Abstract
Increased endothelial cell adhesion molecule (ECAM) expression, leukocyte-endothelial cell adhesive interactions (LECA), platelet-endothelial cell adhesion (PECA), mast cell activation, production of reactive oxygen species (ROS), and microvascular permeability are hallmarks of the inflammatory response. The infiltration of inflammatory phagocytes is associated with myeloperoxidase (MPO)-dependent production of hypochlorous acid (HOCl), a reactive chlorinating species (RCS) that targets membrane lipids to produce halogenated lipids such as 2-chlorohexadecanal (2-ClHDA) and 2-chloropalmitic acid (2-ClPA). Whether these chlorinated lipids contribute to microcirculatory dysfunction is largely unknown. Thus, the objectives of this study were to determine if chlorinated lipids exposure induces such inflammatory responses in an in vitro model employing cultured human intestinal mesenteric vascular endothelial cells (HIMVEC), and in an in vivo model examining responses in small intestinal and mesenteric postcapillary venules of naïve rats. Following the addition of either 2-ClPA or 2-ClHDA to the culture medium, HIMVEC displayed increased platelet and neutrophil adherence that was associated with elevated expression of ECAMs and increased permeability. In vivo, chlorinated lipid exposure significantly increased LECA, PECA, ROS production, and albumin leakage, inflammatory events that were associated with mast cell activation and increased tissue MPO activity and expression. Our data provide proof-of-principle that 2-ClPA and 2-ClHDA induce powerful proinflammatory responses both in vitro and in vivo, suggesting the possibility that these chlorinated lipid products of the MPO/H2O2/chloride system may contribute to inflammation noted in neutrophil-dependent, myeloperoxidase-mediated pathologic states such as ischemia/reperfusion, hemorrhagic shock, and sepsis.
Keywords: Adhesion molecules, electrical resistance, platelet-endothelial cell adhesion, leukocyte-endothelial cell adhesive interactions, mast cell activation, reactive oxygen species, venular protein leakage, myeloperoxidase
INTRODUCTION
A diverse array of pathologic conditions, including ischemia/reperfusion, hemorrhagic shock, rheumatoid arthritis, sepsis, and inflammatory bowel disease, are associated with intense inflammatory responses in the microcirculation. These include elaboration of a wide variety of proinflammatory mediators, mast cell activation, marked increases in adhesion molecule expression and leukocyte-endothelial cell adhesive interactions (LECA), platelet-endothelial cell adhesion (PECA), over-exuberant production of reactive oxygen species (ROS), and endothelial barrier disruption (1–3). Once activated, neutrophils infiltrate into the tissues and release a variety of oxidants and hydrolytic enzymes that can contribute to tissue injury. One of the granular constituents released by activated neutrophils is the green heme protein myeloperoxidase (MPO), an enzyme best known for its antimicrobial actions. MPO catalyzes the formation of the highly reactive chlorinating species (RCS) hypochlorous acid (HOCl) from hydrogen peroxide (H2O2) and chloride (4, 5). HOCl is cytotoxic in its own right but can also oxidize primary amines to form highly reactive chloramines. In addition, recent work has demonstrated that chlorinated lipid products such as 2-chloropalmitaldehyde (2-ClHDA) and other related chlorinated lipids are formed in response to RCS attack on membrane plasmalogens in human atherosclerotic plaques, rodent postischemic myocardium, and in plasma from LPS or Sendai virus treated rodents (6–10).
Plasmalogens are a major phospholipid class in mammals and are present in robust quantities in cells of the cardiovascular system. Plasmalogens possess a vinyl ether linkage between the sn-1 aliphatic group and the glycerol backbone that can be targeted for oxidation by MPO-derived RCS, resulting in the production of 2-chloro fatty aldehydes, including 2-chlorohexadecanal (2-ClHDA). 2-chloro fatty aldehyde has been shown to be a potent chemoattractant in vitro (11) suggesting that it may amplify neutrophil recruitment to sites of inflammation. In addition, exogenous application of 2-ClHDA to cultured human brain microvascular endothelial cells or via intra-carotid perfusion in vivo increased permeability and disrupted the blood-brain-barrier, respectively (9, 12). Although these studies indicate the 2-ClHDA may serve as a chemoattractant and increase microvascular permeability, it is not clear whether this chlorolipid can also induce mast cell activation or provoke the expression of endothelial cell adhesion molecules, LECA, platelet adhesion or the formation of ROS in the microcirculation. In addition, 2-ClHDA promotes the disappearance of IκB in endothelial cells, freeing NF-κB for translocation to the nucleus, where it can bind to promoter regions of genes involved in ECAM expression, various chemokines and other proinflammatory mediators (13). Although 2-ClHDA also provokes the expression of COX-2 and increases production of the anti-inflammatory prostanoid, prostacyclin, it has been suggested that this chlorinated lipid may activate COX-2 associated isomerases to generate proinflammatory eicosanoids in neutrophils and monocytes (13). Moreover, exposing human umbilical cord endothelial cells to low micromolar concentrations of 2-ClHDA (2–10 μM) or hypochorite-modified low density lipoprotein has been reported to decrease eNOS expression and inhibit nitric oxide production (14, 15), effects that should promote leukocyte-endothelial cell adhesive interactions and microvascular barrier disruption (16).
The aforementioned studies led us to propose the general hypothesis that in proinflammatory states such as sepsis or ischemia/reperfusion, leukocytes are recruited and activated by a variety of cytokines, chemokines, and damage associated molecular pattern molecules. As a consequence, activated neutrophils produce reactive oxygen species, such as hydrogen peroxide, which fuels the formation of hypochlorous acid by the enzymatic action of the neutrophilic enzyme myeloperoxidase. Hypochlorous acid can target membrane plasmalogens for oxidation, resulting in the production of 2-chlorolipids. We tested the specific postulate that 2-ClHDA, at doses measured at sites of inflammation (6, 7, 11, 12, 17–21) would elicit elicit ECAM expression, LECA, platelet adhesion and ROS production as well as provoke mast cell activation, disrupt endothelial barrier function, and promote neutrophil accumulation in the tissues. In addition, because it is known that 2-ClHDA can be metabolized to 2-chlorohexadecanoic acid (or 2-chloropalmitic acid, 2-ClPA) we also sought to determine whether this chlorinated lipid metabolite may also exert proinflammatory effects in the microcirculation.
MATERIALS AND METHODS
Mesenteric endothelial cell culture
Human intestinal mesenteric vascular endothelial cells (HIMVEC, Cell Biologics, Chicago, IL, cat number H6055) were grown in EGM-2MV medium and maintained at 37°C in a humidified atmosphere of 95% O2 and 5% CO2. Cells were incubated with chlorinated lipids (2-ClHDA or 2-ClPA, 10 μmol/L) or non-chlorinated lipids (HDA or PA, 10 μmol/L). This dose was selected for study because it falls well within the range of tissue values (~20 μM) measured following neutrophil and monocyte activation (6, 7, 11, 12, 17–21). 2-ClPA was synthesized and prepared as described previously using palmitic acid as precursor (22). 2-ClHDA was prepared by treating 1-O-hexadec-1′-enyl-glycero-3-phosphocholine (100 mg) with freshly prepared hypochlorous acid (final concentration 1.5 mM) in phosphate buffer (pH 4) for 5 min at 37°C (22). All other lipids were obtained from NuChek Prep (Elysian MN). All in vitro studies described below were repeated with at least four separate cell cultures.
In vitro studies in HIMVEC to examine inflammatory responses to chlorinated lipids
Leukocyte adherence to cultured endothelium
Adherence of leukocytes to HIMVEC was assessed as previously described (23) and as authorized by Saint Louis University Institutional Review Board protocol 9952. Briefly, HIMVEC were grown to confluence in a 12-well plate. Cells were treated with 10 μM BSA-conjugated lipids in growth media for 30 min. Leukocytes were isolated from healthy volunteers. Five hundred microliters (4 x 106 cells/mL) were subsequently added to HIMVEC and incubated for 20 min. Unbound leukocytes were washed away with three PBS rinses. The remaining leukocytes were lysed with 0.2% Triton X-100 and scraped into an Eppendorf tube. Tubes were briefly sonicated to ensure complete lysis of cells. To measure myeloperoxidase, 400 μL cell lysate was transferred to a test tube containing phosphate buffer, Hank’s buffer with BSA, 1, 9-dimethyl-methylene blue, and 0.5% hydrogen peroxide. Samples were incubated for 15 min at room temperature. Sodium azide (1%) was added to stop the reaction. Absorbance was measured at 460 nm.
Platelet adherence to cultured endothelium
Adherence of platelets to HIMVEC was assessed as previously described (24) and as authorized by Saint Louis University Institutional Review Board protocol 12369. Briefly, HIMVEC were grown to confluence in a 24-well plate. Cells were treated with 10 μM BSA-conjugated lipids in growth media for 30 min. Platelets were isolated from healthy volunteers. Platelets were stained with Calcein-AM (2.5 μmol/L) for 15 min at 37°C in the dark. Fluorescence-labeled platelets were subsequently added to HIMEC and incubated for 20 min at 37°C. Unbound platelets were washed away with three PBS rinses. The remaining platelets were lysed in lysis buffer for 10 min, and fluorescence was measured with a plate reader (excitation at 492 nm, emission at 535 nm).
Adhesion molecule cell surface expression
HIMVEC grown to confluence in 16-mm culture dishes, were incubated with 10 μmol/L chlorinated lipids (2-ClHDA or 2-ClPA) versus non-chlorinated lipids (HDA or PA), or in media alone for time periods described below at 37°C in 95% O2-5% CO2, respectively. At the end of incubation, buffer was quickly removed, and cells were immediately fixed with ice-cold 1% paraformaldehyde and incubated overnight at 4°C. Cells were washed three times with phosphate-buffered saline (PBS) and then blocked with Tris-buffered saline-Tween supplemented with 0.8% BSA (wt/vol) and 0.5% fish gelatin (wt/vol) for 1 h at 24°C. Appropriate primary antibody (1:50) was used before treatment with horseradish peroxidase-conjugated secondary antibody (1:5000). Subsequently, each well was incubated in the dark with the 3, 3′, 5, 5′-tetramethylbenzidine liquid substrate system. Reactions were stopped by the addition of sulfuric acid, and color development was measured with a microtiter plate spectrophotometer at 450 nm.
For these in vitro studies, endothelial cells were incubated with the lipids of interest for various time periods, according to the following rationale. Preformed P-selectin has been reported to be rapidly upregulated on the endothelial cell surface, following mobilization from Weibel-Palade bodies, reaching a peak within 30 min of exposure to proinflammatory stimuli. After declining expression of this preformed pool, a second protein synthesis-dependent peak in P-selectin expression occurs at 4 hrs, but we did not assess expression of this adhesion molecule at this time. E-selectin rises over a slower time course, but detectable levels are present after 1 hr. ICAM-1 and VCAM-1 are constitutively expressed, but rise to a peak after four hrs of exposure to proinflammatory stimuli by a protein-synthesis dependent mechanism. Thus, assessing P-selectin, E-selectin, ICAM-1 and VCAM-1 at 30 min, 1 hr, 4 hr and 4hr after exposure provided optimal time points for detection of putative effects of the chlorinated lipids on expression of these different adhesion molecules. Following these incubation periods, isolated leukocytes or platelets were then added to endothelial cells and incubated for 15–20 min, after which adhesion was assessed as described. This corresponds to a similar time frame over which adhesion and other inflammatory events (oxidant production, venular protein leakage and mast cell degranulation) were examined in vivo (see below).
Resistance measurements in HIMVEC
Resistance measurements in mesenteric endothelial cells was performed as previously described (23, 25). HIMVEC were grown to confluence on Transwell inserts, and then incubated with chlorinated lipids (2-ClHDA or 2-ClPA) versus non-chlorinated lipids (HDA or PA) (10 μmol/L) or in media alone. Changes in electrical resistance were measured over time using an epithelial volt ohmmeter.
Animals, surgery, and intravital microscopic examination of inflammatory responses to chlorinated lipids
Male Sprague-Dawley rats (250–300 g) were acquired from ENVIGO (Indianapolis, IN), and provided with standard rat chow ad libitum. All animal protocols were approved by the Institutional Animal Care and Use Committee at the University of Missouri-Columbia, and were conducted in accordance with the National Institutes of Health’s “Guide for the Care and Human Use of Laboratory Animals”. All animals were anesthetized by intraperitoneal injection of a mixture of ketamine (90 mg/kg body wt.) and xylazine (10 mg/kg body wt). Once a deep plain of anesthesia was achieved, as assessed by loss of paw pinch and corneal reflexes, the rats were placed on a Plexiglas animal board. Body temperature was maintained between 36.5–37.5°C via use of thermostatically controlled heat lamp. A right side laparotomy was performed and the ileocecal portion of the mesentery was exteriorized and draped over a glass coverslip. Mesenteries were then superfused with physiological salt solutions containing chlorinated lipids (2-ClHDA or 2-ClPA, 10 μmol/L,) versus non-chlorinated lipids (HDA or PA, 10 μmol/L).
The Plexiglas animal board was then mounted on the stage of an inverted microscope (Eclipse TE2000; Nikon) and inflammatory events occurring in postcapillary venules and the surrounding mesentery were observed, typically with 20x magnification (26). Fluorescent images (excitation, 420–490 nm; emission 520 nm) were detected with a charge-coupled device (CCD) camera (Photometrics COOLSNAP ES). Images were projected onto a television monitor (PVM-1953 MD; Sony) and recorded on a DVD recorder (DMR-E50; Panasonic) or captured through Metamorph software version 7.8. A time-date generator (WJ810, Panasonic) displayed this function on the monitor.
Quantification of inflammatory responses in mesenteric vessels and surrounding tissues
In the in vivo experiments, leukocyte-endothelial cell interactions were measure in rat small intestine, while platelet-endothelial cell adhesion, mast cell activation, ROS production, and albumin extravasation were quantified in rat mesenteries superfused at 2 ml/min with physiologic salt solution containing PA (n=6), 2-ClPA (n=6), HDA (n=6), or 2-ClHDA (n=6), each at 10 μM, according to the procedures described below. Pilot studies revealed modest increases in leukocyte rolling and adhesion at 1 μM and very little response to 2-ClHDA at 100 nM. Previous work from our group has shown robust changes in endothelial barrier function and adhesion molecule expression induced by 100 nM – 10 μM 2-ClHDA in pulmonary microvascular endothelial cells while human renal glomerular endothelial cells manifested a less pronounced reduction in transendothelial resistance without any change in adhesion molecule expression when exposed to 0.1–10 μM 2-ClPA. Thus, our results suggest that human mesenteric microvascular endothelial cells exhibit a sensitivity to chlorinated lipids that is intermediate between human pulmonary microvascular endothelial cells and human glomerular endothelial cells. Thus, lung endothelium is at greatest risk for 2-chlorolipid-mediated injury, followed by mesenteric endothelium, with renal glomerular endothelium being the least sensitive.
Leukocyte/platelet-endothelial adhesive interactions
The procedures and fluorescent labeling of leukocytes and platelets were similar to those used previously in our lab (26, 27). Briefly, carboxyfluorescein diacetate succinimidyl ester (CFDA-SE, Molecular Probes, Eugene, OR) was injected through left jugular vein and the small intestine was scanned. At each time point, 10 single unbranched postcapillary venules (20–50 μm in diameter and 100 μm in length) in submucosal layer of the ileum (LECA) or mesentery (PECA) were observed for at least 30 seconds. Cell adhesive interactions (number of rolling leukocytes and number of firmly attached leukocytes) were quantified in 10 postcapillary venules, followed by calculation of the mean for the 10 venules. Rolling cells were defined to be those passing a cross line at a velocity significantly slower than the centerline velocity and were expressed as rolling cells per minute. Leukocytes or platelets were considered to be adherent if they did not move for at least 30 seconds. The numbers of adherent cells were normalized in terms of mm2 surface area.
Mast cell activation
Ruthenium red was mixed to BBS (0.001%) and the uptake of ruthenium red was used as a molecular marker of mast cell activation (28). After being exteriorized, mesenteries were superfused with ruthenium red in BBS for baseline evaluation, then with ruthenium red in chlorinated lipids or non-chlorinated lipids solutions. The number of activated mast cells were counted in 10 fields at each time point and averaged.
ROS production
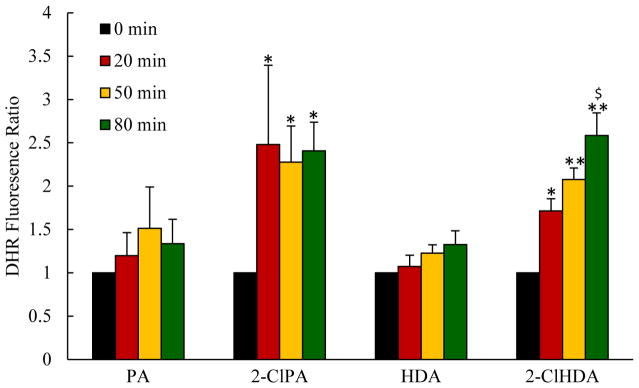
In a second group of rats, the oxidant-sensitive fluorescent probe dihydrorhodamine 123 (DHR 123) was added to BBS (10 μmol/L) to estimate ROS generation in mesenteric postcapillary venular walls. In brief, DHR 123 in BBS was superfused over mesentery and the mesentery was scanned for the baseline assessment, then the perfusate was switched to one containing DHR 123 and either chlorinated lipid or non-chlorinated lipid solutions. At each time point, 10 single unbranched postcapillary venules (20–50 μm in diameter and 100 μm in length) were observed and images were acquired. The fluorescence intensity was measured on the venular wall in 5 regions of interest (25 μm in diameter) (28). Since the baseline fluorescence intensity varies depending on the animal, the ratio of the fluorescence intensity at each time point to the baseline was calculated as DHR fluorescence ratio, as an indicator of redox state in mesenteric postcapillary venular walls (26).
Albumin leakage
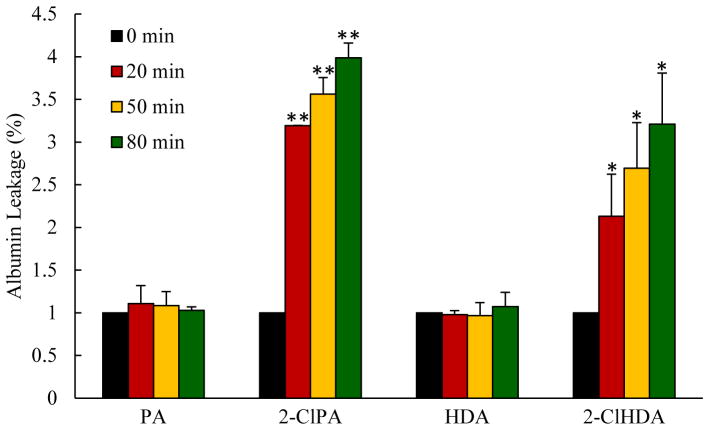
FITC-labeled albumin was used to evaluate the albumin leakage across postcapillary venules in rat mesenteries. In brief, after left jugular vein cannulation, 50 mg/kg of FITC-albumin was injected intravenously. After being exteriorized, mesentery was superfused with BBS first, and then with chlorinated lipids or non-chlorinated lipids solutions. At each time point, 10 single unbranched postcapillary venules (20–50 μm in diameter and 100 μm in length) were selected. Images were captured, and the fluorescence intensity of FITC-albumin in 5 regions of interest (25 μm in diameter) within the venules (Iv) and in the perivenular interstitium within 10–50 μm of the venular wall (Ip) were measured using Metamorph software version 7.8. Albumin leakage was estimated by dividing Ip by Iv at each pair of corresponding circles, and the ratio of albumin leakage at a time point to that of the baseline was designated as the ratio of albumin leakage at that point (26). This method assumes that fluorescent-tagged albumin will move from the microvessel into the tissue space in a manner that reflects the behavior of native albumin.
Tissue MPO activity
Mucosal MPO activity was measured in biopsies obtained from rat small intestine (jejunum) collected at the end of superfusion period, with use of a fluorescence assay kit (Cell Technology, Mountain View, CA). Samples were prepared as per kit manufacturer’s instructions and MPO activity in the samples was quantified by adding the kit detection reagent and measuring the resulting fluorescence (excitation, 530 nm; emission, 590 nm) (29). Values were normalized to sample protein content and MPO activity was expressed as milliunits per milligram protein.
Immunohistochemical staining for MPO
The collected small intestine samples were fixed in 10% (w/v) PBS-buffered formaldehyde and embedded in paraffin. Following dewaxing, endogenous peroxidase was quenched with 3% (v/v) hydrogen peroxide for 5 min. The slides were incubated in 5% (v/v) bovine serum for 20 min to block non-specific binding. Sections were then incubated with anti-MPO antibody (ab9535, 1:50 in PBS, Abcam) for 60 min. Samples were rinsed in wash buffer and incubated with detection system for 30 min. Slides were applied with DAB substrate and stained with Mayer’s hematoxylin for 30 seconds, then dehydrated in graded alcohol and xylene.
Statistical analysis
Data were analyzed using one-way ANOVA followed by post hoc analysis using Dunnett’s test or Newman-Keuls test. Data are means ± SEM. Differences were regarded as significant at P < 0.05 and highly significant at P < 0.01.
RESULTS
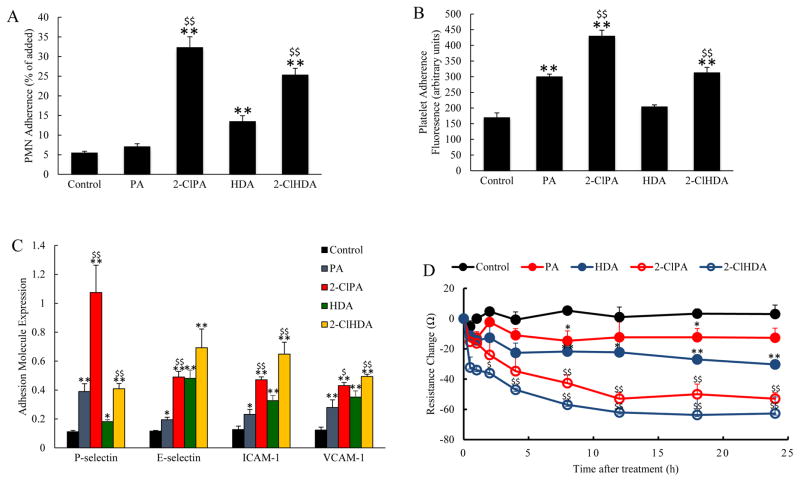
To determine whether exposure to 2-ClHDA or 2-ClPA would induce adherence of inflammatory cells to HIMVEC, we added human PMNs or platelets (2 x 106) to confluent HIMVEC monolayers treated with 10 μM BSA-conjugated lipids (PA; 2-ClPA; HDA; or 2-ClHDA) in separate experiments. We observed increased adherence of PMNs and platelets to the endothelial monolayers after 30 min of chlorinated lipids exposure (Figure 1A, B). It is of interest to note that exposure to non-chlorinated PA increased platelet adherence to a level intermediate between control and 2-ClPA, but was without effect on PMN adhesion. On the other hand, non-chlorinated HDA exposure produced a modest increase in PMN adhesion to HIMVEC, but had no effect on platelet adhesion. Endothelial cell surface expression of adhesion molecules known to be involved in inflammatory cell adherence, including P-selectin, E-selectin, ICAM-1 and VCAM-1, was also increased by exposure to the chlorinated lipids (2-ClPA, 2-ClHDA), while the non-chlorinated lipids (PA, HDA) exerted more modest effects relative to control (Figure 1C). Significant increases in both P-selectin and E-selectin expression were detected when measured after 30 min and 1 hour of chlorinated lipids exposure, respectively, and in ICAM-1 and VCAM-1 expression after 4 hours of exposure (Figure 1C). These observations are consistent with the hypothesis that chlorinated lipids induce surface expression of adhesion molecules on cultured endothelial cells, which is associated with their effect to enhance the adhesion of platelets and PMNs to HIMVEC.
Figure 1.
Increased PMN (Panel A) and platelet (Panel B) adherence to human intestinal mesenteric vascular endothelial cells (HIMVEC) occurred in response to incubation with the chlorinated lipids, 2-ClPA or 2-ClHDA (10 μmol/L), and the non-chlorinated lipids, PA or HDA, respectively (n=6 in each group). Panel C illustrates cell surface expression of adhesion molecules in HIMVEC exposure to these chlorinated and non-chlorinated lipids. Adhesion molecule cell surface expression was measured at indicated times of incubation: P-selectin (30 min), E-selectin (1 hr), ICAM-1 (4 hrs) and VCAM-1 (4hrs) (n=8). Panel D shows the changes in electrical resistance of HIMVEC measured over 24 hr exposure to chlorinated or non-chlorinated lipids (n=8 in each group). *P<0.05 and **P<0.01 when compared to control; $P<0.05 and $$P<0.01 between 2-ClPA and PA, 2-ClHDA and HDA groups at indicated time point.
Figure 1D illustrates the changes in endothelial permeability (as reflected by reductions in electrical resistance across HIMVEC monolayers) induced by chlorinated lipids exposure. We detected a significant decrease in electrical resistance in endothelial cells following exposure to the various lipids (PA, HDA, 2-ClPA, 2-ClHDA) compared to control, with much larger reductions in monolayer electrical resistance exhibited by HIMVC exposed to 2-ClPA or 2-ClHDA compared to measurements obtained in the PA and HDA exposure groups, respectively (Figure 1D). The observed reductions in electrical resistance were detectable within 2–3 hrs exposure to 2-ClPA and 2-ClHDA, reached a nadir after 12 hrs and persisted at these levels over the remaining time course of the experiments. These reductions in electrical resistance induced by chlorinated lipids indicate that these compounds increase endothelial permeability, another hallmark feature of inflammation.
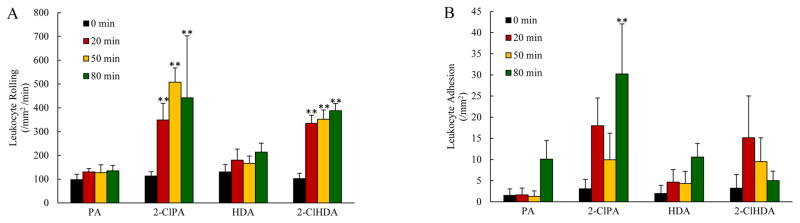
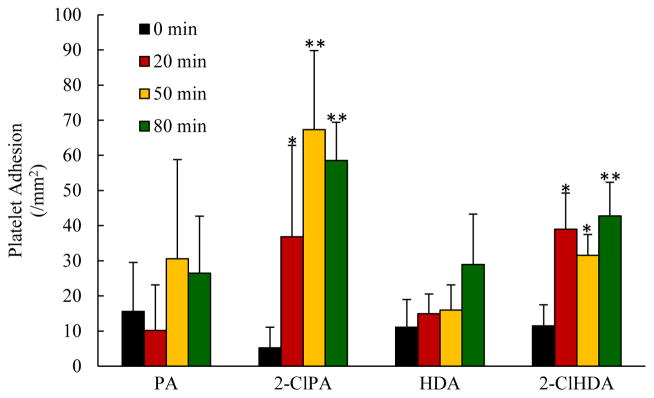
In our next series of experiments, we sought to extend our in vitro observations to the intact, blood-perfused microcirculation in vivo. In these studies, the proinflammatory effects of chlorinated lipids exposure were assessed in the ileal and mesenteric microvasculatures. Superfusion of rat small intestine with physiological salt solutions containing either 2-ClPA or 2-ClHDA promoted marked increases in the numbers of leukocytes rolling along postcapillary venular walls as well as in the numbers of firmly adherent leukocytes, while exposure to the non-chlorinated lipids PA and HDA were without effect on leukocyte rolling or adhesion (Figure 2A and 2B, respectively). In addition to these effects, 2-ClPA or 2-ClHDA superfusion of rat mesenteries also promoted increases in the number of firmly adherent platelet along postcapillary venules, while exposure to the non-chlorinated lipids PA and HDA were without effect on platelet adhesion (Figure 3). The increases in leukocyte-endothelial and platelet-endothelial adhesive interactions produced by superfusion with chlorinated lipids were similar in magnitude to that which we have previously reported in response to ischemia/reperfusion (26, 27, 30, 31).
Figure 2.
Chlorinated lipids promote leukocyte rolling and adhesion in vivo. The number of leukocyte rolling per mm2 per minute were increased by superfusion of small intestine with 2-ClPA and 2-ClHDA but not PA and HDA (Panel A). The number of firmly adherent leukocytes per mm2 venular surface were also increased in response to chlorinated lipids superfusion, while topical application of the non-chlorinated lipids over the small intestine was without effect (Panel B). For each group, n=6; and at each time point for every animal, responses in 10 postcapillary venules from intestine wall were measured and averaged. **P<0.01 when compared to baseline.
Figure 3.
Chlorinated lipids increase platelet adhesion in vivo. The number of firmly adherent platelets per mm2 venular surface were increased by superfusion of mesenteries with 2-ClPA and 2-ClHDA but not PA and HDA. For each group, n=6; and at each time point for every animal, responses in 10 postcapillary venules in jejunal submucosa were measured and averaged. *P<0.05 and **P<0.01 when compared to baseline.
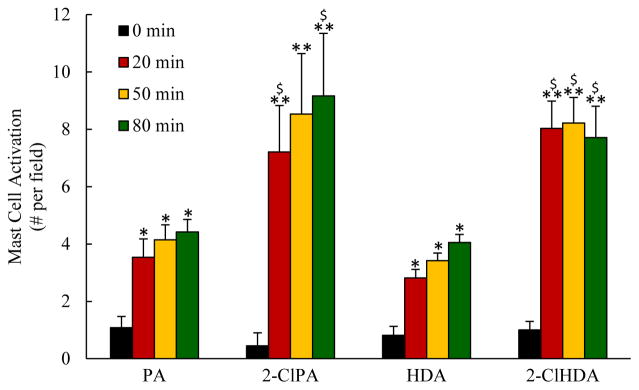
The data depicted in Figures 4 and 5 illustrate the effects of mesenteric superfusion with either 2-ClPA or 2-ClHDA on mast cell activation and postcapillary venular ROS production, respectively, compared to the responses to non-chlorinated PA and HDA. Quantitative analysis revealed that both 2-ClPA and 2-ClHDA elicited mast cell activation within 20 min of exposure and remained elevated for the remainder of the experimental protocol. Mesenteric superfusion with non-chlorinated PA and HDA also induced mast cell activation but to levels lower than that evoked by the chlorinated lipids. Superfusion with these chlorinated lipids also increased DHR fluorescence in mesenteric postcapillary venules. On the contrary, mesenteric exposure to PA or HDA groups were without effect on DHR fluorescence throughout the observation period. The latter data indicate that chlorinated lipids increase ROS production in postcapillary venules.
Figure 4.
Chlorinated lipids induce mast cell activation in vivo. For each group, n=6; and at each time point for every animal, mast cell activation was quantified in 10 fields (20x) surrounding mesenteric venules and averaged. *P<0.05 and **P<0.01 when compared to baseline; $P<0.05 between 2-ClPA and PA, 2-ClHDA and HDA groups at indicated time point.
Figure 5.
Mesenteric superfusion with chlorinated lipids increased ROS production in postcapillary venules, as assessed by DHR fluorescence. For each group (n=6), at each time point for every animal, 10 mesenteric venules were randomly chosen and 5 regions of interest (25 um in diameter) along venule wall were measured, averaged and presented relative to baseline (just prior to lipid superfusion). *P<0.05 and **P<0.01 when compared to baseline, $P<0.05 between 2-ClPA and PA, 2-ClHDA and HDA groups at indicated time point.
To determine whether superfusion with chlorinated lipids would initiate endothelial barrier dysfunction in vivo, we assessed the effect of 2-ClPA or 2-ClHDA on albumin leakage in rat mesenteric postcapillary venules (Figure 6). Albumin leakage was increased with chlorinated lipids challenge, results corroborating our findings in HIMVEC monolayers. Albumin leakage was not detected before lipid treatment in all groups, and this situation persisted throughout the observation period in mesenteries superfused with PA or HDA for 80 min.
Figure 6.
Chlorinated lipids elicit albumin leakage across single postcapillary venules of rat mesentery. For each group (n=6), at each time point for every animal, 10 mesenteric venules were randomly chosen and 5 regions of interest (r=12.5 mm) along venule wall were measured. *P<0.05 and **P<0.01 when compared to baseline.
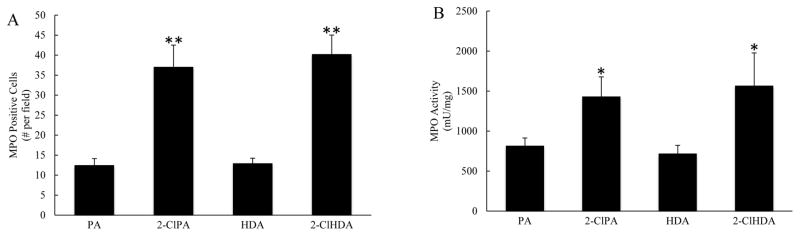
The proinflammatory effects of chlorinated lipids shown above suggested that these RCS might induce significant leukocyte infiltration into the tissues. To address this, we evaluated the effect of the chlorinated lipids on tissue neutrophil content in a final series of experiments. Increased staining of the neutrophil marker MPO was detected in the submucosal layer of the gut wall in samples obtained after mesenteric superfusion with the chlorinated lipids, relative to non-chlorinated PA and HDA (Figure 7A). We also measured MPO activity in gut samples obtained after 80 min of superfusion with the chlorinated or non-chlorinated lipids, as another marker of neutrophil infiltration. Treatment with 2-ClPA and 2-ClHDA resulted in twofold increases in intestinal MPO activity compared with the PA and HDA treatments groups, respectively (Figure 7B). Taken together, the results presented in Figure 6 indicate that chlorinated lipids induce a marked infiltration of neutrophils into the jejunal wall, primarily within the submucosal layer. We also used a mucosal injury scoring system to evaluate the effects of mesenteric exposure to chlorinated lipids on enterocyte damage, but detected no enterocyte injury. This most likely relates to a dilutional effect to reduce the concentration of chlorinated lipids in blood draining the mesentery and entering submucosal vessels of the intestinal wall. In other words, we superfused the 2-chlorolipids over the exposed mesentery and presume their concentration was reduced as they diffused into the bloodstream perfusing the mesenteric microcirculation, which then flowed into the submucosal vessels of intestinal wall. This implies that the concentration was sufficient to invoke modest infiltration of inflammatory phagocytes at this downstream site, but was insufficient to cause significant mucosal damage.
Figure 7.
The effect of mesenteric superfusion with chlorinated (compared to non-chlorinated) lipids increase MPO expression in jejunal submucosa, as assessed by immunohistochemistry (Panel A). Panel B depicts MPO activity values in jejunal samples obtained 80 min after superfusion of the mesentery supplying the same region of jejunum with chlorinated or non-chlorinated lipids. The measurements were repeated three times and mean values were normalized for sample protein content (n=6). *P<0.05 and **P<0.01 between 2-ClPA and PA, 2-ClHDA and HDA groups.
DISCUSSION
Neutrophil infiltration into the tissues is a hallmark feature of a wide variety of inflammatory conditions, including low blood flow states such as ischemia/reperfusion, hemorrhage and sepsis. Once sequestered in the tissues, these inflammatory phagocytes contribute to parenchymal cell injury and repair via mechanisms that are attributable to the formation of reactive oxygen species (ROS) and release of a variety of enzymes (1, 2). Myeloperoxidase (MPO) is an enzyme released from azurophilic granules of activated neutrophils and catalyzes chloride and hydrogen peroxide conversion to HOCl, a major reactive chlorinating species (RCS) produced by these inflammatory phagocytes. RCS contribute to neutrophil-mediated cytotoxicity by a variety of mechanisms, including oxidative bleaching of protein heme and iron sulfur centers and via oxidation of primary amines to form chloramines, which are powerful oxidizing agents in their own right (1, 2, 32–35). Plasmalogens are a major phospholipid found in many cells of the cardiovascular system (and other tissues) throughout the body. Plasmalogens are enriched in intracellular membrane pools including plasmalemma and lipid rafts (36–38). These phospholipids possess a vinyl ether bond (linking the sn-1 aliphatic group to the glycerol backbone) that is susceptible to oxidation by MPO-derived HOCl to produce the 2-chlorofatty aldehyde, 2-chlorohexadecanal (2-ClHDA), which is a potent chemoattractant for neutrophils (11). With the demonstration that 2-ClHDA accumulates in postischemic tissues (6, 7), it has been proposed that this chlorinated lipid may contribute to the proinflammatory effects elicited by conditions such as ischemia/reperfusion, hemorrhagic shock and sepsis. Thus, the major purpose of this study was to determine whether this chlorinated lipids, as well as related chlorinated lipids, exert proinflammatory effects as proof-of-principle that HOCl generated by MPO/H2O2/chloride system could potentially contribute to increased endothelial cell adhesion molecule expression, leukocyte-endothelial cell interactions, platelet-endothelial cell adhesion, mast cell activation, ROS production, and microvascular barrier dysfunction that contribute to the pathogenesis of neutrophil-dependent inflammatory injury. To accomplish this aim, we compared the effects of two different chlorinated lipids, 2-ClPA and 2-ClHDA, which have been shown to increase during neutrophil and monocyte activation (7, 11, 18, 21), to induce these proinflammatory effects, to the non-chlorinated lipids, PA and HDA. We wished to determine the local and direct effects of exposure to chlorinated lipids in the absence of the multitude of confounding proinflammatory mediators and compromise in the function of multiple organs, as occurs in ischemia/reperfusion, hemorrhagic shock or sepsis.
In the first group of studies, we used cultured mesenteric microvascular endothelial cells as a model system to initially characterize proinflammatory responses to chlorinated lipids. We showed that both 2-ClPA and 2-ClHDA increased PMN and platelet adhesion to these cells, adhesive events that were associated with increased expression of the endothelial cell adhesion molecules P-selectin, E-selectin, ICAM-1 and VCAM-1 (Figures 1A–C). The non-chlorinated parent lipids, PA and HDA, induced modest increases in platelet and PMN adhesion, respectively, as well as the expression of all four endothelial adhesion molecules. However, these effects were much lower than produced by the chlorinated lipids. A similar pattern of response was noted with regard to endothelial permeability changes, as assessed by changes in electrical resistance across the monolayers, which were invoked by exposure to the chlorinated and non-chlorinated lipids (Figure 1D). PA and HDA exposure decreased electrical resistance (increased permeability) by about 20% while the chlorinated lipids produced a reduction by almost 60%. These in vitro studies clearly established that chlorinated lipids are capable of inducing powerful pro-inflammatory effects in vitro and do so at a dose (10 μM) that is well within the range of tissue values (~20 μM) measured following neutrophil and monocyte activation (6, 7, 11, 18, 21).
In the next several groups of experiments, we examined the effects of local tissue exposure to chlorinated and non-chlorinated lipids in the intact, blood-perfused microcirculation using intravital microscopy. Superfusion of intestine wall with 2-ClPA or 2-ClHDA produced marked increases in the numbers of rolling and firmly adherent leukocytes as well as platelet adhesion (Figures 2A–B and 3). These proadhesive effects were consistent with our in vitro observations and were similar in magnitude to the increases in rolling and adhesion that we and others have reported for ischemia/reperfusion (26, 27, 30, 31) and septic shock (39). However, in contrast to the modest proinflammatory effects of non-chlorinated lipids we observed in vitro, PA and HDA did not influence leukocyte or platelet adhesion in vivo. This apparent discrepancy is most likely due to the effects of shear stress in vivo and dilutional effects on the concentrations to which blood borne cells are exposed. The increases in leukocyte rolling and adhesion were associated with enhanced leukocyte infiltration into the gut wall, as reflected by increased intestinal MPO expression and activity (Figure 7).
In addition to inducing significant increases in leukocyte-endothelial cell interactions and platelet-endothelial cell adhesion, mesenteric superfusion with either 2-ClPA or 2-ClHDA caused interstitial mast cells activation (Figure 4), increased ROS production in postcapillary venules (Figure 5), and provoked microvascular barrier disruption within 20 min of the onset of exposure (Figure 6). Although not tested as part of these studies, it is tempting to speculate that chlorinated lipids instigate these proinflammatory responses secondary to their effect on mast cells. When these sentinel cells are activated, they release a wide variety of proinflammatory mediators that can induce leukocyte-endothelial cell adhesive interactions, platelet-endothelial cell adhesion and increase venular protein leakage. In addition, mast cells release angiotensin II, which can activate NADPH oxidases to provoke oxidant production. Mast cell activation is also associated with the release of chymase, an enzyme that produces angiotensin II and is likely the main source of this proinflammatory peptide at sites of tissue injury. It is important to note that in addition to potential mast cell-dependent mechanisms, it is likely that direct effects of the chlorinated lipids on endothelial function also contribute, a concept supported by our in vitro data wherein 2-ClPA and 2-ClHDA produced marked inflammatory responses in the absence of mast cells.
In the only other published work on the proinflammatory effects of chlorinated lipids, Ullen and coworkers (9) have presented evidence indicating that 2-ClHDA severely compromised the barrier function of brain microvascular endothelial cells and morphological alterations in tight and adherens junctions, mediated by mitogen-activated protein kinases (MAPK) cascade activation, extracellular signal-regulated kinases (ERK1/2) and JNK cascade. Subsequent work from the same group showed that 2-ClHDA induced endothelial barrier disruption that was associated with endothelial cell apoptosis (12), while treatment with a clickable alkyne analog of 2-ClHDA modified several cytoskeletal proteins associated with tight junction patterning, fibronectin, and proteins involved in induction of oxidative stress responses and regulation of intracellular redox homeostasis (40). Other in vitro work from our laboratory (unpublished observations) has demonstrated the 2-ClHDA provokes an increase in albumin permeability that is accompanied by coincident increase in endothelial stiffness and loss of cadherins.
Until very recently, the mechanisms by which chlorinated lipids induce ECAM expression and LECA have been largely unexplored. In a study just published by our group (19), we report significant new insight regarding how 2-chlorofatty acids induce expression of adhesion molecules in human coronary artery endothelial cells. Using a Click chemistry approach, we demonstrated that 2-chlorofatty acids localize to Weibel-Palade bodies to promote release of von Willebrand factor (VWF). The release of VWF induced by 2-chlorofatty acids was abolished by treatment with a protein kinase C inhibitor or a calcium chelator, and may possibly involve acidification of Weibel-Palade bodies and actomyosin II contracility. VWF mediates platelet adhesion to endothelium via interactions with platelet GPIba. Endothelial surface expression of P-selectin also follows Weibel-Palade body mobilization, which interacts with PSGL-1 on platelets and sialyl-LewisX on neutrophils to promote adhesive interactions. Thus, this recent work suggests that 2-chlorofatty acids may act target Weibel-Palade bodies to promote expression of VWF and P-selectin by a PKC and calcium-dependent mechanism.
Several other studies suggest additional mechanisms that may underlie the effects of chlorolipids to promote adhesion molecule expression. For example, in another study just published by our group (20), we showed that 2-chlorofatty acids promote the release of another Weibel-Palade body resident molecule, angiopoietin-2. Others have shown that angiopoietin-2 promotes leukocyte adhesion by a β2-integrin/ICAM-1-dependent mechanism (41), suggesting that this growth factor may contribute to 2-chlorolipid-induced ECAM expression. Work conducted by other suggests that 2-chlorofatty acids inhibit eNOS signaling, thereby decreasing the bioavailability of nitric oxide (14, 15), a gaseous signaling molecule that normally acts to suppress adhesion molecule expression (24). It is likely that 2-chlorolipid-induced ROS production (Figure 5) contributes to this reduction in bioavailable NO and thus adhesion molecule expression. In addition to these mechanisms, the effect of 2-chlorolipids to promote mast cell degranulation (Figure 4) may play a role, as these cells have been shown to release a variety of proadhesive molecules that promote adhesion molecule expression. 2-ClHDA promotes the disappearance of IκB in endothelial cells, freeing NF-κB for translocation to the nucleus, where it binds to promoter regions of genes involved in ECAM expression, various chemokines and other proinflammatory mediators (13). Clearly, much additional work will be required to directly address these postulated mechanisms.
In summary, the results of this study indicate that chlorinated lipids induce a number of inflammatory responses in rat mesentery, including increased leukocyte-endothelial cell interactions, platelet-endothelial cell adhesion, and neutrophil infiltration into the tissues, mast cell activation, ROS production and disrupted endothelial barrier function. On the contrary, non-chlorinated lipids did not produce these responses in vivo. Similar proinflammatory responses were noted in response to chlorinated lipids in a cultured mesenteric microvascular endothelial cell model in vitro. Although much addition work will be required to elucidate the mechanisms underlying these inflammatory responses, our work provides proof-of-principle that chlorinated lipids that are generated by MPO/H2O2/chloride system in response to ischemia/reperfusion and sepsis could potentially contribute to the proinflammatory responses that occur in these neutrophil-dependent inflammatory conditions.
Acknowledgments
Grant support: This work was supported by a grant from the National Institutes of Health (GM-115553).
References
- 1.Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol. 2012;298:229. doi: 10.1016/B978-0-12-394309-5.00006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Ischemia/reperfusion. Compr Physiol. 2016;7(1):113–170. doi: 10.1002/cphy.c160006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Backer D, Orbegozo Cortes D, Donadello K, Vincent J-L. Pathophysiology of microcirculatory dysfunction and the pathogenesis of septic shock. Virulence. 2014;5(1):73–79. doi: 10.4161/viru.26482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Connick RE. The interaction of hydrogen peroxide and hypochlorous acid in acidic solutions containing chloride ion. J Am Chem Soc. 1947;69(6):1509–1514. [Google Scholar]
- 5.Levinsky R. The Neutrophil: Function and Clinical Disorders. J Med Genet. 1980;17(2):160. [Google Scholar]
- 6.Thukkani AK, Martinson BD, Albert CJ, Vogler GA, Ford DA. Neutrophil-mediated accumulation of 2-ClHDA during myocardial infarction: 2-ClHDA-mediated myocardial injury. Am J Physiol Heart Circ Physiol. 2005;288(6):H2955–H2964. doi: 10.1152/ajpheart.00834.2004. [DOI] [PubMed] [Google Scholar]
- 7.Anbukumar DS, Shornick LP, Albert CJ, Steward MM, Zoeller RA, Neumann WL, Ford DA. Chlorinated lipid species in activated human neutrophils: lipid metabolites of 2-chlorohexadecanal. J Lipid Res. 2010;51(5):1085–1092. doi: 10.1194/jlr.M003673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malle E, Waeg G, Schreiber R, Gröne EF, Sattler W, Gröne HJ. Immunohistochemical evidence for the myeloperoxidase/H2O2/halide system in human atherosclerotic lesions. Eur J Biochem. 2000;267(14):4495–4503. doi: 10.1046/j.1432-1327.2000.01498.x. [DOI] [PubMed] [Google Scholar]
- 9.Üllen A, Singewald E, Konya V, Fauler G, Reicher H, Nusshold C, Hammer A, Kratky D, Heinemann A, Holzer P. Myeloperoxidase-derived oxidants induce blood-brain barrier dysfunction in vitro and in vivo. PLoS One. 2013;8(5):e64034. doi: 10.1371/journal.pone.0064034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albert CJ, Crowley JR, Hsu F-F, Thukkani AK, Ford DA. Reactive Chlorinating Species Produced by Myeloperoxidase Target the Vinyl Ether Bond of Plasmalogens IDENTIFICATION OF 2-CHLOROHEXADECANAL. J Biol Chem. 2001;276(26):23733–23741. doi: 10.1074/jbc.M101447200. [DOI] [PubMed] [Google Scholar]
- 11.Thukkani AK, Hsu F-F, Crowley JR, Wysolmerski RB, Albert CJ, Ford DA. Reactive Chlorinating Species Produced during Neutrophil Activation Target Tissue Plasmalogens PRODUCTION OF THE CHEMOATTRACTANT, 2-CHLOROHEXADECANAL. J Biol Chem. 2002;277(6):3842–3849. doi: 10.1074/jbc.M109489200. [DOI] [PubMed] [Google Scholar]
- 12.Üllen A, Fauler G, Bernhart E, Nusshold C, Reicher H, Leis H-J, Malle E, Sattler W. Phloretin ameliorates 2-chlorohexadecanal-mediated brain microvascular endothelial cell dysfunction in vitro. Free Radic Biol Med. 2012;53(9):1770–1781. doi: 10.1016/j.freeradbiomed.2012.08.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Messner MC, Albert CJ, Ford DA. 2-Chlorohexadecanal and 2-chlorohexadecanoic acid induce COX-2 expression in human coronary artery endothelial cells. Lipids. 2008;43(7):581. doi: 10.1007/s11745-008-3189-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marsche G, Heller R, Fauler G, Kovacevic A, Nuszkowski A, Graier W, Sattler W, Malle E. 2-Chlorohexadecanal Derived From Hypochlorite-Modified High-Density Lipoprotein–Associated Plasmalogen Is a Natural Inhibitor of Endothelial Nitric Oxide Biosynthesis. Arterioscler Thromb Vasc Biol. 2004;24(12):2302–2306. doi: 10.1161/01.ATV.0000148703.43429.25. [DOI] [PubMed] [Google Scholar]
- 15.Nuszkowski A, Gräbner R, Marsche G, Unbehaun A, Malle E, Heller R. Hypochlorite-modified low density lipoprotein inhibits nitric oxide synthesis in endothelial cells via an intracellular dislocalization of endothelial nitric-oxide synthase. J Biol Chem. 2001;276(17):14212–14221. doi: 10.1074/jbc.M007659200. [DOI] [PubMed] [Google Scholar]
- 16.Kubes P, McCafferty D-M. Nitric oxide and intestinal inflammation. Am J Med. 2000;109(2):150–158. doi: 10.1016/s0002-9343(00)00480-0. [DOI] [PubMed] [Google Scholar]
- 17.Thukkani AK, Albert CJ, Wildsmith KR, Messner MC, Martinson BD, Hsu F-F, Ford DA. Myeloperoxidase-derived reactive chlorinating species from human monocytes target plasmalogens in low density lipoprotein. J Biol Chem. 2003;278(38):36365–36372. doi: 10.1074/jbc.M305449200. [DOI] [PubMed] [Google Scholar]
- 18.Wang W-y, Albert CJ, Ford DA. Alpha-Chlorofatty Acid Accumulates in Activated Monocytes and Causes Apoptosis Through Reactive Oxygen Species Production and Endoplasmic Reticulum StressSignificance. Arterioscler Thromb Vasc Biol. 2014;34(3):526–532. doi: 10.1161/ATVBAHA.113.302544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartman CL, Duerr MA, Albert CJ, Neumann WL, McHowat J, Ford DA. 2-Chlorofatty acids induce Weibel-Palade body mobilization. J Lipid Res. 2017:M080200. doi: 10.1194/jlr.M080200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyer NJ, Reilly JP, Feng R, Christie JD, Hazen SL, Albert CJ, Franke JD, Hartman CL, McHowat J, Ford DA. Myeloperoxidase-derived 2-chlorofatty acids contribute to human sepsis mortality via acute respiratory distress syndrome. JCI insight. 2017;2(23) doi: 10.1172/jci.insight.96432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thukkani AK, McHowat J, Hsu F-F, Brennan M-L, Hazen SL, Ford DA. Identification of α-chloro fatty aldehydes and unsaturated lysophosphatidylcholine molecular species in human atherosclerotic lesions. Circulation. 2003;108(25):3128–3133. doi: 10.1161/01.CIR.0000104564.01539.6A. [DOI] [PubMed] [Google Scholar]
- 22.Wildsmith KR, Albert CJ, Anbukumar DS, Ford DA. Metabolism of myeloperoxidase-derived 2-chlorohexadecanal. J Biol Chem. 2006;281(25):16849–16860. doi: 10.1074/jbc.M602505200. [DOI] [PubMed] [Google Scholar]
- 23.Meyer MC, Creer MH, McHowat J. Potential role for mast cell tryptase in recruitment of inflammatory cells to endothelium. Am J Physiol Cell Physiol. 2005;289(6):C1485–C1491. doi: 10.1152/ajpcell.00215.2005. [DOI] [PubMed] [Google Scholar]
- 24.Radomski M, Palmer R, Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet. 1987;330(8567):1057–1058. doi: 10.1016/s0140-6736(87)91481-4. [DOI] [PubMed] [Google Scholar]
- 25.Marentette J, Kolar G, McHowat J. Increased susceptibility to bladder inflammation in smokers: targeting the PAF–PAF receptor interaction to manage inflammatory cell recruitment. Physiol rep. 2015;3(12):e12641. doi: 10.14814/phy2.12641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zuidema MYKR. INtravital microscopic methods to evaluate anti-inflammatory effects and signaling mechanisms evoked by hydrogen sulfide. Methods Enzymol. 2015;555:93–125. doi: 10.1016/bs.mie.2014.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dai H, Wang M, Patel PN, Kalogeris TJ, Liu Y, Durante W, Korthuis RJ. Preconditioning with the BKCa Channel Activator NS-1619 Prevents Ischemia/Reperfusion-Induced Inflammation and Mucosal Barrier Dysfunction: ROS and HO-1. Am J Physiol Heart Circ Physiol. 2017 doi: 10.1152/ajpheart.00620.2016. 00620.02016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steiner DR, Gonzalez NC, Wood JG. Mast cells mediate the microvascular inflammatory response to systemic hypoxia. J Appl Physiol. 2003;94(1):325–334. doi: 10.1152/japplphysiol.00637.2002. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Kalogeris T, Wang M, Zuidema MY, Wang Q, Dai H, Davis MJ, Hill MA, Korthuis RJ. Hydrogen sulfide preconditioning or neutrophil depletion attenuates ischemia-reperfusion-induced mitochondrial dysfunction in rat small intestine. Am J Physiol Gastrointest Liver Physiol. 2012;302(1):G44–G54. doi: 10.1152/ajpgi.00413.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zuidema MY, Peyton KJ, Fay WP, Durante W, Korthuis RJ. Antecedent hydrogen sulfide elicits an anti-inflammatory phenotype in postischemic murine small intestine: role of heme oxygenase-1. Am J Physiol Heart Circ Physiol. 2011;301(3):H888–H894. doi: 10.1152/ajpheart.00432.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zuidema MY, Yang Y, Wang M, Kalogeris T, Liu Y, Meininger CJ, Hill MA, Davis MJ, Korthuis RJ. Antecedent hydrogen sulfide elicits an anti-inflammatory phenotype in postischemic murine small intestine: role of BK channels. Am J Physiol Heart Circ Physiol. 2010;299(5):H1554–H1567. doi: 10.1152/ajpheart.01229.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Albrich JM, McCarthy CA, Hurst JK. Biological reactivity of hypochlorous acid: implications for microbicidal mechanisms of leukocyte myeloperoxidase. Proc Natl Acad Sci U S A. 1981;78(1):210–214. doi: 10.1073/pnas.78.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heinecke JW, Li W, Mueller DM, Bohrer A, Turk J. Cholesterol chlorohydrin synthesis by the myeloperoxidase-hydrogen peroxide-chloride system: potential markers for lipoproteins oxidatively damaged by phagocytes. Biochemistry. 1994;33(33):10127–10136. doi: 10.1021/bi00199a041. [DOI] [PubMed] [Google Scholar]
- 34.Thomas EL, Jefferson MM, Grisham MB. Myeloperoxidase-catalyzed incorporation of amines into proteins: role of hypochlorous acid and dichloramines. Biochemistry. 1982;21(24):6299–6308. doi: 10.1021/bi00267a040. [DOI] [PubMed] [Google Scholar]
- 35.Weiss SJ, Klein R, Slivka A, Wei M. Chlorination of taurine by human neutrophils: evidence for hypochlorous acid generation. J Clin Invest. 1982;70(3):598. doi: 10.1172/JCI110652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pike LJ, Han X, Chung K-N, Gross RW. Lipid rafts are enriched in arachidonic acid and plasmenylethanolamine and their composition is independent of caveolin-1 expression: a quantitative electrospray ionization/mass spectrometric analysis. Biochemistry. 2002;41(6):2075–2088. doi: 10.1021/bi0156557. [DOI] [PubMed] [Google Scholar]
- 37.Gross RW. High plasmalogen and arachidonic acid content of canine myocardial sarcolemma: A fast atom bombardment mass spectroscopic and gas chromatography-mass spectroscopic characterization. Biochemistry. 1984;23(1):158–165. doi: 10.1021/bi00296a026. [DOI] [PubMed] [Google Scholar]
- 38.Post J, Verkleij A, Roelofsen B, de Kamp JO. Plasmalogen content and distribution in the sarcolemma of cultured neonatal rat myocytes. FEBS Lett. 1988;240(1–2):78–82. doi: 10.1016/0014-5793(88)80343-0. [DOI] [PubMed] [Google Scholar]
- 39.Singer G, Stokes KY, Terao S, Granger DN. Sepsis-induced intestinal microvascular and inflammatory responses in obese mice. Shock. 2009;31(3):275–279. doi: 10.1097/SHK.0b013e3181834ab3. [DOI] [PubMed] [Google Scholar]
- 40.Nusshold C, Üllen A, Kogelnik N, Bernhart E, Reicher H, Plastira I, Glasnov T, Zangger K, Rechberger G, Kollroser M. Assessment of electrophile damage in a human brain endothelial cell line utilizing a clickable alkyne analog of 2-chlorohexadecanal. Free Radic Biol Med. 2016;90:59–74. doi: 10.1016/j.freeradbiomed.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scholz A, Lang V, Henschler R, Czabanka M, Vajkoczy P, Chavakis E, Drynski J, Harter PN, Mittelbronn M, Dumont DJ. Angiopoietin-2 promotes myeloid cell infiltration in a β 2-integrin–dependent manner. Blood. 2011;118(18):5050–5059. doi: 10.1182/blood-2011-03-343293. [DOI] [PubMed] [Google Scholar]