Abstract
Male germ cells are transformed from undifferentiated stem cells into spermatozoa through a series of highly regulated steps together termed spermatogenesis. Spermatogonial stem cells undergo mitosis and differentiation followed by two rounds of meiotic division and then proceed through a series of dramatic cell shape changes to form highly differentiated spermatozoa. Using indirect immunofluorescence, we investigated a role for the mitotic kinase, Aurora A (AURKA) in these events through localization of this protein in mouse testis and spermatozoa. AURKA is expressed in several cell types in the testis. Spermatogonia and spermatocytes express AURKA as expected based on the known role of this kinase in cell division. Surprisingly, we also found AURKA localized to spermatids and the flagellum of spermatozoa. Total AURKA and activated AURKA are expressed in different compartments of the sperm flagellum with total AURKA found in the principal piece and its phosphorylated and activated form found in the sperm midpiece. In addition, active AURKA is enriched in the flagellum of motile sperm isolated from cauda epididymis. These results provide evidence for a unique role for AURKA in spermatogenesis and sperm motility. Defining the signaling mechanisms that govern spermatogenesis and sperm cell function is crucial to understanding and treating male infertility as well as for development of new contraceptive strategies.
Keywords: Aurora A Kinase (AURKA), testis, spermatogenesis, spermiogenesis, flagellum, motility
1. Introduction
Spermatogenesis is a complex and tightly regulated developmental process that transforms undifferentiated spermatogonia into streamlined, motile sperm capable of fertilization. Precise control of spermatogenesis is critical to produce viable sperm that can are capable of fertilizing an egg to ensure overall propagation of the species. Spermatogenesis begins with a series of mitotic events to renew the spermatonial stem cell population and produce progeny that initiate differentiation, followed by two meiotic divisions to produce haploid spermatids. A series of striking morphological changes follows whereby male germ cells achieve their final form composed of condensed chromatin packaged into the head, an associated acrosome responsible for egg penetration, and a flagellum to propel the sperm towards the egg.
It is remarkable that much of spermatogenesis occurs in the absence of ongoing transcription and therefore must rely on post-translational modifications (PTMs) to regulate this process. Existing sperm proteins are subject to a wide range of PTMs to modulate their activity including phosphorylation, acetylation, glycosylation, and sumoylation [1, 2]. While well-defined signaling pathways such as MAPK and others are critical for spermatogenesis [3, 4]; this complex developmental program likely involves a unique and varied array of phosphorylation/dephosphorylation pathways to regulate differentiation. Here we describe our discovery of a novel localization for a mitotic kinase, Aurora A (AURKA), in spermatids and spermatozoa.
The family of Aurora kinases (AURKA, AURKB, and AURKC) are related serine-threonine kinases with critical but distinct roles in cell division in somatic and germ cells [5]. AURKA has well-established roles in formation and function of the mitotic spindle. AURKA localizes to the centrosome and spindle fibers and is essential for centrosome maturation, separation, mitotic entry, and bipolar spindle assembly [6]. AURKB is a DNA passenger protein that functions in spindle checkpoint activation and is associated with kinetochores from prophase to anaphase and then is transported to the spindle midzone at telophase [7]. AURKC is expressed primarily in the testis with proposed roles in meiosis [8-10]
In this work we describe a novel localization for AURKA during spermatogenesis. In addition to being found in dividing spermatogonia and spermatocytes, as predicted by its function in dividing cells, we localized AURKA to developing spermatids and spermatozoa. The localization of AURKA in spermatids and spermatozoa has not been described previously and suggests distinctive roles for this kinase in sperm development and motility.
2. Methods and Materials
2.1 Animals
Adult male CD1 mice were purchased from Charles River Laboratories (Raleigh, NC). All use of animals was approved and conducted in accordance with International Animal Care and Use Committee (IACUC) of East Carolina University, protocol #W179f.
2.2 Immunoprecipitation
Mouse testes were collected then homogenized in lysis buffer (10 mM Tris-HCl pH 7.0, 1 mM EGTA, 1 mM EDTA 10mM benzamidine, 1% NP-40) containing 0.1% BME, 1% PMSF and 1% protease inhibitor cocktail. Lysates corresponding to 350 μm total protein were centrifuged twice at 14,000 xg at 4°C for 30 minutes each. Lysates were precleared with 30 μl of Protein G Sepharose beads (BioVision; Milpitas, CA) by rotating for 1 hour at 4°C. The clarified lysates were then collected following centrifugation and complexes formed by rotating the supernatant overnight at 4°C with 2 μg of AURKA antibody (pA5-32035, Thermo-Fisher Scientific; Waltham, MA). Immune complexes were captured by rotating the sample in 30 μl of beads for 1 hour at 4°C. Beads were washed five times in lysis buffer, denatured by boiling in 2X sample buffer for 5 min and proteins analyzed by SDS-PAGE followed by blotting with rabbit anti-total AURKA (1:250 pA5-32035, Thermo-Fisher Scientific; Waltham, MA) and Veriblot anti-rabbit HRP (1:2500; Abcam; Cambridge, MA). The negative control was precleared lysate without antibody.
2.3 Indirect Immunofluorescence
Adult mouse testes were incubated in 4% paraformaldehyde (PFA) for 24 hours, then placed in 30% sucrose for 24 48 hours. Frozen tissues were sliced into 10 μm sections, blocked in 3% BSA for 1 hour at room temperature, and then incubated with anti-Aurora A antibody (IHC, 1:100; Bethyl Laboratories; Montgomery, TX) overnight at 4°C. Sections were washed in TBST 3 times at room temperature for 5 minutes each, then incubated in secondary Alexa Fluor 594 antibody (1:200; Jackson ImmunoResearch Laboratories, West Grove, PA.). Vectashield with DAPI was used to visualize DNA (Vector Laboratories Inc; Burlingame, CA).
Freshly excised epididymides were carefully disassociated with forceps and separated into caput, corpus and cauda sections and placed in pre-warmed phosphate buffered solution and 4-6 cuts were made in each section of the epididymis. Sperm were allowed to swim out for 10 minutes at 37°C and the resulting sperm suspension was smeared onto glass slides for staining. Sperm density was verified under a light microscope then air-dried for at least 30 mins at room temperature. Once completely dry, sperm were fixed in cold 4% PFA for 10 min then stored at 4°C until further use. Before immunostaining, TBST was used to remove any residual PFA. Sperm were blocked in 3% BSA for 1 hour at room temperature then stained with anti-Aurora A antibody (IHC, 1:50; Bethyl Laboratories; Montgomery, TX) or anti-phosphorylated Aurora A (Thr288) (1:50; ThermoFisher; Rochester, NY) along with PNA-lectin conjugated to Alexa Fluor 488 (1:200; ThermoFisher; Rochester, NY) to visualize the acrosome. DAPI was used to visualize the nucleus. Images for both testes and epididymis were observed with a Nikon E600 fluorescence microscope fitted with the appropriate filters using 40X and 100X objectives were captured with an Orca II CCD camera (Hamamatsu). Fluorescent intensity of AURKA staining in adult spermatozoa was quantified using Metamorph image analysis and acquisition software (Universal Imaging Corporation, Downingtown, PA).
2.4 Statistical Analysis
Statistical analyses were performed using JMP Version 13.1. Two-tailed student’s t-tests were used to determine significance and reported as mean plus SEM.
Results and Discussion
3.1 Aurora A kinase is expressed in multiple male germ cell types in the testis
We first determined if AURKA kinase is present in the testis and if its expression changes as spermatogenesis progresses towards the formation of spermatozoa. We took advantage of the fact that different germ cell types appear at specific time points after birth to estimate the germ cell type(s) that express AURKA [11]. For example, only mitotically dividing spermatogonia are found in the epithelia at 7 days postpartum (dpp) while spermatocytes appear in the epithelium at 14 dpp and undergo meiosis to form haploid spermatids at 21 dpp. Spermatids then undergo cytodifferentiation and all cell types populate the seminiferous epithelium in the adult testis, including immotile spermatozoa prior to spermiation. Therefore, we isolated protein from testis of the above aged animals and probed with an AURKA specific antibody. AURKA expression remained constant during the first wave of spermatogenesis (Figure 1A) demonstrating that AURKA is present in all spermatogenic cell types including spermatogonia, spermatocytes and spermatids (Figure 1A).
Figure 1. Aurora A kinase is expressed in multiple male germ cell types in the testis.
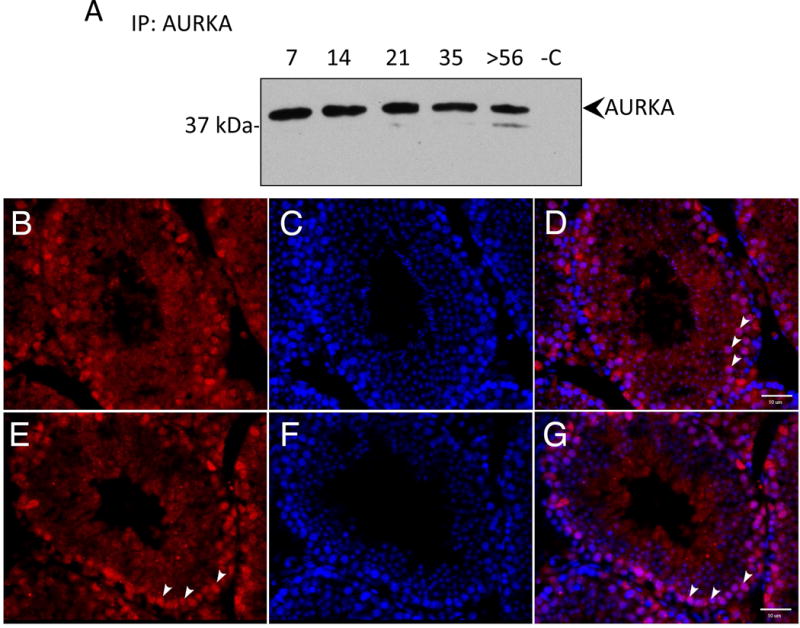
(A) Protein lysates were prepared from testes of postnatal day 7, 14, 21, 35 and adult mice (>56 dpp). AURKA was precipitated with an AURKA specific antibody and detected with the same antibody. Proteins in lane -C where precipitated in the absence of antibody as control. AURKA expression was detected during the first wave of spermatogenesis in the testis when specific cell types appear at defined times after birth. All cell types can be detected in the adult seminiferous tubule. (B-G) Frozen testes sections were stained with AURKA (red in B and E) and DNA with DAPI (blue in C and F) with the merged image in D and G. (B-D) AURKA localized to spermatogonia (representative spermatogonia indicated with arrowheads). (E-G) In other tubules, AURA localized to spermatocytes (representative spermatocytes indicated with arrowheads). Scale bars indicate 10 μm.
To further investigate AURKA expression in testicular germ cells, adult mouse testes sections were stained for AURKA. AURKA is expressed in multiple cell types in the testis and localizes to spermatogonia and spermatocytes (Figure 1B-G). Cells along the basement membrane were identified as spermatogonia and those located one cell layer closer to the lumen were identified as spermatocytes. It is notable that not all spermatogonia stain with the AURA antibody perhaps due to the heterogeneity of this cell population. AURKA is a kinase with crucial roles in cell division, therefore localization to dividing spermatogonia and spermatocytes in the testis was expected; surprisingly however, AURKA was found associated with the nucleus in elongating spermatids (Figure 2A-F). This site of expression suggests that AURKA functions in events associated with morphogenesis of the sperm head or formation of the sperm flagellum. AURKA was also localized to the lumen of certain tubules containing sperm flagella, which appear as striations in the center of the tubule (arrowheads, Figure 2G-H). This arrangement of staining is consistent with labeling of sperm flagella prior to release from the epithelium. This novel localization pattern suggests that AURKA may have functions in flagella biogenesis and remodeling of spermatids as well as sculpting of the sperm head.
Figure 2. AURKA is associated with elongated spermatids and spermatozoa in the seminiferous epithelia.
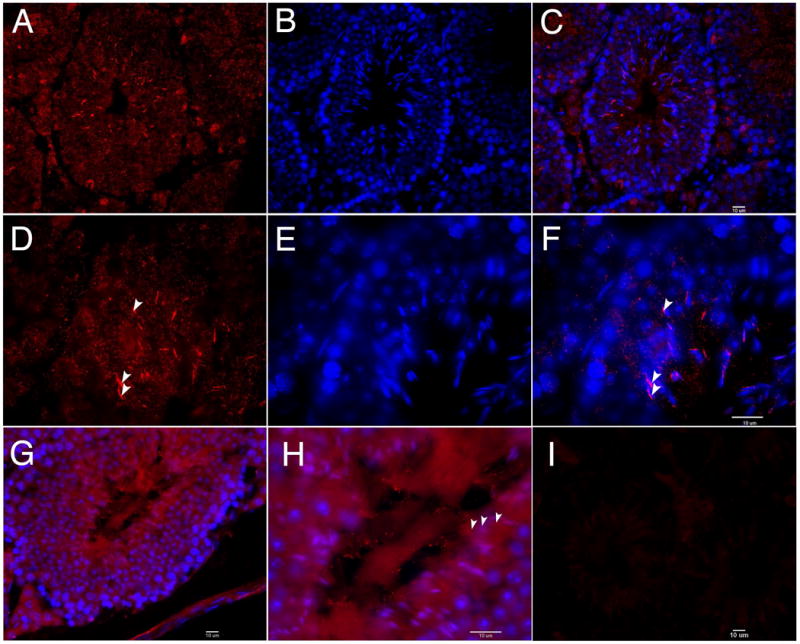
(A-H) Frozen testis sections were stained for AURKA (red in A and D) and DAPI (blue in B and E) with merged images in C and F. Images were taken with a 40X objective (A-C, G) or a 100X objective (D-F, H). In tubules containing abundant elongating spermatids, AURKA was associated with the heads of elongating spermatids during the process of spermiogenesis (arrowheads, D and F). (G-H) AURKA also localized to the flagella of sperm within the lumen of the tubule (arrowheads in H). These are the flagella of immotile sperm undergoing spermiation. Negative control incubated without primary antibody is shown in I. Scale bars indicate 10μm.
3.2 Aurora A Kinase localizes to the flagellum of epididymal sperm
After release from the seminiferous epithelium, sperm are stored and mature in the epididymis. Motility is acquired during their transit through the epididymis and motile sperm are stored in the cauda epididymis [12, 13]. To further characterize AURKA in spermatozoa, sperm were isolated from the different functional and structural compartments of the epididymis; the caput, corpus and cauda. Sperm were stained with an AURKA antibody, and dye conjugated PNA-lectin to visualize the acrosome. AURKA localized to the principal piece of the spermatozoa from the caput (Figure 3A), corpus (Figure 3B) and cauda (Figure 3C). The staining appeared concentrated at the annulus, a specialized structure separating the midpiece from the principal piece, before distributing throughout the principal piece, with expression decreasing caudally. AURKA was not observed in the midpiece, nucleus or acrosome. Further examination revealed AURKA expression as two parallel stripes along the length of the principal piece of the flagella (insets, Figure 3), suggesting specific localization to the longitudinal columns of the fibrous sheath. AURKA expressing as indicated by fluorescent intensity was greatest in spermatozoa of the caput compared to corpus and cauda, although this differential expression pattern was not statistically significant (Figure 3D).
Figure 3. AURKA localizes to the flagellum of epididymal sperm.
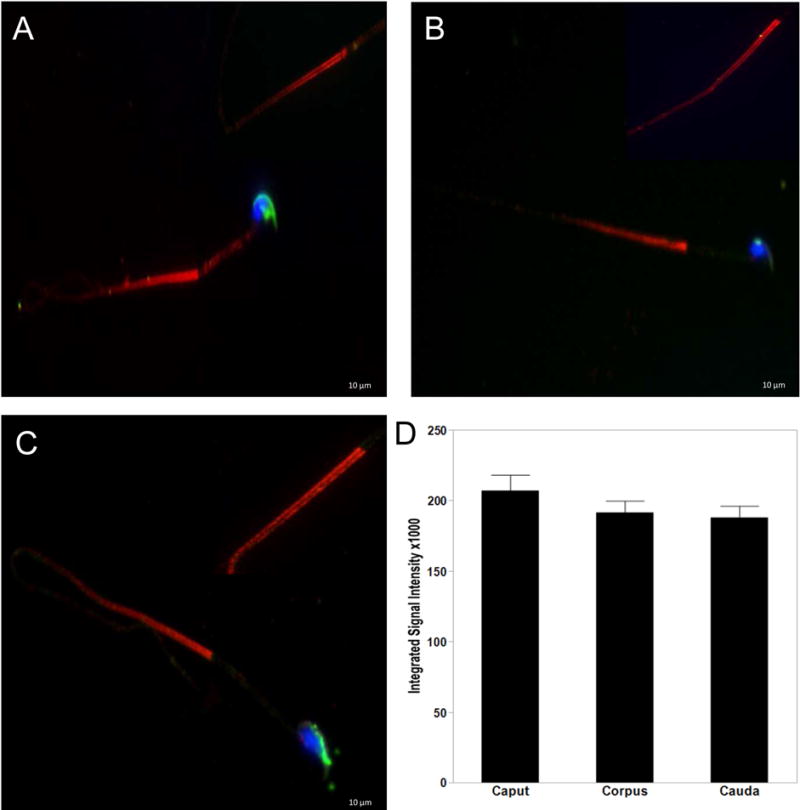
Sperm collected from the caput (A), corpus (B) and cauda (C) were triple stained with antibodies to AURKA (red), conjugated PNA-Lectin AF488 (green) for the acrosome and DAPI to visualize the nuclei (blue). AURKA is concentrated at the annulus and expressed throughout the principle piece of the flagellum as two parallel stripes (indicated by arrows in A inset). AURKA was not observed associated with the nucleus, acrosome or midpiece. AURKA expression decreased as sperm traveled through the epididymis, which is graphically represented in (D). Scale bars indicate 10μM.
The principal piece of the sperm flagellum is composed of a microtubulebased axoneme surrounded by outer dense fibers overlaid by a fibrous sheath [14-16]. These cytoskeletal structures are specific to the sperm flagella and provide stability to the flagella during motility. In addition to a structural role, the fibrous sheath contains A-kinase anchoring proteins thought to act as a scaffold for signaling proteins regulating sperm motility and hyperactivation [17]. Localization of AURKA to parallel structures along the sperm principal piece is reminiscent of the longitudinal columns of the fibrous sheath surrounding the axoneme and places this kinase in a localization containing signaling molecules with roles in regulating sperm maturation, motility, capacitation, and hyperactivated motility [14]. The association of AURKA with the sperm principal piece is consistent with our hypothesis that AURKA regulates proteins with roles in sperm motility.
3.3 Activated Aurora A Kinase is found in the midpiece of the sperm flagellum
AURKA kinase is activated prior to mitosis by phosphorylation of threonine 288 in the activation loop [18, 19]. We next examined the localization of activated AURKA in the flagella using a phosphor-Thr288 specific antibody (Figure 4). Sperm from the caput (Figure 4A), corpus (Figure 4B) and cauda (Figure 4C) were triple stained with antibodies to phospho-AURKA (Thr288), PNA-Lectin AF488 and DAPI. Activated AURKA was primarily found in the sperm midpiece with lesser staining in the acrosome and principal piece. Phospho-AURKA also orients in two parallel lines, but only in the midpiece (Figure 4A-C inlays). Interestingly, expression of active AURKA was significantly increased in motile cauda sperm when compared to both the caput p<0.0019 and corpus p<0.0237 containing primarily immotile sperm (Figure 4D) suggesting that AURKA plays a role in sperm motility.
Figure 4. Phospho-Aurora A localizes to the flagellum of mature spermatozoa.
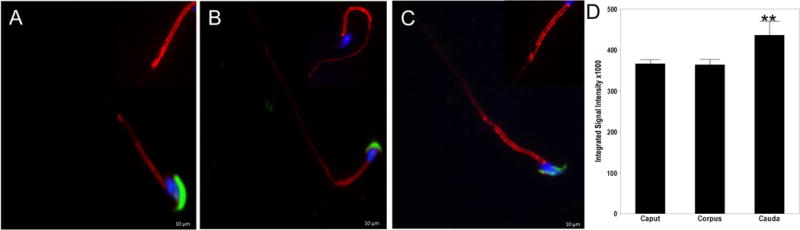
Sperm collected from the caput (A), corpus (B) and cauda (C) were triple stained with phosphor-Aurora A (Thr288) (red), conjugated PNA-Lectin AF488 (green) and DAPI to visualize the nuclei (blue). Active AURKA is also arranged into two parallel lines. However, expression is localized to the midpiece of epididymal sperm. (D) Expression of active AURKA was significantly increased in cauda sperm when compared to both the caput p<0.0019 and corpus p<0.0237 (D). Scale bars indicate 10μM.
Sperm maturation in the epididymis and acquisition of motility is achieved through a cAMP (PKA) dependent tyrosine phosphorylation cascade [20]. Our results indicate that activated AURKA is localized to the midpiece of the sperm flagella of motile sperm collected from the cauda epididymis. This pattern of expression parallels an increase in protein tyrosine phosphorylation of proteins of the midpiece [17]. AURKA is a known substrate of PKA, suggesting that this kinase functions in the signaling pathway responsible for initiation of sperm motility [19].
In this study, we show that AURKA is not only localized to dividing cells of the seminiferous epithelia (as expected due to its role in spindle formation) but also in spermatids and the sperm flagellum suggesting a unique role for AURKA in spermatogenesis, spermiogenesis, and sperm flagella function. Our research is the first to report an extra-mitotic function for AURKA. Sperm flagella formation and acquisition of motility are critically important to male fertility. Infertility affects approximately 48.5 million couples globally with 50% of overall cases attributed to sperm flagella defects resulting in defects in motility (Agarwal et al 2015). Uncovering the molecular processes that control formation, differentiation and motility of mammalian sperm is essential for the advancement of treatments for male infertility as well as for the development of non-hormonal approaches to contraception.
Highlights.
Aurora A kinase is a previously identified mitotic kinase.
Developing male germ cells express Aurora A including spermatogonia, spermatocytes, and spermatids.
Aurora A kinase and its activated form are localized to the flagella of sperm.
Activated Aurora A kinase is enriched in motile sperm of the epididymis suggesting a role in sperm motility.
Acknowledgments
The Authors acknowledge funding from the National Institutes of Health (HD030151) to A.O.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The Authors declare no conflict of interest.
(1) All third-party financial support for the work in the submitted manuscript. No third-party financial support
(2) All financial relationships with any entities that could be viewed as relevant to the general area of the submitted manuscript. No financial relationships
(3) All sources of revenue with relevance to the submitted work who made payments to you, or to your institution on your behalf, in the 36 months prior to submission. No payments to me.
(4) Any other interactions with the sponsor of outside of the submitted work should also be reported. No interactions to report
(5) Any relevant patents or copyrights (planned, pending, or issued). No patents or copywrites
(6) Any other relationships or affiliations that may be perceived by readers to have influenced, or give the appearance of potentially influencing, what you wrote in the submitted work. No other relationships.
References
- 1.Freitas MJ, Vijayaraghavan S, Fardilha M. Signaling mechanisms in mammalian sperm motility. Biol Reprod. 2017;96:2–12. doi: 10.1095/biolreprod.116.144337. [DOI] [PubMed] [Google Scholar]
- 2.Baker MA. Proteomics of post-translational modifications of mammalian spermatozoa. Cell Tissue Res. 2016;363:279–287. doi: 10.1007/s00441-015-2249-x. [DOI] [PubMed] [Google Scholar]
- 3.Almog T, Naor Z. The role of Mitogen activated protein kinase (MAPK) in sperm functions. Mol Cell Endocrinol. 2010;314:239–243. doi: 10.1016/j.mce.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Li MW, Mruk DD, Cheng CY. Mitogen-activated protein kinases in male reproductive function. Trends Mol Med. 2009;15:159–168. doi: 10.1016/j.molmed.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crane R, Gadea B, Littlepage L, Wu H, Ruderman JV. Aurora A, meiosis and mitosis. Biol Cell. 2004;96:215–229. doi: 10.1016/j.biolcel.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Marumoto T, Zhang D, Saya H. Aurora-A a guardian of poles. Nature reviews Cancer. 2005;5:42–50. doi: 10.1038/nrc1526. [DOI] [PubMed] [Google Scholar]
- 7.Carmena M, Earnshaw WC. The cellular geography of aurora kinases. Nat Rev Mol Cell Biol. 2003;4:842–854. doi: 10.1038/nrm1245. [DOI] [PubMed] [Google Scholar]
- 8.Yang KT, Tang CJ, Tang TK. Possible Role of Aurora-C in Meiosis. Front Oncol. 2015;5:178. doi: 10.3389/fonc.2015.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kimmins S, Crosio C, Kotaja N, Hirayama J, Monaco L, Hoog C, van Duin M, Gossen JA, Sassone-Corsi P. Differential Functions of the Aurora-B and Aurora-C Kinases in Mammalian Spermatogenesis. Mol Endocrinol. 2007;21:726–739. doi: 10.1210/me.2006-0332. [DOI] [PubMed] [Google Scholar]
- 10.Tang CJ, Lin CY, Tang TK. Dynamic localization and functional implications of Aurora-C kinase during male mouse meiosis. Dev Biol. 2006;290:398–410. doi: 10.1016/j.ydbio.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 11.Bellve AR. Purification, culture, and fractionation of spermatogenic cells. Methods Enzymol. 1993;225:84–113. doi: 10.1016/0076-6879(93)25009-q. [DOI] [PubMed] [Google Scholar]
- 12.Syntin P, Cornwall GA. Immunolocalization of CRES (Cystatin-related epididymal spermatogenic) protein in the acrosomes of mouse spermatozoa. Biol Reprod. 1999;60:1542–1552. doi: 10.1095/biolreprod60.6.1542. [DOI] [PubMed] [Google Scholar]
- 13.Tulsiani DR, Nagdas SK, Cornwall GA, Orgebin-Crist MC. Evidence for the presence of high-mannose/hybrid oligosaccharide chain(s) on the mouse ZP2 and ZP3. Biol Reprod. 1992;46:93–100. doi: 10.1095/biolreprod46.1.93. [DOI] [PubMed] [Google Scholar]
- 14.Eddy EM. The scaffold role of the fibrous sheath. Soc Reprod Fertil Suppl. 2007;65:45–62. [PubMed] [Google Scholar]
- 15.Eddy EM, O’Brien DA, Fenderson BA, Welch JE. Intermediate filament–like proteins in the fibrous sheath of the mouse sperm flagellum. Ann N Y Acad Sci. 1991;637:224–239. doi: 10.1111/j.1749-6632.1991.tb27312.x. [DOI] [PubMed] [Google Scholar]
- 16.Oko R. Comparative analysis of proteins from the fibrous sheath and outer dense fibers of rat spermatozoa. Biol Reprod. 1988;39:169–182. doi: 10.1095/biolreprod39.1.169. [DOI] [PubMed] [Google Scholar]
- 17.Urner F, Sakkas D. Protein phosphorylation in mammalian spermatozoa. Reproduction. 2003;125:17–26. doi: 10.1530/rep.0.1250017. [DOI] [PubMed] [Google Scholar]
- 18.Littlepage LE, Wu H, Andresson T, Deanehan JK, Amundadottir LT, Ruderman JV. Identification of phosphorylated residues that affect the activity of the mitotic kinase Aurora-A. Proc Natl Acad Sci U S A. 2002;99:15440–15445. doi: 10.1073/pnas.202606599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walter AO, Seghezzi W, Korver W, Sheung J, Lees E. The mitotic serine/threonine kinase Aurora2/AIK is regulated by phosphorylation and degradation. Oncogene. 2000;19:4906–4916. doi: 10.1038/sj.onc.1203847. [DOI] [PubMed] [Google Scholar]
- 20.Leclerc P, de Lamirande E, Gagnon C. Cyclic adenosine 3′,5′monophosphatedependent regulation of protein tyrosine phosphorylation in relation to human sperm capacitation and motility. Biol Reprod. 1996;55:684–692. doi: 10.1095/biolreprod55.3.684. [DOI] [PubMed] [Google Scholar]


