Summary
Dickeya dadantii 3937 secretes pectate lyases (Pels) to degrade plant cell walls. Previously, we have demonstrated that EGcpB and EcpC function as bis‐(3′,5′)‐cyclic dimeric guanosine monophosphate (c‐di‐GMP)‐specific phosphodiesterases (PDEs) to positively regulate Pel production. However, the diguanylate cyclase (DGC) responsible for the synthesis of c‐di‐GMP and the dichotomous regulation of Pel has remained a mystery. Here, we identified GcpA as the dominant DGC to negatively regulate Pel production by the specific repression of pelD gene expression. Quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR) assays revealed that the expression levels of histone‐like, nucleoid‐structuring protein encoding gene hns and post‐transcriptional regulator encoding genes rsmA and rsmB were significantly affected by GcpA. Deletion of hns or rsmB in the gcpAD418A site‐directed mutant restored its Pel production and pelD expression, demonstrating that H‐NS and RsmB contribute to the GcpA‐dependent regulation of Pel in D. dadantii. In addition, RsmB expression was subject to positive regulation by H‐NS. Thus, we propose a novel pathway consisting of GcpA–H‐NS–RsmB–RsmA–pelD that controls Pel production in D. dadantii. Furthermore, we showed that H‐NS and RsmB are responsible for the GcpA‐dependent regulation of motility and type III secretion system (T3SS) gene expression, respectively. Of the two PDEs involved in the regulation of Pels, only EGcpB regulates pelD expression through the same pathway as GcpA.
Keywords: c‐di‐GMP, motility, pectate lyase, soft‐rot pathogen, type III secretion system, virulence
Introduction
Dickeya dadantii 3937 is an Enterobacterium that causes soft‐rot disease in a wide range of economically important crops (Czajkowski et al., 2011; Ma et al., 2007). Pectate lyases (Pels), which degrade the plant cell wall, are one of the major virulence factors that contribute to the pathogenesis of D. dadantii (Collmer and Keen, 1986). The production of Pel is controlled by a sophisticated regulatory mechanism that includes modifications of DNA topology, quorum sensing and other regulatory systems associated with bacterial physiological status or environmental stimuli (Charkowski et al., 2012; Hugouvieux‐Cotte‐Pattat et al., 1996; Reverchon and Nasser, 2013). During the early stages of infection, several transcriptional repressors, such as FIS, H‐NS, KdgR, PecS, PecT, Fur and the PhoP/Q two‐component regulatory system, negatively regulate the expression of pel genes in response to initially encountering the oxidative and acidic environment in plant intercellular spaces (Franza et al., 2002; Hérault et al., 2014; Llama‐Palacios et al., 2005; Ouafa et al., 2012; Reverchon et al., 1991). In addition, the post‐transcriptional regulator RsmA/RsmB inhibits Pel via an unknown mechanism (Wu et al., 2014; Yang et al., 2008). RsmA facilitates specific mRNA degradation, whereas RsmB is an untranslated regulatory RNA that binds to RsmA and neutralizes its effect on target gene expression (Liu et al., 1997). After adaptation to the intracellular spaces, D. dadantii secretes a massive amount of Pel into the plant apoplast following the inactivation of the previously mentioned Pel repressors and the activation of Pel inducers, which include the GacS/A two‐component system, MfbR, CRP and the Vfm quorum sensing system (Charkowski et al., 2012; Franza et al., 2002; Hugouvieux‐Cotte‐Pattat et al., 1996; Nasser et al., 2013; Reverchon and Nasser, 2013; Reverchon et al., 2010; Yang et al., 2008).
Bis‐(3′,5′)‐cyclic dimeric guanosine monophosphate (c‐di‐GMP) is a common bacterial second messenger found in most major bacterial phyla (Römling et al., 2013). It was first discovered as an allosteric activator for cellulose synthase in Gluconacetobacter xylinus (Ross et al., 1987). It is now established that c‐di‐GMP is involved in the regulation of many cellular activities, including biofilm formation, motility, the cell cycle, antibiotic production and virulence (Cotter and Stibitz, 2007; Hengge, 2009; Jenal et al., 2017; Tamayo et al., 2007). The synthesis and hydrolysis of c‐di‐GMP are catalyzed by diguanylate cyclase (DGC) and c‐di‐GMP‐specific phosphodiesterase (PDE) enzymes, respectively. DGC activity is associated with the GGDEF domain, which converts two molecules of guanosine‐5′‐triphosphate (GTP) to one molecule of c‐di‐GMP (Paul et al., 2004; Whiteley and Lee, 2015). PDE activity is associated with either an EAL or HD‐GYP domain, which degrades c‐di‐GMP to 5′‐phosphoguanylyl‐(3′‐5′)‐guanosine (pGpG) or two molecules of guanosine monophosphate (GMP) (Ryan et al., 2006; Schmidt et al., 2005; Tamayo et al., 2005). The sophisticated c‐di‐GMP‐mediated signalling network includes transcriptional, post‐transcriptional and post‐translational regulation. The regulatory function of c‐di‐GMP is exerted through the binding of c‐di‐GMP to a variety of cellular effectors, such as PilZ domain proteins, transcription factors, enzymatically inactive GGDEF, EAL or HD‐GYP domain proteins and RNA riboswitches (Jenal et al., 2017; Orr et al., 2016; Römling et al., 2013; Ryan et al., 2012).
It has been revealed that GGDEF and EAL domain proteins are abundantly present in many Gram‐negative bacteria. For example, Escherichia coli K‐12 contains 29 genes and Vibrio cholerae contains 53 (Povolotsky and Hengge, 2012; Waters et al., 2008). In D. dadantii, we found 12 gcp (GGDEF domain‐containing protein), four ecp (EAL domain‐containing protein) and two egcp (EAL‐GGDEF domain‐containing protein) genes in the genome using the Pfam program (pfam.xfam.org) (Fig. S1, see Supporting Information). Our previous studies have demonstrated that two c‐di‐GMP‐specific PDEs, EGcpB and EcpC, positively regulate swimming motility, Pel production and type III secretion system (T3SS) gene expression, but negatively regulate biofilm formation (Yi et al., 2010; Yuan et al., 2015). EGcpA, a homologue of E. coli CsrD, negatively regulates Pel production and T3SS gene expression by modulating the expression of RsmB (Wu et al., 2014). Nevertheless, the function of other Gcp and Ecp proteins in D. dadantii and the molecular mechanism of c‐di‐GMP signalling in the regulation of diverse virulence factors remain unclear.
In this study, we first analysed the regulatory roles of 18 GGDEF and/or EAL domain proteins in Pel production. GcpA was identified to be the major DGC that negatively regulated Pel production by repression of the expression of the pelD gene. We then demonstrated that GcpA regulates pelD through the H‐NS–RsmB–RsmA pathway. Although EGcpB and EcpC are the two major PDEs that up‐regulate Pel production, only EGcpB positively regulates pelD gene expression through the same regulatory pathway as GcpA. Furthermore, we demonstrated that GcpA was involved in the regulation of swimming motility and T3SS gene expression through diverse mechanisms that were independent of its regulation of Pel. Together, our studies define a comprehensive signalling network that links c‐di‐GMP signalling and multiple virulence factors in D. dadantii.
Results
The GGDEF domain protein GcpA negatively regulates Pel production in D. dadantii
GGDEF and EAL domains are responsible for the enzymatic activities of DGCs and PDEs, respectively. In D. dadantii, 18 genes were found to encode proteins containing putative GGDEF and/or EAL domains at their C‐terminal regions (Fig. S1A), implying that a complicated c‐di‐GMP signalling network exists for the regulation of diverse cell behaviour. Our results showed that four proteins, GcpA, GcpD, GcpF and EGcpB, contained two types of sensory domain, GAF (cGMP phosphodiesterase, Adenyl cyclase, FhlA domain) and PAS (Per/Arnt/Sim), at their N‐terminus (Fig. S1A). Nine proteins (GcpB, GcpC, GcpG, GcpH, GcpJ, GcpK, GcpL, EGcpA and EcpD) contained one or multiple N‐terminal transmembrane domains (Fig. S1A). Amino acid sequence alignments between the known GGDEF and EAL domains from Caulobacter crescentus, V. cholerae, Pseudomonas aeruginosa and D. dadantii revealed that most of the GGDEF domains in D. dadantii contained an active site (A‐site) that is involved in GTP binding (Römling et al., 2013). Eight GGDEF domains from GcpA‐H were annotated with an inhibition site or I‐site (RxxD motif), a secondary c‐di‐GMP binding site that represses the cyclase activity of DGC enzymes (Christen et al., 2006) (Fig. S1B).
To fully investigate the network of c‐di‐GMP signalling in D. dadantii involved in Pel regulation, the impacts of each Gcp and Ecp protein on Pel were investigated. Nine gcp and ecp gene deletion mutants were newly constructed (Table S1, see Supporting Information). However, several attempts to delete gcpA were not successful, suggesting that gcpA might be essential for the viability of D. dadantii. We then disrupted the predicted A‐site motif (SGDEF) in the GGDEF domain of GcpA by replacing the essential aspartic acid residue with alanine (SGAEF), resulting in a gcpAD418A site‐directed mutant. This mutant was not defective for growth when compared with the wild‐type strain (data not shown). Therefore, together with eight mutants that had been constructed previously, we expanded the mutant library to cover each individual gcp, ecp or egcp gene (Table S1). Pel activity was measured in the 18 mutants and the wild‐type strain. As reported previously, deletion of egcpB and ecpC led to reduced Pel activities, whereas ΔegcpA showed enhanced Pel activity relative to the wild‐type strain (Wu et al., 2014; Yi et al., 2010) (Fig. 1A). No difference in Pel activity was observed in ΔecpA, ΔecpD or ΔecpE (Fig. 1A). Interestingly, amongst the 12 gcp gene deletion mutants, only gcpAD418A exhibited increased Pel activity compared with the wild‐type strain (Fig. 1A). Complementation assays confirmed that the in trans expression of gcpA drastically reduced Pel activity in gcpAD418A (Fig. S2, see Supporting Information). These findings suggest that GcpA negatively regulates Pel production in D. dadantii.
Figure 1.
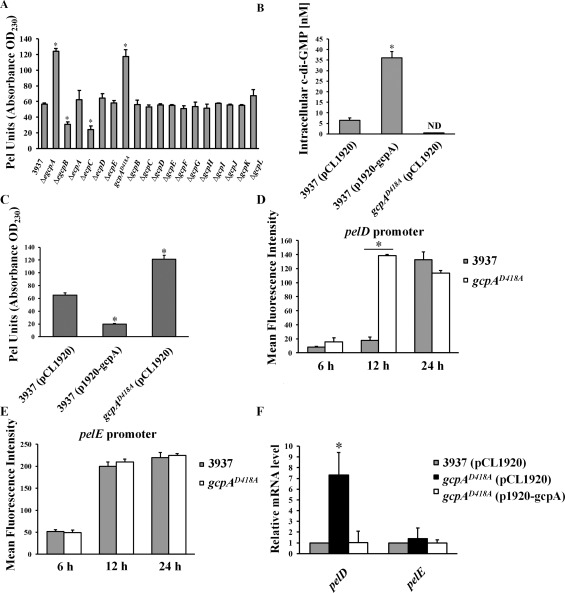
GcpA synthesizes bis‐(3′,5′)‐cyclic dimeric guanosine monophosphate (c‐di‐GMP) to negatively regulate pectate lyase (Pel) production and pelD gene expression in Dickeya dadantii. (A) Pel production of wild‐type D. dadantii and GGDEF and/or EAL deletion mutant strains cultured in minimal medium (MM) + 0.1% polygalacturonic acid (PGA) for 12 h at 28 °C. OD230, optical density at 230 nm. Measurement of intracellular c‐di‐GMP (B) and Pel (C) production in wild‐type D. dadantii harbouring empty vector pCL1920, wild‐type harbouring pCL1920‐gcpA and gcpAD418A harbouring pCL1920‐gcpA. pelD (D) and pelE (E) promoter activities were measured in the parental strain D. dadantii and gcpAD418A. Cells cultured in MM + 0.1% PGA were harvested at 6, 12 and 24 h to measure the mean fluorescence intensity (MFI) by flow cytometry. (F) Quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR) analysis of mRNA levels of pelD and pelE in D. dadantii strains. The data represent the expression levels of each gene relative to that in the wild‐type, which was mathematically designated as unity. The rplU gene was used as an endogenous control for the calculation. All results are from one representative experiment. Three independent experiments were performed and three replicates were used for each experiment. Error bars indicate standard errors of the means. ND represents not detectable. Asterisks indicate statistically significant differences of the means (P < 0.05 by Student's t‐test).
The DGC activity of GcpA is essential for its regulation of Pel production
As mutation of the GcpA A‐site enhanced the production of Pel and a functional A‐site is required for GGDEF domain activity, we hypothesized that GcpA is an active DGC and regulates Pel production through c‐di‐GMP signalling. To test this, the intracellular concentrations of c‐di‐GMP were compared in the wild‐type strain, gcpAD418A and wild‐type expressing gcpA using ultra‐performance liquid chromatography coupled with tandem mass spectrometry (UPLC‐MS‐MS). The results showed that the c‐di‐GMP concentration in the strain over‐expressing gcpA was about six‐fold higher than that in the same strain carrying the empty vector (Fig. 1B). Alternatively, the c‐di‐GMP concentration was below the detection limit in gcpAD418A (Fig. 1B). These data confirm that GcpA is an active DGC. We also observed that the overexpression of gcpA drastically reduced Pel activity in the wild‐type strain (Fig. 1C). Together, these results support the conclusion that GcpA synthesizes c‐di‐GMP in D. dadantii to negatively regulate the production of Pel.
The expression of pelD is enhanced in the gcpAD418A A‐site mutant
To reveal the underlying mechanism of the inhibition of Pel production by GcpA, we analysed the effects of GcpA on two major Pel genes, pelD and pelE. In the presence of polygalacturonic acid (PGA), the promoter activities of pelD and pelE were induced by 22‐ and 4.5‐fold, respectively, at 24 h (Fig. S3, see Supporting Information), suggesting that these pel promoter‐GFP transcriptional fusions are sensitive to the addition of pectin catabolic products. Next, the promoter activities of pelD and pelE were determined in wild‐type and gcpAD418A strains cultured in the presence of PGA for 6, 12 and 24 h. At 12 h, a significant increase in pelD promoter activity was observed in the gcpAD418A mutant compared with the wild‐type strain (Fig. 1D). Interestingly, no significant change was observed for pelE promoter activity (Fig. 1E). To confirm the negative effect of GcpA on pelD, the mRNAs of pelD and pelE were measured by quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR) in wild‐type, gcpAD418A and the complemented strain containing the p1920‐gcpA plasmid. Consistent with the change in pelD promoter activity, pelD transcript levels increased by seven‐fold in gcpAD418A relative to the wild‐type strain (Fig. 1F). The elevated mRNA level of pelD in gcpAD418A was restored to wild‐type levels by the complementation plasmid p1920‐gcpA (Fig. 1F). In contrast, pelE transcript levels were not altered in the tested strains (Fig. 1F). Thus, these results suggest that GcpA negatively regulates Pel production by repression of the expression of pelD in D. dadantii.
The expression of Pel regulators in the gcpAD418A mutant
To assess whether the regulation of Pel by GcpA is through any known Pel regulators, we determined the expression of six negative regulators and three positive regulators in wild‐type and gcpAD418A strains using qRT‐PCR assays (Fig. 2A). Mutation of gcpA resulted in increased RNA levels of rsmB, hns, fur, pecS and kdgR, and decreased RNA levels of rsmA. No significant difference was observed for pecT, fis and crp. Given that Fur, PecS and KdgR are pel gene repressors, we reasoned that the enhanced Pel activity in gcpAD418A was not caused by the increased expression of these genes. As hns, rsmA, and rsmB showed the highest fold changes by qRT‐PCR analysis, we questioned whether there were any changes at the level of promoter activity. These promoters were examined using transcriptional fusions to a green fluorescent protein (GFP) reporter in wild‐type and gcpAD418A strains. None of the promoter activities were significantly influenced in the gcpAD418A mutant (Fig. S4, see Supporting Information), suggesting that GcpA regulates the expression of these genes at the post‐transcriptional level. A Western blot assay confirmed that the protein levels of RsmA were reduced in the gcpAD418A mutant (Fig. 2B). Together, these results imply that the control of Pel production by GcpA might rely on its positive regulation of RsmA and negative regulation of RsmB and H‐NS at the post‐transcriptional level.
Figure 2.
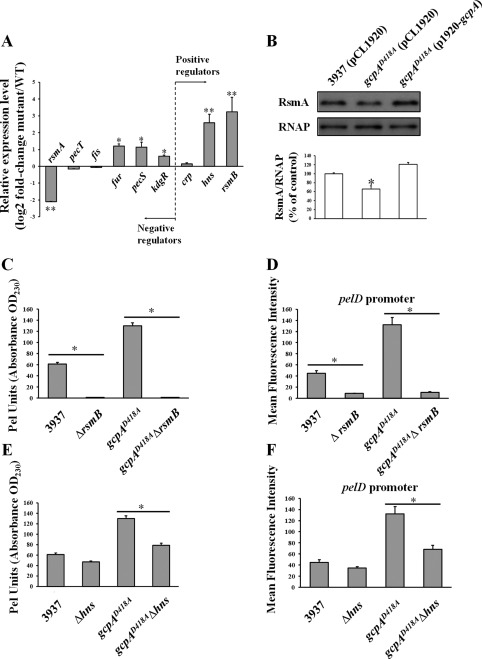
RsmB and H‐NS play important roles in GcpA‐dependent pectate lyase (Pel) regulation. (A) Quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR) analysis of RNA levels of rsmA, pecT, fis, fur, pecS, kdgR, crp, hns and rsmB in Dickeya dadantii wild‐type (WT) and gcpAD418A. The mutant/WT ratio for each gene expression was calculated as described in Experimental procedures. (B) Western blot analysis of RsmA protein in D. dadantii strains. Pel production (C, E) and pelD promoter activities (D, F) were tested in D. dadantii strains. Values are representative of three independent experiments. Three replicates were used in each experiment. Error bars indicate standard errors of the means. Asterisks indicate statistically significant differences of the means (*P < 0.05 or **P < 0.01 by Student's t‐test). OD230, optical density at 230 nm.
GcpA regulates Pel through the Rsm system
A significant increase in rsmB and decrease in rsmA in gcpAD418A strongly suggest that the Rsm system may play an important role in the GcpA‐dependent signalling pathway for Pel regulation. To investigate this hypothesis, we constructed an rsmB deletion in the wild‐type and gcpAD418A strains, and tested the Pel‐related phenotypes. We found that deletion of rsmB resulted in severe defects in Pel production and pelD promoter activity in both backgrounds (Fig. 2C,D). These results were in agreement with previous studies showing that RsmB is a positive regulator of Pel (Yang et al., 2008). More importantly, deletion of rsmB in gcpAD418A (gcpAD418AΔrsmB) drastically reduced both Pel production and pelD promoter activity to a level similar to that of the ΔrsmB mutant (Fig. 2C,D). This result suggests that RsmB plays a predominant role in controlling Pel in D. dadantii, and that the repression of Pel production by GcpA is possibly through the regulation of RsmB. Considering that RsmB functions mainly by titration of the effect of the RNA‐binding protein RsmA, we speculated that the over‐expression of rsmA might result in similar phenotypes. To test this hypothesis, we constructed a plasmid to express rsmA in trans and transformed it into the gcpAD418A mutant. As expected, the over‐expression of rsmA reduced Pel production and pelD promoter activity to near wild‐type levels in the gcpAD418A mutant (Fig. S5, see Supporting Information). Therefore, we conclude that the RsmA/RsmB system plays an important role in the c‐di‐GMP signalling pathway mediated by GcpA to regulate Pel activity.
H‐NS is involved in the GcpA–Rsm pathway
It has been shown previously that H‐NS positively modulates Pel synthesis in D. dadantii (Nasser and Reverchon, 2002). Interestingly, we did not observe a significant reduction in Pel production or pelD promoter transcription when hns was deleted in the wild‐type strain (Fig. 2E,F). However, when hns was deleted in the gcpAD418A mutant, Pel production and pelD promoter activity were dramatically reduced (Fig. 2E,F). These results, together with the observation that the expression of hns was elevated in the gcpAD418A mutant (Fig. 2A), suggest that GcpA also represses Pel through H‐NS.
As we have shown that both H‐NS and the Rsm system are involved in GcpA‐dependent Pel regulation, we hypothesized that there might be a genetic link between these two regulators. To address this hypothesis, the RNA levels of rsmB were determined in wild‐type, gcpAD418A, Δhns and the gcpAD418AΔhns double mutant using qRT‐PCR. In contrast with the approximate four‐fold increase in rsmB in the gcpAD418A mutant, the relative level of rsmB was decreased by about four‐fold in the Δhns mutant (Fig. 3). More importantly, rsmB expression was recovered to near wild‐type levels in the gcpAD418AΔhns double mutant. The difference in rsmB RNA levels in the different strains was further confirmed by Northern blot analysis (data not shown). Together, our data indicate that GcpA regulates Pel through the GcpA–H‐NS–RsmB–RsmA–pelD pathway.
Figure 3.
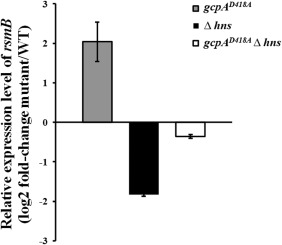
H‐NS is involved in the GcpA‐dependent regulation of rsmB. rsmB RNA levels were examined in Dickeya dadantii using quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR). The mutant/wild‐type (WT) ratio for rsmB gene expression was calculated as described in Experimental procedures. One representative experiment was chosen, and three independent experiments were performed. Error bars indicate standard errors of the means. Asterisks indicate statistically significant differences of the means (P < 0.05 by Student's t‐test).
EGcpB and EcpC differentially affect Pel production
Previously, we have shown that the PDEs EGcpB and EcpC increase Pel production in D. dadantii by reducing c‐di‐GMP, which is in contrast with GcpA, which synthesizes c‐di‐GMP to inhibit Pel production (Yi et al., 2010; Yuan et al., 2015). Therefore, we asked whether these two PDEs control Pel through the same regulatory pathways as GcpA. To address this question, we first examined the promoter activities of pelD in ΔegcpB and ΔecpC. As expected, deletion of either egcpB or ecpC in the wild‐type strain strongly reduced the expression of pelD (Fig. 4A). Next, we compared the RNA levels of hns and rsmB in the ΔegcpB and ΔecpC mutants with those in the wild‐type strain using qRT‐PCR. Interestingly, although a strong reduction in rsmB transcripts was detected in both ΔegcpB and ΔecpC, the hns transcript levels were only reduced in ΔegcpB, but not in ΔecpC (Fig. 4B). These data suggest that EGcpB might regulate Pel through the H‐NS–RsmB–RsmA–pelD pathway, whereas EcpC might regulate rsmB expression through a different mechanism. To further investigate their genetic interactions, the egcpB or ecpC gene was deleted in gcpAD418A, resulting in the double mutants gcpAD418AΔegcpB and gcpAD418AΔecpC. We then detected rsmB transcripts in the wild‐type strain and the single and double mutants by Northern blots. Consistent with the qRT‐PCR results, both ΔegcpB and ΔecpC mutants showed reduced levels of rsmB (Fig. 4C). Interestingly, rsmB expression was recovered to near wild‐type level in the gcpAD418AΔegcpB double mutant, whereas it appeared to be even more reduced in the gcpAD418AΔecpC double mutant than in the ΔecpC mutant. Moreover, the expression levels of pelD in various mutants were detected by qRT‐PCR assays. The results showed that the expression of pelD was down‐regulated in both ΔegcpB and ΔecpC (Fig. 4D), which is consistent with the reduced rsmB levels in these mutants. As expected, pelD expression was recovered to near wild‐type levels in the gcpAD418AΔegcpB double mutant, but remained low in gcpAD418AΔecpC (Fig. 4D). In summary, our results suggest that GcpA and EGcpB inversely regulate pelD gene expression through the same regulatory pathway, whereas EcpC utilizes a different mechanism.
Figure 4.
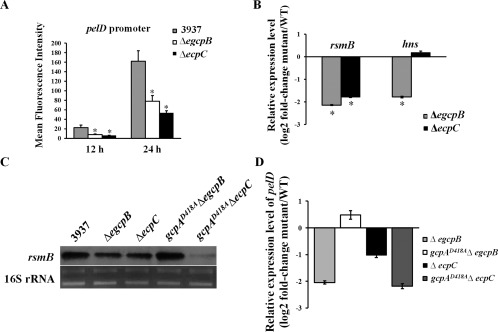
EGcpB and EcpC positively regulate pelD gene expression via different pathways. (A) The promoter activity of pelD was examined in Dickeya dadantii. (B) RNA levels of rsmB and hns were examined using quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR). The mutant/wild‐type (WT) ratio of each gene was calculated as described in Experimental procedures. (C) Northern blot analysis of rsmB mRNA in D. dadantii strains. (D) mRNA levels of pelD in mutant strains relative to that in the wild‐type strain. Values are representative of three independent experiments. Three replicates were used in each experiment. Error bars indicate standard errors of the means. Asterisks indicate statistically significant differences of the means (P < 0.05 by Student's t‐test).
GcpA negatively regulates the virulence, swimming motility and T3SS gene expression of D. dadantii
As the mutation of gcpA showed an opposite effect on Pel production to the mutation of either egcpB or ecpC, and the virulence of ΔegcpB and ΔecpC on host plants was reduced, we further determined whether the virulence of gcpAD418A was altered. As shown in Fig. 5A, gcpAD418A exhibited a two‐fold increase in maceration area relative to the wild‐type strain in the leaves of the host plant Chinese cabbage (Brassica campestris). Furthermore, in comparison with the reduced maceration areas caused by ΔegcpB and ΔecpC, double mutants of both gcpAD418AΔegcpB and gcpAD418AΔecpC partially restored the virulence phenotype, but not to the extent of the wild‐type strain (Fig. 5A). This result was noteworthy for gcpAD418AΔecpC, as its pelD expression was not restored at all (Fig. 4D), indicating that GcpA might regulate other virulence factors in addition to Pel in D. dadantii.
Figure 5.
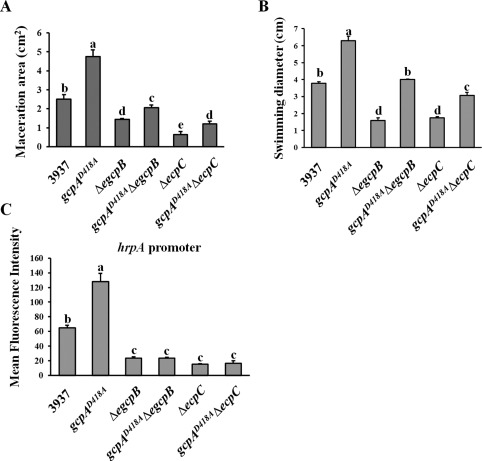
Effects of GcpA on swimming motility, type III secretion system (T3SS) gene expression and virulence. (A) Bacterial cells of Dickeya dadantii were inoculated in the leaves of Chinese cabbage (Brassica campestris). The maceration areas were measured at 16 h post‐inoculation. The swimming motility (B) and T3SS gene hrpA promoter activity (C) were examined. Values are representative of three independent experiments. Three replicates were used in each experiment. Error bars indicate standard errors of the means. Different lowercase letters above the bars indicate statistically significant differences between treatments [Fisher's least significant difference (LSD), P < 0.05].
Thus, we further tested the swimming motility and expression of the T3SS gene, hrpA, in the gcpAD418A mutant, both of which are virulence factors previously shown to be regulated by c‐di‐GMP signalling in D. dadantii (Yi et al., 2010). The results showed that the swimming motility and hrpA promoter activity were significantly enhanced in the gcpAD418A mutant compared with the wild‐type strain (Fig. 5B,C). More interestingly, mutation of gcpA in the ΔegcpB mutant fully recovered its swimming motility (Fig. 5B), but not its hrpA promoter activity (Fig. 5C). The gcpAD418AΔecpC mutant exhibited low hrpA promoter activity that was equivalent to that of the ΔecpC mutant (Fig. 5C), whereas its swimming motility was partially restored from ΔecpC (Fig. 5B). These results could explain the partial restoration of virulence in the gcpAD418AΔegcpB and gcpAD418AΔecpC double mutants, suggesting that all three virulence factors, including Pel production, swimming motility and T3SS, are essential for the full virulence of D. dadantii in host plants.
H‐NS and Rsm play different roles in the GcpA‐dependent regulation of swimming motility and T3SS
As we have proposed a novel regulatory pathway in which GcpA controls Pel production via H‐NS–RsmB–RsmA, we asked whether the same regulatory pathway was involved in the GcpA‐dependent regulation of swimming motility and T3SS gene expression. As shown in Fig. 6A, Δhns and ΔrsmB mutants exhibited wild‐type levels of swimming motility; however, deletion of hns, but not rsmB, in the gcpAD418A mutant fully recovered its swimming motility to the wild‐type level. This result, together with the observation that GcpA negatively regulates H‐NS (Fig. 2A), suggests that H‐NS is essential for GcpA to control swimming motility. Next, hrpA promoter activity was determined, and the results indicated that H‐NS is not involved in the regulation of T3SS in either the D. dadantii wild‐type or gcpAD418A strain (Fig. 6B). Deletion of rsmB drastically decreased the promoter activity of hrpA relative to the wild‐type, and deletion of rsmB in gcpAD418A reduced its hrpA promoter activity to the ΔrsmB level (Fig. 6B). To investigate how H‐NS and RsmB contribute to virulence through various GcpA‐regulated virulence factors, a virulence assay was performed in the host plant B. campestris. The maceration ability of Δhns was reduced compared with that of the wild‐type strain. In addition, deletion of hns in gcpAD418A significantly reduced its maceration ability to nearly wild‐type levels (Fig. 6C). However, despite the hyperswimming motility phenotype observed in the gcpAD418AΔrsmB double mutant (Fig. 6A), deletion of rsmB in either the wild‐type strain or the gcpAD418A mutant resulted in a non‐pathogenic phenotype (Fig. 6C). Together, we conclude that the molecular mechanisms of GcpA that control various virulence factors are diverse; H‐NS and RsmB are essential for the GcpA‐dependent regulation of swimming motility and T3SS, respectively. However, the H‐NS–RsmB–RsmA pathway is not a major component modulating these two virulence factors in a GcpA‐dependent manner.
Figure 6.
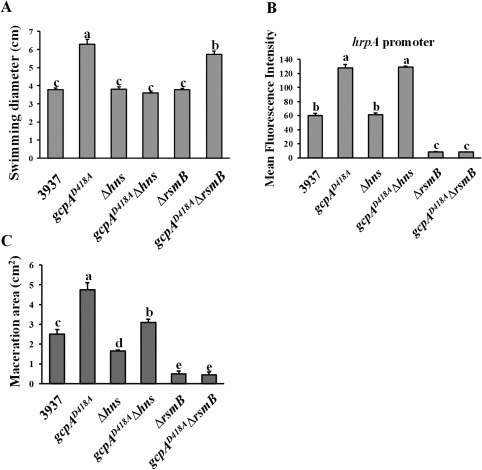
Effects of H‐NS and RsmB on swimming motility, type III secretion system (T3SS) gene expression and virulence. The swimming motility (A), T3SS gene hrpA promoter activity (B) and maceration area on the leaves of Chinese cabbage (C) were examined. Values are representative of three independent experiments. Three replicates were used in each experiment. Error bars indicate standard errors of the means. Different lowercase letters above the bars indicate statistically significant differences between treatments [Fisher's least significant difference (LSD), P < 0.05].
Discussion
Dickeya dadantii produces Pels to degrade plant cell walls, and the production of this virulence factor is negatively regulated by c‐di‐GMP (Yi et al., 2010; Yuan et al., 2015). In this study, we propose a unique regulatory model that connects c‐di‐GMP regulation of bacterial virulence to both the global transcriptional and post‐transcriptional regulatory systems in D. dadantii. To our knowledge, this is the first report implicating the H‐NS–Rsm systems in the c‐di‐GMP signalling network for the negative regulation of pelD.
We confirmed that GcpA, which contains conserved A‐ and I‐sites, is a genuine DGC (Fig. 1B). More importantly, our findings clearly demonstrated that GcpA relies on its DGC activity to regulate Pel (Fig. 1B,C). Moreover, the unsuccessful attempt to delete gcpA suggests that GcpA may be essential for bacterial viability and may play an additional role to its DGC activity. Interestingly, no other Gcp or Ecp proteins were shown to affect Pel production, suggesting that there might be specificity in different c‐di‐GMP signalling pathways.
PelD and PelE, which share high homology (89% similarity in amino acid sequence), are the most important Pels for the virulence of D. dadantii (Boccara et al., 1988). The expression of pelD and pelE is differentially regulated in both plant tissues and media (Hugouvieux‐Cotte‐Pattat et al., 1992; Masclaux et al., 1996; Tardy et al., 1997). Here, we observed that the expression of pelD was much more significantly induced (22‐fold) than that of pelE (4.5‐fold) when PGA was supplied in minimal medium (MM), probably because the basal expression level of pelE is higher than that of pelD (Fig. S3). These results are in agreement with previous studies, which indicate that a high basal level of pelE expression is essential to initiate rapid pectin degradation, whereas a high induced expression of pelD is necessary for the maximum production of Pels during infection in the plant (Ouafa et al., 2012; Robert‐Baudouy et al., 2000). Furthermore, the transcription of pelD was monitored together with growth in MM supplemented with PGA, and the results showed that the expression of the pelD gene was only strongly induced in the mid‐logarithmic, but not in the early logarithmic, phase of growth (Fig. S6, see Supporting Information). This result suggests that the transcription of the pelD gene is growth phase dependent. Interestingly, we observed that the expression of pelD at both transcriptional and post‐transcriptional levels was significantly enhanced in the gcpAD418A A‐site mutant relative to the wild‐type at 12 h, which corresponded to the early logarithmic phase, whereas the expression level of pelE was unaltered (Fig. 1D–F). A recent study has demonstrated that the transcriptional start site shift after gene duplication might be one of the reasons for the different expression patterns of pelD and pelE (Duprey et al., 2016). However, the upstream regulatory mechanism remains unclear. Our results imply that the GcpA‐mediated c‐di‐GMP signalling pathway is involved in the mechanism of differential expression of pelD and pelE.
The post‐transcriptional regulatory system, RsmA/RsmB, has been shown to regulate several virulence factors, including Pel, in the soft‐rot pathogens D. dadantii and Pectobacterium carotovorum, but how RsmA/RsmB regulates pel gene expression remains unclear (Chatterjee et al., 1995; Mukherjee et al., 1996; Yang et al., 2008). Deletion of rsmA was lethal to D. dadantii 3937, unlike previous results with P. carotovorum. Therefore, we were unable to generate a rsmA mutant to examine its direct effect on Pel production. Instead, we observed that the over‐expression of rsmA significantly repressed pelD expression and Pel production (data not shown). Nevertheless, our results demonstrate that GcpA regulates the expression of RsmB and RsmA (Fig. 2). This regulation was further determined to be essential for GcpA‐dependent Pel regulation, as either deletion of rsmB or overexpression of rsmA in the gcpAD418A mutant drastically reduced pelD promoter activity and Pel production (Figs 2C,D and S5). In P. aeruginosa, the RsmY and RsmZ sRNAs sequester the mRNA‐binding protein RsmA in a mechanism similar to that of the RsmA/RsmB system (Lapouge et al., 2008). Several studies have revealed that different DGCs negatively regulate RsmA activity through RsmY or RsmZ to control biofilm formation (Colley et al., 2016; Moscoso et al., 2014; Valentini et al., 2016). Thus, our findings strongly suggest that the regulation of c‐di‐GMP signalling through the Rsm system might be common in different bacterial species.
H‐NS is a nucleoid‐associated protein that functions as a global transcriptional regulator in many Gram‐negative bacteria (Castang et al., 2008; Falconi et al., 1998; Yu and DiRita, 2002). In D. dadantii, it has been shown that H‐NS positively regulates swimming motility and Pel production, but negatively regulates EPS synthesis (Nasser et al., 2001). Our findings demonstrated that GcpA negatively regulates hns expression at the post‐transcriptional level (Figs 2A and S4). Strikingly, deletion of hns in the gcpAD418A mutant not only restored its pelD promoter activity and Pel production (Fig. 2E,F), but also restored rsmB RNA to the wild‐type level (Fig. 3). Taken together, we propose a regulatory pathway in which GcpA represses pelD gene expression via H‐NS–RsmB–RsmA. In addition to its direct function as a repressor of pelD gene expression, H‐NS is also known to be a positive regulator of Pel synthesis because of its negative impacts on PecT production (Nasser and Reverchon, 2002). As we did not observe significant changes in pecT expression in the gcpAD418A mutant (Fig. 2A), this regulatory pathway might not be related to PecT.
It is of interest to note that bacteria use multiple GGDEF and/or EAL domain proteins to regulate the same cellular behaviour in a sophisticated manner (Lindenberg et al., 2013; Valentini et al., 2016). Our findings demonstrated that the Rsm system plays an essential role in c‐di‐GMP signalling and the regulation of Pel production in D. dadantii. Although EGcpB, EcpC and GcpA have been shown to modulate the expression of rsmB at a post‐transcriptional level (Figs 2A and 4C), our results indicate that their regulatory mechanisms might be different. GcpA and EGcpB may respond to similar environmental signals via their GAF and PAS sensory domains (Fig. S1A), and modulate the same c‐di‐GMP pool to control pelD gene expression through the H‐NS–RsmB–RsmA pathway (Fig. 7A). In Acetobacter xylinum, Qi et al. (2009) have reported that the PAS domain of the DGC AxDGC2 enhances its cyclase activity by binding to the flavin adenine dinucleotide (FAD) cofactor under redox conditions. Similarly, oxygen levels may play a role in the modulation of c‐di‐GMP metabolism in D. dadantii. EcpC, the sole‐EAL domain protein, probably modulates a different c‐di‐GMP pool that targets RsmB directly, bypassing H‐NS. This convergence and divergence in c‐di‐GMP signalling are also supported by the regulation of swimming motility and T3SS gene expression in D. dadantii (Fig. 7B,C). We have shown here that GcpA and EGcpB inversely modulate swimming motility through H‐NS, which is different from EcpC. It is worth noting that our data indicate that the regulatory mechanism of GcpA on T3SS gene expression might be different from that of EGcpB and EcpC, for which previous reports have indicated that these two PDEs positively regulate RpoN at the post‐transcriptional level to control the T3SS master regulator HrpL. Indeed, we observed that only the transcript of hrpL, not rpoN, was increased in the gcpAD418A A‐site mutant compared with the wild‐type strain (Fig. S7, see Supporting Information). Finally, our virulence assay further confirmed that swimming motility, T3SS gene expression and Pel production are essential for D. dadantii to express full virulence in plants. Nevertheless, the environmental signals triggering c‐di‐GMP‐dependent regulation, the expression patterns and localization of different DGCs and PDEs, and the c‐di‐GMP effectors that contribute to the signalling specificity of diverse cellular behaviours remain to be determined.
Figure 7.
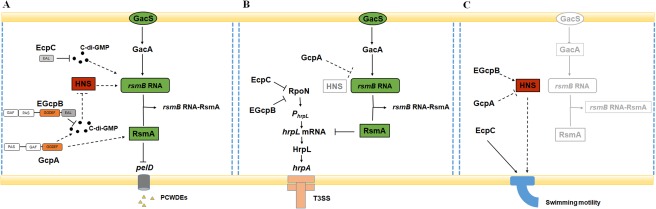
Working model for the bis‐(3′,5′)‐cyclic dimeric guanosine monophosphate (c‐di‐GMP) signalling pathway in Dickeya dadantii. (A) The regulation of c‐di‐GMP signalling on pectate lyase (Pel) production in D. dadantii is complex, involves several Gcp and Ecp proteins, and takes place at both transcriptional and post‐transcriptional levels. The Rsm system is a central component in c‐di‐GMP‐related Pel regulation. GcpA and EGcpB modulate the same c‐di‐GMP pool to control pelD gene expression through the H‐NS–rsmB–RsmA pathway. EcpC modulates a different c‐di‐GMP pool that directly targets RsmB, bypassing H‐NS. GcpA positively regulates RsmA at the post‐transcriptional level. PCWDE, plant cell wall degrading enzyme. (B) The regulation of GcpA on type III secretion system (T3SS) gene expression is dependent on its impact on RsmB, which controls the expression of hrpL at the post‐transcriptional level. This regulation is different from that of EGcpB and EcpC, which regulate hrpL at the transcriptional level via the RpoN–hrpL pathway. (C) GcpA and EGcpB regulate swimming motility through H‐NS, whereas EcpC is different. ⊥, negative control; →, positive control. The broken lines indicate the regulatory mechanisms identified in this study.
Overall, this multilevel regulation of c‐di‐GMP signalling of diverse virulence factors ensures the accurate control of the virulence process for the infection of D. dadantii. It provides evidence of the complexity and specificity of c‐di‐GMP signalling in bacteria and sheds light on the understanding of D. dadantii infection strategies under various environmental conditions or during different infection status.
Experimental Procedures
Bacterial strains, plasmids, primers and media
The bacterial strains and plasmids used in this study are listed in Table S1. Dickeya dadantii strains were grown in Luria–Bertani (LB) medium (1% tryptone, 0.5% yeast extract and 1% NaCl), mannitol–glutamic acid (MG) medium (1% mannitol, 0.2% glutamic acid, 0.05% potassium phosphate monobasic, 0.02% NaCl and 0.02% MgSO4) or M9 minimal medium (MM) supplemented with 0.1% PGA at 28 °C (Yang et al., 2007). Escherichia coli strains were grown in LB at 37 °C. Antibiotics were added to the media at the following concentrations: ampicillin (100 μg/mL), kanamycin (Km, 50 μg/mL), chloramphenicol (20 μg/mL) and spectinomycin (100 μg/mL). The D. dadantii 3937 genome sequence was retrieved from a systematic annotation package for community analysis of genomes (ASAP) (https://asap.ahabs.wisc.edu/asap/home.php). Primers used for cloning and qPCR in this study are listed in Table S2 (see Supporting Information).
Mutant construction and complementation
The GGDEF and/or EAL domain encoding genes, hns, rsmB and pecT, were deleted from the genome by allelic exchange mutagenesis (Yang et al., 2002). In brief, upstream and downstream fragments flanking each target gene were amplified by PCR with specific primers (Table S2). The Km cassette was amplified from the pKD4 plasmid (Datsenko and Wanner, 2000) and cloned between two flanking regions using three‐way cross‐over PCR. The PCR construct was inserted into the suicide plasmid pWM91, and the resulting plasmid was transformed into D. dadantii 3937 by conjugation using E. coli strain S17‐1 λ‐pir. Recombinants that grew on Km medium were plated onto 10% sucrose plates to select strains with chromosomal deletions. Cells that were resistant to sucrose because of the loss of SacB‐mediated toxicity were then plated onto an ampicillin plate, and the ampicillin‐sensitive cells were confirmed by PCR using outside primers. Mutations were confirmed by sequencing.
To construct the site‐specific point mutation in the GGDEF motif of GcpA, single nucleotide substitution was performed using the QuikChange XL Site‐Directed Mutagenesis Kit (Agilent, Santa Clara, CA, USA). Briefly, a primer set, gcpA‐D418A‐1 and gcpA‐D418A‐2 (Table S2), was used to generate gcpA D418A, in which the SGDEF motif was changed to SGAEF. Substitution was confirmed by sequencing. The gcpAD418A fragment was then amplified using the primer set, gcpA‐A‐SacI and gcpA‐B (Table S2), and cloned upstream of the Km cassette, followed by the downstream fragment flanking gcpA, using three‐way cross‐over PCR. The construct was inserted into pWM91, and the resulting plasmid was transferred into D. dadantii by conjugation using E. coli strain S17‐1 λ‐pir. The above described allelic exchange mutagenesis was conducted to replace wild‐type gcpA with gcpAD418A. Mutation was confirmed by sequencing using outside primers.
To construct double mutants, rsmB, hns, egcpB and ecpC were allelic exchanged in a gcpAD418A unmarked mutant strain. In brief, the pFLP2 plasmid encoding the FLP (flipase) recombinase enzyme was transferred into the gcpAD418A::Km strain by conjugation using E. coli S17‐1 λ‐pir. Two FLP recombinase target (FRT) sites flanking the Km cassette allowed for flipase‐mediated excision of Km. Transconjugants that were sensitive to Km and a high concentration of sucrose were then confirmed using outside primers and sequencing. To generate complemented strains, the promoter and open reading frame (ORF) regions of target genes were amplified and cloned into the low‐copy‐number plasmid pCL1920 (Table S1). The resulting plasmids were then confirmed by sequencing and electroporated into mutant strains.
Swimming motility assay
Swimming motility was examined by inoculation of 10 µL of overnight bacterial culture [optical density at 600 nm (OD600) = 1.0] onto the centre of MG plates containing 0.2% agar. The inoculated plates were incubated at 28 °C for 16 h. The diameter of radial growth was measured (Antúnez‐Lamas et al., 2009).
Pel activity assay
Extracellular Pel activity was measured by spectrometry as described previously (Matsumoto et al., 2003). Bacterial cells were cultured in MM supplemented with 0.1% PGA at 28 °C for 16 h; 1 mL of bacterial culture was then centrifuged at 13 000 g for 2 min, and the supernatant was collected; 10 μL of supernatant were added to 990 μL of reaction buffer [0.05% PGA, 0.1 m Tris‐HCl (pH 8.5) and 0.1 mm CaCl2, prewarmed to 30 °C]. Pel activity was monitored at A 230 for 3 min and calculated on the basis of one unit of Pel activity being equal to an increase of 1 × 10−3 OD230 in 1 min.
GFP reporter plasmid construction and flow cytometry assay
To generate the reporter plasmids pAT‐pelE, pAT‐rsmA and pAT‐hns, the promoter regions of each gene were PCR amplified and cloned into the promoter probe vector pPROBE‐AT, which contains a ribosomal binding site upstream of the gfp gene (Leveau and Lindow, 2001; Miller et al., 2000). The reporter plasmids pAT‐pelD, pAT‐hrpA and pAT‐rsmB had been constructed previously following the same procedure (Li et al., 2015; Peng et al., 2006; Yang et al., 2007). Promoter activity was monitored by measurement of the GFP intensity through flow cytometry (BD Biosciences, San Jose, CA, USA), as described previously (Peng et al., 2006). Briefly, bacterial cells with the reporter plasmid were grown in LB medium overnight and inoculated 1 : 100 into MM with or without 0.1% PGA. Samples were collected at 6, 12 and 24 h, and promoter activity was quantified by detection of the GFP intensity using flow cytometry.
Determination of the intracellular c‐di‐GMP concentration
Intracellular c‐di‐GMP concentrations were determined using UPLC‐MS‐MS as described previously (Massie et al., 2012). Briefly, overnight bacterial cultures were inoculated 1 : 1000 into 50 mL of LB medium in a flask. After OD600 of the bacterial culture had reached about 0.8, corresponding to mid‐ to late‐exponential growth, all cells were centrifuged in 50‐mL polystyrene centrifuge tubes for 30 min at 1500 g. The supernatant was then removed, and the pellet was resuspended in 1.5 mL of extraction buffer (40% acetonitrile and 40% methanol in 0.1 M formic acid). To lyse the cells and release intracellular c‐di‐GMP, cells resuspended in extraction buffer were dried by speed‐vac, resuspended in 100 µL of HPLC grade water, centrifuged for 5 min at 21 000 g in a tabletop centrifuge to pellet insoluble debris, filtered through a Titan syringe filter [polyvinylidene difluoride (PVDF), 0.45 μm, 4 mm] and analysed by UPLC‐MS‐MS.
Western blot analysis
Dickeya dadantii cells were grown in MM broth supplemented with 0.1% PGA at 28 °C for 12 h during the exponential growth phase, and 1‐mL samples were taken. Cells were then resuspended in phosphate‐buffered saline (PBS) and lysed by sonication. The protein in crude lysates was quantified using the Bradford protein assay (Bio‐Rad, Hercules, CA, USA). Samples were boiled before loading onto 12% sodium dodecyl sulfate polyacrylamide gels. Proteins were then transferred onto a PVDF membrane (Millipore, Bedford, MA, USA). Blots were washed with PBS containing 0.05% Tween‐20 and probed with an anti‐RsmA antibody (Proteintech, Rosemont, IL, USA). Anti‐RNA polymerase monoclonal antibody (Neoclone, Madision, WI, USA) was used as a control. The resulting blots were incubated for 1 min in enhanced chemiluminescence reagent (GE Healthcare, Chicago, IL, USA) and detected using O‐MAT X‐ray film.
Northern blot analysis
To measure the RNA levels of rsmB in D. dadantii strains, bacterial cells grown in MM supplemented with 0.1% PGA for 12 h were harvested, and total RNA was isolated using TRI reagent (Sigma‐Aldrich, St Louis, MO, USA). The residual DNA was removed with a Turbo DNA‐free DNase kit (Invitrogen, Austin, TX, USA). Northern blot analysis was performed using biotin‐labelled probe and a biotin detection system (BrightStar Psolaren‐Biotin and Bright Star BioDetect, Ambion, Carlsbad, CA, USA). 16S rRNA was used as an internal control.
qRT‐PCR analysis
The mRNA levels of pelD, pelE, rsmA, rsmB, pecT, pecS, fis, fur, kdgR, crp, hns, hrpL and rpoN were measured by qRT‐PCR. Briefly, bacterial cells cultured in MM broth supplemented with 0.1% PGA for 12 h were harvested, and total RNA was extracted using a PureLink RNA Mini Kit (Ambion) according to the manufacturer's instructions. On‐column DNase treatment (Invitrogen, Carlsbad, CA, USA) was performed. cDNA was synthesized using the QuantiTect Reverse Transcription Kit (Qiagen, Hilden, Germany). The cDNA level of different samples was quantified by real‐time PCR using a PowerUp SYBR Green Master Mix (Life Technologies, Carlsbad, CA, USA). The relative levels of gene expression were determined using the 2–ΔΔCT method (Livak and Schmittgen, 2001), with the rplU gene as the internal control (Mah et al., 2003). Three technical replicates were used each time.
Virulence assay
The local leaf maceration assay was performed using the leaves of Chinese cabbage (B. campestris), as described previously (Yuan et al., 2015). In brief, 10 µL of bacterial suspension at 107 colony‐forming units (CFU)/mL were inoculated into the wounds punched with a sterile pipette on the leaves. Five leaves were used for each strain. Inoculated Chinese cabbage leaves were kept in a growth chamber at 28 °C with 100% relative humidity for 16 h before photographs were taken. To evaluate disease symptoms, American Physiological Society ASSESS 1.0 software (Image Analysis Software for Plant Disease Quantification) was used to determine the leaf maceration areas.
Statistical analysis
Means and standard deviations of experimental results were calculated using Excel, and the statistical analysis was performed using a two‐tailed Student's t‐test (Microsoft, Redmond, WA, USA) or Fisher's least significant difference (LSD) test with a DPS data processing system (http://www.dpsw.cn/dps_eng).
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Fig. S1 Analysis of GGDEF and/or EAL domain proteins in Dickeya dadantii. (A) Summary of GGDEF and/or EAL domain proteins. Proteins are shown with the encoded gene names and protein length. Protein domains were predicted by the simplified modular architecture research tool (SMART). ORF, open reading frame. (B) Amino acid sequence alignment for the GGDEF domains in D. dadantii. PleD and WspR are two active diguanylate cyclases (DGCs) from Caulobacter crescentus and Pseudomonas aeruginosa, respectively. The inhibition site RxxD motif (I‐site) and enzymatic activity site GGDEF motif (A‐site) are marked. (C) Amino acid sequence alignment for the EAL domains in D. dadantii. VC1086 is an active phosphodiesterase (PDE) from Vibrio cholerae. The arrow indicates the glutamate residue in the EAL motif. ‘*’, residues identical in all sequences in the alignment; ‘:’, conserved substitutions; ‘.’, semi‐conserved substitutions.
Fig. S2 Effect of complementation of gcpAD418A on pectate lyase (Pel) production. Values are representative of three independent experiments. Three replicates were used in each experiment. Error bars indicate standard errors of the means. Asterisks indicate statistically significant differences of the means (P < 0.05 by Student's t‐test). OD230, optical density at 230 nm.
Fig. S3 Polygalacturonic acid (PGA) induces the transcription of pelD and pelE genes. (A, B) The promoter activities of pelD and pelE in Dickeya dadantii were examined at 12 and 24 h. The experiments were repeated three independent times with similar results. The figure represents the results from one experiment which includes three technical replicates. Error bars indicate standard errors of the means. Asterisks indicate statistically significant differences of the means (P < 0.05 by Student's t‐test).
Fig. S4 Promoter activities of rsmA, rsmB and hns are not affected in gcpAD418A. (A–C) The promoter activities of rsmA, rsmB and hns in Dickeya dadantii strains. One representative experiment was chosen, and three independent experiments were performed. Assays were performed as described in Experimental procedures. Error bars indicate standard errors of the means.
Fig. S5 Expression of rsmA restores pectate lyase (Pel) in the gcpAD418A A‐site mutant. (A, B) Pel production and pelD promoter activity were measured in Dickeya dadantii. Three independent experiments were performed with three replicates in each experiment. Values are from one representative experiment. Error bars indicate standard errors of the means. Asterisks indicate statistically significant differences of the means (*P < 0.05 by Student's t‐test). OD230, optical density at 230 nm.
Fig. S6 pelD gene transcription is growth phase dependent. The promoter activity of the pelD gene was determined throughout growth in minimal medium (MM) supplemented with 0.1% polygalacturonic acid (PGA) at 28 °C. The experiments were repeated twice independently with similar results. Three replicates were used for each experiment. Error bars indicate standard errors of the means. Open squares, OD600 (optical density at 600 nm). Filled triangles, mean fluorescence intensity.
Fig. S7 Impact of GcpA on the expression of hrpL and rpoN. mRNA levels of hrpL and rpoN in gcpAD418A relative to that in the wild‐type, which was mathematically designated as unity. All results are from one representative experiment. Three independent experiments were performed and three replicates were used for each experiment. Error bars indicate standard errors of the means. Asterisk indicates statistically significant difference of the means (P < 0.05 by Student's t‐test).
Table S1 Strains and plasmids used in this study.
Table S2 Primers used in this study.
Acknowledgements
X.Y. was supported by the Postdoctoral Workstation of Jiangsu Academy of Agricultural Sciences. This work was funded by the following: US Department of Agriculture‐National Institute of Food and Agriculture‐Agriculture and Food Research Initiative‐Exploratory Research Program (2016–67030‐24856) and the Research Growth Initiative of the University of Wisconsin‐Milwaukee awarded to C.‐H.Y; National Key Research and Development Program of China (2017YFC200604) awarded to F.L.; China Scholarship Council (201503250007) awarded to F.T.; National Science Foundation of China (31370160; 31671990) awarded to C.H.; National Institutes of Health grant GM109259 awarded to C.M.W.
Contributor Information
Fengquan Liu, Email: fqliu20011@sina.com.
Ching‐Hong Yang, Email: chyang@uwm.edu.
References
- Antúnez‐Lamas, M. , Cabrera‐Ordonez, E. , Lopez‐Solanilla, E. , Raposo, R. , Trelles‐Salazar, O. , Rodriguez‐Moreno, A. and Rodriguez‐Palenzuela, P. (2009) Role of motility and chemotaxis in the pathogenesis of Dickeya dadantii 3937 (ex Erwinia chrysanthemi 3937). Microbiology, 155, 434–442. [DOI] [PubMed] [Google Scholar]
- Boccara, M. , Diolez, A. , Rouve, M. and Kotoujansky, A. (1988) The role of individual pectate lyases of Erwinia chrysanthemi strain 3937 in pathogenicity on saintpaulia plants. Physiol. Mol. Plant Pathol. 33, 95–104. [Google Scholar]
- Castang, S. , McManus, H.R. , Turner, K.H. and Dove, S.L. (2008) H‐NS family members function coordinately in an opportunistic pathogen. Proc. Natl. Acad. Sci. USA, 105, 18 947–18 952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charkowski, A. , Blanco, C. , Condemine, G. , Expert, D. , Franza, T. , Hayes, C. , Hugouvieux‐Cotte‐Pattat, N. , Solanilla, E.L. , Low, D. , Moleleki, L. , Pirhonen, M. , Pitman, A. , Perna, N. , Reverchon, S. , Rodríguez Palenzuela, P. , San Francisco, M. , Toth, I. , Tsuyumu, S. , van der Waals, J. , van der Wolf, J. , Van Gijsegem, F. , Yang, C.‐H. and Yedidia, I. (2012) The role of secretion systems and small molecules in soft‐rot Enterobacteriaceae pathogenicity. Annu. Rev. Phytopathol. 50, 425–449. [DOI] [PubMed] [Google Scholar]
- Chatterjee, A. , Cui, Y. , Liu, Y. , Dumenyo, C.K. and Chatterjee, A.K. (1995) Inactivation of rsmA leads to overproduction of extracellular pectinases, cellulases, and proteases in Erwinia carotovora subsp. carotovora in the absence of the starvation/cell density‐sensing signal, N‐(3‐oxohexanoyl)‐L‐homoserine lactone. Appl. Environ. Microbiol. 61, 1959–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christen, B. , Christen, M. , Paul, R. , Schmid, F. , Folcher, M. , Jenoe, P. , Meuwly, M. and Jenal, U. (2006) Allosteric control of cyclic di‐GMP signaling. J. Biol. Chem. 281, 32 015–32 024. [DOI] [PubMed] [Google Scholar]
- Colley, B. , Dederer, V. , Carnell, M. , Kjelleberg, S. , Rice, S.A. and Klebensberger, J. (2016) SiaA/D interconnects c‐di‐GMP and RsmA signaling to coordinate cellular aggregation of Pseudomonas aeruginosa in response to environmental conditions. Front. Microbiol. 7, 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collmer, A. and Keen, N.T. (1986) The role of pectic enzymes in plant pathogenesis. Annu. Rev. Phytopathol. 24, 383–409. [Google Scholar]
- Cotter, P.A. and Stibitz, S. (2007) c‐di‐GMP‐mediated regulation of virulence and biofilm formation. Curr. Opin. Microbiol. 10, 17–23. [DOI] [PubMed] [Google Scholar]
- Czajkowski, R. , Perombelon, M.C. , van Veen, J.A. and van der Wolf, J.M. (2011) Control of blackleg and tuber soft rot of potato caused by Pectobacterium and Dickeya species: a review. Plant Pathol. 60, 999–1013. [Google Scholar]
- Datsenko, K.A. and Wanner, B.L. (2000) One‐step inactivation of chromosomal genes in Escherichia coli K‐12 using PCR products. Proc. Natl. Acad. Sci. USA, 97, 6640–6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprey, A. , Nasser, W. , Léonard, S. , Brochier‐Armanet, C. and Reverchon, S. (2016) Transcriptional start site turnover in the evolution of bacterial paralogous genes—the pelE‐pelD virulence genes in Dickeya . FEBS J. 283, 4192–4207. [DOI] [PubMed] [Google Scholar]
- Falconi, M. , Colonna, B. , Prosseda, G. , Micheli, G. and Gualerzi, C.O. (1998) Thermoregulation of Shigella and Escherichia coli EIEC pathogenicity. A temperature‐dependent structural transition of DNA modulates accessibility of virF promoter to transcriptional repressor H‐NS. EMBO J. 17, 7033–7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franza, T. , Michaud‐Soret, I. , Piquerel, P. and Expert, D. (2002) Coupling of iron assimilation and pectinolysis in Erwinia chrysanthemi 3937. Mol. Plant–Microbe Interact. 15, 1181–1191. [DOI] [PubMed] [Google Scholar]
- Hengge, R. (2009) Principles of c‐di‐GMP signalling in bacteria. Nat. Rev. Microbiol. 7, 263–273. [DOI] [PubMed] [Google Scholar]
- Hérault, E. , Reverchon, S. and Nasser, W. (2014) Role of the LysR‐type transcriptional regulator PecT and DNA supercoiling in the thermoregulation of pel genes, the major virulence factors in Dickeya dadantii . Environ. Microbiol. 16, 734–745. [DOI] [PubMed] [Google Scholar]
- Hugouvieux‐Cotte‐Pattat, N. , Dominguez, H. and Robert‐Baudouy, J. (1992) Environmental conditions affect transcription of the pectinase genes of Erwinia chrysanthemi 3937. J. Bacteriol. 174, 7807–7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugouvieux‐Cotte‐Pattat, N. , Condemine, G. , Nasser, W. and Reverchon, S. (1996) Regulation of pectinolysis in Erwinia chrysanthemi . Annu. Rev. Microbiol. 50, 213–257. [DOI] [PubMed] [Google Scholar]
- Jenal, U. , Reinders, A. and Lori, C. (2017) Cyclic di‐GMP: second messenger extraordinaire. Nat. Rev. Microbiol. 15, 271–284. [DOI] [PubMed] [Google Scholar]
- Lapouge, K. , Schubert, M. , Allain, F.H.T. and Haas, D. (2008) Gac/Rsm signal transduction pathway of γ‐proteobacteria: from RNA recognition to regulation of social behaviour. Mol. Microbiol. 67, 241–253. [DOI] [PubMed] [Google Scholar]
- Leveau, J.H. and Lindow, S.E. (2001) Predictive and interpretive simulation of green fluorescent protein expression in reporter bacteria. J. Bacteriol. 183, 6752–6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , Hutchins, W. , Wu, X. , Liang, C. , Zhang, C. , Yuan, X. , Khokhani, D. , Chen, X. , Che, Y. , Wang, Q. and Yang, C.‐H. (2015) Derivative of plant phenolic compound inhibits the type III secretion system of Dickeya dadantii via HrpX/HrpY two‐component signal transduction and Rsm systems. Mol. Plant Pathol. 16, 150–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberg, S. , Klauck, G. , Pesavento, C. , Klauck, E. and Hengge, R. (2013) The EAL domain protein YciR acts as a trigger enzyme in a c‐di‐GMP signalling cascade in E. coli biofilm control. EMBO J. 32, 2001–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, M.Y. , Gui, G. , Wei, B. , Preston, J.F. , Oakford, L. , Yüksel, Ü. , Giedroc, D.P. and Romeo, T. (1997) The RNA molecule CsrB binds to the global regulatory protein CsrA and antagonizes its activity in Escherichia coli . J. Biol. Chem. 272, 17 502–17 510. [DOI] [PubMed] [Google Scholar]
- Livak, K.J. and Schmittgen, T.D. (2001) Analysis of relative gene expression data using real‐time quantitative PCR and the 2−ΔΔCT method. Methods, 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Llama‐Palacios, A. , Lopez‐Solanilla, E. and Rodriguez‐Palenzuela, P. (2005) Role of the PhoP‐PhoQ system in the virulence of Erwinia chrysanthemi strain 3937: involvement in sensitivity to plant antimicrobial peptides, survival at acid pH, and regulation of pectolytic enzymes. J. Bacteriol. 187, 2157–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, B. , Hibbing, M.E. , Kim, H.‐S. , Reedy, R.M. , Yedidia, I. , Breuer, J. , Breuer, J. , Glasner, J.D. , Perna, N.T. , Kelman, A. and Charkowski, A.O. (2007) Host range and molecular phylogenies of the soft rot enterobacterial genera Pectobacterium and Dickeya . Phytopathology, 97, 1150–1163. [DOI] [PubMed] [Google Scholar]
- Mah, T.‐F. , Pitts, B. , Pellock, B. , Walker, G.C. , Stewart, P.S. and O'Toole, G.A. (2003) A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature, 426, 306–310. [DOI] [PubMed] [Google Scholar]
- Masclaux, C. , Hugouvieux‐Cotte‐Pattat, N. and Expert, D. (1996) Iron is a triggering factor for differential expression of Erwinia chrysanthemi strain 3937 pectate lyases in pathogenesis of African violets. Mol. Plant–Microbe Interact. 9, 198–205. [Google Scholar]
- Massie, J.P. , Reynolds, E.L. , Koestler, B.J. , Cong, J.‐P. , Agostoni, M. and Waters, C.M. (2012) Quantification of high‐specificity cyclic diguanylate signaling. Proc. Natl. Acad. Sci. USA, 109, 12 746–12 751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto, H. , Muroi, H. , Umehara, M. , Yoshitake, Y. and Tsuyumu, S. (2003) Peh production, flagellum synthesis, and virulence reduced in Erwinia carotovora subsp. carotovora by mutation in a homologue of cytR. Mol. Plant–Microbe Interact. 16, 389–397. [DOI] [PubMed] [Google Scholar]
- Miller, W.G. , Leveau, J.H. and Lindow, S.E. (2000) Improved gfp and inaZ broad‐host‐range promoter‐probe vectors. Mol. Plant–Microbe Interact. 13, 1243–1250. [DOI] [PubMed] [Google Scholar]
- Moscoso, J.A. , Jaeger, T. , Valentini, M. , Hui, K. , Jenal, U. and Filloux, A. (2014) The diguanylate cyclase SadC is a central player in Gac/Rsm‐mediated biofilm formation in Pseudomonas aeruginosa . J. Bacteriol. 196, 4081–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee, A. , Cui, Y. , Liu, Y. , Dumenyo, C.K. and Chatterjee, A.K. (1996) Global regulation in Erwinia species by Erwinia carotovora rsmA, a homologue of Escherichia coli csrA: repression of secondary metabolites, pathogenicity and hypersensitive reaction. Microbiology, 142, 427–434. [DOI] [PubMed] [Google Scholar]
- Nasser, W. and Reverchon, S. (2002) H‐NS‐dependent activation of pectate lyase synthesis in the phytopathogenic bacterium Erwinia chrysanthemi is mediated by the PecT repressor. Mol. Microbiol. 43, 733–748. [DOI] [PubMed] [Google Scholar]
- Nasser, W. , Faelen, M. , Hugouvieux‐Cotte‐Pattat, N. and Reverchon, S. (2001) Role of the nucleoid‐associated protein H‐NS in the synthesis of virulence factors in the phytopathogenic bacterium Erwinia chrysanthemi . Mol. Plant–Microbe Interact. 14, 10–20. [DOI] [PubMed] [Google Scholar]
- Nasser, W. , Dorel, C. , Wawrzyniak, J. , Van Gijsegem, F. , Groleau, M.‐C. , Déziel, E. and Reverchon, S. (2013) Vfm a new quorum sensing system controls the virulence of Dickeya dadantii . Environ. Microbiol. 15, 865–880. [DOI] [PubMed] [Google Scholar]
- Orr, M.W. , Galperin, M.Y. and Lee, V.T. (2016) Sustained sensing as an emerging principle in second messenger signaling systems. Curr. Opin. Microbiol. 34, 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouafa, Z.‐A. , Reverchon, S. , Lautier, T. , Muskhelishvili, G. and Nasser, W. (2012) The nucleoid‐associated proteins H‐NS and FIS modulate the DNA supercoiling response of the pel genes, the major virulence factors in the plant pathogen bacterium Dickeya dadantii . Nucleic Acids Res. 40, 4306–4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul, R. , Weiser, S. , Amiot, N.C. , Chan, C. , Schirmer, T. , Giese, B and Jenal, U. (2004) Cell cycle‐dependent dynamic localization of a bacterial response regulator with a novel di‐guanylate cyclase output domain. Genes Dev. 18, 715–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, Q. , Yang, S. , Charkowski, A.O. , Yap, M.‐N. , Steeber, D.A. , Keen, N.T. and Yang, C.‐H. (2006) Population behavior analysis of dspE and pelD regulation in Erwinia chrysanthemi 3937. Mol. Plant–Microbe Interact. 19, 451–457. [DOI] [PubMed] [Google Scholar]
- Povolotsky, T.L. and Hengge, R. (2012) ‘Life‐style’ control networks in Escherichia coli: signaling by the second messenger c‐di‐GMP. J. Biotechnol. 160, 10–16. ‘ [DOI] [PubMed] [Google Scholar]
- Qi, Y. , Rao, F. , Luo, Z. and Liang, Z.‐X. (2009) A flavin cofactor‐binding PAS domain regulates c‐di‐GMP synthesis in Ax DGC2 from Acetobacter xylinum . Biochemistry, 48, 10 275–10 285. [DOI] [PubMed] [Google Scholar]
- Reverchon, S. and Nasser, W. (2013) Dickeya ecology, environment sensing and regulation of virulence programme. Environ. Microbiol. Rep. 5, 622–636. [DOI] [PubMed] [Google Scholar]
- Reverchon, S. , Nasser, W. and Robert‐Baudouy, J. (1991) Characterization of kdgR, a gene of Erwinia chrysanthemi that regulates pectin degradation. Mol. Microbiol. 5, 2203–2216. [DOI] [PubMed] [Google Scholar]
- Reverchon, S. , Van Gijsegem, F. , Effantin, G. , Zghidi‐Abouzid, O. and Nasser, W. (2010) Systematic targeted mutagenesis of the MarR/SlyA family members of Dickeya dadantii 3937 reveals a role for MfbR in the modulation of virulence gene expression in response to acidic pH. Mol. Microbiol. 78, 1018–1037. [DOI] [PubMed] [Google Scholar]
- Robert‐Baudouy, J. , Nasser, W. , Condemine, G. , Reverchon, S. , Shevchik, V.E. and Hugouvieux‐Cotte‐Pattat, N. (2000) Pectic enzymes of Erwinia chrysanthemi, regulation and role in pathogenesis In: Plant Microbe Interactions, Vol. 5 (Stacey G. and Keen N. T., eds), pp. 221–268. St. Paul, MN: APS Press. [Google Scholar]
- Römling, U. , Galperin, M.Y. and Gomelsky, M. (2013) Cyclic di‐GMP: the first 25 years of a universal bacterial second messenger. Microbiol. Mol. Biol. Rev. 77, 1–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross, P. , Weinhouse, H. , Aloni, Y. , Michaeli, D. , Weinberger‐Ohana, P. , Mayer, R. , Braun, S. , de Vroom, E. , van der Marel, G.A. , van Boom, J.H. and Benziman, M. (1987) Regulation of cellulose synthesis in Acetobacter xylinum by cyclic diguanylic acid. Nature, 325, 279–281. [DOI] [PubMed] [Google Scholar]
- Ryan, R.P. , Fouhy, Y. , Lucey, J.F. , Crossman, L.C. , Spiro, S. , He, Y.‐W. , Zhang, L.‐H. , Heeb, S. , Camara, M. , Williams, P. and Dow, J.M. (2006) Cell–cell signaling in Xanthomonas campestris involves an HD‐GYP domain protein that functions in cyclic di‐GMP turnover. Proc. Natl. Acad. Sci. USA, 103, 6712–6717. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ryan, R.P. , Tolker‐Nielsen, T. and Dow, J.M. (2012) When the PilZ don't work: effectors for cyclic di‐GMP action in bacteria. Trends Microbiol. 20, 235–242. [DOI] [PubMed] [Google Scholar]
- Schmidt, A.J. , Ryjenkov, D.A. and Gomelsky, M. (2005) The ubiquitous protein domain EAL is a cyclic diguanylate‐specific phosphodiesterase: enzymatically active and inactive EAL domains. J Bacteriol. 187, 4774–4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamayo, R. , Tischler, A.D. and Camilli, A. (2005) The EAL domain protein VieA is a cyclic diguanylate phosphodiesterase. J. Biol. Chem. 280, 33 324–33 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamayo, R. , Pratt, J.T. and Camilli, A. (2007) Roles of cyclic diguanylate in the regulation of bacterial pathogenesis. Annu. Rev. Microbiol. 61, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardy, F. , Nasser, W. , Robert‐Baudouy, J. and Hugouvieux‐Cotte‐Pattat, N. (1997) Comparative analysis of the five major Erwinia chrysanthemi pectate lyases: enzyme characteristics and potential inhibitors. J. Bacteriol. 179, 2503–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentini, M. , Laventie, B.‐J. , Moscoso, J. , Jenal, U. and Filloux, A. (2016) The diguanylate cyclase HsbD intersects with the HptB regulatory cascade to control Pseudomonas aeruginosa biofilm and motility. PLoS Genet. 12, e1006354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters, C.M. , Lu, W. , Rabinowitz, J.D. and Bassler, B.L. (2008) Quorum sensing controls biofilm formation in Vibrio cholerae through modulation of cyclic di‐GMP levels and repression of vpsT . J. Bacteriol. 190, 2527–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteley, C.G. and Lee, D.‐J. (2015) Bacterial diguanylate cyclases: structure, function and mechanism in exopolysaccharide biofilm development. Biotechnol. Adv. 33, 124–141. [DOI] [PubMed] [Google Scholar]
- Wu, X. , Zeng, Q. , Koestler, B.J. , Waters, C.M. , Sundin, G.W. , Hutchins, W. and Yang, C.‐H. (2014) Deciphering the components that coordinately regulate virulence factors of the soft rot pathogen Dickeya dadantii . Mol. Plant–Microbe Interact. 27, 1119–1131. [DOI] [PubMed] [Google Scholar]
- Yang, C.‐H. , Gavilanes‐Ruiz, M. , Okinaka, Y. , Vedel, R. , Berthuy, I. , Boccara, M. , Chen, J.W.‐T. , Perna, N.T. and Keen, N.T. (2002) hrp genes of Erwinia chrysanthemi 3937 are important virulence factors. Mol. Plant–Microbe Interact. 15, 472–480. [DOI] [PubMed] [Google Scholar]
- Yang, S. , Zhang, Q. , Guo, J. , Charkowski, A.O. , Glick, B.R. , Ibekwe, A.M. , Cooksey, D.A. and Yang, C.‐H. (2007) Global effect of indole‐3‐acetic acid biosynthesis on multiple virulence factors of Erwinia chrysanthemi 3937. Appl. Environ. Microbiol. 73, 1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, S. , Peng, Q. , Zhang, Q. , Yi, X. , Choi, C.J. , Reedy, R.M. , Charkowski, A.O. and Yang, C.‐H. (2008) Dynamic regulation of GacA in type III secretion, pectinase gene expression, pellicle formation, and pathogenicity of Dickeya dadantii (Erwinia chrysanthemi 3937). Mol. Plant–Microbe Interact. 21, 133–142. [DOI] [PubMed] [Google Scholar]
- Yi, X. , Yamazaki, A. , Biddle, E. , Zeng, Q. and Yang, C.H. (2010) Genetic analysis of two phosphodiesterases reveals cyclic diguanylate regulation of virulence factors in Dickeya dadantii . Mol. Microbiol. 77, 787–800. [DOI] [PubMed] [Google Scholar]
- Yu, R.R. and DiRita, V.J. (2002) Regulation of gene expression in Vibrio cholerae by ToxT involves both antirepression and RNA polymerase stimulation. Mol. Microbiol. 43, 119–134. [DOI] [PubMed] [Google Scholar]
- Yuan, X. , Khokhani, D. , Wu, X. , Yang, F. , Biener, G. , Koestler, B.J. , Raicu, V. , He, C. , Waters, C.M. , Sundin, G.W. , Tian, F. and Yang, C.‐H. (2015) Cross‐talk between a regulatory small RNA, cyclic‐di‐GMP signalling and flagellar regulator FlhDC for virulence and bacterial behaviours. Environ. Microbiol. 17, 4745–4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Fig. S1 Analysis of GGDEF and/or EAL domain proteins in Dickeya dadantii. (A) Summary of GGDEF and/or EAL domain proteins. Proteins are shown with the encoded gene names and protein length. Protein domains were predicted by the simplified modular architecture research tool (SMART). ORF, open reading frame. (B) Amino acid sequence alignment for the GGDEF domains in D. dadantii. PleD and WspR are two active diguanylate cyclases (DGCs) from Caulobacter crescentus and Pseudomonas aeruginosa, respectively. The inhibition site RxxD motif (I‐site) and enzymatic activity site GGDEF motif (A‐site) are marked. (C) Amino acid sequence alignment for the EAL domains in D. dadantii. VC1086 is an active phosphodiesterase (PDE) from Vibrio cholerae. The arrow indicates the glutamate residue in the EAL motif. ‘*’, residues identical in all sequences in the alignment; ‘:’, conserved substitutions; ‘.’, semi‐conserved substitutions.
Fig. S2 Effect of complementation of gcpAD418A on pectate lyase (Pel) production. Values are representative of three independent experiments. Three replicates were used in each experiment. Error bars indicate standard errors of the means. Asterisks indicate statistically significant differences of the means (P < 0.05 by Student's t‐test). OD230, optical density at 230 nm.
Fig. S3 Polygalacturonic acid (PGA) induces the transcription of pelD and pelE genes. (A, B) The promoter activities of pelD and pelE in Dickeya dadantii were examined at 12 and 24 h. The experiments were repeated three independent times with similar results. The figure represents the results from one experiment which includes three technical replicates. Error bars indicate standard errors of the means. Asterisks indicate statistically significant differences of the means (P < 0.05 by Student's t‐test).
Fig. S4 Promoter activities of rsmA, rsmB and hns are not affected in gcpAD418A. (A–C) The promoter activities of rsmA, rsmB and hns in Dickeya dadantii strains. One representative experiment was chosen, and three independent experiments were performed. Assays were performed as described in Experimental procedures. Error bars indicate standard errors of the means.
Fig. S5 Expression of rsmA restores pectate lyase (Pel) in the gcpAD418A A‐site mutant. (A, B) Pel production and pelD promoter activity were measured in Dickeya dadantii. Three independent experiments were performed with three replicates in each experiment. Values are from one representative experiment. Error bars indicate standard errors of the means. Asterisks indicate statistically significant differences of the means (*P < 0.05 by Student's t‐test). OD230, optical density at 230 nm.
Fig. S6 pelD gene transcription is growth phase dependent. The promoter activity of the pelD gene was determined throughout growth in minimal medium (MM) supplemented with 0.1% polygalacturonic acid (PGA) at 28 °C. The experiments were repeated twice independently with similar results. Three replicates were used for each experiment. Error bars indicate standard errors of the means. Open squares, OD600 (optical density at 600 nm). Filled triangles, mean fluorescence intensity.
Fig. S7 Impact of GcpA on the expression of hrpL and rpoN. mRNA levels of hrpL and rpoN in gcpAD418A relative to that in the wild‐type, which was mathematically designated as unity. All results are from one representative experiment. Three independent experiments were performed and three replicates were used for each experiment. Error bars indicate standard errors of the means. Asterisk indicates statistically significant difference of the means (P < 0.05 by Student's t‐test).
Table S1 Strains and plasmids used in this study.
Table S2 Primers used in this study.


