Correction to: Scientific Reports 10.1038/s41598-017-08324-z, published online 22 August 2017
The Article mentions but does not include control data demonstrating that a random mutation in the cDNA sequence of DNAJB6b used in the study does not affect the behaviour of its protein product. Specifically, the cDNA sequence of DNAJB6b WT and DNAJB6b H31Q in the plasmids used for all the experiments reported in this study contained a mutation (TTC to TCC) leading to the F46S mutation in the translated proteins, which was randomly introduced during the cloning. The mutation did not change the localisation or ability to inhibit α-syn aggregation in HEK293T-α-syn-dsred cells, as shown in Figure 1 below.
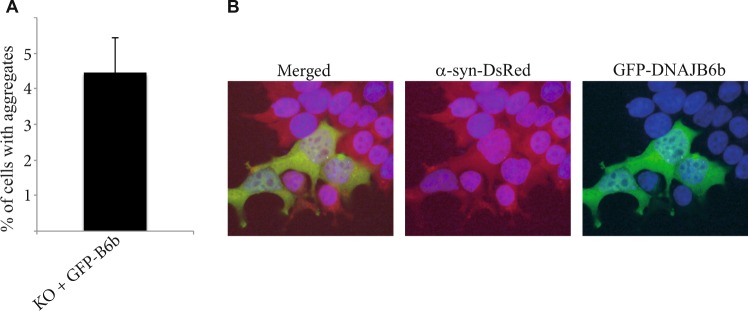
Figure 1.
Quantification of aggregates in α-syn-DsRed expressing HEK293 DNAJB6 KO cells transfected with GFP-DNAJB6b expression construct (n = 3) (A). Experiment showing that α-syn-DsRed aggregation is suppressed in DNAJB6 KO cells transfected with GFP-DNAJB6b as well as the localization of the GFP-DNAJB6b fusion protein (B).
This does not affect the conclusions of the Article.