Abstract
Impaired social interaction is a key feature of several major psychiatric disorders including depression, autism and schizophrenia. While, anatomically, the prefrontal cortex (PFC) is known as a key regulator of social behavior, little is known about the cellular mechanisms that underlie impairments of social interaction. One etiological mechanism implicated in the pathophysiology of the aforementioned psychiatric disorders is cellular stress and consequent adaptive responses in the endoplasmic reticulum (ER) that can result from a variety of environmental and physical factors. The ER is an organelle that serves essential roles in protein modification, folding, and maturation of proteins, however, the specific role of ER stress in altered social behavior is unknown. In this study, treatment with tunicamycin, an ER stress inducer enhanced the phosphorylation level of inositol-requiring ER-to-nucleus signal kinase 1 (IRE1) and increased X-box-binding protein 1 (XBP1) mRNA splicing activity in the mouse PFC, whereas inhibition of IRE1/XBP1 pathway in PFC by a viral particle approach attenuated social behavioral deficits caused by tunicamycin treatment. Reduced estrogen receptor beta (ERβ) protein levels were found in the PFC of male mice following tunicamycin treatment. Pretreatment with an ERβ specific agonist, ERB-041 significantly attenuated tunicamycin-induced deficits in social behavior, and activation of IRE1/XBP1 pathway in mouse PFC. Moreover, ERB-041 inhibited tunicamycin-induced increases in functional connectivity between PFC and hippocampus in male mice. Together, these results show that ERβ agonist attenuates ER stress-induced deficits in social behavior through the IRE-1/XBP1 pathway.
Keywords: ER stress, social behavior, estrogen, IRE1, connectivity
Introduction
Impaired social interaction is a key feature of several major psychiatric disorders including depression, autism and schizophrenia [1]. Previous studies have shown the role of various genetic as well as epigenetic factors in social behaviors in both nonhuman primates and rodents [2]. Convergent research from animal models to human disease implicates the prefrontal cortex (PFC) as a key regulator in social behavior [3]. Postmortem studies have linked changes in the expression of various genes in PFC involved in synaptic plasticity to social behavior alterations in the above psychiatric conditions [4,5,6]. Furthermore, functional magnetic resonance imaging (fMRI) studies have shown altered functional connectivity between cortical regions across a variety of diseases with social behavior deficits, including autism [7], schizophrenia [8], and major depression [9]. The above findings are further supported by in vivo electrophysiology studies in rodents linking abnormal PFC-hippocampus connectivity to social behavior deficits [10]. In addition, other animal studies suggest that changes in social behavior are sex-specific. Ovariectomized rats spent less time interacting with a novel rat than familiar ones [11]. Additional studies suggest a potential role of sex hormones, in particular estrogen in social behavior [12, 13, 14]. For example, social behavioral deficits have been reported in estrogen receptor β (ERβ) KO mice [15] whereas estradiol replacement improves social memory in ovariectomized animals [16]. While the studies described above suggest the role of sex hormones in social behaviors, the cellular mechanisms mediating these effects are unknown.
The endoplasmic reticulum (ER) is an intracellular organelle that serves key functions involving in the biosynthesis of membrane and secretory proteins, synthesis of lipids, and maintenance of intracellular calcium homeostasis [17]. A number of pathophysiological stimuli such as viral infections, environmental toxins, inflammatory cytokines, and genetic mutations such as H246N and Y251S, in the gene-encoding synaptic cell adhesion molecule-1 can impose stress on the ER and subsequently interrupt the protein folding process in the ER, leading to accumulation of unfolded or misfolded proteins in the ER lumen called ER stress [18, 19]. Three ER-resident proteins have been identified as sensors of ER stress: IRE1 (inositol-requiring protein 1), PERK [PKR (double-stranded-RNA-dependent protein kinase)-like ER kinase] and ATF6 (activating transcription factor [20]. IRE1 is a type 1 transmembrane serine/threonine receptor protein kinase which functions as a sensor for misfolded/unfolded proteins in the ER lumen. Activated IRE1 induces the splicing of XBP1 (X-box-binding protein 1) mRNA by cleaving off its intron [21]. PERK is a type 1 transmembrane protein kinase that transmits stress signals in response to the perturbation of protein folding [22]. When activated, PERK phosphorylates theα subunit of eIF2 (eukaryotic initiation factor 2) leading to the translation of ATF4 and activation of the C/EBP homologous protein (CHOP) promoter [23]. ER stress activates ATF6 by translocating it from the ER to Golgi complex, where it is cleaved by the Golgi-resident serine proteases S1P and S2P (site 1 and site 2 proteases respectively) resulting in the activation of the transcription of unfolded protein response (UPR) targets such as 78 kDa glucose-regulated protein (GRP78), CHOP and XBP1 [24]. The UPR is generally a pro-survival mechanism, mediated by translation arrest and the induction of a number of transcription factors and chaperone proteins that function to restore ER homeostasis and help the cells adapt to ER stress conditions. However, when ER stress is prolonged or the degree of ER stress is too severe, UPR signaling can initiate programmed cell death by activating stress-induced pro-apoptotic factors [25, 26]. A number of studies have demonstrated that ER stress is involved in autism [27], depression [28] and schizophrenia [29]. However, it remains unclear whether elevated ER stress leads specifically to impaired social interaction.
To investigate the role of ER stress in social behavior, we induced ER stress in mice by tunicamycin administration. Tunicamycin is an inhibitor of the UDP-N-acetylglucosamine-dolichol phosphate N-acetylglucosamine-1-phosphate transferase (GPT), therefore blocking the initial step of glycoprotein biosynthesis in the ER leading to ER stress [30]. We found that tunicamycin treatment induces social interaction deficits and alterations in functional brain connectivity in male mice. We observed a significant reduction in ERβ protein levels in the PFC of male mice following tunicamycin treatment, while pretreatment with ERβ agonist, ERB-041 attenuated tunicamycin–induced social interaction deficits and changes in brain connectivity. Indeed, pretreatment with ERB-041 significantly decreased the ER stress-induced increase in IRE1 activation and CHOP (a downstream target of IRE-1/XBP1 pathway) mRNA levels in mouse PFC suggesting that inhibition of IRE1/XBP1 signaling could be a potential mechanism involved in the ERβ signaling mediated rescue of impaired social behavior.
Materials and Methods
Animals
Adult (8–10 week old) C57BL/6J male and female mice were purchased from Charles River Laboratories (Wilmington, MA, USA). Mice ovariectomized at 4-weeks of age were purchased from Jackson Laboratory (Bar Harbor, Maine, USA). Mice were housed in groups of 4 mice in standard polypropylene cages in 12-h light-dark cycle. All behavior experiments were performed at 8–10 weeks of age. Separate cohorts of animals were used for different behavioral analyses due to the acute response of tunicamycin and the length of time needed to perform behavioral testing. The same animals that were used for behavioral analysis were used for molecular studies.
Drug treatment
Mice were injected intraperitoneally with 1mg/kg tunicamycin (catalog #T7765; Sigma, St. Louis, Missouri) dissolved in DMSO (vehicle control). Tunicamycin is known to cross blood brain barrier [31] and previous studies have shown that tunicamycin administration (1 mg/kg; IP) induces increases in ER stress markers in mouse brain [32, 33]. The behavioral effects of ERB-041 are less well characterized than those of other ER subtype agonists, such as propyl pyrazole triol (PPT) and diarylpropionitrile (DPN). The few behavioral studies used 0.9 mg/kg of DPN and PPT; and showed that both drugs enhanced novel object recognition [34] and object placement [35]. Given the high affinity and selectivity of ERB-041 for ERβ [36], we chose 1 mg/kg to use in our study. It is known that ERB-041 reaches maximum brain levels 30 minutes after injection [36]. Therefore, ERB-041 (catalog #PZ0183; Sigma, St. Louis, Missouri) was administered 30 minutes before tunicamycin injection.
Surgery and EEG recordings
Anesthesia was induced with ketamine-xylazine (80mg/kg–5mg/kg) in sterile distilled water. The mouse was then placed in a stereotaxic frame with heating pad (Kopf, Tujunga, California). The skull was exposed and perfluoroalkoxy-coated tungsten wire electrodes were placed into bur holes in mPFC (1.8mm anterior, 0.5mm lateral, 1.5mm deep), dorsal hippocampus (1.9mm posterior, 1.4mm lateral, 1.35mm deep), and ventral hippocampus (3.2mm posterior, 3.1mm lateral, 3.85mm deep) relative to bregma. Reference and ground screws attached to electrodes were placed at neutral locations superficial to parietal and occipital cortex, respectively. Electrodes were soldered to a 7 pin adaptor and fixed to the skull with dental cement (Co-oral-lte Dental Manufacturing Company, Diamond Springs, California). Once the cement had dried, animals were removed from the stereotaxic instrument, injected intraperitoneally with 0.1 mL saline and buprenorphine (0.2 mg/kg) and returned to a clean cage and singly housed. Mice were given an additional buprenorphine injection (0.2 mg/kg) the following day. Mice were monitored daily for 2 weeks to assure proper recovery. Experiments were performed 2 weeks post-surgery. Recording quality was tested and animals with poor signals were excluded from the study.
EEG recordings were performed 12 hours after vehicle (DMSO, i.p.), tunicamycin (1mg/kg, i.p.), or ERB-041 (1mg/kg, i.p.) + tunicamycin (1mg/kg i.p.), between 9am and 12pm. For recording purposes wireless Neurologger 2a (Evolocus LLC, Tarrytown, NY) was used to monitor EEG activity. The Neurologger 2a apparatus was attached to the mounted 7 pin adaptor of the animal and allowed recording from up to 4 channels at a sampling rate of 1600Hz (reference assignment 2:2; oversampling x4). Recordings were downloaded offline to a PC for analysis. Data were analyzed in MATLAB (MathWorks, Natick, Massachusetts) using custom scripts.
Stereotaxic Injection of Lentivirus or drugs
IRE1 shRNA (m) Lentiviral (LV) particles and its control shRNA LV particles were purchased from Santa Cruz, CA, USA. LV-IRE1-shRNA is a pool of concentrated, transduction-ready viral particles containing 4 target-specific constructs that encode 19–25 nt (plus hairpin) shRNA designed to knock down gene expression. shRNA lentiviral particles frozen stock contains a concentration of 1.0 × 106 infectious units of virus in Dulbecco’s Modified Eagle’s Medium with 25 mM HEPES pH 7.3. Lentiviral particles were infused into mouse PFC (anterior-posterior AP=+1.8 mm, mediolateral ML= 0 mm; dorso-ventral DV= −2.5 mm) or hippocampus (coordinates: 2.3 mm posterior to bregma, 1.3 mm lateral to the midline, and 2.0 mm below dura) by stereotaxic microinjection at a rate of 0.2 µl/min at each site (Stoelting Co) [37]. In separate set of experiments, mice were stereotaxically microinjected with vehicle or tunicamycin into PFC under anesthesia as described above. In the pretreatment groups, ERB041 or vehicle was injected into PFC 30 min before tunicamycin administration. Behavior studies were performed 12 h post tunicamycin treatment.
Behavior experiments
Behavioral testing was performed in a room with constant background sound and ambient lighting approximately 25–30 Lux (lumen/m2) unless noted. Temperature and pressure in behavioral rooms are monitored and kept constant. Animals are transferred in their home cages to behavioral rooms at least 1 hour before testing and allowed to habituate to the testing room. All behavioral experiments were scored blind to treatment.
Three Chamber Test
This test was performed to measure sociability and social deficits. The test mouse was placed in a box with 3 chambers. Each chamber is 19 cm × 45 cm × 22cm and the dividing walls are made from clear Plexiglas®, with openings on each wall for free access to the other two chambers. Two identical wire containers that were large enough to house a single mouse were placed vertically inside the apparatus with one in each side chamber and weighted down. The test mouse was habituated to the apparatus for 5 minutes while freely exploring. After the habituation period, the stranger mouse was placed in one of the wire containers while the test mouse was still allowed to freely move outside of the container. The wire containers allow air exchange between the interior and exterior, but the holes are small enough to prevent direct physical contact between the stranger mouse and test mouse. The free test mouse was allowed to interact through the wire container with the stranger mouse for 5 minutes. During this time, time spent in chambers (stranger mouse, empty cage, and center) was recorded by an examiner with a stopwatch. The stranger mouse chamber is defined as the chamber containing the wire container with the stranger mouse inside. The empty cage chamber is the chamber containing an empty wire container. The stranger mouse was a mouse of similar age, same sex, and similar weight as the test mouse.
Reciprocal Social Interaction Test
This test was performed to measure sociability and social deficits. The test mouse was placed in a neutral box (57cm × 45cm × 22cm) made from clear Plexiglas® and allowed to habituate for 5 minutes. After habituation, a stranger mouse was placed in the box and the test mouse was allowed to freely interact with the stranger mouse. Interaction is defined as close physical contact, nose to nose sniffing, anogenital sniffing, and grooming. Time spent interacting (initiated by the test mouse) was recorded by an examiner with a stopwatch. The stranger mouse was a mouse of similar age, same sex, and similar weight as the test mouse.
Western blotting
Animals were sacrificed by cervical dislocation after being anesthetized using isoflurane. PFC tissue was homogenized in a tissue lysis buffer containing 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1.0% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 2 mM EDTA, 6 µM PMSF, and 1.0% Triton X-100 supplemented with protease inhibitor cocktail (Sigma, St. Louis, Missouri). The homogenate was centrifuged at 13,000 rpm for 10 min at 4°C and the supernatant was used for protein estimation by the bicinchoninic acid method (BCA Protein Assay Kit, Sigma, St. Louis, Missouri). Samples (30 µg) were subjected to SDS-PAGE and transferred onto a nitrocellulose membrane. The membrane was blocked for 1 hour in PBS with Tween 20 and 5%–10% non-fat milk followed by overnight incubation with a primary antibody. Blots were incubated in the appropriate primary antibody specific for IRE1 (Santa Cruz Biotechnology, Dallas, TX, catalog #SC-20790, 1:200 and Cell Signaling, Boston, MA, catalog #3294, 1:500), phosphoIRE1 (Novus Biologicals, Littleton, CO, catalog#NB100-2323, 1:1000), tubulin (Cell Signaling, Boston, MA, catalog#2144, 1:10,000), ERβ (Abcam, Cambridge, UK; 3576; 1:1,000); ERα (Santa Cruz Biotechnology, Dallas, Texas; 71064; 1:1,000); and developed with the SuperSignal West Pico Chemiluminescent substrate system (Thermo Fisher Scientific, West Columbia, SC). Optical densities of the bands were analyzed using ImageJ software (NIH). For analysis, protein levels were normalized to tubulin levels, and then expressed as a fold change of that in control animals. For figure panels, contrasts have been adjusted linearly for easier viewing of bands.
Quantitative reverse transcriptase PCR (qRT-PCR)
RNA was purified using a commercially available kit (SV RNA Isolation, Promega, Madison, WI, USA), qRT-PCR was performed on a MasterCycler (Eppendorf, Hamburg, Germany) using a SuperScript III Platinum SYBR Green One-Step qRT-PCR kit (Invitrogen, Carlsbad, CA, USA). Gene-specific primers were synthesized by Integrated DNA Technologies. Primers used are: CHOP-FP: 5’- CATACACCACCACACCTGAAAG -3’, CHOP-RP: 5’- CCGTTTCCTAGTTCTTCCTTGC -3’, sXBP1-FP: 5’- CTGAGTCCGAATCAGGTGCAG-3′, sXBP1-RP: 5’- GTCCATGGGAAGATGTTCTGG-3’, RPS3-FP: 5’- AATGAACCGAAGCACACCATA-3’, and RPS3-RP: 5’- ATCAGAGAGTTGACCGCAGTT-3’. Ct values of genes of interest were normalized to that of housekeeping gene (RPS3).
Statistical analysis
All data are presented as mean ± s.e.m. (error bars). Statistical results, along with tests used, are summarized in Supplementary Table 1. p < 0.05 was considered significant. For behavioral studies we used a two-way ANOVA or one-way ANOVA with a Bonferroni multiple comparison post-hoc test unless otherwise specified in the figure legend or Supplementary Table 1. For quantification of western blots or RT-PCR experiments, we used a one-way ANOVA with Bonferroni’s post-hoc test or Student’s t-test. To compare changes in group means in frequency in EEG recordings, a one-way ANOVA with Bonferroni’s post-hoc test was used. All analyses were performed using SPSS Statistics 20 software (IBM).
Results
IRE1/XBP1 pathway mediates ER stress-induced social interaction deficits in male mice
The IRE1/XBP1 pathway is the most conserved ER stress-response pathway. The activation of IRE1 results in non-conventional splicing of the mRNA encoding the transcription factor XBP1, generating a spliced active form of XBP1 (sXBP1) to initiate a major UPR program. To determine whether IRE1/XBP1 pathway is altered in PFC following ER stress, we first examined the phosphorylation status of IRE1 in PFC of mice treated with tunicamycin (Figure 1a). We found significant increase in phospho-IRE1 levels in the PFC of tunicamycin-treated mice indicating activation of IRE1 following ER stress (Figure 1b). Also, we found significant increase in splicing of XBP1 as determined by the mRNA levels of sXBP1 in PFC of tunicamycin-treated mice (Figure 1c).
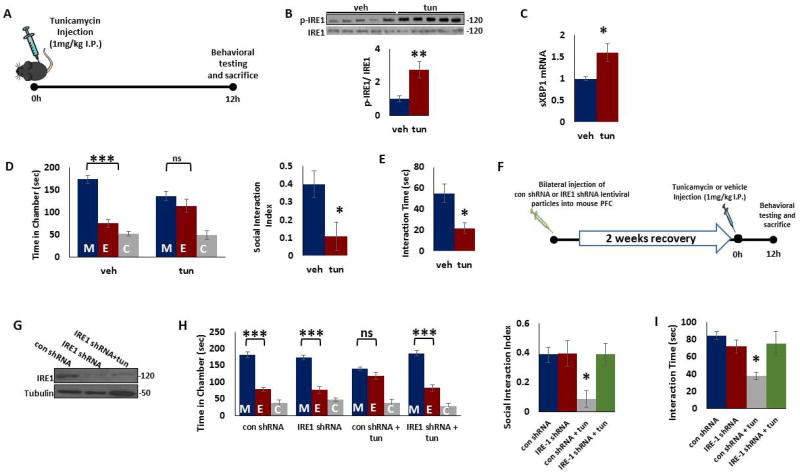
Figure 1. IRE-1 in PFC mediates ER stress-induced deficits in social behavior in male mice.
A) Treatment paradigm. Young adult male mice were injected intraperitoneally with tunicamycin (1mg/kg in dimethyl sulfoxide, DMSO) or vehicle (DMSO). Behavior tests were performed 12 h after tunicamycin injection. B) Tunicamycin treatment induced activation of IRE1. Phospho-IRE1 and IRE1 protein levels were determined in the mouse prefrontal cortex (PFC) 12 h after tunicamycin injection. Top. Representative blot. Bottom. Quantification of phospho-IRE1 to IRE1 ratio. Protein levels were measured by western blot analysis. **p < 0.01; Student's t-test. C) Tunicamycin treatment induced increase in spliced XBP1 (sXBP1) mRNA levels. mRNA levels of sXBP1 were determined by qRT-PCR in the mouse PFC 12 h after tunicamycin injection. The Cycle threshold (Ct) values were normalized to ribosomal protein S3 (RPS3). *p < 0.05; Student's t-test. D–E) Tunicamycin treatment induced deficits in social behavior. D) The three-chamber social interaction test. Left, time in chamber. ***p<0.001 vs. stranger mouse chamber. Two-way ANOVA (n=7 per group). Right, the discrimination index calculated as the difference in the time spent in the social and non-social chambers, divided by the sum of the time spent in both chambers. *p<0.05; Student's t-test (n=7 per group). E) Reciprocal social interaction test. *p<0.05; Student's t-test (n=7 per group). F) Schematic representation of stereotaxic injection of control or IRE1 shRNA lentiviral particles into mouse PFC followed by tunicamycin treatment for 12 h. G) Representative immunoblot data showing decrease in IRE1 expression in the PFC of mice injected with IRE1 shRNA particles in the presence or absence of tunicamycin. Tubulin was used as loading control. H–I) IRE1 shRNA administration attenuated tunicamycin-induced deficits in social behavior. H) The three-chamber social interaction test. Left, time in chamber. ***p<0.001 vs. stranger mouse chamber. Two-way ANOVA (n=6 per group). Right, the discrimination index calculated as the difference in the time spent in the social and non-social chambers, divided by the sum of the time spent in both chambers. *p<0.05 vs. con shRNA group; One-way ANOVA (n=6 per group). I) Reciprocal social interaction test. *p<0.05 vs. con shRNA group; One-way ANOVA (n=6 per group). Data are expressed as mean ±s.e.m. M, chamber housing stranger mouse; E, chamber housing an empty cage; C, center. ns, non-significant.
To determine the effects of ER stress on social behavior, we performed three chamber test and reciprocal interaction test in mice treated with tunicamycin or vehicle. In Three-chamber test, we found that whereas vehicle-injected mice spent more time in the chamber housing stranger mouse than the empty cage chamber, tunicamycin-injected mice had no preference for either chamber (Figure 1d). In Reciprocal Social Interaction test, tunicamycin-injected mice showed decreased interaction with a stranger mouse when compared with those from vehicle-treated group (Figure 1e).
To determine the direct role of IRE1 in ER stress-induced social interaction deficits in male mice, we silenced IRE1 expression in mouse PFC using lentiviral vectors expressing IRE1 shRNA (Figure 1f). A significant reduction in IRE1 protein levels was found in mouse PFC following IRE1 shRNA administration (Figure 1g). We found that tunicamycin induced deficits in three chamber test (Figure 1h) and reciprocal interaction test (Figure 1i) in control shRNA-treated mice, but not in IRE1 shRNA-injected mice. However, IRE1 shRNA administration into hippocampus failed to attenuate tunicamycin-induced social behavior in three chamber (Figure S1a) and reciprocal interaction tests (Figure S1b). Together, these results indicate the important role of IRE1 in PFC in ER stress-induced changes in social behavior.
Tunicamycin treatment does not induce social behavior deficits in female mice
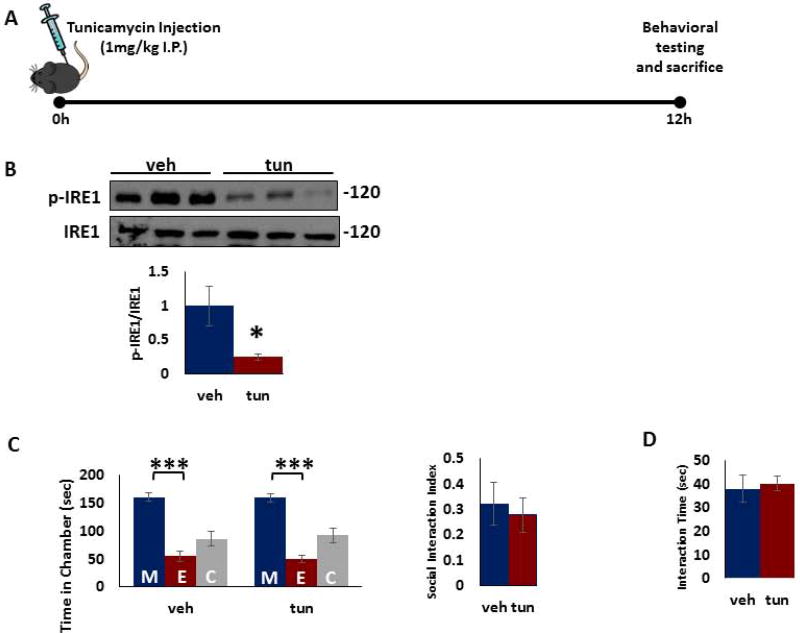
Next, we examined the effects of tunicamycin treatment on IRE-1 phosphorylation and social interaction in female mice (Figure 2a). Tunicamycin treatment induced a significant decrease in IRE1 phosphorylation in PFC of female mice (Figure 2b). In behavior tests, tunicamycin treatment did not result in any significant changes in three chamber test (Figure 2c) and reciprocal interaction test (Figure 2d) in female mice as compared to vehicle treated mice.
Figure 2. ER stress by tunicamycin treatment does not induce social behavior deficits in female mice.
A) Treatment paradigm. Young adult female mice were injected intraperitoneally with tunicamycin (1mg/kg in dimethyl sulfoxide, DMSO) or vehicle (DMSO) and behavioral testing was performed 12 hours after injection. B) Tunicamycin treatment induced decrease in phospho-IRE1 protein levels. Phospho-IRE1 and IRE1 protein levels were determined in the mouse PFC 12 h after tunicamycin injection. Top. Representative blot. Bottom. Quantification of phospho-IRE1 to IRE1 ratio. Protein levels were measured by western blot analysis. *p < 0.05; Student's t-test. C–D) No change in social behavior following tunicamycin treatment. C) The three-chamber social interaction test. Left, time in chamber. ***p<0.001 vs. stranger mouse chamber. Two-way ANOVA. Right, the discrimination index calculated as the difference in the time spent in the social and non-social chambers, divided by the sum of the time spent in both chambers. D) Reciprocal social interaction test. Data are expressed as mean ±s.e.m (n=6–8 per group). M, chamber housing stranger mouse; E, chamber housing an empty cage; C, center.
Tunicamycin treatment induces decrease in ERβ protein levels in PFC of male mice
Since we did not find any significant effect of tunicamycin on social behavior in female mice, we examined whether ovariectomy influences the behavior in mice. Ovariectomized mice showed significant deficits in the social behavior in three chamber test (Figure 3a) and reciprocal interaction test (Figure 3b) suggesting a potential role of ovarian hormones in social behavior in mice. In order to examine whether tunicamycin treatment influences estrogen signaling, we examined the levels of the key estrogen receptors, ERα and ERβ in male mouse PFC following tunicamycin treatment. ERβ total protein levels were found to be significantly decreased in PFC (Figure 3c) of mice treated with tunicamycin as compared to vehicle treated mice. No change in ERα protein levels was found in PFC following tunicamycin treatment (Figure 3d). We did not find any significant change in ERβ protein levels in the PFC of female mice (Fig 3e).
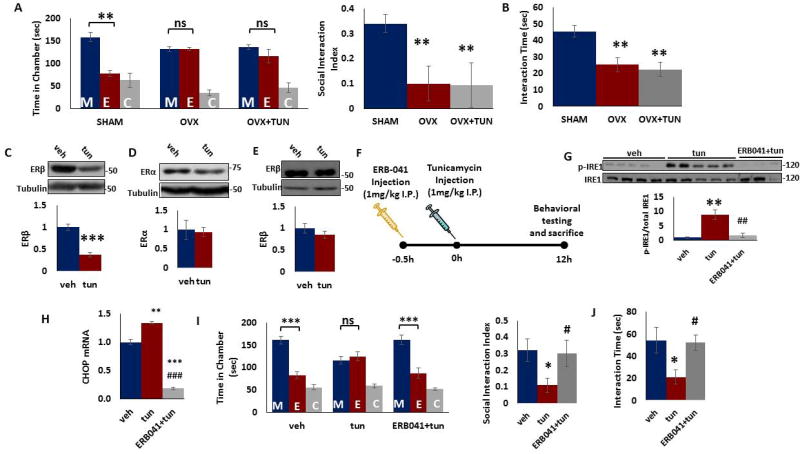
Figure 3. Peripheral administration of ERβ agonist ERB-041 attenuates ER stress-induced social behavior deficits in male mice.
A–B) Ovariectomy induces changes in social behavior. Adult sham–operated (SHAM), ovariectomized (OVX) mice and OVX-mice treated intraperitoneally with tunicamycin (1mg/kg) were tested for social behavior. A) The three-chamber social interaction test. Left, time in chamber. **p<0.01 vs. stranger mouse chamber. Two-way ANOVA (n=5 per group). Right, the discrimination index calculated as the difference in the time spent in the social and non-social chambers, divided by the sum of the time spent in both chambers. **p<0.01 vs. SHAM; One-way ANOVA (n=5 per group). B) Reciprocal social interaction test. **p<0.01 vs. SHAM; One-way ANOVA (n=5 per group). C–D) Adult male mice were injected intraperitoneally with tunicamycin (1mg/kg in DMSO) or vehicle (DMSO). Tunicamycin treatment induced decrease in (C) ERβ, but not (D) ERα protein levels in mouse PFC 12 h after injection. Top. Representative blot. Bottom. Quantification of ERβ protein. Protein levels were measured by western blot analysis and normalized to tubulin. ***p<0.001 vs. vehicle; Student's t test. E) No change in ERβ protein levels in PFC of female mice following tunicamycin injection. Top. Representative blot. Bottom. Quantification of ERβ or ERα protein. Protein levels were measured by western blot analysis and normalized to tubulin. F) Treatment paradigm. Adult male mice were injected intraperitoneally with tunicamycin (1mg/kg in dimethyl sulfoxide, DMSO), vehicle (DMSO), or ERB-041 (1mg/kg in DMSO, 30 minutes before tunicamycin injection) and tunicamycin (1mg/kg in DMSO). Behavior was performed 12 hours after tunicamycin injection. G) ERB-041 pretreatment attenuated tunicamycin-induced IRE1 activation. Phospho-IRE1 and IRE1 protein levels were determined in the mouse PFC 12 h after tunicamycin injection. Top. Representative blot. Bottom. Quantification of phospho-IRE1 to IRE1 ratio. Protein levels were measured by western blot analysis. **p<0.01 vs. vehicle, ##p<0.01 vs. tunicamycin group. One-way ANOVA. H) ERB-041 pretreatment attenuated tunicamycin-induced increase in CHOP (CCAAT/enhancer-binding protein homologous protein) mRNA levels. mRNA levels of CHOP were determined by qRT-PCR in the mouse prefrontal cortex (PFC) 12 h after tunicamycin injection. The Ct values were normalized to RPS3. **p<0.01 and ***p<0.001 vs. vehicle; ###p<0.001 vs. tunicamycin group. One-way ANOVA. Data are expressed as mean ±s.e.m. I–J) ERB-041 pretreatment attenuated tunicamycin-induced deficits in social behavior. I) The three-chamber social interaction test. Left, time in chamber. ***p<0.001 vs. stranger mouse chamber. Two-way ANOVA (n=8–10 per group). Right, the discrimination index calculated as the difference in the time spent in the social and non-social chambers, divided by the sum of the time spent in both chambers. *p<0.05 vs. vehicle, #p<0.01 vs. tunicamycin group; One-way ANOVA (n=8–10 per group). J) Reciprocal social interaction test. *p<0.05 vs. vehicle, #p<0.01 vs. tunicamycin group. One-way ANOVA (n=8–10 per group). Data are expressed as mean ±s.e.m. M, chamber housing stranger mouse; E, chamber housing an empty cage; C, center. ns, non-significant.
ERβ agonist ERB-041 attenuates ER stress-induced social interaction deficits in male mice
If a reduction in ERβ signaling mediates the tunicamycin-induced deficits in social behavior in mice, activation of ERβ should ameliorate the behavioral signs. ERB-041 (2-[3-fluoro-4-hydroxyphenyl]-7-vinyl-1,3-benzoxazol-5-ol) is a selective ERβ agonist. Adult male mice were treated with ERB-041 30 min prior to tunicamycin injection and behavioral tests were performed 12 h later (Figure 3f). We examined the effects of ERB-041 on IRE1/XBP1 pathway in PFC samples from tunicamycin-treated male mice. We found that ERB-041 pretreatment significantly attenuated tunicamycin-induced increase in IRE1 activation in PFC of tunicamycin-treated mice (Figure 3g). It is known that CHOP is a downstream target of IRE1/sXBP1 pathway and is induced following ER stress [24, 38]. To determine whether tunicamycin-induced increase in IRE1 pathway is accompanied by increase in CHOP levels, we examined CHOP mRNA levels in mouse PFC following tunicamycin administration. We found significant increases in CHOP mRNA levels in the PFC of male mice treated with tunicamycin (Figure 3h). Moreover, CHOP mRNA levels in ERB-041+tunicamycin-treated mice were significantly lower than those of both tunicamycin and vehicle-treated groups (Figure 3h). In the behavioral tests, the tunicamycin-induced deficits in social behavior in three chamber test (Figure 3i) and reciprocal interaction test (Figure 3j) were significantly reduced by ERB-041 pre-treatment. To further understand the relationship between IRE1and ERβ in tunicamycin-induced ER stress, we examined whether silencing IRE1 in PFC prevents tunicamycin-induced reduction in ERβ protein levels. We found that IRE1 shRNA failed to attenuate the tunicamycin-induced reduction in ERβ protein levels in mouse PFC (Fig S2).
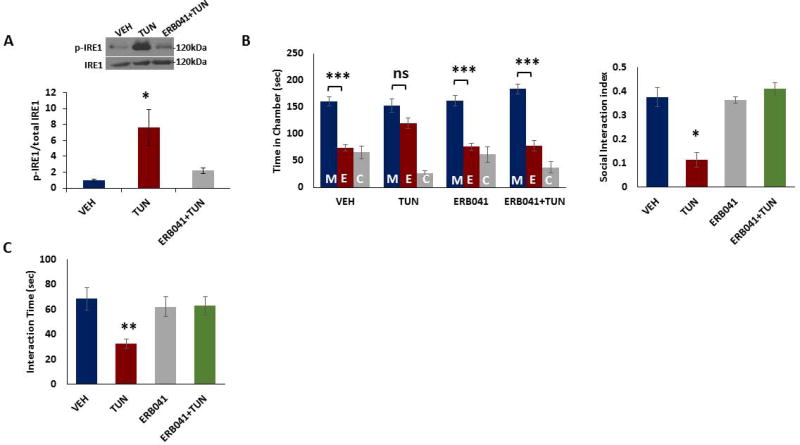
To determine whether ER stress in the PFC is necessary for social behavior deficits, we injected tunicamycin into PFC and social behavior was examined. We found significant increase in phospho-IRE1 levels in the PFC of tunicamycin-injected mice (Figure 4a). Whereas vehicle-injected mice spent more time in the chamber housing stranger mouse than the empty cage chamber, tunicamycin-injected mice had no preference for either chamber (Figure 4b). In addition, tunicamycin-injected mice showed decreased interaction with a stranger mouse when compared with those from vehicle-treated group (Figure 4c). Furthermore, ERB-041 pretreatment into PFC could significantly attenuate tunicamycin-induced increase in phospho-IRE1 levels (Figure 4a) and deficits in social behavior (Figure 4b,c) in mice. Together, these results strongly suggest that reduced ERβ signaling in PFC is involved in the social interaction deficits observed in tunicamycin-treated mice.
Figure 4. ERB-041 administration into PFC attenuates ER stress-induced social behavior deficits in male mice.
Tunicamycin (1mg/kg in dimethyl sulfoxide, DMSO), vehicle (DMSO), or ERB-041 (1mg/kg in DMSO, 30 minutes before tunicamycin injection) and tunicamycin (1mg/kg in DMSO) was injected into mouse prefrontal cortex (PFC), and social behavior was examined at 12 h after tunicamycin administration. A) ERB-041 pretreatment attenuated tunicamycin-induced IRE1 activation. Phospho-IRE1 and IRE1 protein levels were determined in the mouse PFC 12 h after tunicamycin injection. Top. Representative blot. Bottom. Quantification of phospho-IRE1 to IRE1 ratio. Protein levels were measured by western blot analysis. *p < 0.05; On-way ANOVA. B–C) ERB-041 pretreatment attenuated tunicamycin-induced deficits in social behavior. B) The three-chamber social interaction test. Left, time in chamber. ***p<0.001 vs. stranger mouse chamber. Two-way ANOVA (n=7–8 per group). Right, the discrimination index calculated as the difference in the time spent in the social and non-social chambers, divided by the sum of the time spent in both chambers. *p<0.05 vs.vehicle; One-way ANOVA (n=7–8 per group). C) Reciprocal social interaction test. **p<0.01 vs. vehicle; One-way ANOVA (n=7–8 per group). Data are expressed as mean ±s.e.m. M, chamber housing stranger mouse; E, chamber housing an empty cage; C, center. ns, non-significant.
ERβ agonist ERB-041 attenuates ER stress-induced PFC-hippocampus hyperconnectivity in male mice
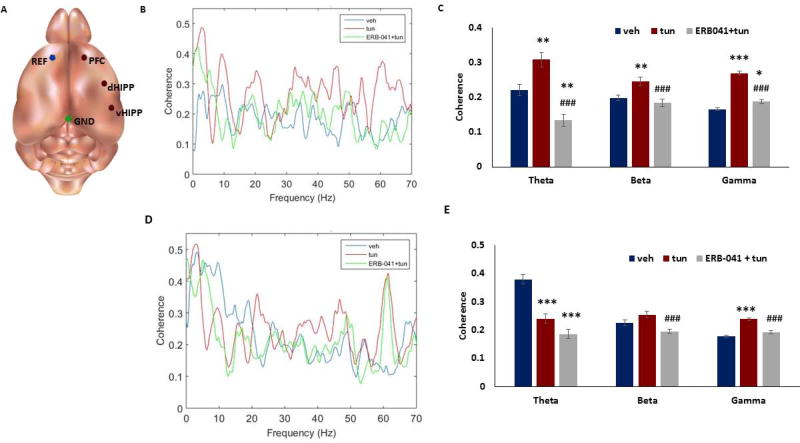
Social behavior deficits are known to be associated with alterations in brain functional connectivity. Since we found deficits in social behavior in male mice following tunicamycin treatment, we examined whether ER stress induces changes in brain functional connectivity. Male mice (3 months old) were implanted with depth electrodes, and local field potentials (LFPs) were recorded with a wireless Neurologger 2A device mounted on the head of the animal (Figure 5a). To quantify long-range functional connectivity, we measured the coherence of LFPs between the medial PFC (mPFC) and dorsal hippocampus (dHIPP) in mice treated with tunicamycin or vehicle. Coherence spectra indicated a significant increase in functional connectivity between mPFC and dHIPP in the theta (4–12 Hz), beta (15–25 Hz) and gamma (26–70 Hz) bands (Figure 5b–c). Interestingly, ERB-041 pretreatment could significantly attenuate tunicamycin-induced increase in mPFC-dHIPP coherence (Figure 5b–c). Also, we examined whether tunicamycin alters brain connectivity between mPFC and ventral HIPP (vHIPP) in mice. We found a decrease in coherence in theta band, but increase in gamma band between mPFC-vHIPP in mice treated with tunicamycin (Figure 5d–e). No significant change was found in mPFC-vHIPP beta band between vehicle and tunicamycin-treated mice. Moreover, the ERB-041 pretreatment significantly reduced the coherence in beta and gamma bands in tunicamycin-treated mice (Figure 5d–e).
Figure 5. ERβ agonist ERB-041 attenuates ER stress-induced brain hyperconnectivity in male mice.
Adult male mice were injected intraperitoneally with tunicamycin (1mg/kg in dimethyl sulfoxide, DMSO), vehicle (DMSO), or ERB-041 (1mg/kg in DMSO, 30 minutes before tunicamycin injection) and tunicamycin (1mg/kg in DMSO). Local field potential (LFP) recordings were performed 12 hours after injection. A) Electrode diagram. REF = reference electrode, mPFC = medial prefrontal cortex electrode, dHIPP = dorsal hippocampal electrode, vHIPP = ventral hippocampal electrode, GND = ground electrode. (B) Coherence v. frequency of mPFC-dorsal HIPP. (C) Coherence in theta (4–12 Hz), beta (15–25 Hz) and gamma (26–70 Hz) bands in mPFC-dorsal HIPP. (D) Coherence v. frequency of mPFC-ventral HIPP. (E) Coherence in theta, beta and gamma bands in mPFC-ventral HIPP. Coherence was determined using values from 3 seconds before and 3 seconds after (for a total of 6 seconds) a novel social interaction with a stranger mouse. *p<0.05, **p<0.01 and ***p<0.001 vs. vehicle; #p<0.05, ##p<0.01 and ###p<0.001 vs. tunicamycin groups. One-way ANOVA. Data are expressed as mean ±s.e.m.
Discussion
Emerging evidence suggests that ER stress may be one potential mechanism of the synaptic deficits and social behavioral alterations that are observed in several neuropsychiatric disorders including schizophrenia, depression and ASD [27, 28, 29, 39, 40]. Moreover, increases in ER stress markers have been found in restraint stress [10] and learned helpless [28] rodent models of depression. In the present study, we used the glycosylation inhibitor, tunicamycin to induce ER stress [41, 42] in mice to determine if induction of this cellular response results in social behavior deficits. Glycosylation is of great physiological significance since changes in glycans significantly alter the structure and function of polypeptide parts of glycoproteins [43]. Proper glycosylation of membrane receptors is critical for adaptive properties of the cell and affects communication between cells [44].
The lack of deficits in social behavior in female mice following tunicamycin treatment suggests that ER stress induced deficits in social behavior in mice are sex specific. Our data on the social behavior deficits in ovariectomized mice further suggest a potential role of estrogen in social behavior. Moreover, the above data are in agreement with a number of previous studies where ovariectomized rats were shown to spend less time interacting with a novel rat than familiar ones did with their intact counterparts [11]. In addition, estradiol replacement has been shown to improve social memory in ovariectomized animals [16]. The decrease in ERβ protein levels in the PFC of tunicamycin-treated male mice observed in this study is consistent with the increasing evidence for the role of estrogen signaling in the social deficits seen in schizophrenia and ASD [12, 13, 14]. In rodent studies, social behavioral deficits have been reported in ERβ KO mice [15]. ERβ mediates some of the effects of estrogens on anxiety, locomotor activity, fear responses, and learning behavior [45]. ERβ knockout mice showed defects of neuronal migration [46], and ERβ knockdown abolished E2-induced reductions in depressive behavior in mice [47]. Moreover, administration of an ERβ agonist has been shown to reduce anxiety and depressive-like behavior in rats [47]. Our data from experiments using an ERβ agonist (ERB-041) further strengthened the role of ERβ in social behavior.
Our data indicating a significant decrease in IRE1 phosphorylation in the PFC of female mice following tunicamycin treatment are intriguing. It is known that IRE1 activation is inhibited in unstressed ER lumen through its interaction with GRP78, an ER molecular chaperone, whereas during ER stress GRP78 releases IRE1 leading to its activation [48]. Interestingly, 17β-estradiol has been shown to protect cells from ER stress-induced apoptosis by promoting GRP78 induction [49]. Therefore, it is possible that in females, high estradiol levels (as compared to males) increases the protein levels of GRP78 and/or promotes GRP78 interaction with IRE-1, which prevents its dissociation leading to inhibition of the IRE1 pathway. However, additional studies are warranted to test this mechanism.
We found hyperconnectivity as indicated by an increase in coherence between mPFC and dorsal hippocampus in theta, beta and gamma bands in tunicamycin-treated mice. Brain hyperconnectivity has been linked to impairments in social behavior. For example, ASD children with greater connectivity exhibited more severe impairment in the social domain [50]. In addition, a number of studies have shown neural network oscillatory abnormalities such as abnormal gamma oscillations in ASD and schizophrenia [51, 52]. Gamma oscillations are instrumental for the synchronization of neuronal discharges in cortical networks and sensory processing [53]. Hyperconnectivity has been observed among the frontal, temporal, and subcortical regions in gamma frequency ranges in subjects with ASD or schizophrenia [54, 55]. The hyperconnectivity in tunicamycin-treated mice in our study could be a result of aberrant balance of excitation and inhibition in local neural circuits [56]. An imbalance between excitation and inhibition has been postulated as a neurophysiological mechanism underlying the social behavior deficits [56]. Together, the brain-behavior relationship found in mice following ER stress suggests that aberrant functional connectivity may underlie the deficits in social behavior.
An important question is: How does ERB041 protect mice from ER stress-induced deficits in social behavior? Activation of ER stress transducer IRE1α is known to produce the spliced form of XBP1 [21]. We investigated whether ERB041 treatment is associated with inhibition of ER stress-induced activation of IRE1. Indeed, pretreatment with ERB-041 significantly decreased the ER stress-induced increase in IRE1 activation in mouse PFC suggesting that inhibition of IRE1/XBP1 signaling could be a potential mechanism involved in ERβ signaling mediated rescue of deficits in social behavior (Figure 6). Moreover, we found a significant reduction in the ER stress-induced increase in CHOP mRNA levels in mice pretreated with ERB-041. It is important to note that ERB-041 not only blocks tunicamycin-induced increases in CHOP mRNA, but it decreases the levels significantly below those of the vehicle-treated group. These data suggest that ERB-041 may act through protective mechanisms in addition to those associated with the tunicamycin-induced signaling pathway. At the cellular level, ER stress via IRE1 signaling has been implicated in mitochondrial dysfunction and oxidative stress [57, 58]. Mitochondria act as a platform for integrating nucleotide-binding domain and leucine-rich repeat containing (NLRP3) inflammasome activation and innate immune signaling [59]. Interestingly, estrogen has been shown to preserve mitochondrial function via increased/enhanced oxidative phosphorylation and reduced ATPase activity [60]. In particular, ERβ activation leads to the translocation of ERβ to the mitochondria and increased expression of mtDNA-encoded cytochrome oxidase I, a critical subunit of the electron transport chain terminal in the brain [61]. Thus, the observed protective effects of ERB-041 in social behavior could be a result of ERβ-mediated improvements in the functional efficiency of mitochondria and reduced oxidative damage. ER stress activates NLRP3 inflammasomes via thioredoxin-interacting protein (TXNIP), leading to increases in proinflammatory cytokine levels [62]. Also, ER stress is known to induce transglutaminase 2, an enzyme involved in proinflammatory signaling [63] and depressive behavior [37] in rodents. Estrogens have been shown to modulate all subsets of T cells that include CD4+ (Th1, Th2, Th17, and Tregs) and CD8+cells [64]. It is important to note that IRE1 shRNA failed to prevent the tunicamycin-induced reduction in ERβ levels in the mouse PFC. Such a data suggest that ERβ functions upstream of IRE1 in the tunicamycin-induced signaling pathway. This model is further supported by our data showing that activation of ERβ could attenuate the tunicamycin-induced increase in IRE1 activation.
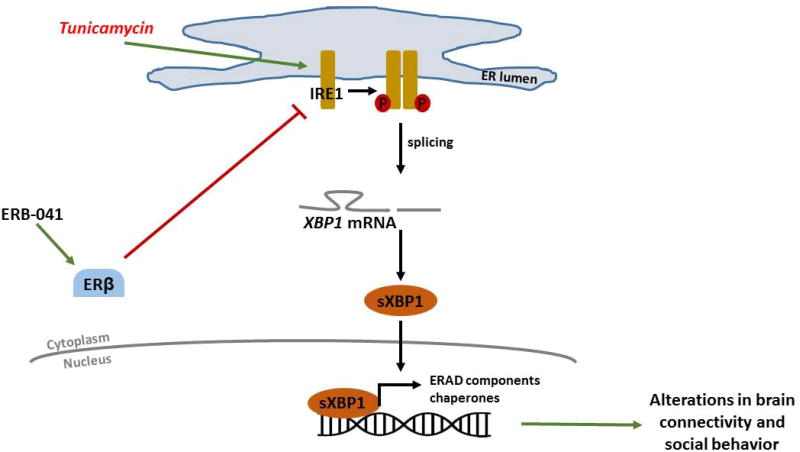
Figure 6. Schematic Diagram of ERβ regulation of the IRE/XBP1 pathway in social behavior.
Tunicamycin treatment leads to the activation of IRE1 that promotes splicing of XBP1 mRNA. This results in increased levels of the transcription factor sXBP1, which triggers the expression of unfolded protein response (UPR) genes that induces alterations in brain connectivity and social behavior. The activation of ERβ signaling inhibits IRE1 activation, downregulates sXBP1 and attenuates ER stress-induced changes in brain connectivity and social behavior.
Collectively, these studies suggest that ERβ signaling plays an important role in ER-stress induced changes in brain connectivity and social behavior. It is possible that the lack of effects of tunicamycin on social behavior in females could be due to the difference in the rate at which tunicamycin is metabolized in females compared to males. Future studies should examine whether higher dose of tunicamycin could induce changes in social behavior in female mice. Although the above data on the effects of pharmacological induction of ER stress on social behavior deficits in adult mice are interesting, further studies are warranted to understand the role of ER stress during neurodevelopment on social behavior. While ERβ is known to be present in neurons and glial cells [65, 66], and our study found a protective role of ERB-041 in ER stress-induced social behavior, the cell type/s involved in ERβ signaling needs further investigation. Also, it is important to determine whether ER stress induces changes in connectivity between PFC and brain regions other than hippocampus such as amygdala which is also implicated in social behavior. At the translational level, while selective ERβ agonists have estrogen-like effects in the brain including improvement in cognitive performance and social behavior, they appear to be relatively free of estrogenic side effects.
Supplementary Material
Figure S1. IRE1 in hippocampus does not mediate tunicamycin-induced deficits in social behavior in mice. Control or IRE1 shRNA lentiviral particles were stereotaxically administered into mouse hippocampus, and tunicamycin (1mg/kg; i,.p) was administered 2 weeks following shRNA administration. Social behavior was examined at 12 h after tunicamycin treatment. IRE1 shRNA administration failed to attenuate tunicamycin-induced deficits in social behavior. A) The three-chamber social interaction test. Left, time in chamber. ***p<0.001 vs. stranger mouse chamber. Two-way ANOVA. Right, the discrimination index calculated as the difference in the time spent in the social and non-social chambers, divided by the sum of the time spent in both chambers. *p<0.05 vs. con shRNA group; One-way ANOVA. B) Reciprocal social interaction test. *p<0.05 vs. con shRNA group; One-way ANOVA. Data are expressed as mean ±s.e.m (n=4 per group). M, chamber housing stranger mouse; E, chamber housing an empty cage; C, center. ns, non-significant.
Figure S2. IRE1 shRNA in PFC does not attenuate tunicamycin-induced decrease in ERβ protein levels. Control or IRE1 shRNA lentiviral particles were stereotaxically administered into mouse prefrontal cortex (PFC), and tunicamycin (1mg/kg; i.p) was administered 2 weeks following shRNA administration. ERβ protein levels were determined in mouse PFC 12 h after tunicamycin injection. Top. Representative blot. Bottom. Quantification of ERβ protein. Protein levels were measured by western blot analysis and normalized to tubulin. Data are expressed as mean ±s.e.m. *p<0.05 vs. con shRNA group; One-way ANOVA.
Table S1. Statistical results.
Acknowledgments
Funding
We acknowledge the funding support from US National Institute of Mental Health (MH 097060) to A.P. The funding agencies had no involvement in the research other than financial support.
Footnotes
Compliance with Ethical Standards
All experiments were in compliance with the US National Institute of Health guidelines and approved by Augusta University animal welfare guidelines.
Conflict of Interest
The authors declare that they have no conflicts of interest.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 2.Kundakovic M, Champagne FA. Early-life experience, epigenetics, and the developing brain. Neuropsychopharmacology. 2015;40:141–153. doi: 10.1038/npp.2014.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bicks LK, Koike H, Akbarian S, Morishita H. Prefrontal Cortex and Social Cognition in Mouse and Man. Front Psychol. 2015;6:1805. doi: 10.3389/fpsyg.2015.01805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calabrese F, Riva MA, Molteni R. Synaptic alterations associated with depression and schizophrenia: potential as a therapeutic target. Expert Opin Ther Targets. 2016;20:1195–1207. doi: 10.1080/14728222.2016.1188080. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Yu S, Fu Y, Li X. Synaptic proteins and receptors defects in autism spectrum disorders. Front Cell Neurosci. 2014;8:276. doi: 10.3389/fncel.2014.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duric V, Banasr M, Stockmeier CA, Simen AA, Newton SS, Overholser JC, et al. Altered expression of synapse and glutamate related genes in post-mortem hippocampus of depressed subjects. Int J Neuropsychopharmacol. 2013;16:69–82. doi: 10.1017/S1461145712000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dichter GS. Functional magnetic resonance imaging of autism spectrum disorders. Dialogues Clin Neurosci. 2012;14:319–351. doi: 10.31887/DCNS.2012.14.3/gdichter. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyer-Lindenberg AS, Olsen RK, Kohn PD, Brown T, Egan MF, Weinberger DR, et al. Regionally specific disturbance of dorsolateral prefrontal-hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatry. 2005;62:379–86. doi: 10.1001/archpsyc.62.4.379. [DOI] [PubMed] [Google Scholar]
- 9.Marchand WR, Lee JN, Suchy Y, Johnson S, Thatcher J, Gale P. Aberrant functional connectivity of cortico-basal ganglia circuits in major depression. Neurosci Lett. 2012;514:86–90. doi: 10.1016/j.neulet.2012.02.063. [DOI] [PubMed] [Google Scholar]
- 10.Zhan Y, Paolicelli RC, Sforazzini F, Weinhard L, Bolasco G, Pagani F, et al. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat Neurosci. 2014;17:400–6. doi: 10.1038/nn.3641. [DOI] [PubMed] [Google Scholar]
- 11.Vetter-O'Hagen CS, Spear LP. The effects of gonadectomy on sex- and age-typical responses to novelty and ethanol-induced social inhibition in adult male and female Sprague-Dawley rats. Behav Brain Res. 2012;227:224–32. doi: 10.1016/j.bbr.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crider A, Pillai A. Estrogen Signaling as a Therapeutic Target in Neurodevelopmental Disorders. J Pharmacol Exp Ther. 2017;360:48–58. doi: 10.1124/jpet.116.237412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crider A, Thakkar R, Ahmed AO, Pillai A. Dysregulation of estrogen receptor beta (ERβ), aromatase (CYP19A1), and ER co-activators in the middle frontal gyrus of autism spectrum disorder subjects. Mol Autism. 2014;5:46. doi: 10.1186/2040-2392-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong J, Weickert CS. Transcriptional interaction of an estrogen receptor splice variant and ErbB4 suggests convergence in gene susceptibility pathways in schizophrenia. J Biol Chem. 2009;284:18824–32. doi: 10.1074/jbc.M109.013243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choleris E, Ogawa S, Kavaliers M, Gustafsson JA, Korach KS, Muglia LJ, et al. Involvement of estrogen receptor alpha, beta and oxytocin in social discrimination: A detailed behavioral analysis with knockout female mice. Genes Brain Behav. 2006;5:528–539. doi: 10.1111/j.1601-183X.2006.00203.x. [DOI] [PubMed] [Google Scholar]
- 16.Tang AC, Nakazawa M, Romeo RD, Reeb BC, Sisti H, McEwen BS. Effects of long-term estrogen replacement on social investigation and social memory in ovariectomized C57BL/6 mice. Horm Behav. 2005;47:350–7. doi: 10.1016/j.yhbeh.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Castillo K, Rojas-Rivera D, Lisbona F, Caballero B, Nassif M, Court FA, et al. BAX inhibitor-1 regulates autophagy by controlling the IRE1α branch of the unfolded protein response. EMBO J. 2011;30:4465–4478. doi: 10.1038/emboj.2011.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang K, Kaufman RJ. Signaling the Unfolded Protein Response from the Endoplasmic Reticulum. J Biol Chem. 2004;279:25935–25938. doi: 10.1074/jbc.R400008200. [DOI] [PubMed] [Google Scholar]
- 19.Fujita E, Dai H, Tanabe Y, Zhiling Y, Yamagata T, Miyakawa T, et al. Autism spectrum disorder is related to endoplasmic reticulum stress induced by mutations in the synaptic cell adhesion molecule, CADM1. Cell Death Dis. 2010;1:e47. doi: 10.1038/cddis.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schröder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res. 2005;569:29–63. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 21.Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, et al. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92–96. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- 22.Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 23.Dey S, Baird TD, Zhou D, Palam LR, Spandau DF, Wek RC. Both transcriptional regulation and translational control of ATF4 are central to the integrated stress response. J Biol Chem. 2010;285:33165–33174. doi: 10.1074/jbc.M110.167213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshida H, Okada T, Haze K, Yanagi H, Yura T, Negishi M, et al. ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol Cell Biol. 2000;20:6755–67. doi: 10.1128/mcb.20.18.6755-6767.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ai D, Baez JM, Jiang H, Conlon DM, Hernandez-Ono A, Frank-Kamenetsky M, et al. Activation of ER stress and mTORC1 suppresses hepatic sortilin-1 levels in obese mice. J Clin Invest. 2012;122:1677–1687. doi: 10.1172/JCI61248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindholm D, Wootz H, Korhonen L. ER stress and neurodegenerative diseases. Cell Death Differ. 2006;13:385–92. doi: 10.1038/sj.cdd.4401778. 2006. [DOI] [PubMed] [Google Scholar]
- 27.Momoi T, Fujita E, Senoo H, Momoi M. Genetic factors and epigenetic factors for autism: endoplasmic reticulum stress and impaired synaptic function. Cell Biol Int. 2010;34:13–19. doi: 10.1042/CBI20090250. [DOI] [PubMed] [Google Scholar]
- 28.Timberlake MA, 2nd, Dwivedi Y. Altered Expression of Endoplasmic Reticulum Stress Associated Genes in Hippocampus of Learned Helpless Rats: Relevance to Depression Pathophysiology. Front Pharmacol. 2016;6:319. doi: 10.3389/fphar.2015.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubio MD, Wood K, Haroutunian V, Meador-Woodruff JH. Dysfunction of the ubiquitin proteasome and ubiquitin-like systems in schizophrenia. Neuropsychopharmacology. 2013;38:1910–20. doi: 10.1038/npp.2013.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elbein AD. Inhibitors of the biosynthesis and processing of N-linked oligosaccharide chains. Annu Rev Biochem. 1987;56:497–534. doi: 10.1146/annurev.bi.56.070187.002433. [DOI] [PubMed] [Google Scholar]
- 31.Steele KE, Seth P, Catlin-Lebaron KM, Schoneboom BA, Husain MM, Grieder F, et al. Tunicamycin enhances neuroinvasion and encephalitis in mice infected with Venezuelan equine encephalitis virus. Vet Pathol. 2006;43:904–13. doi: 10.1354/vp.43-6-904. [DOI] [PubMed] [Google Scholar]
- 32.Lee S, Shang Y, Redmond SA, Urisman A, Tang AA, Li KH, et al. Activation of HIPK2 Promotes ER Stress-Mediated Neurodegeneration in Amyotrophic Lateral Sclerosis. Neuron. 2016;91:41–55. doi: 10.1016/j.neuron.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jo F, Jo H, Hilzendeger AM, Thompson AP, Cassell MD, Rutkowski DT, et al. Brain endoplasmic reticulum stress mechanistically distinguishes the saline-intake and hypertensive response to deoxycorticosterone acetate-salt. Hypertension. 2015;65:1341–1348. doi: 10.1161/HYPERTENSIONAHA.115.05377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walf AA, Rhodes ME, Frye CA. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiol Learn Mem. 2006;86:35–46. doi: 10.1016/j.nlm.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frye CA, Duffy CK, Walf AA. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol Learn Mem. 2007;88:208–216. doi: 10.1016/j.nlm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pisani SL, Neese SL, Katzenellenbogen JA, Schantz SL, Korol DL. Estrogen Receptor-Selective Agonists Modulate Learning in Female Rats in a Dose- and Task-Specific Manner. Endocrinology. 2016;157:292–303. doi: 10.1210/en.2015-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pandya CD, Hoda N, Crider A, Peter D, Kutiyanawalla A, Kumar S, et al. Transglutaminase 2 overexpression induces depressive-like behavior and impaired TrkB signaling in mice. Mol Psychiatry. 2016 Sep 13; doi: 10.1038/mp.2016.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goodall JC, Wu C, Zhang Y, McNeill L, Ellis L, Saudek V, et al. Endoplasmic reticulum stress-induced transcription factor, CHOP, is crucial for dendritic cell IL-23 expression. Proc Natl Acad Sci U S A. 2010;107:17698–17703. doi: 10.1073/pnas.1011736107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Etherton MR, Tabuchi K, Sharma M, Ko J, Südhof TC. An autism-associated point mutation in the neuroligin cytoplasmic tail selectively impairs AMPA receptor-mediated synaptic transmission in hippocampus. EMBO J. 2011;30:2908–2919. doi: 10.1038/emboj.2011.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitsuda T, Omi T, Tanimukai H, Sakagami Y, Tagami S, Okochi M, et al. Sigma-1Rs are upregulated via PERK/eIF2α/ATF4 pathway and execute protective function in ER stress. Biochem Biophys Res Commun. 2011;415:519–25. doi: 10.1016/j.bbrc.2011.10.113. [DOI] [PubMed] [Google Scholar]
- 41.Hwang HJ, Jung TW, Ryu JY, Hong HC, Choi HY, Seo JA, et al. Dipeptidyl petidase-IV inhibitor (gemigliptin) inhibits tunicamycin-induced endoplasmic reticulum stress, apoptosis and inflammation in H9c2 cardiomyocytes. Mol Cell Endocrinol. 2014;392:1–7. doi: 10.1016/j.mce.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 42.Quan X, Wang J, Liang C, Zheng H, Zhang L. Melatonin inhibits tunicamycin-induced endoplasmic reticulum stress and insulin resistance in skeletal muscle cells. Biochem Biophys Res Commun. 2015;463:1102–1107. doi: 10.1016/j.bbrc.2015.06.065. [DOI] [PubMed] [Google Scholar]
- 43.Skropeta D. The effect of individual N-glycans on enzyme activity. Bioorg Med Chem. 2009;17:2645–2653. doi: 10.1016/j.bmc.2009.02.037. [DOI] [PubMed] [Google Scholar]
- 44.Dennis JW, Lau KS, Demetriou M, Nabi IR. Adaptive regulation at the cell surface by N-glycosylation. Traffic. 2009;10:1569–1578. doi: 10.1111/j.1600-0854.2009.00981.x. [DOI] [PubMed] [Google Scholar]
- 45.Bodo C, Rissman EF. New roles for estrogen receptor beta in behavior and neuroendocrinology. Front Neuroendocrinol. 2006;27:217–32. doi: 10.1016/j.yfrne.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 46.Rocha Ba, Fleischer R, Schaeffer JM, Rohrer SP, Hickey GJ. 17 Beta-estradiol-induced antidepressant-like effect in the forced swim test is absent in estrogen receptor-beta knockout (BERKO) mice. Psychopharmacology. 2005;179:637–43. doi: 10.1007/s00213-004-2078-1. [DOI] [PubMed] [Google Scholar]
- 47.Walf AA, Frye CA. Administration of estrogen receptor beta-specific selective estrogen receptor modulators to the hippocampus decrease anxiety and depressive behavior of ovariectomized rats. Pharmacol Biochem Behav. 2007;86:407–414. doi: 10.1016/j.pbb.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 48.Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- 49.Guo YS, Sun Z, Ma J, Cui W, Gao B, Zhang HY, et al. 17β-Estradiol inhibits ER stress-induced apoptosis through promotion of TFII-I-dependent Grp78 induction in osteoblasts. Lab Invest. 2014;94:906–916. doi: 10.1038/labinvest.2014.63. [DOI] [PubMed] [Google Scholar]
- 50.Grice SJ, Spratling MW, Karmiloff-Smith A, Halit H, Csibra G, de Haan M, et al. Disordered visual processing and oscillatory brain activity in autism and Williams syndrome. Neuroreport. 2001;12:2697–2700. doi: 10.1097/00001756-200108280-00021. [DOI] [PubMed] [Google Scholar]
- 51.Gandal MJ, Edgar JC, Ehrlichman RS, Mehta M, Roberts TP, Siegel SJ. Validating γ oscillations and delayed auditory responses as translational biomarkers of autism. Biol Psychiatry. 2010;68:1100–1106. doi: 10.1016/j.biopsych.2010.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McNally JM, McCarley RW. Gamma band oscillations: a key to understanding schizophrenia symptoms and neural circuit abnormalities. Curr Opin Psychiatry. 2016;29:202–10. doi: 10.1097/YCO.0000000000000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ye AX, Leung RC, Schäfer CB, Taylor MJ, Doesburg SM. Atypical resting synchrony in autism spectrum disorder. Hum Brain Mapp. 2014;35:6049–66. doi: 10.1002/hbm.22604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anticevic A, Hu X, Xiao Y, Hu J, Li F, Bi F, et al. Early-course unmedicated schizophrenia patients exhibit elevated prefrontal connectivity associated with longitudinal change. J Neurosci. 2015;35:267–286. doi: 10.1523/JNEUROSCI.2310-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Delmonte S, Gallagher L, O’Hanlon E, McGrath J, Balsters JH. Functional and structural connectivity of frontostriatal circuitry in Autism Spectrum Disorder. Front Hum Neurosci. 2013;7:430. doi: 10.3389/fnhum.2013.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O'Shea DJ, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–8. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–6. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 58.Win S, Than TA, Fernandez-Checa JC, Kaplowitz N. JNK interaction with Sab mediates ER stress induced inhibition of mitochondrial respiration and cell death. Cell Death Dis. 2014;5:e989. doi: 10.1038/cddis.2013.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Subramanian N, Natarajan K, Clatworthy MR, Wang Z, Germain RN. The adaptor MAVS promotes NLRP3 mitochondrial localization and inflammasome activation. Cell. 2013;153:348–61. doi: 10.1016/j.cell.2013.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nilsen J, Brinton RD. Mitochondria as therapeutic targets of estrogen action in the central nervous system. Curr Drug Targets CNS Neurol Disord. 2004;3:297–313. doi: 10.2174/1568007043337193. [DOI] [PubMed] [Google Scholar]
- 61.Irwin RW, Yao J, To J, Hamilton RT, Cadenas E, Brinton RD. Selective oestrogen receptor modulators differentially potentiate brain mitochondrial function. J Neuroendocrinol. 2012;24:236–248. doi: 10.1111/j.1365-2826.2011.02251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oslowski CM, Hara T, O'Sullivan-Murphy B, Kanekura K, Lu S, Hara M, et al. Thioredoxin-interacting protein mediates ER stress-induced β cell death through initiation of the inflammasome. Cell Metab. 2012;16:265–73. doi: 10.1016/j.cmet.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim SY. Transglutaminase 2 in inflammation. Front Biosci. 2006;11:3026–35. doi: 10.2741/2030. [DOI] [PubMed] [Google Scholar]
- 64.Lélu K, Laffont S, Delpy L, Paulet PE, Périnat T, Tschanz SA, et al. Estrogen receptor α signaling in T lymphocytes is required for estradiol-mediated inhibition of Th1 and Th17 cell differentiation and protection against experimental autoimmune encephalomyelitis. J Immunol. 2011;187:2386–93. doi: 10.4049/jimmunol.1101578. [DOI] [PubMed] [Google Scholar]
- 65.Spence RD, Wisdom AJ, Cao Y, Hill HM, Mongerson CR, Stapornkul B, et al. Estrogen mediates neuroprotection and anti-inflammatory effects during EAE through ERα signaling on astrocytes but not through ERβ signaling on astrocytes or neurons. J Neurosci. 2013;33:10924–33. doi: 10.1523/JNEUROSCI.0886-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saijo K, Collier JG, Li AC, Katzenellenbogen JA, Glass CK. An ADIOL-ERβ-CtBP transrepression pathway negatively regulates microglia-mediated inflammation. Cell. 2011;145:584–95. doi: 10.1016/j.cell.2011.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. IRE1 in hippocampus does not mediate tunicamycin-induced deficits in social behavior in mice. Control or IRE1 shRNA lentiviral particles were stereotaxically administered into mouse hippocampus, and tunicamycin (1mg/kg; i,.p) was administered 2 weeks following shRNA administration. Social behavior was examined at 12 h after tunicamycin treatment. IRE1 shRNA administration failed to attenuate tunicamycin-induced deficits in social behavior. A) The three-chamber social interaction test. Left, time in chamber. ***p<0.001 vs. stranger mouse chamber. Two-way ANOVA. Right, the discrimination index calculated as the difference in the time spent in the social and non-social chambers, divided by the sum of the time spent in both chambers. *p<0.05 vs. con shRNA group; One-way ANOVA. B) Reciprocal social interaction test. *p<0.05 vs. con shRNA group; One-way ANOVA. Data are expressed as mean ±s.e.m (n=4 per group). M, chamber housing stranger mouse; E, chamber housing an empty cage; C, center. ns, non-significant.
Figure S2. IRE1 shRNA in PFC does not attenuate tunicamycin-induced decrease in ERβ protein levels. Control or IRE1 shRNA lentiviral particles were stereotaxically administered into mouse prefrontal cortex (PFC), and tunicamycin (1mg/kg; i.p) was administered 2 weeks following shRNA administration. ERβ protein levels were determined in mouse PFC 12 h after tunicamycin injection. Top. Representative blot. Bottom. Quantification of ERβ protein. Protein levels were measured by western blot analysis and normalized to tubulin. Data are expressed as mean ±s.e.m. *p<0.05 vs. con shRNA group; One-way ANOVA.
Table S1. Statistical results.