccination with VLPs. Antibody levels against the vaccine were determined by HI assay before booster vaccination and before challenge. For the HI assay, we used recombinant H5 antigens corresponding to A/VN and A/Hubei. The antigen corresponding to A/Egypt showed poor hemagglutination with RBC from turkey, chicken, goose, guinea pig, sheep, or horse, so was not included in the HI analysis. As shown in Table 2. the highest titers of HI antibody to A/VN and to A/Hubei were observed in group 1 that were vaccinated i.m. with BPL-untreated H5 VLPs. In this group, HI titers to both A/VN and A/Hubei increased 2-4 times after the boost (Table 2). In contrast, ferrets in groups 2 and 3 that were vaccinated with BPL-treated VLPs, HI titers to A/VN or A/Hubei antigens were low and showed minimal, if any, booster effect. Low immunogenicity of BPL treated VLPs were observed in both i.m. (group 2) and i.n. (group 3) vaccinated animals. In the latter group, several animals did not seroconvert as determined by HI assay (Table 2). In the group 3 animals that received i.n. vaccination of BPL-treated VLPs, the HI titers to A/VN virus were higher as compared to HI titers to A/Hubei virus suggesting that immune response may depend on the particular strain of vaccine antigen or the route of vaccination. As expected, control animals (group 4) did not show any detectable HI titers to either A/VN or A/Hubei antigens. These results suggested that treatment with BPL can result in lower immunogenicity of VLPs and lower immune responses in ferrets, especially following i.n. delivery of vaccine.
Table 2.
Immunogenicity of multi-clade H5 VLPs by HI assay.
| Anti-A/VN | Anti-A/Hubei | |||
|---|---|---|---|---|
|
|
||||
| pre-boost | post-boost | pre-boost | post-boost | |
| Gr1. H5 VLP i.m. | 4/4 (40-80) | 4/4 (80-320) | 4/4 (20-80) | 4/4 (80-320) |
| Gr2. H5 VLP-BPL i.m. | 4/4 (10-40) | 4/4 (10-40) | 1/4 (<10-20 | ) 4/4 (10-80) |
| Gr3. H5 VLP-BPL i.n. | 4/5 (<10-40) | 4/5 (<10-10) | 2/5 (<10-20 | ) 1/5 (<10-20) |
| Gr4. PBS | 0/5 (<10) | 0/5 (<10) | 0/5 (<10) | 0/5 (<10) |
HI assay was performed using 0.5% turkey red blood cells with 4 HA units of recombinant H5 antigen A/VN and A/Hubei. Ratio of seroconverted/total animals is shown. Range of HI titers in individual ferrets is shown in parentheses.
3.5. Protective efficacy of multi-clade H5N1 VLPs by using H5N1 HPAI challenge
To determine if variable immunogenicity of VLPs treated with BLP affected in vivo protection, multi-clade H5N1 VLPs were tested either in BPL-treated or untreated formulations for the ability to protect animals against fatal H5 virus challenge. Four weeks after the booster vaccination with H5N1 VLPs, all animals were challenged with A/VN HPAI virus, which was homologous to A/VN H5 antigens included into multi-clade H5N1 VLP vaccine while heterologous in relation to H5 from A/Hubei, H5 from A/Egypt, and N1 from A/Indonesia. A/VN virus causes severe clinical symptoms and lethal disease in ferrets [26]. Vaccine-mediated protection from challenge was measured by overall survival, as well as morbidity as measured by reduction of viral titer in nasal washes following challenge (Fig. 4). and changes in body weight (Fig. 5). On day 2 post-challenge, viral titers were comparable in all groups including controls, while on day 4 after challenge, animals vaccinated via the i.m. route, including BPL-treated vaccine, showed a sharp reduction in viral titers (Fig. 4). On days 6 and 8, all vaccinated animals had low virus titers including in the i.n. group, while control animals still had high titers of replicating virus. Interestingly, animals that received i.n. vaccination of BPL-treated VLPs, cleared the virus faster as compared to i.m. vaccinated animals with the same BPL-treated VLPs (Fig. 3). This may suggest local immune response such as IgA following i.n. administration, although additional studies are needed.
Fig. 4.
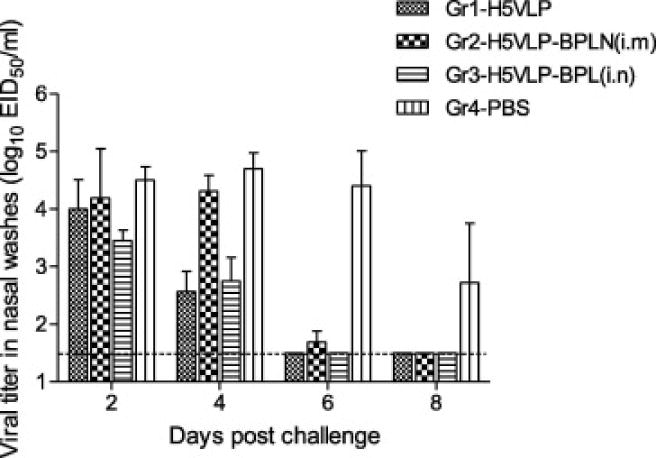
Virus titers in nasal washes of ferrets following H5N1 virus challenge. Ferrets were vaccinated on days 0 and 28 intramuscularly (i.m.) with untreated (Gr1) or BPL-treated (Gr2) VLPs. One group (Gr3) was vaccinated with BPL-treated VLPs intranasally (i.n.) Control animals (Gr4) received PBS as placebo. Ferrets were challenged i.n. on day 56 (28 days after boost) with 106 EID50 of influenza A/VN (clade 1) virus, and nasal wash titers are reported. Limit of detection, 101.5 EID50/ml. Statistical significance (P<0.05) was detected for all vaccinated groups as determined by Student’s t-test.
Fig. 5.
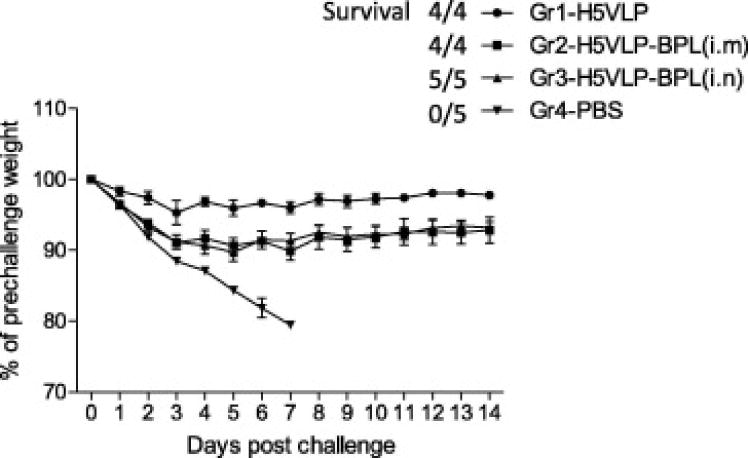
Weight loss in ferrets after H5N1 challenge. Vaccination groups Gr1-Gr4 are indicated in the legend to Fig. 4. Ferrets were challenged i.n. on day 28 after boost with 106 EID50 of influenza A/VN (clade 1) virus. Weight of animals was determined daily. Error bars indicate standard deviations. Survival (survived/total ratio) of animals is indicated.
Animals in group 1 that was vaccinated with H5 VLPs with no BPL treatment showed a minimal weight loss (Fig. 5). In contrast, ferrets vaccinated i.m (group 2) or i.n. (group 3) with BPL-treated VLPs displayed up to 10% weight loss. These results were consistent with virus titration experiments (Fig. 4). and suggest that BPL treatment affects immunogenicity of VLPs or reduces adjuvant effects of live rBV that is present in VLP preparations. All animals in vaccine groups 1, 2, and 3 survived challenge including i.n. vaccinated animals that did not seroconvert as detected by HI assay. In contrast, all control placebo-vaccinated animals showed 100% mortality and died by day 7 post challenge. These data indicate a protective effect of vaccination with either BPL-treated or BPL-untreated multi-clade H5N1 VLP vaccine against A/VN HPAI virus challenge.
4. Discussion
Broad protection against multiple strains of influenza is a desirable feature for influenza vaccines, especially for pre-pandemic strains, because reliable prediction of the future pandemic strain is currently not possible [35]. An ideal vaccine to counter H5N1 influenza viruses should provide efficient protection against multiple strains including newly emerging variants and should not depend on manufacturing in eggs because of poor growth of avian viruses and adaptive mutations [1,12,36,37]. Eggs are used for manufacturing other vaccines such as yellow fever 17D, measles and mumps vaccines [38,39] and the supply of eggs used for vaccine manufacturing could be limited, especially in the case of an outbreak of HPAI or other agricultural disease that affects chicken flocks [27]. The first non-egg influenza vaccines, Flucelvax and FluBlok, have been approved by the FDA [40,41]. Several promising approaches are being tested that allow induction of broad immunity to highly conserved or “consensus” viral protein regions [42–44]. However, additional data are needed to establish safety, immunogenicity and efficacy of these approaches.
Recombinant VLPs represent a proven experimental strategy for prevention of influenza including HPAI [1,17,18,45]. VLPs have advantages in safety, efficacy, and manufacturing because they circumvent problems like slow virus growth, unpredictable yields, and host-adaptive mutations in eggs. Previous studies have shown that VLPs induce broader immunity against divergent strains than standard influenza vaccines, especially if administered mucosally [20,27,32,46]. Furthermore, we have previously described that multi-subtype VLP designs, in which several distinct subtypes of HA are co-localized within the same VLP structure, can confer protection in vivo against multiple challenge viruses [19,20,30,47]. Co-localization of HA subtypes within VLPs was confirmed by immunoprecipitation and immunoelectron microscopy experiments [19,22]. Another previous study showed that VLPs co-localizing H5, H7, H9 and H10 subtypes elicited subtype-specific immune responses, with no HI antibody detected to H1 or H3 viruses [20]. Detection of HI antibodies to H5, H7, H9 and H10 antigens suggested expression of all four subtypes from the quadri-HA gene expression cassette, with no detectable gradient of expression related to the gene location within the rBV [20,22]. In the same previous study, H5/H7/H9/H10 VLPs and monovalent H10 VLPs elicited comparable levels of protection from experimental challenge with H10 virus [20]. Multi-subtype vaccines have the advantage of inducing broad-range, yet traditional virus-ne utralizing immune responses directed against several influenza subtypes. This protection is likely due to the presence of HA molecules derived from distinct strains or subtypes. In addition, VLPs that contained NA without HA also provided protection against the homologous challenge [23].
The presence of residual infectious rBV, which is often present in the antigens prepared using baculovirus expression systems, may provide an adjuvant effect [25]. However, rBV is capable of DNA transfer to human cells and is used in gene therapy applications [48,49]. Therefore this residual rBV represents a contaminant in vaccine products and may need to be removed by using purification or inactivation strategies. BPL is often used for preparation of inactivated virus vaccines [29,50]. However, it has been reported that BPL causes modifications of viral proteins that can potentially affect protein function [34]. There are limited data available on the effects of BPL treatment on rBV inactivation and immunogenicity and protective efficacy of VLPs.
Here we initiated studies towards the development of broadly protective, multi-clade H5N1 vaccine by studying the effects of BPL treatment on rBV inactivation, as well as on immunogenicity and efficacy of H5 multi-clade VLPs. Here we report preparation of multi-clade H5 VLPs that co-localize three clades of H5 proteins, as well as N1 from the fourth clade of the H5N1 virus. In the current study, three distinct H5 proteins were derived from HPAI H5N1 viruses A/VietNam/1203/2004, A/Egypt/3300-NAMRU3/2008, and A/Hubei/1/2010, all recommended by the WHO for H5N1 vaccine development [51]. VLPs also included N1 derived from A/Indonesia/5/2005 that has been reported to provide protection from homologous H5N1 virus [23]. VLPs also included BIV retrovirus Bgag as a structural component of VLPs [22]. H5 proteins that were expressed in VLPs had 92-94% homology, with clade-specific mutations mostly within the fusion domain of H1 fragment of HA [52], suggesting that multi-clade H5N1 VLPs may result in a broadly protective H5N1 vaccine. For vaccinations, VLPs were purified and administered to ferrets with or without BPL treatment. We found that VLPs with no BPL treatment were immunogenic and induced HI responses to A/VN and A/Hubei antigens. Immunogenicity to A/Egypt antigen was not evaluated due to poor binding to RBC. However, induction of HI antibody to all HA components and protection against multiple viruses with the multi-HA VLPs has been shown in the previous studies [19,20,30]. Protection against A/VN challenge was likely mediated by the presence of homologous A/VN H5 HA in the VLP formulation, as other H5 proteins and N1 were heterologous respective to challenge virus. However, potential contribution of H5 and N1 proteins from heterologous clades of H5N1 virus to immunogenicity and protection from A/VN challenge is not excluded. The dose of 90 μg of HA was similar to that of the stockpiled clade 1 A/VN H5N1 vaccine; however, assuming equivalent expression of H5 clades in multi-clade VLPs, the administered dose of A/VN to ferrets was 30 μg. BPL-treated VLPs showed reduced immunogenicity in comparison with BPL-untreated VLPs. However, all animals survived challenge despite weight loss in BPL-treated groups. Notably, groups 1 and 3 showed differences in weight loss but did not show substantial differences in viral titers suggesting potential involvement of additional immune mechanisms such as mucosal immunity or cytokines. The results suggest that BPL treatment does not preclude elicitation of protective immunity and that development of a single-dose VLP vaccination regimen for human immunization may be possible. In addition, virus neutralization assays using reassortant H5N1 viruses can provide additional immunological data.
It was previously shown that i.n. vaccination resulted in broader protection against heterologous challenge, which can potentially be associated with local immune response at the site of vaccine administration [20,32,46]. Therefore, we tested two routes of VLP administration for BPL-treated VLPs, i.m. and i.n. Interestingly, i.n. immunization with BPL-treated experimental vaccine resulted in faster clearance of the virus, with all animals protected from lethal challenge with A/VN virus. One conclusion of this study is that in the cases when removal of rBV is required, the effects of inactivation procedures on VLP vaccine need to be carefully evaluated. The reason for reduction of immunogenicity is not clear. Possible explanations include potential alteration of immunogenic influenza epitopes [34], as well as possible interference with the adjuvant effect associated with live rBV [25]. Potentially, reduction of BPL concentration can result in improvement of VLP immunogenicity. The effects of other inactivating agents, such as formalin, on HA and rBV within multi-clade H5N1 VLPs also remains to be studied.
Taken together, the data suggest that BPL treatment of purified multi-subtype VLPs reduces immunogenicity of VLPs, while still protecting the animals following vaccination by either i.m. or i.n. delivery. Further studies are warranted to examine the protective efficacy of this vaccine preparation against other strains of H5 virus, including challenges with heterologous H5 influenza strains.
Acknowledgments
We thank Alex Tibbens and Raphael Prather for technical assistance. This research was supported in part by the NIH NIAD grant R01AI111532. The findings and conclusions in this report are those of the authors and do not necessarily reflect the views of the NIH or Centers for Disease Control and Prevention/ATSDR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kang SM, Pushko P, Bright RA, Smith G, Compans RW. Influenza virus-like particles as pandemic vaccines. Curr Top Microbiol Immunol. 2009;333:269–89. doi: 10.1007/978-3-540-92165-3_14. [DOI] [PubMed] [Google Scholar]
- 2.Creanga A, Hang NLK, Cuong VD, Nguyen HT, Phuong HVM, Thanh LT, et al. Highly Pathogenic Avian Influenza A(H5N1) Viruses at the Animal-Human Interface in Vietnam, 2003-2010. J Infect Dis. 2017 Sep 15;216(suppl_4):S529–S38. doi: 10.1093/infdis/jix003. [DOI] [PubMed] [Google Scholar]
- 3.Belser JA, Johnson A, Pulit-Penaloza JA, Pappas C, Pearce MB, Tzeng WP, et al. Pathogenicity testing of influenza candidate vaccine viruses in the ferret model. Virology. 2017 Nov;511:135–41. doi: 10.1016/j.virol.2017.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antigenic and genetic characteristics of zoonotic influenza viruses and development of candidate vaccine viruses for pandemic preparedness. Releve epidemiologique hebdomadaire/Section d’hygiene du Secretariat de la Societe des Nations = Weekly epidemiological record/Health Section of the Secretariat of the League of Nations. 2012 Mar 16;87(11):97–108. [PubMed] [Google Scholar]
- 5.WHO. Antigenic genetic characteristics of A(H5N1), A(H7N3), A(H9N2) and variant influenza viruses and candidate vaccine viruses developed for potential use in human vaccines. 2013 [Google Scholar]
- 6.Baz M, Luke CJ, Cheng X, Jin H, Subbarao K. H5N1 vaccines in humans. Virus research. 2013 May 28; doi: 10.1016/j.virusres.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.FDA. Vaccines Licensed for Use in the United States. 2017 [cited October 24, 2017] Available from: https://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm093833.htm.
- 8.Oxford JS, Lambkin R, Elliot A, Daniels R, Sefton A, Gill D. Scientific lessons from the first influenza pandemic of the 20th century. Vaccine. 2006 Nov 10;24(44–46):6742–6. doi: 10.1016/j.vaccine.2006.05.101. [DOI] [PubMed] [Google Scholar]
- 9.Levine MZ, Holiday C, Liu F, Jefferson S, Gillis E, Bellamy AR, et al. Cross-Reactive Antibody Responses to Novel H5Nx Influenza Viruses Following Homologous and Heterologous Prime-Boost Vaccination with a Prepandemic Stockpiled A(H5N1) Vaccine in Humans. J Infect Dis. 2017 Sep 15;216(suppl_4):S555–S9. doi: 10.1093/infdis/jix001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Vries RD, Altenburg AF, Rimmelzwaan GF. Universal influenza vaccines: a realistic option? Clin Microbiol Infect. 2016 Dec 01;22(Suppl 5):S120–S4. doi: 10.1016/j.cmi.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Nachbagauer R, Krammer F. Universal influenza virus vaccines and therapeutic antibodies. Clin Microbiol Infect. 2017 Apr;23(4):222–8. doi: 10.1016/j.cmi.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palese P. Making better influenza virus vaccines? Emerg Infect Dis. 2006 Jan;12(1):61–5. doi: 10.3201/eid1201.051043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pushko P, RB T, Tumpey G. Smith Engineering Better Influenza Vaccines: Traditional and New Approaches. In: Khudyakov YE, editor. Medicinal Protein Engineering. Boca Raton: CRC Press; 2009. pp. 169–204. 2009. [Google Scholar]
- 14.Ray R, Dos Santos G, Buck PO, Claeys C, Matias G, Innis BL, et al. A review of the value of quadrivalent influenza vaccines and their potential contribution to influenza control. Hum Vaccin Immunother. 2017 Jul 03;13(7):1640–52. doi: 10.1080/21645515.2017.1313375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin H, Subbarao K. Live attenuated influenza vaccine. Curr Top Microbiol Immunol. 2015;386:181–204. doi: 10.1007/82_2014_410. [DOI] [PubMed] [Google Scholar]
- 16.Wu NC, Zost SJ, Thompson AJ, Oyen D, Nycholat CM, McBride R, et al. A structural explanation for the low effectiveness of the seasonal influenza H3N2 vaccine. PLoS Pathog. 2017 Oct 23;13(10):e1006682. doi: 10.1371/journal.ppat.1006682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pushko P, Pumpens P, Grens E. Development of virus-like particle technology from small highly symmetric to large complex virus-like particle structures. Intervirology. 2013;56(3):141–65. doi: 10.1159/000346773. [DOI] [PubMed] [Google Scholar]
- 18.Pushko P, Tumpey TM, Bu F, Knell J, Robinson R, Smith G. Influenza virus-like particles comprised of the HA, NA, and M1 proteins of H9N2 influenza virus induce protective immune responses in BALB/c mice. Vaccine. 2005 Dec 30;23(50):5751–9. doi: 10.1016/j.vaccine.2005.07.098. [DOI] [PubMed] [Google Scholar]
- 19.Pushko P, Pearce MB, Ahmad A, Tretyakova I, Smith G, Belser JA, et al. Influenza virus-like particle can accommodate multiple subtypes of hemagglutinin and protect from multiple influenza types and subtypes. Vaccine. 2011 Aug 11;29(35):5911–8. doi: 10.1016/j.vaccine.2011.06.068. [DOI] [PubMed] [Google Scholar]
- 20.Pushko P, Sun X, Tretyakova I, Hidajat R, Pulit-Penaloza JA, Belser JA, et al. Mono- and quadri-subtype virus-like particles (VLPs) containing H10 subtype elicit protective immunity to H10 influenza in a ferret challenge model. Vaccine. 2016 Sep 20;34(44):5235–42. doi: 10.1016/j.vaccine.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pushko P, Tretyakova I, Hidajat R, Zsak A, Chrzastek K, Tumpey TM, et al. Virus-like particles displaying H5, H7, H9 hemagglutinins and N1 neuraminidase elicit protective immunity to heterologous avian influenza viruses in chickens. Virology. 2017 Jan 15;501:176–82. doi: 10.1016/j.virol.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tretyakova I, Hidajat R, Hamilton G, Horn N, Nickols B, Prather RO, et al. Preparation of quadri-subtype influenza virus-like particles using bovine immunodeficiency virus gag protein. Virology. 2016 Jan;487:163–71. doi: 10.1016/j.virol.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith GE, Sun X, Bai Y, Liu YV, Massare MJ, Pearce MB, et al. Neuraminidase-based recombinant virus-like particles protect against lethal avian influenza A(H5N1) virus infection in ferrets. Virology. 2017 Sep;509:90–7. doi: 10.1016/j.virol.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carvalho SB, Freire JM, Moleirinho MG, Monteiro F, Gaspar D, Castanho MA, et al. Bioorthogonal Strategy for Bioprocessing of Specific-Site-Functionalized Enveloped Influenza-Virus-Like Particles. Bioconjug Chem. 2016 Oct 07; doi: 10.1021/acs.bioconjchem.6b00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heinimaki S, Tamminen K, Malm M, Vesikari T, Blazevic V. Live baculovirus acts as a strong B and T cell adjuvant for monomeric and oligomeric protein antigens. Virology. 2017 Nov;511:114–22. doi: 10.1016/j.virol.2017.08.023. [DOI] [PubMed] [Google Scholar]
- 26.Bright RA, Carter DM, Crevar CJ, Toapanta FR, Steckbeck JD, Cole KS, et al. Cross-clade protective immune responses to influenza viruses with H5N1 HA and NA elicited by an influenza virus-like particle. PLoS One. 2008;3(1):e1501. doi: 10.1371/journal.pone.0001501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bright RA, Carter DM, Daniluk S, Toapanta FR, Ahmad A, Gavrilov V, et al. Influenza virus-like particles elicit broader immune responses than whole virion inactivated influenza virus or recombinant hemagglutinin. Vaccine. 2007 May 10;25(19):3871–8. doi: 10.1016/j.vaccine.2007.01.106. [DOI] [PubMed] [Google Scholar]
- 28.Sasaki Y, Yoshino N, Sato S, Muraki Y. Analysis of the beta-propiolactone sensitivity and optimization of inactivation methods for human influenza H3N2 virus. J Virol Methods. 2016 Sep;235:105–11. doi: 10.1016/j.jviromet.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 29.Pawar SD, Murtadak VB, Kale SD, Shinde PV, Parkhi SS. Evaluation of different inactivation methods for high and low pathogenic avian influenza viruses in egg-fluids for antigen preparation. J Virol Methods. 2015 Sep 15;222:28–33. doi: 10.1016/j.jviromet.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 30.Tretyakova I, Pearce MB, Florese R, Tumpey TM, Pushko P. Intranasal vaccination with H5, H7 and H9 hemagglutinins co-localized in a virus-like particle protects ferrets from multiple avian influenza viruses. Virology. 2013 Jul 20;442(1):67–73. doi: 10.1016/j.virol.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 31.WHO. Recommendations laboratory procedures for detection of avian influenza A(H5N1) virus in specimens from suspected human cases. 2007 [cited October 24, 2017] Available from: http://www.who.int/influenza/resources/documents/RecAIlabtestsAug07.pdf.
- 32.Perrone LA, Ahmad A, Veguilla V, Lu X, Smith G, Katz JM, et al. Intranasal vaccination with 1918 influenza virus-like particles protects mice and ferrets from lethal 1918 and H5N1 influenza virus challenge. J Virol. 2009 Jun;83(11):5726–34. doi: 10.1128/JVI.00207-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nayak DP, Hui EK, Barman S. Assembly and budding of influenza virus. Virus Res. 2004 Dec;106(2):147–65. doi: 10.1016/j.virusres.2004.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.She YM, Cheng K, Farnsworth A, Li X, Cyr TD. Surface modifications of influenza proteins upon virus inactivation by beta-propiolactone. Proteomics. 2013 Dec;13(23–24):3537–47. doi: 10.1002/pmic.201300096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morse SS, Mazet JA, Woolhouse M, Parrish CR, Carroll D, Karesh WB, et al. Prediction and prevention of the next pandemic zoonosis. Lancet. 2012 Dec 01;380(9857):1956–65. doi: 10.1016/S0140-6736(12)61684-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cox MM. Cell-based protein vaccines for influenza. Current opinion in molecular therapeutics. 2005 Feb;7(1):24–9. [PubMed] [Google Scholar]
- 37.Quan FS, Vunnava A, Compans RW, Kang SM. Virus-like particle vaccine protects against 2009 H1N1 pandemic influenza virus in mice. PloS one. 5(2):e9161. doi: 10.1371/journal.pone.0009161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Betakova T, Svetlikova D, Gocnik M. Overview of measles and mumps vaccine: origin, present, and future of vaccine production. Acta Virol. 2013;57(2):91–6. doi: 10.4149/av_2013_02_91. [DOI] [PubMed] [Google Scholar]
- 39.Graham BS. Advances in antiviral vaccine development. Immunol Rev. 2013 Sep;255(1):230–42. doi: 10.1111/imr.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cox MM, Izikson R, Post P, Dunkle L. Safety, efficacy, and immunogenicity of Flublok in the prevention of seasonal influenza in adults. Ther Adv Vaccines. 2015 Jul;3(4):97–108. doi: 10.1177/2051013615595595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manini I, Domnich A, Amicizia D, Rossi S, Pozzi T, Gasparini R, et al. Flucelvax (Optaflu) for seasonal influenza. Expert Rev Vaccines. 2015 Jun;14(6):789–804. doi: 10.1586/14760584.2015.1039520. [DOI] [PubMed] [Google Scholar]
- 42.Schotsaert M, De Filette M, Fiers W, Saelens X. Universal M2 ectodomain-based influenza A vaccines: preclinical and clinical developments. Expert review of vaccines. 2009 Apr;8(4):499–508. doi: 10.1586/erv.09.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang TT, Palese P. Universal epitopes of influenza virus hemagglutinins? Nature structural & molecular biology. 2009 Mar;16(3):233–4. doi: 10.1038/nsmb.1574. [DOI] [PubMed] [Google Scholar]
- 44.Wei CJ, Boyington JC, McTamney PM, Kong WP, Pearce MB, Xu L, et al. Induction of Broadly Neutralizing H1N1 Influenza Antibodies by Vaccination. Science (New York, NY) Jul 15; doi: 10.1126/science.1192517. [DOI] [PubMed] [Google Scholar]
- 45.Tang XC, Lu HR, Ross TM. Baculovirus-produced influenza virus-like particles in mammalian cells protect mice from lethal influenza challenge. Viral Immunol. 2011 Aug;24(4):311–9. doi: 10.1089/vim.2011.0016. [DOI] [PubMed] [Google Scholar]
- 46.Schwartzman LM, Cathcart AL, Pujanauski LM, Qi L, Kash JC, Taubenberger JK. An Intranasal Virus-Like Particle Vaccine Broadly Protects Mice from Multiple Subtypes of Influenza A Virus. MBio. 2015;6(4):e01044. doi: 10.1128/mBio.01044-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kapczynski DR, Tumpey TM, Hidajat R, Zsak A, Chrzastek K, Tretyakova I, et al. Vaccination with virus-like particles containing H5 antigens from three H5N1 clades protects chickens from H5N1 and H5N8 influenza viruses. Vaccine. 2016 Mar 18;34(13):1575–81. doi: 10.1016/j.vaccine.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Airenne KJ, Hu YC, Kost TA, Smith RH, Kotin RM, Ono C, et al. Baculovirus: an insect-derived vector for diverse gene transfer applications. Mol Ther. 2013 Apr;21(4):739–49. doi: 10.1038/mt.2012.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kost TA, Kemp CW. Fundamentals of Baculovirus Expression and Applications. Adv Exp Med Biol. 2016;896:187–97. doi: 10.1007/978-3-319-27216-0_12. [DOI] [PubMed] [Google Scholar]
- 50.Roberts A, Lamirande EW, Vogel L, Baras B, Goossens G, Knott I, et al. Immunogenicity and protective efficacy in mice and hamsters of a beta-propiolactone inactivated whole virus SARS-CoV vaccine. Viral Immunol. 2010 Oct;23(5):509–19. doi: 10.1089/vim.2010.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.WHO. Antigenic genetic characteristics of influenza A(H5N1) and influenza A(H9N2) viruses and candidate vaccine viruses developed for potential use in human vaccines. 2012 [cited; Available from: http://www.who.int/influenza/vaccines/virus/characteristics_virus_vaccines/en/index.html.
- 52.Thoennes S, Li ZN, Lee BJ, Langley WA, Skehel JJ, Russell RJ, et al. Analysis of residues near the fusion peptide in the influenza hemagglutinin structure for roles in triggering membrane fusion. Virology. 2008 Jan 20;370(2):403–14. doi: 10.1016/j.virol.2007.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]


