Abstract
Objectives
Prior research has revealed an age-related shift in how individuals recall events from their personal past, with older adults reporting events that are more positive than young adults. We recently showed that age-by-valence interactions may be partially driven by a prefrontally-mediated control mechanism recruited by older adults during retrieval of negative laboratory events to reduce phenomenological richness. Specifically, age was associated with greater increases in prefrontal recruitment during retrieval of negative relative to positive events, with this recruitment linked to decreases in hippocampal activity and subjective vividness ratings. In the current study, we examined whether older adults may rely on a similar mechanism during retrieval of a complex, highly emotional real-world event.
Methods
Participants (n=58, ages 18–87) were presented with images related to the 2013 Boston Marathon Bombings and were asked to retrieve a memory associated with each image. Images cued participants with either negative (associated with fear, destruction, sadness) or positive (images of hope, resilience, support) features.
Results
This study replicated prior episodic memory tasks: age was associated with more negative hippocampal connectivity with dorsomedial prefrontal regions during retrieval of memories triggered by negative relative to positive cues.
Conclusions
Such findings suggest that older adults may be recruiting a similar regulatory mechanism during retrieval of both negative laboratory stimuli and highly negative events from their past. These findings are discussed in relation to prior work showing that young and older adults interact differently with the negative details related to a highly negative event.
Keywords: Memory, Emotion, Aging, fMRI, Prefrontal Cortex
How we think about memories from our past can shape how we view ourselves, our relationships, and the world around us1. We recently provided evidence that age can significantly alter memory retrieval processes, meaning that young and older adults recall their pasts in distinctly different ways2–4. Specifically, older adults showed greater increases in prefrontal recruitment for negative relative to positive event retrieval.4 This prefrontally-mediated mechanism may reduce the vividness of older adults’ negative memories2–4. In this prior study of episodic retrieval, activation of the dorsomedial prefrontal cortex (dmPFC) was unrelated to positive event vividness across the adult lifespan, but showed age-related reversals for negative event vividness. In young adults, dmPFC recruitment was associated with increased vividness, suggesting a supportive role of this region in detail retrieval, but in older adults, recruitment was associated with decreased vividness ratings3. Functional connectivity analyses were conducted to examine a potential mechanism for age-related decreases in vividness. These analyses found that dmPFC recruitment was associated with decreased hippocampal involvement during older adults’ negative event retrieval, but increased involvement during young adults’ negative event retrieval and positive event retrieval at any age4. These findings suggest that the dmPFC typically works together with the hippocampus to support detail retrieval, but the relation breaks down, and is functionally reversed, when older adults retrieve negative information. Such a mechanism may enable older adults to remember past negative events without re-experiencing them vividly, consistent with frequently reported age-related positivity effects in memory, where older adults exhibit reduced retrieval of negative relative to positive information compared to young adults5–6.
In addition to supporting a prefrontally-mediated affective mechanism, this prior study also was inconsistent with an alternate hypothesis for older adults’ decreased negative event vividness that would explain the effects based on age differences in sensory recruitment. A prevailing view in the literature is that older adults recruit posterior visual regions less than young adults during memory retrieval due to sensory processing deficits7–8. As negative event retrieval relies more heavily on these regions than positive9, such deficits could produce the selective decreases in negative event vividness seen in healthy aging. However, our recent results10, showed that this reduced recruitment was limited to the initial search phase of retrieval. When the entire retrieval trial was examined, an age-by-phase interaction was identified in sensory regions, revealing age-related increases in these same sensory regions later in the memory retrieval process. Critically, recruitment of these regions, regardless of when that recruitment occurred, was associated with memory vividness. These results demonstrate that older adults can recruit sensory regions in service of memory retrieval, and that they do so for negative as well as positive events, making a global sensory deficit an unlikely explanation for age-related decreases in negative memory vividness.
The analyses reported above focused on age-related differences during episodic retrieval of recently-learned emotional word-image pairs. It is unclear whether age-related effects during retrieval of these simple emotional episodic events can be generalized to retrieval of real-world events. Autobiographical memories are richer, more emotional, more self-relevant, and can vary substantially both in how long ago they were encoded and how many times they have been retrieved since encoding. It is possible that older adults will be less motivated, or less able, to reduce negative event vividness when the event is more significant and self-relevant, leading to a decrease or elimination of the age-by-valence effects reported previously. Alternatively, older adults may approach retrieval of all negative events in the same way, leading to similar findings for autobiographical memory retrieval as for episodic memory retrieval.
The current research extends our prior findings by examining age-related effects during autobiographical memory retrieval. Specifically, the goal of the current analysis was to replicate prior findings from episodic memory research in support of a prefrontal regulatory mechanism4 and arguing against a sensory deficit explanation3. To argue against a sensory deficit explanation, the current analysis would have to show a) that age was not associated with greater decreases in posterior sensory recruitment during negative relative to positive event retrieval, and b) that age-related decreases in recruitment were limited to the initial search phase of retrieval. To provide additional support for a prefrontal regulatory mechanism, the current analysis examines connectivity between the same hippocampal and dmPFC regions that were reported in the prior episodic memory study. If the same age-by-valence interactions in connectivity are identified in the current dataset, that would suggest that older adults are recruiting this prefrontal regulatory mechanism during retrieval of negative episodic and real-world events.
Methods
Participants
Data from fifty-eight healthy adults (Mage= 46.21, SD= 20.37; Medu= 16.26, SD= 2.56; 31 females) are reported. The gender distribution did not differ across decades (x2(7, N=58)=5.41, p= .61), and age was not correlated with education (p= .88). Participants were right-handed native English speakers without psychiatric illness or neurological disorde1 and were recruited from the greater Boston area. All participants were paid for their participation ($20/hour) and gave written informed consent in accordance with the requirements of the Institutional Review Board at Boston College. All participants completed the Geriatric Depression Scale11 to evaluate symptoms of depression. In addition, participants engaged in a series of tests intended to examine general cognitive ability, vocabulary, verbal fluency, and working memory. Healthy aging was associated with decreased performance on the digit/symbol task (r= −.51 and r= −.63 for the 60- and 90-second measures, respectively). In addition, although all participants received scores of 27 or higher on the Mini-Mental State Exam 212, older adults’ scores were significantly lower than young adults’ scores (r= −.30; see Supplementary Table 1).
Materials and Methods
Retrieval cues were 60 color images presented across six retrieval runs. Twenty of these images were neutral Boston-related images while the remaining 40 images were selected from a sample of 62 images related to the Boston Marathon bombings (see Supplementary Materials). Based on pilot data, these images were assigned to negative (15 images), positive (15 images), or complex conditions (10 images). The current manuscript focuses on differences between positive and negative images, so neutral and emotionally complex images will not be discussed further. Six retrieval runs consisted of 10 memory retrieval trials each. The 20 neutral images were presented in Runs 1 and 6, while the 40 marathon-related images were presented across Runs 2–5. The order of images was randomized for each participant. On each trial, participants were presented with an image and asked to retrieve an associated specific memory (i.e., an event situated in a particular place and time). Participants were given the entire trial (20s) to retrieve a memory, but were instructed to hit a button with their index finger as soon as a memory came to mind. Following the button press, participants spent the rest of the trial elaborating on the retrieved memory in as much detail as possible. At the end of each trial, participants rated the positive and negative emotion associated with the memory, reported their personal involvement in the event, and rated the extent to which the event was related to the marathon bombings. Participants were given up to 5 seconds for each rating, with the trial progressing to the next rating once an answer was given. All four ratings were given on a scale of 1–5. After being removed from the scanner, participants were re-presented with all 40 marathon-related images in a post-retrieval survey.
Data Acquisition
Participants’ heads were stabilized in a Siemens Tim Trio 3 Tesla scanner. A localizing scan and auto-align scout were followed by a high resolution multi-echo T1 structural scan for anatomical visualization (176 1mm slices, TR=2530ms, TE1=1.64ms, TE2= 3.5ms, TE3= 5.36ms, TE4= 7.22ms). Six runs of whole brain, gradient-echo, echo planar images (31 3mm slices aligned along the line between the anterior and posterior commissures, 20% skip, TR=2.5s, TE=30ms, Flip angle=90) were acquired during memory retrieval using interleaved slice acquisition. A diffusion weighted scan was collected but will not be discussed. Response data were collected using a magnet-safe button response box.
Preprocessing and Data Analysis
Images were preprocessed and analyzed using SPM8 software (Wellcome Department of Cognitive Neurology, London, UK) implemented in MATLAB. Images were co-registered, realigned, normalized, and smoothed using a Gaussian 8 mm kernel. First level models were generated for all participants. For each of the four event types (complex, negative, positive, and neutral), memory search was modeled at cue presentation and elaboration was at button press; all events were assigned a duration of 2.5s (the TR). Subject maps of recruitment for all eight conditions were entered into a random-effects analysis, with age included as a continuous variable of interest, which allowed for examination of age-by-valence and age-by-phase interactions in recruitment. Follow-up conjunction analyses were conducted to identify neural regions exhibiting age related increases (i.e., age effects during search and elaboration) or decreases (negative effects of age during search and elaboration) across both phases. A third conjunction analysis, based on prior findings, interrogated the age-by-phase interaction by identifying regions in which age was associated with decreased recruitment during search and increased recruitment during elaboration. The significance threshold for all analyses was set at p < .005 (uncorrected). Monte Carlo simulations13, run with the normalized voxel size of 3×3×3, determined that a 19-voxel extent corrected results to p < .05. Parameter estimates of activity were extracted from regions identified in the third conjunction analysis (i.e., age-related decreases in recruitment during search and increases in recruitment during elaboration) using the REX toolbox for visualization purposes.
To determine if the age-by-valence interactions in hippocampal connectivity identified in a prior episodic memory paradigm4 replicate during retrieval of highly emotional real-world events, we examined functional connectivity between the same hippocampal sphere and dmPFC cluster examined in the prior work. The SPM8 gPPI toolbox14 (gPPI; http://brainmap.wisc.edu/PPI) was used to estimate whole-brain functional connectivity with a 6mm volume of interest around a left hippocampal voxel identified in a prior study4 (−24, −28, −6) for all eight memory conditions. As was done with recruitment, subject maps of connectivity for all eight conditions were entered into a random-effects analysis with age included as a continuous variable of interest. A mask was generated that included dmPFC clusters exhibiting age-by-valence interactions in hippocampal connectivity (specifically, a greater negative effect of age during negative relative to positive event retrieval) in our prior study at p< .05. This inclusive mask was applied to our current data, also at p<.05, to identify dorsomedial prefrontal regions exhibiting the same age-by-valence interactions in hippocampal connectivity across the two datasets. Parameter estimates of connectivity were extracted the region identified in this conjunction analysis using the REX toolbox for visualization purposes and to confirm patterns reported in the whole-brain analysis. Specifically, these additional analyses were used to determine whether age-by-valence interactions (i.e., more positive age effects for positive relative to negative event retrieval) were driven by age-related increases in connectivity during positive event retrieval and/or age-related decreases in connectivity during negative event retrieval.
Results (See Supplementary Table 2)
Interactive effects of age with emotion and phase on neural recruitment
No regions exhibited age-by-valence interactions on neural recruitment, meaning that no regions exhibited greater valence effects in young relative to older adults. Replicating prior episodic memory findings3, age-by-phase interactions were identified in posterior sensory regions (see Figure 1), driven by greater age-related decreases in recruitment during search relative to elaboration. In fact, conjunction analyses identified two sub-regions in which age was associated with significant decreases in recruitment during search and significant increases in recruitment during elaboration (Figure 1). Parameter estimates of activity were extracted from the lingual gyrus cluster for visualization purposes.
Figure 1.
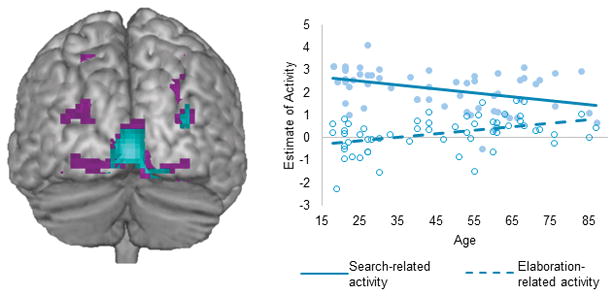
Posterior regions exhibiting age-by-phase interactions in recruitment at p< .005, k ≤ 20 (violet). Regions showing significant (p<.005) age-related decreases in recruitment during search (a, solid line) and age-related increases during elaboration (a, dashed line) are shown in cyan. The pattern in these regions is inconsistent with a sensory deficit account of age-related retrieval because activity averaged across search and elaboration is relatively stable with age and it is only the timing of the engagement that changes.
No regions exhibited age-related increases or decreases across both memory phases. Age-by-phase-by-valence interactions were identified in right middle frontal gyrus and left putamen.
Interactive effects of age and emotion hippocampal-dmPFC connectivity
The hippocampal connectivity analysis identified one dmPFC region (see Figure 2) that exhibited the same age-by-valence interactions in both the current study and the prior episodic memory study4. Parameter estimates of activity were extracted from this cluster for visualization purposes and to confirm patterns reported in the whole-brain analysis. Follow-up analyses conducted on the parameter estimates extracted from this cluster confirmed an age-by-valence interaction (F(1,56)=4.93, p=.03, ηp2=.08), driven by a significant age-related decrease in hippocampal connectivity during negative (r= −.39, p=.003) but not positive (r= −.07, p=.61) event retrieval. See Supplementary Table 2 for whole-brain analyses.
Figure 2.
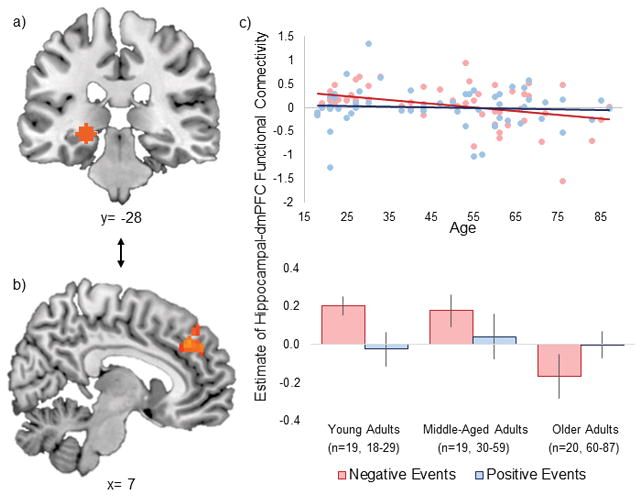
Estimates of functional connectivity between a) a left hippocampal sphere used in a recent episodic memory study analysis (Ford, Morris, & Kensinger, 2014) and b) a dmPFC cluster exhibiting significant age-by-valence interactions in hippocampal connectivity (at p<.05) in both this prior analysis and the current dataset. Graphs (c) depict age-by-valence interactions from the current data.
Discussion
The current autobiographical memory findings extend recent reports2,4 that older adults recruit a prefrontally-mediated negativity-decreasing mechanism during episodic memory retrieval. This replication is striking, as it suggests that older adults engage in similar regulatory processes during retrieval of self-irrelevant laboratory-learned images and highly emotional, personal events. This consistency suggests that research that uses controlled laboratory studies to identify and examine factors that strengthen or diminish this effect in older adults will have generalizable relevance to the way older adults remember events in their daily life. Further, understanding the mechanisms supporting age-related reductions in negativity will help to pinpoint affective, mnemonic, and executive processes that are preserved in healthy aging, and may pave the way for development of cognitive training protocols that could act at retrieval to enhance wellbeing.
The age-by-valence interactions reported here are consistent with research showing that older adults are motivated to regulate their emotional state to a greater extent than young adults, potentially altering the way in which they interact with emotional content5. These age effects may, therefore, reflect increased emotional regulation when older adults encounter negative stimuli. The dmPFC has been implicated in both automatic and voluntary emotion regulation15–16. The socioemotional selectivity theory (SST)17–19 proposes that a limited future time perspective—such as can be seen in healthy aging—shifts priorities away from acquiring new knowledge and toward emotional meaningful goals20. These shifting priorities may cause older adults to recruit dorsomedial prefrontal regions in the service of downregulating negative affective experience during retrieval. Consistent with a motivation account, prior work from our lab has shown stronger negative connectivity between mPFC and medial temporal lobe regions in older adults with the greatest structural integrity in white matter tracts connecting these regions2. In other words, older adults rely on this prefrontal mechanism when they are able to do so, rather than it being a by-product of age-related degeneration.
The current study was the first to show neural evidence of an age-related prefrontally-mediated regulatory mechanism during retrieval of highly negative personal events. Further, we provided evidence for preserved posterior visual recruitment during negative event retrieval. Together these findings point to prefrontal regulatory mechanism, rather than an underlying deficit, as an explanation for age-related positivity effects in autobiographical memory retrieval.
Supplementary Material
Acknowledgments
The authors would like to thank Tala Berro, Maria Box, Haley DiBiase, Marissa DiGirolamo, and Katherine Grisanzio for their help collecting and analyzing data for this project. Magnetic resonance data were collected at the Harvard Center for Brain Science. We thank the staff there, particularly Tammy Moran and Ross Mair, for their assistance with data collection and quality assurance. This work was supported by an NIH grant MH080833 (EAK) and by funding from Boston College.
Footnotes
Handedness was evaluated using the Edinburgh Handedness Inventory (Oldfield, 1971)
Disclosure statement: The authors report no actual or potential conflicts of interest.
References
- 1.Pillemer DB. Remembering personal circumstances: A functional analysis. In: Winograd E, Neisser U, editors. Affect and accuracy in recall: Studies of “flashbulb” memories. 4. New York: Cambridge University Press; 1992. pp. 236–264. Emory symposia in cognition. [Google Scholar]
- 2.Ford JH, Kensinger EA. The relation between structural and functional connectivity depends on age and on task goals. Frontiers in Human Neuroscience. 2014;8:307. doi: 10.3389/fnhum.2014.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ford JH, Kensinger EA. Prefrontally-mediated alterations in the retrieval of negative events: Links to memory vividness across the adult lifespan. Neuropsychologia. 2017;102:82–94. doi: 10.1016/j.neuropsychologia.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ford JH, Morris JA, Kensinger EA. Neural recruitment and connectivity during emotional memory retrieval across the adult life span. Neurobiology of Aging. 2014;35(12):2770–2784. doi: 10.1016/j.neurobiolaging.2014.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mather M, Carstensen LL. Aging and motivated cognition: The positivity effect in attention and memory. Trends in Cognitive Sciences. 2005;9:496–502. doi: 10.1016/j.tics.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Reed AE, Carstensen LL. The theory behind the age-related positivity effect. Front Psychol. 2012;3:339. doi: 10.3389/fpsyg.2012.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The posterior-anterior shift in aging. Cerebral Cortex. 2008;18:1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grady CL, Maisog JM, Horwitz B, Ungerleider LG, Mentis MJ, Salerno JA, Pietrini P, Wagner E, Haxby JV. Age-related changes in cortical blood flow activation during visual processing of faces and location. Journal of Neuroscience. 1994;14:1450–1462. doi: 10.1523/JNEUROSCI.14-03-01450.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bowen HJ, Kark SM, Kensinger EA. NEVER forget: Negative emotional valence enhances recapitulation. Psychonomic Bulletin and Review. doi: 10.3758/s13423-017-1313-9. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ford JH, Kensinger EA. Age-related reversals in neural recruitment across memory retrieval phases. Journal of Neuroscience. 2017;37:5172–5182. doi: 10.1523/JNEUROSCI.0521-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheikh JI, Yesavage JA. Clinical Gerontology : A Guide to Assessment and Intervention. NY: The Haworth Press; 1986. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. [Google Scholar]
- 12.Folstein MF, Folstein SE, White T, Messer MA. MMSE-2 Mini-Mental State Examination. 2. Psychological Assessment Resources Inc; 2010. User’s Manual. [Google Scholar]
- 13.Slotnick SD, Moo LR, Segal JB, Hart J. Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Cognitive Brain Research. 2003;17:75–82. doi: 10.1016/s0926-6410(03)00082-x. [DOI] [PubMed] [Google Scholar]
- 14.McLaren DG, Ries ML, Xu G, Johnson SC. A generalized from of context-dependent psychophysiological interactions (gPPI): A comparison to standard approaches. NeuroImage. 2012;61:1277–1286. doi: 10.1016/j.neuroimage.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: Implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular Psychiatry. 2008;13:833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ochsner KN, Gross JJ. The neural architecture of emotion regulation. In: Gross JJ, editor. Handbook of Emotion Regulation. Guilford Press; New York: 2007. [Google Scholar]
- 17.Carstensen LL. Motivation for social contact across the life span: A theory of socioemotional selectivity. In: Jacobs J, editor. Nebraska symposium on motivation: Developmental perspectives on motivation. Vol. 40. Lincoln: University of Nebraska Press; 1993. pp. 209–254. [PubMed] [Google Scholar]
- 18.Carstensen LL, Isaacowitz DM, Charles ST. Taking time seriously: A theory of socioemotional selectivity. American Psychologist. 1999;54:165–181. doi: 10.1037//0003-066x.54.3.165. [DOI] [PubMed] [Google Scholar]
- 19.Carstensen LL, Pasupathi M, Mayr U, Nesselroade J. Emotion experience in the daily lives of older and younger adults. Journal of Personality and Social Psychology. 2000;79:1–12. [PubMed] [Google Scholar]
- 20.Lang FR, Carstensen LL. Time counts: Future time perspective, goals, and social relations. Psychology and Aging. 2002;17:125–139. doi: 10.1037/0882-7974.17.1.125. [DOI] [PubMed] [Google Scholar]
- 21.Oldfield RC. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


