SUMMARY
Preclinical work has long focused on male animals, though biological sex clearly influences risk for certain diseases, including many psychiatric disorders. Such disorders are often treated by drugs targeting the CNS norepinephrine system. Despite roles for noradrenergic neurons in behavior and neuropsychiatric disease models, their molecular characterization has lagged. We profiled mouse noradrenergic neurons in vivo, defining over 3,000 high-confidence transcripts expressed therein, including druggable receptors. We uncovered remarkable sex differences in gene expression, including elevated expression of the EP3 receptor in females—which we leverage to illustrate the behavioral and pharmacologic relevance of these findings—and of Slc6a15 and Lin28b, both major depressive disorder (MDD)-associated genes. Broadly, we present a means of transcriptionally profiling locus coeruleus under baseline and experimental conditions. Our findings underscore the need for preclinical work to include both sexes and suggest that sex differences in noradrenergic neurons may underlie behavioral differences relevant to disease.
Graphical abstract
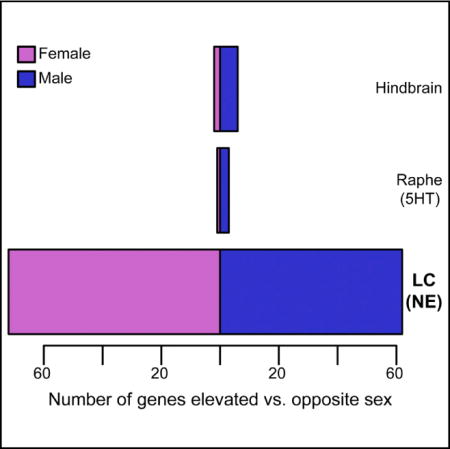
In Brief: Mulvey et al. present gene expression data from adult mouse norepinephrine neurons of the locus coeruleus (LC). They discover that over 100 genes are sex-differentially expressed in LC, including receptors, and that these receptor expression differences are substantial enough to have sex-specific consequences for LC neurons and the behaviors they control.
INTRODUCTION
Numerous neuropsychiatric and neurodevelopmental diseases demonstrate a skew in incidence between sexes, including a female predominance of major depressive disorder and generalized anxiety disorder (MDD and GAD, respectively) (Kessler et al., 2005), and a male predominance of attention-deficit/hyperactivity disorder and autism spectrum disorders (ADHD and ASDs, respectively) (Christensen et al., 2016; Fombonne, 2009). Sex differences in reproductive behavior have been thoroughly attributed to sexually dimorphic brain regions; however, questions remain as to whether more modest behavioral differences—especially those relevant to common psychiatric disorders—are mediated by transcriptional sex differences in key neuronal populations.
The locus coeruleus (LC)—a small nucleus of neuromodulatory neurons whose projections release norepinephrine (NE) throughout the CNS—is implicated in a broad range of functions, including learning, novelty detection, arousal, anxiety, and fever (Sara, 2009). Given these extensive neurobehavioral roles, it is perhaps unsurprising that the LC-NE system has been broadly implicated in psychiatric disorders and animal models of them. For example, depression is often modeled in rodents using pro-inflammatory compounds including interleukins and lipopolysaccharide (LPS). LPS, besides inducing fever, is known to induce substantial activation of the LC and other noradrenergic cell types (established by Hare et al., 1995). Interleukin-6, a common upstream pro-inflammatory messenger, was recently found to directly trigger tonic firing of the LC, eliciting depressive behaviors via activation of alpha-adrenergic receptors (Kurosawa et al., 2016). Tonic firing of the LC in response to corticotropin-releasing factor (CRF) or optogenetic stimulation can also induce acute aversive and anxiety-like behaviors (McCall et al., 2015, 2017; Seo and Bruchas, 2017). Sex differences in behavioral responses to stress have been attributed to molecular-level differences in CRF signaling via the LC (Bangasser et al., 2011; Valentino and Bangasser, 2016). In the clinical setting, evidence-based practices offer a robust demonstration of the involvement of LC dysregulation—or its ability to normalize dysregulation occurring elsewhere—in psychiatric disease, given the use of NE-modulating drugs in depression, anxiety, attention-deficit/hyperactivity disorder, and addiction. Altogether, a comprehensive transcriptomic profile of the LC is of substantial interest for understanding, and potentially targeting, the molecular functions of these cells.
To date, sex differences in the rodent LC have been observed at both the single-gene and the structural level. Sex differences in stress response have been attributed to differential CRF sensitivity and CRF receptor trafficking in mouse LC (Curtis et al., 2006; Valentino et al., 2012), including sex-differential effects of CRF1 agonism on LC excitability (Bangasser et al., 2016). μ opioid receptors are also highly expressed in the LC; μ agonism completely suppresses firing of the LC in male, but not female, mice (Guajardo et al., 2016). Reports of structural dimorphism in the rodent LC have been ambiguous, with reports of the LC being larger in either sex, depending on the strain of rat (compare Babstock et al., 1997; Bangasser et al., 2011; and Luque et al., 1992). These repeated demonstrations of sex differences in particular aspects of LC structure and function compelled us to study both sexes in our pursuit of characterizing LC gene expression.
In order to transcriptionally profile the LC, we generated a translating ribosome affinity purification (TRAP) line. We identified dozens of potential LC-specific drug targets and validated a subset of these with independent methods. We identified differentially expressed genes (DEGs) in the LC following LPS stimulation, demonstrating the utility of this line and method to detect pharmacologically mediated changes in gene expression in the LC. To our surprise, we discovered a comparable number of DEGs between sexes as well. In order to demonstrate that these transcript-level sex differences in the LC correspond to consequent physiologic differences, we modulated a receptor upregulated in female LC, EP3 (encoded by Ptger3). Using electrophysiology and behavior experiments in cannulated mice, we demonstrate that the EP3 agonist sulprostone acts more strongly in female mice to suppress tonic firing of LC neurons ex vivo and to specifically inhibit an LC-mediated stress response in females in vivo. Thus, we demonstrate previously unidentified sex differences in gene expression in NE neurons at a magnitude capable of influencing neurophysiology and pharmacologic responses. These molecular sex differences at the level of LC neurons may guide future investigations into models, mechanisms, or treatments for sex-skewed psychiatric diseases.
RESULTS
Generation and Validation of Reagents for Transcriptional Profiling of Noradrenergic Neurons
We generated a mouse line for transcriptional profiling of noradrenergic neurons by expressing EGFP/RPL10A from a NE reuptake transporter (Slc6a2) bacterial artificial chromosome (BAC). Neuroanatomical characterization revealed robust transgene expression in the A4 and A6 subdivisions of the LC (Figures 1A–1C), where EGFP/RPL10A perfectly co-localized with the LC-specific NE-synthesizing protein, DßH (Figure 1D), along with reasonably robust co-labeling in the A5 and A7 groups. EGFP/RPL10A labeling was weak and sparse in more caudal DßH+ populations (e.g., A1 and A2), consistent with prior immunofluorescence studies of SLC6A2 expression (Schroeter et al., 2000). Little EGFP expression was seen in ependymal cells, except rarely in cells caudal to the fourth ventricle (data not shown), in contrast to previously reported ependymal expression of SLC6A2. In total, this anatomical characterization asserts that mRNA collected by TRAP will be from the most robustly labeled and populous cells: the A4–A7 groups, predominantly the LC.
Figure 1. Characterization of Noradrenergic bacTRAP Lines.
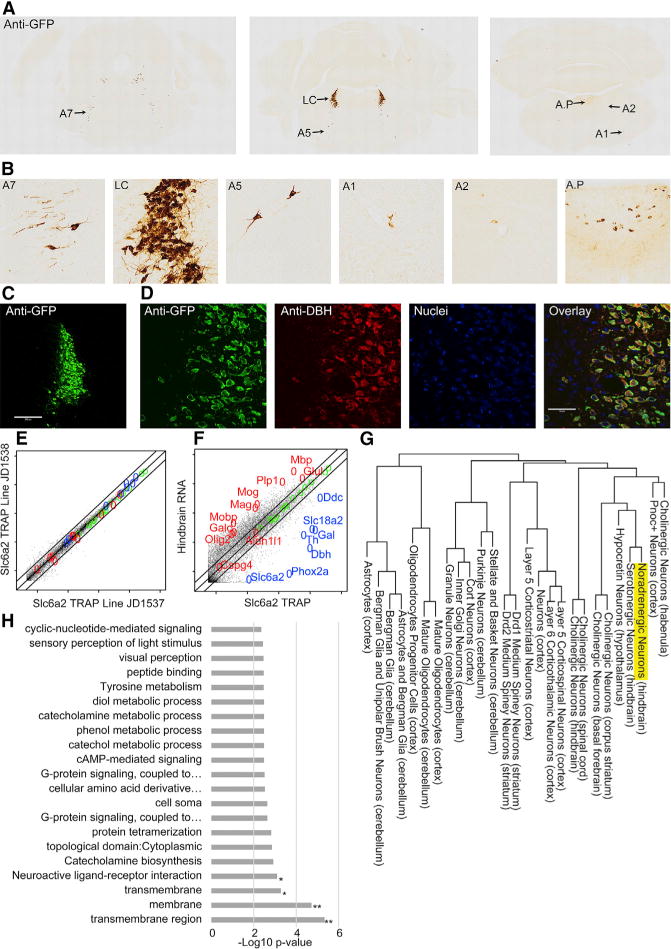
(A) Anti-GFP immunohistochemistry demonstrates EGFP/Rpl10a labeling in the hindbrain.
(B) Anti-GFP staining is most robust in anterior groups (A4–A7), especially LC.
(C) Immunofluorescence for GFP (green) labels entire LC. Scale bar, 200 mM.
(D) GFP co-labels completely with dopamine-β-hydroxylase (DBH) (red). Scale bar, 50 mM.
(E) Comparison of two Slc6a2 TRAP lines demonstrates reproducibility.
(F) Slc6a2 TRAP mRNA versus total hindbrain mRNA enriches NE neuron markers (blue) and depletes unrelated cell-type (glial) markers (red). (E and F) Lines indicate 0.5-, 1-, and 2-fold. Log10 scale.
(G) Hierarchical clustering of Slc6a2 neurons.
(H) Slc6a2 TRAP enriches for transmembrane proteins and receptors. Hypergeometric test, Benjamini-Hochberg corrected; *p < 0.06; **p < 0.01.
See also Table S1B.
We then performed TRAP on two Slc6a2 founder lines to evaluate consistency, to confirm enrichment of known LC-specific transcripts by TRAP, and to identify transcripts enriched in LC compared to the hindbrain. Reproducibility was strong between the lines (Pearson correlation > 0.99; Figure 1E). Relative to whole-hindbrain RNA from the same mice, TRAP enriched for genes with known specificity and functionality in the LC (Figure 1F), including (1) enzymes related to NE turnover (Th, Ddc, Maoa, and Dbh), (2) vesicular monoamine (Slc18a2) and NE (Slc6a2) transporters, (3) galanin (Gal) and its receptor (GalR1), and (4) a transcriptional regulator of LC development (Phox2a). After conservative filtering for expression and background, at least 3,139 transcripts were detected with high confidence in NE neurons; 526 were enriched >2-fold compared to hindbrain.
Gene Ontology (GO) analysis was applied to broadly characterize NE neuron-enriched transcripts, revealing enrichment of transmembrane receptors and ligands (Figure 1H), consistent with prior observations that CNS cell-type-specific genes often include receptors (Doyle et al., 2008). These LC-enriched receptors are highlighted (Table S1B), given the importance of the LC—and extrinsic modulation thereof—in behavior. Next, transcriptional profiles of LC and other CNS cell subtypes previously characterized by TRAP were compared to predict genes specific to the LC compared to other cells in the brain and the “transcriptional ontology” of the LC using our previously described pSI (specificity index p value) algorithm (see Experimental Procedures). Compared to all other available cell types, 162 genes scored as LC enriched (pSI < 0.05), with 78 highly so (pSI < 0.005) (Figure 2A; Table S1A). Hierarchical clustering placed noradrenergic cells with other neuromodulatory populations (Figure 1G), including serotonergic, hypocretinergic (Hcrt), and forebrain cholinergic neurons. The shared transcriptional relationship with Hcrt neurons is striking; despite their anatomic separation and the use of distinct neurotransmitters, they form reciprocal connections and share functional roles in control of sleep and arousal (reviewed in Carter et al., 2013), supporting the notion that gene expression in neuronal cell subtypes is a strong predictor of a subtype’s functional roles.
Figure 2. Transcript and Protein Expression in LC Neurons.
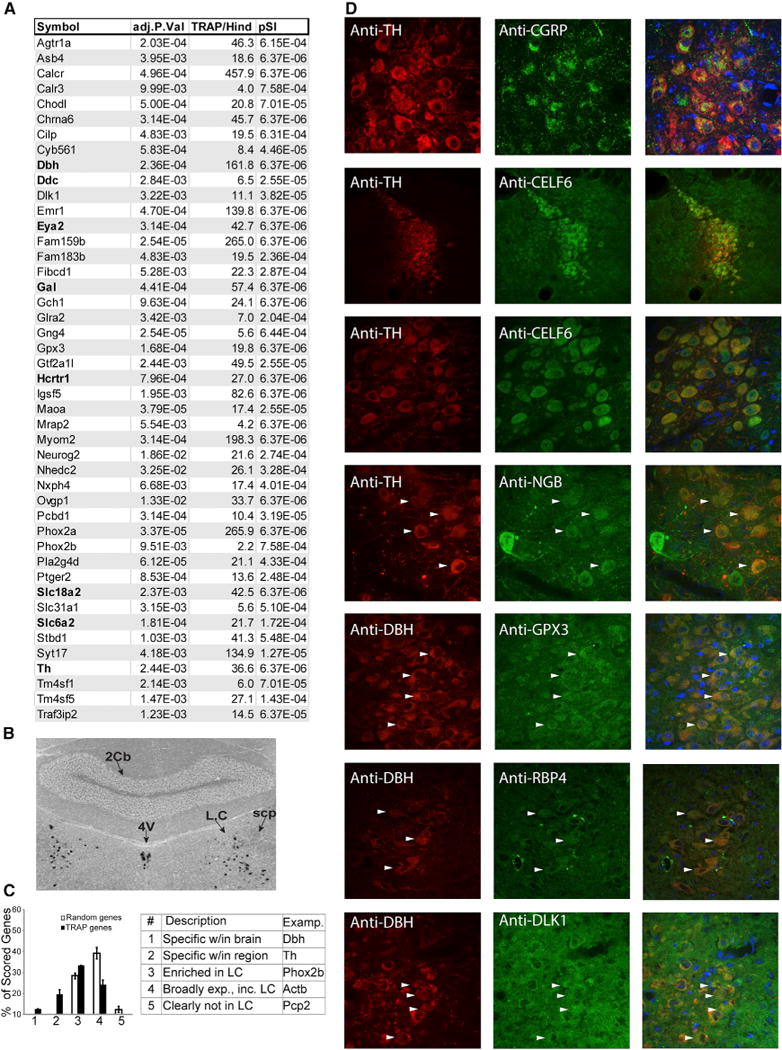
(A) Top 45 named genes enriched by TRAP over hindbrain (adjusted p value), fold change (TRAP/Hind), and specificity index p value (pSI) comparing Slc6a2 to all cell populations from Figure 1G (see also Table S1A).
(B) ISH confirms LC enrichment of calcitonin receptor. 4V, fourth ventricle; scp, superior cerebellar peduncle; 2cb, cerebellar vermis.
(C) Blind comparison of TRAP-identified and random genes confirms TRAP transcript presence in LC (p < 2.7E–38, chi-square test, normalized to number of n = 53 TRAP-identified transcripts and 94 randomly selected transcripts). Error bars are ± SEM for two independent, blinded scorers (see also Figure S1).
(D) Immunofluoresence confirms translation of TRAP-identified transcripts (green) in LC cells (arrowheads), labeled by either TH or DBH (red).
See also Figure S1 and Table S1A.
Transcripts identified as LC enriched (Figure 2A) were then validated using standard RNA and protein detection methods in wild-type mice. In situ hybridization (ISH) for Calcr selectively and robustly stained the LC (Figure 2B), consistent with microar-ray results, suggesting very high enrichment of Calcr in the LC compared to hindbrain (over 300-fold). As the characteristic “quarter-moon” anatomy of the LC could be readily discerned by ISH, additional transcripts were systematically evaluated for enrichment using The Allen Brain Atlas (Figures 2C and S1). 70% of TRAP transcripts showed enriched in situ staining in the LC; over 19% scored as having “marker-like” expression. Finally, we confirmed protein translation in the the LC of several identified genes using immunofluorescence (Figure 2D).
Transcriptional Responses of NE Neurons to LPS Can Be Identified with Slc6a2 TRAP
Having verified that TRAP characterizes baseline transcriptional features of the LC, we next sought to demonstrate the utility of this mouse line for profiling changes consequent to stimulation of the LC. LPS is a well-characterized example of an LC-activating stimulus: it strongly increases FOS expression in the LC, and the LPS-induced febrile response is lost with LC ablation (Almeida et al., 2004). Therefore, we injected individual TRAP mice with LPS or vehicle in a sex-balanced design. Whole-hind-brain RNA from the same lysates served as controls, as did a parallel TRAP experiment with Slc6a4 TRAP mice targeting hind-brain serotonergic neurons (Figure 3).
Figure 3. Differentially Expressed Genes by Sex.
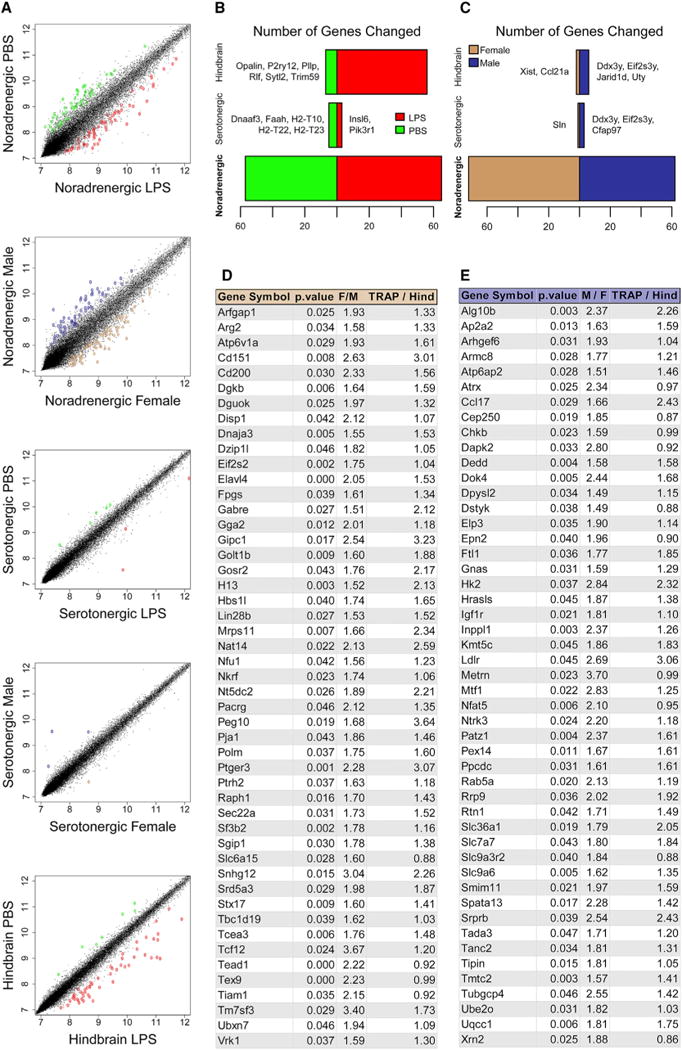
(A–C) Scatterplots contrasting transcriptional data between LPS and vehicle (PBS) or sex (A); DEGs are indicated by color. (B and C) Number of DEGs after LPS
(B) (red, up; green, down) or between vehicle-treated sexes (C) in each sample type (see also Tables S1C–S1G).
(D and E) Top 50 named sex-DEGs in LC of females (D) or males (E). M/F or F/M represents fold change between sexes; TRAP/Hind represents fold change, TRAP versus hindbrain.
See also Figure S2 and Tables S1C–S1G.
We first examined whole-hindbrain changes in response to LPS (Figure 3B); 56 genes showed a response, predominantly upregulation (Table S1C). GO analysis identified a significant increase of interferon-induced transmembrane proteins (Figure S2), consistent with a broad pro-inflammatory transcriptional response. However, examination of Slc6a2 TRAP revealed an even greater response (Figure 3C; Tables S1D and S1E), largely distinct from that of the hindbrain (only two genes were shared between hindbrain and LC). In contrast, response of serotonin neurons was limited (Figure 3B). Given the multitude of psychopharmacotherapeutics and environmental stimuli—including inflammation, pain, and acute stress—known to modulate the LC, these findings establish a useful system for identifying LC-specific molecular responses to whole-animal manipulations.
Noradrenergic Transcriptional Profiles Reveal Robust Sex Differences
To our surprise, sex-stratified analyses of these same experimental data revealed substantial sex differences in noradrenergic neurons. First, analysis of transcriptional profiles of the whole hindbrain varied remarkably little between sexes, with the exception of a few sex chromosomal genes (Figure 3C). Likewise, serotonin neurons showed no appreciable differences outside of sex chromosomal transcripts (e.g., Ddx3y and Eif2s3y). In contrast, noradrenergic neurons showed substantial molecular sex differences: a total of 152 transcripts were DEGs, most of which are autosomal (Figures 3D and 3E; Tables S1F and S1G), and these did not overlap with the serotonin neuron differential transcripts. These transcripts also do not significantly overlap with those stimulated by LPS, nor are they characterized by similar functional categories (Figure S2). This indicates that the observed molecular divergence is not likely due to sex differences in baseline activity level of the LC but rather reflects a more complex molecular distinction between the sexes. Motivated by the clear role of sex as a risk factor for psychiatric disorders, we focused on these sex differences for additional study.
Sex-Differential LC Genes and Putative cis-Regulators Implicated in Disease and Behavior
As a preliminary investigation into possible gene-regulatory mechanisms underlying sex differences in LC gene expression, we characterized DNA sequence motifs in cis with these 152 genes. Performing de novo motif discovery for DEGs from each sex, we identified 12 motifs (Table S2). Compared to their frequency near 1,000 randomly selected genes, six of these were significantly enriched near the DEGs. Thus, at least a portion of the sex differences in gene expression could be explained by conserved cis-regulatory elements in the surrounding genome. Known transcription factor binding sites predicted in these motifs included OTX2, NR2F6, and MTF1 (Table S2).
Molecular Differences Predict Functional Differences between Sexes
Finally, we noted LC specificity and female LC enrichment (>2-fold; Table S1E) of the Ptger3 gene, encoding the prostaglandin E2 (PGE2) receptor EP3. Given the specificity of this gene’s expression to the LC (Table S1A) within the hindbrain and the existence of a known, selective agonist, sulprostone, we selected EP3 to pharmacologically test whether the magnitudes of detected sex differences in receptor gene expression were adequate to alter LC electrophysiology and/or behavior.
We first assessed whether pharmacologic manipulation of the EP3 receptor resulted in sex-differential electrophysiologic responses by performing whole-cell recordings from LC neurons in ex vivo slices. EP3 presence in the LC was confirmed—and subsequently manipulated—by bath application of sulprostone (agonist), followed by L798,106 (antagonist) to displace sulpro-stone, halting its effects. Sulprostone suppressed baseline tonic firing of LC neurons in both sexes, but with a greater magnitude and duration of hyperpolarization in female LC compared to male LC (Figures 4A and 4B). Voltage-clamp recordings from a second cohort of mice revealed a larger outward current from female LC neurons (Figures 4C and 4D), verifying that the magnitude of LC inhibition by EP3 corresponds to sex differences in Ptger3 expression.
Figure 4. Sex Differences in Ptger3 Expression Can Be Reflected in LC-Mediated Behavior.
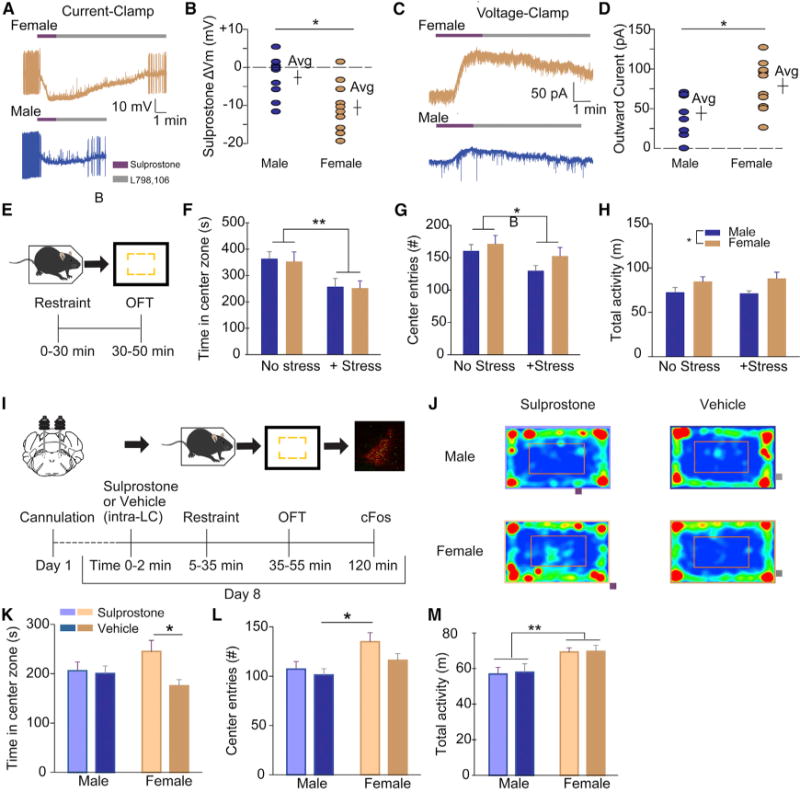
(A–C) Representative current-clamp (A) and voltage-clamp (C) traces from LC slices exposed to sulprostone (200 nM), followed by L798,106 (300 nM).
(B) Maximum change in membrane voltage (mV) after sulprostone, by sex (−10.5 ± 2.1 mV, n = 10 cells from 5 females; −2.6 ± 1.8 mV, n = 9 cells from 4 males; p < 0.05, Mann-Whitney).
(D) Maximum change in outward current (pA) after sulprostone, by sex (78.5 ± 9.7 pA, n = 10 cells from 3 females; 44.4 ± 8.9 pA, n = 9 cells from 3 males; p < 0.05, Mann-Whitney).
(E) Schematic of validated stress-anxiety paradigm. (F–H) Effects of restraint stress on OFT task performance (n = 6–7).
(F) Time in center zone.
(G) Center entries.
(H) Total activity.
(I) Timeline of LC pharmacology-behavior experiments.
(J) Representative OFT traces from each sex and treatment condition.
(K and L) Sulprostone administration prevents stress-induced anxiety in female (ns = 17 and 17) but not male (ns = 16 and 14) mice in OFT; time (K) and center zone entries (L) (see also Figure S4 for estrous-stage-specific behaviors).
(M) Time in center was unaffected by sulprostone for both sexes.
*p < 0.05; **p < 0.01. All error bars indicate SEM.
See also Figure S4.
We previously showed in male mice that LC silencing with Gi-coupled designer receptors exclusively activated by designer drugs (DREADDs) prevents anxiety-like behavior in the open-field task (OFT) after restraint stress (McCall et al., 2015). This behavioral paradigm provided a robust model system in which we could activate the LC in vivo, and subsequently attempt to suppress the LC pharmacologically, with behavior as the outcome measure. We first validated that restraint stress robustly induces anxiety-like behavior (avoidance of center) in the OFT in mice of both sexes (Figures 4E and 4H). Restraint stress did not impact total activity or the sex difference therein but clearly induced avoidance of center in both sexes. Thus, we hypothesized that EP3 agonists could be used to reduce stress-induced anxiety, specifically in wild-type female mice. Indeed, administration of sulprostone via cannula to male and female LC immediately before restraint stress and OFT resulted in selectively reduced anxiety-like behavior in females (Figures 4I–4L). Post hoc analysis of phosphorylated-FOS (p-FOS) expression in TH+ neurons of the LC in the same animals revealed similar staining intensity of p-FOS and numbers of double-positive cells between sexes (data not shown). The robust immediate-early response in both conditions is consistent with intact stimulation of LC neurons by stress, suggesting that sulprostone inhibits noradrenergic output. Sulprostone did not affect the baseline sex difference in total ambulatory activity. (Figure 4M).
DISCUSSION
Characterizing gene expression in noradrenergic neurons—regardless of whether they are at the etiologic root of neuropsychiatric diseases and disease models—is key to narrowing down possible mechanisms by which NE signaling may be dysregulated in disease states. We have presented a mouse line enabling transcriptional profiling of LC neurons at baseline and after physiologic manipulations (LPS). In characterizing this line, we discover and herein report a breadth of previously unidentified sex differences in molecular features of the mouse LC. These findings highlight the LC as an area of focus for future studies in neuropsychiatry, especially in domains where sex differences are observed in modeled behaviors or diseases. In contrast, we find that serotonergic neurons show few sex differences in gene expression, despite their hypothesized role in behavior and psychiatric disease. Sex-differential expression of one such receptor, PTGER3, was adequate in magnitude to sex-differentially affect electrophysiologic and behavioral pharmacologic responses. This independent verification of our transcriptomic findings suggests that sex-differentially expressed genes in the LC (1) may underlie sex differences in behavior and behavioral pathology and (2) can be targeted to sex-specifically modulate LC-mediated behaviors. Thus, we conclude that the LC is an interesting candidate for mediating sex differences in mono-amine-associated psychiatric phenotypes. We further envision that this mouse line could provide an invaluable tool in studies aimed at identifying mechanisms of existing NE-targeting drugs at the transcriptomic level and enable prioritization of new, precise drug targets aimed at the same transcriptional endpoints.
Our profiling extends previous work illustrating discrete molecular sex differences in the LC. Perhaps best characterized is the trafficking of receptor CRF1 and response to its ligand, CRF (Bangasser et al., 2016; Curtis et al., 2006; Valentino et al., 2012). Likewise, the expression of the μ-opioid receptor and response to opioid agonism in the LC shows a sex difference (Guajardo et al., 2016). Estrogen regulates genes required for NE synthesis in a sex-specific fashion (discussed later). Structural dimorphism in the rat LC has also been observed, though the direction of effect depends on the strain of rat (compare Babstock et al., 1997; Bangasser et al., 2011; and Luque et al., 1992). Finally, postnatal citalopram exposure in rats causes ectopic projection of LC fibers into the neocortex and increased LC excitability in males, but not females (Darling et al., 2011). We expand upon this body of research by identifying thousands of genes expressed in the LC and sex differences therein, as well as recurrent, conserved motifs in cis with these genes.
Among the transcripts with sex differences identified in the LC, we identified a number of genes and putative cis-regulators notable for their previous implications in behavior and brain development. Putative regulators in cis with the DEGs included three striking candidates: OTX2, NR2F6, and MTF1. OTX2 was recently shown to regulate depression-related consequences of early life stress in male mice (females were untested) through actions in dopaminergic neurons (Peña et al., 2017). MTF1 is notable for its role in binding and responding to heavy metals, perturbations of which have been implicated in ASDs (Arora et al., 2017; Hagmeyer et al., 2015); furthermore, this transcription factor was itself enriched in the LC of males, providing hints of a potential regulator of some of our observed sex differences. Finally, NR2F6 is a nuclear receptor known to be required for LC differentiation, consistent with LC enrichment of the genes used for motif analysis. As the motif analysis only utilized conserved regions of mammalian sequence near these genes, these regulatory mechanisms—and, thus, sex differences—may be conserved in humans.
Intriguingly, we also noted a previously unidentified female enrichment PGE2 receptor Ptger3 (EP3) in the LC. PGE2 and PTGER family receptors are known to mediate sexually dimorphic neurodevelopment in the preoptic area of the hypothalamus (Amateau and McCarthy, 2004; Wright et al., 2008); sex-differential expression of these receptors in a separate, adult brain region was, thus, intriguing. The enrichment of PGE2 receptors is interesting in the context of LPS, which we used here to stimulate the LC, but also stimulates fever; PGE2 and the LC are major effectors of LPS-induced fever via the EP3 and EP4 receptors (Almeida et al., 2004; Oka et al., 2000). Follow-up studies are merited to explore whether the EP3 receptor plays a role in fever effects on behavior via the LC and whether its differential expression is the cause or consequence of the broader transcriptomic sex differences presented here.
This expression difference was sufficient to modulate behavior in a sex-specific way, which we validated by LC-targeted pharmacologic manipulation. Using sulprostone to agonize EP3, we identified strong inhibitory effects on LC firing in female LC neurons, consistent with the pattern of its increased female expression. We then utilized restraint stress as a validated means of activating the LC and triggering LC-mediated behavioral changes (McCall et al., 2015, 2017; Seo and Bruchas, 2017; Uematsu et al., 2017). In turn, we aimed to suppress restraint-driven LC activation by administering sulprostone beforehand, ameliorating behavioral signs of stress-induced LC activity in female, but not male, mice. Thus, we demonstrate that the sex differences in receptor expression measured in the LC by TRAP are of an adequate magnitude to manipulate an LC-regulated behavior in a sex-specific manner.
Our stress paradigm was only used to robustly activate the LC, rather than to investigate stress per se. Whether EP3 plays a role in—or undergoes transcriptional or translational regulation in response to—physiologic sex differences in the stress response of the LC remains unclarified. We also note that the mice in which DEGs were identified were singly housed (potentially a stressor, i.e., social isolation), and housed at an unstressful, thermonormal 30°C for fever experiments. We note, however, that our electro-physiologic and behavioral findings regarding PTGER3 were consistent with the observed expression changes, despite the mice for the later experiments being group-housed at a normal room temperature. Using these TRAP mice to deliberately study sex-specific transcriptional/translational responses to stress will be an interesting application of this mouse line in future investigations.
It is interesting to speculate that sex differences in LC gene expression may specifically influence increased female risk of disorders like GAD and MDD, where NE-modulating drugs have seen use for decades. If higher baseline expression of some genes in the female LC promotes risk for MDD, then common variants that elevate the expression of those same genes may likewise confer depression risk. Indeed, when we examined the 15 documented MDD-associated loci (Hyde et al., 2016) for the presence of sex-differential LC genes, we found two genes associated with MDD are enriched in LC of female mice: Slc6a15 and Lin28b. This striking coincidence may imply that certain sex- and variant-mediated MDD risk factors converge in the LC. Future research is warranted to explore whether sex- and disease-associated regulatory variants concordantly affect gene expression and psychiatric disease risk via LC and other monoamingergic cell populations.
Overall, the marked molecular sex differences present interesting areas for future inquiry. Most notably, the mechanism of establishing these sex differences (hormonal-developmental, sex-chromosomal, or post-pubertal hormonal) remains unclarified. Previous work has shown that estrogen regulates the expression of Th and Dbh—and, thus, NE synthesis—in a sex-differential manner in adult rodents (Serova et al., 2002; Thanky et al., 2002). In the present study, estrous cycling was not examined for effects on transcriptional sex differences. Regarding behavior, we note that estrous cycling does not appear to play a role in most behaviors (Prendergast et al., 2014), including center time in the open field task for C57BL6 mice (Meziane et al., 2007); binning behavior data by estrus stage revealed no substantial differences (Figure S4). Another possible mechanism, the perinatal masculinizing hormonal surge in male rodents that organizes other dimorphic regions, might be equally important in the LC—indeed, structural differences in female rat LC can be attenuated by perinatal testosterone administration (Guillamón et al., 1988). Overall, it is possible that multiple mechanisms contribute to the molecular sex differences we detected. Thus, future studies are warranted, both focusing on identifying these mechanisms and examining the potential conservation of these differences.
EXPERIMENTAL PROCEDURES
Animal Research Statement
All procedures involving animals were approved by the institutional animal care and use committees of Rockefeller University, Case Western Reserve University, and Washington University in St. Louis, MO.
Immunofluorescence Microscopy
Paraformaldehyde (PFA)-perfused mouse brains were dissected, cryoprotected, and cut by cryostat into floating sections for immunostaining with primary antibodies and Alexa fluorophore-labeled secondary antibodies. Antibodies used are described in Table S1J.
TRAP for Initial Description of LC
Replicate pools of five mixed-sex adult mice from each of the Slc6a2 lines were sacrificed. Brains were removed for collection of hindbrain posterior to the pontine/hypothalamic junction (discarding the cerebellum).
All array data were analyzed in R using Bioconductor packages. GCRMA was used to normalize within replicates and to biotinylated spike-in probes (green dots, Figure 1F) between conditions. Fold change, specificity index (SI), and pSI were calculated for genes expressed above non-specific background, defined as at least 2 SDs above the mean TRAP:hindbrain ratio of negative control transcripts (Figure 1F, red dots). LC-enriched transcripts were identified using the empirical Bayesian statistic with false discovery rate (FDR) correction in limma. The pSI algorithm was used with default settings to compare LC TRAP cells to other cells profiled by TRAP (Figure 1G). Hierarchical clustering across cell types was conducted in R, utilizing expression values from genes with pSI < 0.01 in any cell type.
Scoring of The Allen Brain Atlas for LC Gene Specificity
Any transcript that scored between 1 and 3 (examples in Figure S1) was considered to be marker-like. A chi-square test was performed, comparing the observed counts of each score with expected counts (based on the random gene set) for each score.
Single-Animal TRAP for Sex-Differential and LPS-Responsive Gene Expression
Samples were prepared and hybridized in two batches counterbalanced for mouse strain, sex, and LPS. After processing, one Slc6a4 TRAP sample and two hindbrain samples were excluded due to poor hybridization. Remaining samples were normalized using the lumi package. Appropriate clustering of replicates was confirmed with multidimensional scaling (MDS) plots (Figure S5). Differential expression was defined as p < 0.05 on a paired t test (paired on batch and covariate), and log2 fold change of ± 0.585 across 3 of 4 paired comparisons. For balance, the single Slc6a4 replicate was used twice to replace the low-quality sample. (Results are given in Tables S1C–S1G.)
To validate this analytic approach, we performed standard differential expression analysis in limma. Resulting p values for genes identified in the former analysis in Tables S1C–S1G. Full array results from this analysis are given in Table S3. The two analyses and locations of their results are also given in Table S1K.
Motif Analysis of Peri-TSS Sequences of Sex-DEGs in LC
For each sex-differentially expressed gene identified in the LC (Tables S1F and S1G), mm10 genomic sequences 10 kb 5′ and 10 kb 3′ to the transcription start site (TSS) were acquired. Exonic bases in non-conserved regions of sequence (based on PhyloP scores) were masked out. Masked flanking sequences were submitted to MEME (Bailey et al., 2015) for de novo discovery of motifs 8–20 bp long. The associated tool, TomTom, was used to compare motifs to known transcription factor (TF) binding sites (Kulakovskiy et al., 2018; Mathelier et al., 2016; Weirauch et al., 2014). Motif frequency, consensus sequence, and predicted TFs discussed in the text are provided (Table S2). To assess motif enrichment near LC transcripts, 1,000 random protein-coding TSSs were selected and processed identically. These were searched for motif matches using FIMO at the same p cutoff for a “match” used by MEME during discovery. The number of unique loci containing ≥ 1-motif match among all loci was then compared using chi-square analysis, followed by Benjamini-Hochberg correction.
Electrophysiology of LC Neurons Exposed to PTGER3 Agonist/Antagonist
Whole-cell recordings were made using an Axopatch 200B amplifier (Molecular Devices). LC neurons were identified by location, capacitance > 40 pF, an input resistance < 100 MΩ, and a tonic firing rate of 0.5–4 Hz.
Stereotaxic Cannulation of LC
Mice were allowed to recover from surgery 7–9 days prior to behavioral testing. Animals were also habituated to handling and connection to tubing for 3 consecutive days prior to behavioral testing.
Stress-Induced Anxiety Behavioral Paradigm
Anymaze was used for video recording of animal movements for center and periphery analysis. The center zone was defined as a concentric rectangle comprising 50% of the OFT area. Cannula placement was confirmed by cryostat sectioning of perfused brains (Figure S3) to determine mice for inclusion in the final behavioral and p-FOS analyses.
c-FOS Quantification in LC following Sulprostone/Vehicle, Restraint, and OFT
Gain, light intensity, and exposure time were identical for all prepared microscope slides. Using ImageJ, background was subtracted, ROIs were made around the LC based on tyrosine hydroxylase (TH) staining, and average pixel intensity for p-FOS fluorescence was measured. p-FOS-TH double-positive cells were counted manually by a blinded experimenter.
Supplementary Material
Highlights.
>3,000 genes highly expressed in adult mouse locus coeruleus (LC) are identified
Norepinephrine neurons of LC sex-differentially express >100 genes
PGE2 receptor Ptger3 is more highly expressed in female LC
PTGER3 agonism inhibits LC firing and LC-driven anxiety behavior only in female mice
Acknowledgments
The authors thank R. Jaswany, E. Park, C. Jakes, K. McCullough, and L. Broestl for assistance in performing lab experiments; K. Nygaard for editorial assistance; T.C. Mazer for discussion of potential hormonal mechanisms of these findings; and the Rockefeller University Genomics Resource Center and Bioimaging Cores. This work was supported by the NIH (5R01HG008687, 4R00NS067239, 5R21DA038458, R01DA035821, and R01NS095809), the Simons Foundation, the Brain and Behavior Research Foundation, Ludwig Cancer Research, BBSRC (BB/M001873/1), and a CDI Microgrant from the Washington University Center for Cellular Imaging (WUCCI).
Footnotes
DATA AND SOFTWARE AVAILABILITY
The accession number for the transcriptomic data reported in this paper is GEO: GSE100005 (https://www.ncbi.nlm.nih.gov/geo/).
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures, five figures, and three tables and can be found with this article online at https://doi.org/10.1016/j.celrep.2018.04.054.
AUTHOR CONTRIBUTIONS
Conceptualization: J.D.D., N.H., and B.M; Methodology: J.D.D., S.K., N.H.; Validation: J.D.D., B.M., and D.L.B.; Formal Analysis: J.D.D., A.M.L., and B.M.; Investigation: J.D.D., B.M., D.L.B., S.K., S.G., and C.P.F.; Resources: J.D.D., M.R.B., C.P.F., and N.H.; Data Curation: J.D.D. and A.M.L.; Writing: B.M. and J.D.D.; Visualization: J.D.D., D.L.B., B.M., A.M.L., and C.P.F.; Supervision: N.H., J.D.D., and M.R.B.; Project Administration: J.D.D., B.M., and M.R.B.; Funding Acquisition: N.H., M.R.B., and J.D.D.
DECLARATION OF INTERESTS
J.D.D. has previously received royalties for patents related to TRAP technology. The remaining authors declare no competing interests.
References
- Almeida MC, Steiner AA, Coimbra NC, Branco LGS. Thermoeffector neuronal pathways in fever: a study in rats showing a new role of the locus coeruleus. J Physiol. 2004;558:283–294. doi: 10.1113/jphysiol.2004.066654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amateau SK, McCarthy MM. Induction of PGE2 by estradiol mediates developmental masculinization of sex behavior. Nat Neurosci. 2004;7:643–650. doi: 10.1038/nn1254. [DOI] [PubMed] [Google Scholar]
- Arora M, Reichenberg A, Willfors C, Austin C, Gennings C, Berggren S, Lichtenstein P, Anckarsäter H, Tammimies K, Bölte S. Fetal and postnatal metal dysregulation in autism. Nat Commun. 2017;8:15493. doi: 10.1038/ncomms15493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babstock D, Malsbury CW, Harley CW. The dorsal locus coeruleus is larger in male than in female Sprague-Dawley rats. Neurosci Lett. 1997;224:157–160. doi: 10.1016/S0304-3940(97)13462-0. [DOI] [PubMed] [Google Scholar]
- Bailey TL, Johnson J, Grant CE, Noble WS. The MEME suite. Nucleic Acids Res. 2015;43(W1):W39–W49. doi: 10.1093/nar/gkv416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Zhang X, Garachh V, Hanhauser E, Valentino RJ. Sexual dimorphism in locus coeruleus dendritic morphology: a structural basis for sex differences in emotional arousal. Physiol Behav. 2011;103:342–351. doi: 10.1016/j.physbeh.2011.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Wiersielis KR, Khantsis S. Sex differences in the locus coeruleus-norepinephrine system and its regulation by stress. Brain Res. 2016;1641(Pt B):177–188. doi: 10.1016/j.brainres.2015.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter ME, de Lecea L, Adamantidis A. Functional wiring of hypocretin and LC-NE neurons: implications for arousal. Front Behav Neurosci. 2013;7:43. doi: 10.3389/fnbeh.2013.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen DL, Baio J, Van Naarden Braun K, Bilder D, Charles J, Constantino JN, Daniels J, Durkin MS, Fitzgerald RT, Kurzius-Spencer M, et al. Centers for Disease Control and Prevention (CDC) Prevalence and characteristics of autism spectrum disorder among children aged 8 years–Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2012. MMWR Surveill Summ. 2016;65:1–23. doi: 10.15585/mmwr.ss6503a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis AL, Bethea T, Valentino RJ. Sexually dimorphic responses of the brain norepinephrine system to stress and corticotropin-releasing factor. Neuropsychopharmacology. 2006;31:544–554. doi: 10.1038/sj.npp.1300875. [DOI] [PubMed] [Google Scholar]
- Darling RD, Alzghoul L, Zhang J, Khatri N, Paul IA, Simpson KL, Lin RCS. Perinatal citalopram exposure selectively increases locus ceruleus circuit function in male rats. J Neurosci. 2011;31:16709–16715. doi: 10.1523/JNEUROSCI.3736-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle JP, Dougherty JD, Heiman M, Schmidt EF, Stevens TR, Ma G, Bupp S, Shrestha P, Shah RD, Doughty ML, et al. Application of a translational profiling approach for the comparative analysis of CNS cell types. Cell. 2008;135:749–762. doi: 10.1016/j.cell.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fombonne E. Epidemiology of pervasive developmental disorders. Pediatr Res. 2009;65:591–598. doi: 10.1203/PDR.0b013e31819e7203. [DOI] [PubMed] [Google Scholar]
- Guajardo HM, Snyder K, Ho A, Valentino RJ. Sex differences in μ-opioid receptor regulation of the rat locus coeruleus and their cognitive consequences. Neuropsychopharmacology. 2016;42:1295–1304. doi: 10.1038/npp.2016.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillamón A, de Blas MR, Segovia S. Effects of sex steroids on the development of the locus coeruleus in the rat. Brain Res. 1988;468:306–310. doi: 10.1016/0165-3806(88)90143-5. [DOI] [PubMed] [Google Scholar]
- Hagmeyer S, Mangus K, Boeckers TM, Grabrucker AM. Effects of trace metal profiles characteristic for autism on synapses in cultured neurons. Neural Plast. 2015;2015 doi: 10.1155/2015/985083. Article ID 985083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare AS, Clarke G, Tolchard S. Bacterial lipopolysaccharide-induced changes in FOS protein expression in the rat brain: correlation with thermoregulatory changes and plasma corticosterone. J Neuroendocrinol. 1995;7:791–799. doi: 10.1111/j.1365-2826.1995.tb00716.x. [DOI] [PubMed] [Google Scholar]
- Hyde CL, Nagle MW, Tian C, Chen X, Paciga SA, Wendland JR, Tung JY, Hinds DA, Perlis RH, Winslow AR. Identification of 15 genetic loci associated with risk of major depression in individuals of European descent. Nat Genet. 2016;48:1031–1036. doi: 10.1038/ng.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kulakovskiy IV, Vorontsov IE, Yevshin IS, Sharipov RN, Fedorova AD, Rumynskiy EI, Medvedeva YA, Magana-Mora A, Bajic VB, Papatsenko DA, et al. HOCOMOCO: towards a complete collection of transcription factor binding models for human and mouse via large-scale ChIP-Seq analysis. Nucleic Acids Res. 2018;46(D1):D252–D259. doi: 10.1093/nar/gkx1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosawa N, Shimizu K, Seki K. The development of depression-like behavior is consolidated by IL-6-induced activation of locus coeruleus neurons and IL-1b-induced elevated leptin levels in mice. Psychopharmacology (Berl) 2016;233:1725–1737. doi: 10.1007/s00213-015-4084-x. [DOI] [PubMed] [Google Scholar]
- Luque JM, de Blas MR, Segovia S, Guillamón A. Sexual dimorphism of the dopamine-β-hydroxylase-immunoreactive neurons in the rat locus ceruleus. Brain Res Dev Brain Res. 1992;67:211–215. doi: 10.1016/0165-3806(92)90221-h. [DOI] [PubMed] [Google Scholar]
- Mathelier A, Fornes O, Arenillas DJ, Chen CY, Denay G, Lee J, Shi W, Shyr C, Tan G, Worsley-Hunt R, et al. JASPAR 2016: a major expansion and update of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 2016;44(D1):D110–D115. doi: 10.1093/nar/gkv1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall JG, Al-Hasani R, Siuda ER, Hong DY, Norris AJ, Ford CP, Bruchas MR. CRH engagement of the locus coeruleus noradrenergic system mediates stress-induced anxiety. Neuron. 2015;87:605–620. doi: 10.1016/j.neuron.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall JG, Siuda ER, Bhatti DL, Lawson LA, McElligott ZA, Stuber GD, Bruchas MR. Locus coeruleus to basolateral amygdala noradrenergic projections promote anxiety-like behavior. eLife. 2017;6:2837. doi: 10.7554/eLife.18247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meziane H, Ouagazzal AM, Aubert L, Wietrzych M, Krezel W. Estrous cycle effects on behavior of C57BL/6J and BALB/cByJ female mice: implications for phenotyping strategies. Genes Brain Behav. 2007;6:192–200. doi: 10.1111/j.1601-183X.2006.00249.x. [DOI] [PubMed] [Google Scholar]
- Oka T, Oka K, Scammell TE, Lee C, Kelly JF, Nantel F, Elmquist JK, Saper CB. Relationship of EP(1-4) prostaglandin receptors with rat hypothalamic cell groups involved in lipopolysaccharide fever responses. J Comp Neurol. 2000;428:20–32. doi: 10.1002/1096-9861(20001204)428:1<20::aid-cne3>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Peña CJ, Kronman HG, Walker DM, Cates HM, Bagot RC, Purushothaman I, Issler O, Loh YE, Leong T, Kiraly DD, et al. Early life stress confers lifelong stress susceptibility in mice via ventral tegmental area OTX2. Science. 2017;356:1185–1188. doi: 10.1126/science.aan4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast BJ, Onishi KG, Zucker I. Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci Biobehav Rev. 2014;40:1–5. doi: 10.1016/j.neubiorev.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci. 2009;10:211–223. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- Schroeter S, Apparsundaram S, Wiley RG, Miner LH, Sesack SR, Blakely RD. Immunolocalization of the cocaine- and antidepressant-sensitive l-norepinephrine transporter. J Comp Neurol. 2000;420:211–232. [PubMed] [Google Scholar]
- Seo DO, Bruchas MR. Polymorphic computation in locus coeruleus networks. Nat Neurosci. 2017;20:1517–1519. doi: 10.1038/nn.4663. [DOI] [PubMed] [Google Scholar]
- Serova L, Rivkin M, Nakashima A, Sabban EL. Estradiol stimulates gene expression of norepinephrine biosynthetic enzymes in rat locus coeruleus. Neuroendocrinology. 2002;75:193–200. doi: 10.1159/000048237. [DOI] [PubMed] [Google Scholar]
- Thanky NR, Son JH, Herbison AE. Sex differences in the regulation of tyrosine hydroxylase gene transcription by estrogen in the locus coeruleus of TH9-LacZ transgenic mice. Brain Res Mol Brain Res. 2002;104:220–226. doi: 10.1016/s0169-328x(02)00383-2. [DOI] [PubMed] [Google Scholar]
- Uematsu A, Tan BZ, Ycu EA, Cuevas JS, Koivumaa J, Junyent F, Kremer EJ, Witten IB, Deisseroth K, Johansen JP. Modular organization of the brainstem noradrenaline system coordinates opposing learning states. Nat Neurosci. 2017;20:1602–1611. doi: 10.1038/nn.4642. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Bangasser DA. Sex-biased cellular signaling: molecular basis for sex differences in neuropsychiatric diseases. Dialogues Clin Neurosci. 2016;18:385–393. doi: 10.31887/DCNS.2016.18.4/rvalentino. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Reyes B, Van Bockstaele E, Bangasser D. Molecular and cellular sex differences at the intersection of stress and arousal. Neuropharmacology. 2012;62:13–20. doi: 10.1016/j.neuropharm.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weirauch MT, Yang A, Albu M, Cote AG, Montenegro-Montero A, Drewe P, Najafabadi HS, Lambert SA, Mann I, Cook K, et al. Determination and inference of eukaryotic transcription factor sequence specificity. Cell. 2014;158:1431–1443. doi: 10.1016/j.cell.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CL, Burks SR, McCarthy MM. Identification of prostaglandin E2 receptors mediating perinatal masculinization of adult sex behavior and neuroanatomical correlates. Dev Neurobiol. 2008;68:1406–1419. doi: 10.1002/dneu.20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


