Abstract
Previously, we genetically engineered a Salmonella Typhi bacterial ghost (STG) as a novel inactivated vaccine candidate against typhoid fever. The underlying mechanism employed by the ghost in stimulating the adaptive immune response remains to be investigated. In this study, we aimed to evaluate the immunostimulatory effect of STG on mouse bone marrow-derived dendritic cells (BMDCs) and its activation of the adaptive immune response in vitro. Immature BMDCs were stimulated with STG, which efficiently stimulated maturation events in BMDCs, as indicated by upregulated expressions of CD40, CD80, and major histocompatibility complex class II molecules on CD11+ BMDCs. Immature BMDCs responded to STG stimulation by significantly increasing the expression of interleukin (IL)-6, which might indicate the induction of dendritic cell maturation in vivo (p < 0.05). In addition, ghost-stimulated murine BMDCs showed significant expressions of interferon gamma and IL-4, which can drive the development of Th1 and Th2 cells, respectively, in co-cultured CD4+ T cells in vitro. These results suggest that STG can effectively stimulate maturation of BMDCs and facilitate subsequent immune responses via potent immunomodulatory cytokine responses.
Keywords: Salmonella Typhi, dendritic cells, innate immunity
Introduction
Salmonella enterica serovar Typhi (S. Typhi), responsible for causing typhoid fever in human populations, has posed significant public health burdens in developing countries [8,18]. Typhoid fever is human-restricted and causes a significant disease burden, mostly in children in developing countries with poor sanitation and a lack of clean water [3]. S. Typhi, a facultative intracellular pathogen, is characterized by the intrinsically invasive traits of fast dissemination and long survival within a host [26]. The commercially available Ty21a (oral) and Vi polysaccharide (parenteral) vaccines against typhoid fever have exhibited 30% to 70% effectiveness [1]. An effective vaccine against Salmonella infection needs to elicit both innate and adaptive immune defenses, particularly those involving T-cell immunity in susceptible hosts [4]. Development of a bacterial ghost vaccine against S. Typhi represents a promising approach because of its enhanced immunogenic and biosafety characteristics [17]. Developed S. Typhi bacterial ghosts (BGs) are non-living bacteria inactivated by the single lysis E gene of the DNA phage φX174 [17]. The expression of gene E facilitates formation of transmembrane tunnels on the surface of the bacteria through which cell contents are expelled due to osmotic pressure [10]. Morphologically, BGs are devoid of all cytoplasmic and nucleoplasmic contents, but they maintain intact cell envelopes containing outer membrane proteins, adhesins, and pili [10]. We previously showed that an S. Typhi ghost (STG) vaccine effectively induces humoral and cellular immune responses [15]. Both non-typhoid and typhoid Salmonella ghosts have been utilized in a new, inactivated vaccine platform that efficiently induces humoral and cell-mediated immune responses in immunized hosts [12,13,14]. Protection against S. Typhi requires the effective induction of cellular immune responses [4,7]. Therefore, interactions between the S. Typhi BG vaccine and antigen presenting cells (APCs) and the vaccine's effect on immune development are critically important to determine the suitability of the BG as a vaccine candidate. APCs such as macrophages, dendritic cells (DCs), and B lymphocytes are responsible for initiating and modulating the type of host immune response against invading pathogens [30]. In particular, DCs provide a crucial bridge between innate and adaptive immunity and are directly activated by pathogen-associated molecular patterns encountered by their pattern recognition receptors [27]. Mature DCs collect and process protein antigens through the major histocompatibility complex (MHC) pathway (which involves other co-stimulatory molecules), resulting in antigenic peptide presentation to naive T-cell populations.
As the complex role of DCs in the immune response stimulated by S. Typhi BGs remains unexplored, we evaluated whether BMDCs function as efficient APCs following stimulation with STG. Pulsed DCs were incubated with CD4+ T-cell subpopulations isolated from isogenic naive mice. Profiles of co-stimulatory and cytokine molecules expressed in the primed DCs and in the CD4+ T cells were measured in order to evaluate the suitability of the S. Typhi BG as a vaccine against typhoid fever.
Materials and Methods
Preparation and production of S. Typhi bacterial ghosts
The preparation of S. Typhi ghosts has been previously described [15]. Briefly, the plasmid pJHL187-LTB was electroporated to JOL1498, resulting in production of JOL1502. The E gene harbored in pJHL187-LTB was controlled under the convergent promoter component. JOL1502 cells were grown to mid-logarithmic phase in LB broth in the presence of 0.2% L-arabinose at 28°, which is the optimal condition for the repression of E gene-mediated lysis. The cells were collected by centrifugation at 1,200 × g and then washed three times with sterile phosphate-buffered saline (PBS). To induce E gene-mediated lysis, bacterial cells were cultured in LB broth without arabinose at 42℃ with a slight agitation. After 48 h of incubation, the lysed cells were harvested and resuspended in PBS. Ghost cell preparations were stored at −80℃ until further use. Bacterial strains and plasmids used in this study are listed in Table 1.
Table 1. Bacterial strains and plasmids used in this study.
S. Typhi, Salmonella enterica serovar Typhi.
Dendritic cell preparation
DCs were isolated from the bone marrow of 5-week-old C57BL/6 mice following a previously reported protocol [19]. Briefly, bone marrow-derived dendritic cells (BMDCs) generated from the femurs and tibias of the mice were adjusted to a concentration of 2 × 107 cells/mL in R10 medium (RPMI-1640 containing 10% heat-inactivated fetal bovine serum, 50 µM 2-mercaptoethanol, 1 U/mL of penicillin, 1 µg/mL streptomycin, and 1% L-glutamine). The cells were incubated in a six-well cell culture plate at 37℃ in a 5% CO2 atmosphere overnight. Following the incubation, non-adherent cells in the plate were harvested from the bone marrow culture. The purified cells were cultured in fresh R10 medium supplemented with 100 U/mL of recombinant murine GM-CSF (rmGM-CSF; Biolegend, USA) and 100 µg/mL recombinant murine interleukin-4 (rmIL; Biolegend) for 7 days. On day 8, the DCs were collected and prepared for antigen stimulation. Cells isolated from murine bone marrow were grown in vitro for 8 days in R10 medium with rmIL-4 and rmGM-CSF to trigger the differentiation of monocytes into DCs [30]. All animal experimentation activities were approved by the Chonbuk National University Animal Ethics Committee (CBNU2015-00085) and were carried out according to the guidelines of the Korean Council on Animal Care and the Korean Animal Protection Law (2007), Article 13 (Experiments with Animals).
Stimulation of cells with the Salmonella ghost Following the 7-day incubation period, harvested
Following the 7-day incubation period, harvested DCs (adjusted to 1 × 106 cells/well) were placed in a six-well cell culture plate. Subsequently, the DCs were incubated with 1 × 107 cells/well of the lysed STG at a multiplicity of infection of 10 in order to determine the stimulatory capacity of STG to activate immature DCs. The number of STG particles used for the stimulation was determined based on a protocol described in previous studies [5,9]. Upon lipopolysaccharide (LPS) stimulation, DC maturation was efficiently triggered; thus, LPS-pulsed DCs were used as a positive control. The control DCs were stimulated with 2 µg/mL of Escherichia coli LPS (serotype O127:B8, Sigma-Aldrich, USA). All cell cultures were incubated in duplicate at 37℃ under 5% CO2. After 24 h of incubation, the cells were used for flow cytometry analysis, RNA extraction, and stimulation with naive CD4+ T cells.
Fluorescence-activated cell sorting (FACS) analysis
The surface markers expressed in the stimulated DCs were assessed by using a FACS method. The proportion of DCs expressing co-stimulatory surface markers was determined in CD11c-gated DCs. DCs (1 × 106) pulsed with STG, LPS, or PBS as a negative control were stained with the following monoclonal antibodies: FITC anti-mouse CD11c (eBioscience, USA; clone N418e), APC anti-mouse MHC-II (eBioscience; clone M5/114.15.2), and CD80 and PE anti-mouse CD40 (eBioscience; clone 1C10). The stained cells were washed three times with FACS running buffer (Miltenyi Biotec, Germany). Subsequently, the antigen-pulsed DCs were sorted with a MACSQuant Analyzer (Miltenyi Biotec).
Confirmation of morphological differentiation
Cells were observed under an inverted microscope equipped with a digital imaging system (Leica DMi1; Leica Microsystems, Germany) to evaluate the extent of morphological differentiation of the DCs.
CD4+ T-cell preparation
Splenocytes were aseptically isolated from non-immunized mice and seeded in cell culture dishes at a density of 1 × 106 cells/well. CD4+ T cells were purified from the splenocytes by positive selection using CD4 (L3T4) microbeads with a MidiMACS Separator (Miltenyi Biotech), following the manufacturer's instructions. Subsequently, the sorted naive CD4+ T cells were co-incubated with the antigen-pulsed DCs for 24 h at 37℃ in a 5% CO2 atmosphere. After incubation, the cell cultures were harvested and prepared for cytokine assays. CD4+ T cells incubated with LPS-stimulated DCs were prepared as a positive control.
Quantitative real-time polymerase chain reaction (qRT-PCR) analysis of mRNA levels
Cytokine mRNA transcripts produced in the antigen-stimulated DCs and the primed CD4+ T cells were measured by using qRT-PCR. Total RNA was extracted from the cells with Trizol reagent and GeneAll Hybrid-RTM (GeneAll Biotechnology, Korea). Extracts were then converted into cDNA by using a ReverTra Ace qPCR RT Kit (FSQ-101; TOYOBO, Japan). Cytokine transcripts produced by the cells were amplified by qRT-PCR with SYBR Green Real-Time PCR Master Mix (QPK-201; TOYOBO), following the manufacturer's instructions. The IL-6, IL-10, IL-12p40, and tumor necrosis factor alpha (TNF-α) genes were probed in the cDNA synthesized from the stimulated DCs. For the primed CD4+ T-cell cDNA, the IL-2, IL-4, and interferon gamma (IFN-γ) genes were probed. The housekeeping gene β-actin was used as an endogenous control. The primers and reaction method used in this study for quantification of copy number were previously validated in mouse samples in an earlier study [21]. The relative expression levels of the target genes in the stimulated cells were calculated using the comparative Ct value method, using values of negative controls (unstimulated DCs or CD4+ T cells) as a comparison.
Statistical analysis
A non-parametric Mann-Whitney test within the STATA software system (Stata Corporation, USA) was applied to determine differences between the immune responses of the pulsed cells and the controls. A p value less than 0.05 was considered statistically significant.
Results
Development of immature and activated BMDC populations
On day 7, non-stimulated BMDCs exhibited signs of maturation such as the presence of dendritic processes or veils (arrows; panel B in Fig. 1), although some cells were still rounded in appearance. To assess whether antigen stimulation facilitated differentiation of mature DCs, DCs were pulsed with STG or LPS for 48 h. After stimulation, most cells displayed distinctive morphological features such as large veils or multiple branches on the cell surface (arrows; panels C and D in Fig. 1), typical signs of fully differentiated DCs.
Fig. 1. Morphological observation of bone marrow-derived dendritic cells (BMDCs) during the differentiation procedures. The pictures were captured by inverted microscope equipped with a digital imaging system (Leica DMi1; Leica Microsystems, Germany). (A) BMDCs observed at day 2 of culture. (B) Morphology of the non-stimulated BMDCs (arrows) at day 7 of culture. (C) Morphology of DCs (arrows) pulsed with lipopolysaccharide at day 9 of culture. (D) Morphology of DCs (arrows) stimulated with Salmonella Typhi ghost at day 9 of culture. Scale bars = 50 µm (A–D).

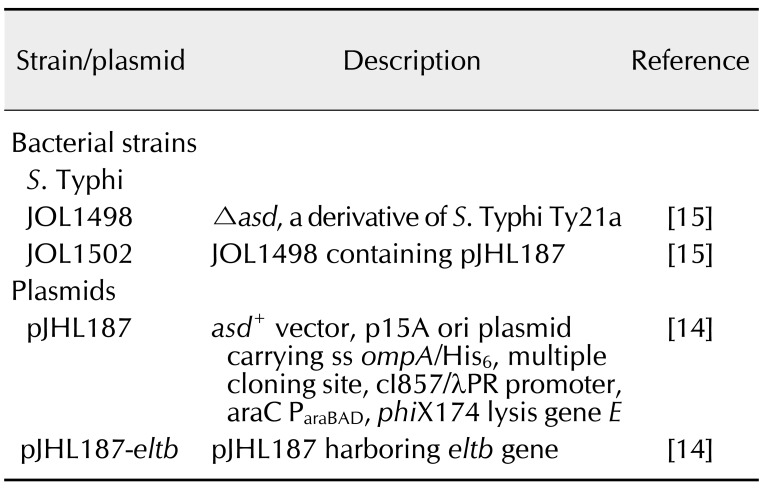
Co-stimulatory molecules expressed on differentiated DCs
Expression of the co-stimulatory molecules CD40, CD80, and MHCII (hallmarks of DC maturation) highlights the ability of DCs to potently induce an acquired immune response via the CD4+ T-cell-dependent effector pathway. By applying FACS analysis, expression levels of cell surface markers were measured in purified DCs pulsed with STG, LPS, or culture medium alone. The proportion of CD11c+CD80+ cells was markedly increased in DCs stimulated with STG compared the level in those pulsed with LPS (panel B in Fig. 2). On average, the sub-fractions of CD11c+CD40+, CD11c+CD80+, and CD11c+MHCII+ cells in JOL1502-stimulated DCs were augmented by 5.76%, 13.57%, and 3.2%, respectively, compared to the same sub-fractions in non-stimulated DCs. These results suggest an upregulation of these cell activation markers in vitro and a concomitant increase in antigen presentation in the JOL1502-stimulated DCs.
Fig. 2. Co-stimulatory molecule expressions on dendritic cells (DC) surfaces was assessed by gating on CD11c+ cell populations using fluorescence-activated cell sorting (FACS). (A) Representative FACS histogram of the surface marker-positive cell population. (B) Percentages of co-stimulatory molecule-positive DCs. NS, non-stimulated; LPS, lipopolysaccharide. *p < 0.05 (vs. non-stimulated DCs).
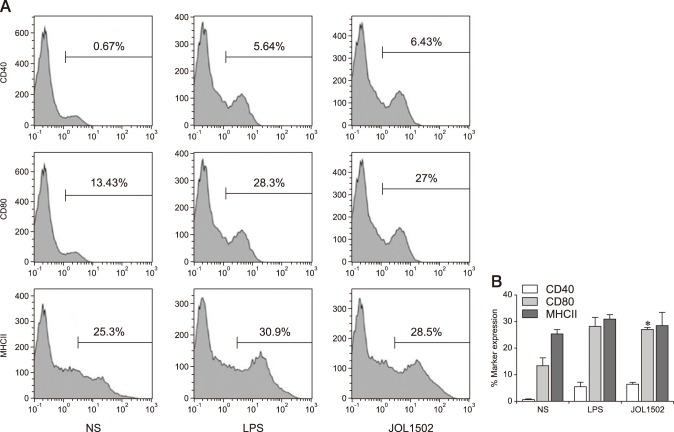
Cytokine assays
In the developmental stages of DC growth, production of proinflammatory cytokines (such as IL-6, IL-10, IL-12p40, and TNF-α) indicates DC activation and profoundly affects the induction of T-cell priming. In DCs stimulated with JOL1502, mRNA levels of IL-6 and IL-10 showed significant increases (on average 3.2- and 3.8-fold, respectively) compared to the LPS-pulsed DCs (Fig. 3). Considering that IL-6 regulates DC maturation in vivo, these results indicate that STG was efficiently internalized by the DCs, where it potently induced DC differentiation. The mRNA levels of IL-12 and TNF-α were slightly increased in the DCs stimulated with JOL1502 compared to the DCs pulsed with LPS. These results suggest that the DCs were properly activated when they encountered STG in vitro.
Fig. 3. Cytokine mRNA upregulated in dendritic cells co-cultured with lipopolysaccharide (LPS) or Salmonella Typhi ghost (JOL1502 ghost). Relative fold changes were calculated based on 2−ΔΔCt method. IL, interleukin; TNF-α, tumor necrosis factor alpha. *p < 0.05.
CD4+ T-cell cytokine assays
To evaluate the potency of STG-pulsed DCs as immune-stimulators that can prime naive CD4+ T cells, stimulated DCs were incubated for 24 h with CD4+ T cells sorted from the splenocytes of non-immunized mice. After incubation, mRNA levels were measured for IL-2, IFN-γ (both proinflammatory cytokines driving the differentiation of naive CD4+ T cells to Th1 cells), and IL-4 (produced by Th2-differentiated CD4+ T cells). In CD4+ T cells pulsed with STG-stimulated DCs, a significant increase in IL-4 mRNA expression was observed compared to that in the positive control (Fig. 4). The mRNA expression of IFN-γ showed an approximately 1.7-fold increase in the CD4+ T cells pulsed with STG-stimulated DCs relative to the expression level in the positive control. IL-2 mRNA was not detectable in the DC-stimulated CD4+ T cells or in the positive control cells compared to the non-stimulated cells. These results show that cytokine gene expression was upregulated in CD4+ T cells upon stimulation with DCs, which implies that DCs can elicit differentiation of CD4+ T cells into Th1 and Th2 subsets.
Fig. 4. Expression of T-cell-derived cytokines induced by indirect activation of naive CD4+ T cells by dendritic cells primed with the JOL1502 ghost (Salmonella Typhi ghost) or lipopolysaccharide (LPS). Relative fold changes were assessed by using the 2−ΔΔCt method. IL, interleukin; INF-β, interferon gamma. *p < 0.05.

Discussion
Antigens are captured, processed, and presented by DCs to undifferentiated T-cell populations [28]. The initiation of the adaptive immune response by a host against the invasion of S. Typhi depends on the antigen processing capacity of DCs and the resulting level of T-cell activation [27]. To activate naive T-cell subsets, exogenous or endogenous antigens initially need to be recognized and processed by dendritic cells. Antigen-derived peptides loaded onto MHC class II (MHC II) molecules are expressed on the surface of mature DCs. Simultaneously, the co-stimulatory molecules CD40 and CD80 (expressed on CD11c+ DCs) provide immune-stimulatory signals to T cells via a respective interaction with the CD40 ligand (CD40L, on the surface of CD4+ T cells) and with CD28 (on the surface of CD8+ T cells) [20]. Previous studies have shown that BGs generated by the φX174 phage lysis gene contributed to a robust activation of DCs [5,9,16]. Major antigenic components preserved on BGs, such as LPS and flagellin, serve as PAMPs. These PAMPs are recognized by toll-like receptors (TLRs), resulting in activation of mature DCs and subsequent induction of T-cell-related immune responses. The ability of BGs to stimulate maturity in naive DCs demonstrates the intrinsic immunostimulatory effect of BGs [10,11]. In this study, a ghost of S. Typhi (JOL1502) stimulated the in vitro maturation of mouse BMDCs. After 48 h of co-incubating immature DCs with STG, morphological alteration of the DCs occurred. Expression of surface markers CD40, CD80, and MHCII on the DCs was also significantly upregulated. The enhanced expression of surface markers demonstrated that STG efficiently induces DC activation in vitro. During the maturation of DCs exposed to antigens, variation in proinflammatory cytokine expression determines whether a primed DC will associate with Th1- or Th2-biased immune activity. IL-12 generated by mature DCs has a critical role in Th1 cell activation, which is required for the clearance of intracellular pathogens [31]. In immature DCs, upregulation of certain cytokines relies on the interaction of TLRs with bacterial components. The differential release of IL-12 is facilitated by activation of TLR-2 on binding to bacterial lipoprotein or peptidoglycan [29]. TNF-α is required to promote DC maturation in the early stages and to stimulate Th2 cells for B-cell activation [23]. IL-6 and IL-10 are also characterized as Th2-skewing cytokines [6]. In the present study, the mRNA expression of IL-6 and IL-10 was significantly augmented in DCs stimulated with STG. To a lesser extent, the mRNA expression of IL-12 and TNF-α was also increased in the DCs. The enhanced production of immunostimulatory cytokines (such as IL-6, IL-10, IL-12, and TNF-α) in mature DCs implies that STG provides significant antigenic stimuli to naive DCs [22]. Further, Bermudez-Brito et al. [2] previously reported the immunomodulatory effects of a live S. Typhi CECT725 strain on human peripheral blood dendritic cells, leading to an increased secretion of proinflammatory cytokines such as IL-1β, IL-6, IL-8, IL-12p40, and TNF-α compared to that in the controls. These results indicated that STG, which maintains cellular morphology and surface antigenicity, may have a potential to induce maturation and activation of human-derived DCs.
Th1-mediated cellular immune responses are crucial for protection against typhoid fever, given that S. Typhi is an enteroinvasive pathogen that can multiply within intestinal epithelial cells [7]. Acquired cellular immunity involves CD4+ and CD8+ T lymphocytes. Naive CD4+ T-cell activation is initiated by binding of a peptide-MHC II complex to T-cell receptors on CD4+ T cells [27]. The capacity of primed DCs to activate naive T-cell proliferation illustrates the efficiency with which DCs process target antigenic peptides, and also the magnitude of the T-cell-related immune response elicited by the peptide complex. In this study, mRNA expressions of IL-4 and IFN-γ (secreted by Th2 and Th1 cells, respectively) were markedly increased. These results suggest that the primed DCs activated naive CD4+ T cells and induced their differentiation into Th1 and Th2 subpopulations. In contrast, IL-2 (which promotes the differentiation of T cells) was not detected in CD4+ T cells stimulated by DCs. Sojka et al. [25] have reported that secretion of IL-2 transiently occurs 1 to 2 h following stimulation. Therefore, although the expression of IL-2 mRNA was not detected in the pulsed CD4+ T cells following the 24 h incubation, a peak in this cytokine's response might have occurred at an earlier time. The increased expression level of IFN-γ in CD4+ T cells stimulated with DCs coincided with an enhanced level of IL-12 in STG-pulsed DCs. This result might imply that IL-12 (an inducer cytokine) from DCs elicits the expression of IFN-γ (an effector cytokine) in CD4+ T cells [24].
In summary, the S. Typhi ghost constructed using the E gene of phage φX174 efficiently induced activation of immature BMDCs, which in turn upregulated co-stimulatory molecules and immunostimulatory cytokines. Furthermore, interaction of the primed DCs with naive CD4+ T cells elicited immunomodulatory cytokines. The results of this study suggest that STG has a sufficient capacity to stimulate both innate and acquired immunity, showing its potential as a vaccine candidate against typhoid fever.
Acknowledgments
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korea government (MISP) (No. 2013R1A4A1069486).
Footnotes
Conflict of Interest: The authors declare no conflicts of interest.
References
- 1.Anwar E, Goldberg E, Fraser A, Acosta CJ, Paul M, Leibovici L. Vaccines for preventing typhoid fever. Cochrane Database Syst Rev. 2014:CD001261. doi: 10.1002/14651858.CD001261.pub3. [DOI] [PubMed] [Google Scholar]
- 2.Bermudez-Brito M, Muñoz-Quezada S, Gomez-Llorente C, Matencio E, Bernal MJ, Romero F, Gil A. Cell-free culture supernatant of Bifidobacterium breve CNCM I-4035 decreases pro-inflammatory cytokines in human dendritic cells challenged with Salmonella typhi through TLR activation. PLoS One. 2013;8:e59370. doi: 10.1371/journal.pone.0059370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Organ. 2004;82:346–353. [PMC free article] [PubMed] [Google Scholar]
- 4.de Jong HK, Parry CM, van der Poll T, Wiersinga WJ. Host-pathogen interaction in invasive Salmonellosis. PLoS Pathog. 2012;8:e1002933. doi: 10.1371/journal.ppat.1002933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eko FO, Mania-Pramanik J, Pais R, Pan Q, Okenu DM, Johnson A, Ibegbu C, He C, He Q, Russell R, Black CM, Igietseme JU. Vibrio cholerae ghosts (VCG) exert immunomodulatory effect on dendritic cells for enhanced antigen presentation and induction of protective immunity. BMC Immunol. 2014;15:584. doi: 10.1186/s12865-014-0056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gal-Mor O, Boyle EC, Grassl GA. Same species, different diseases: how and why typhoidal and non-typhoidal Salmonella enterica serovars differ. Front Microbiol. 2014;5:391. doi: 10.3389/fmicb.2014.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garmory HS, Brown KA, Titball RW. Salmonella vaccines for use in humans: present and future perspectives. FEMS Microbiol Rev. 2002;26:339–353. doi: 10.1111/j.1574-6976.2002.tb00619.x. [DOI] [PubMed] [Google Scholar]
- 9.Hajam IA, Dar PA, Appavoo E, Kishore S, Bhanuprakash V, Ganesh K. Bacterial ghosts of Escherichia coli drive efficient maturation of Bovine Monocyte-Derived Dendritic Cells. PLoS One. 2015;10:e0144397. doi: 10.1371/journal.pone.0144397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hajam IA, Dar PA, Won G, Lee JH. Bacterial ghosts as adjuvants: mechanisms and potential. Vet Res. 2017;48:37. doi: 10.1186/s13567-017-0442-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haslberger AG, Kohl G, Felnerova D, Mayr UB, Fürst-Ladani S, Lubitz W. Activation, stimulation and uptake of bacterial ghosts in antigen presenting cells. J Biotechnol. 2000;83:57–66. doi: 10.1016/s0168-1656(00)00298-4. [DOI] [PubMed] [Google Scholar]
- 12.Hur J, Lee JH. A new enterotoxigenic Escherichia coli vaccine candidate constructed using a Salmonella ghost delivery system: comparative evaluation with a commercial vaccine for neonatal piglet colibacillosis. Vet Immunol Immunopathol. 2015;164:101–109. doi: 10.1016/j.vetimm.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Jawale CV, Chaudhari AA, Lee JH. Generation of a safety enhanced Salmonella Gallinarum ghost using antibiotic resistance free plasmid and its potential as an effective inactivated vaccine candidate against fowl typhoid. Vaccine. 2014;32:1093–1099. doi: 10.1016/j.vaccine.2013.12.053. [DOI] [PubMed] [Google Scholar]
- 14.Jawale CV, Lee JH. Development of a biosafety enhanced and immunogenic Salmonella Enteritidis ghost using an antibiotic resistance gene free plasmid carrying a bacteriophage lysis system. PLoS One. 2013;8:e78193. doi: 10.1371/journal.pone.0078193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim B, Won G, Lee JH. Construction of an inactivated typhoid vaccine candidate expressing Escherichia coli heat-labile enterotoxin B subunit and evaluation of its immunogenicity in a murine model. J Med Microbiol. 2017;66:1235–1243. doi: 10.1099/jmm.0.000543. [DOI] [PubMed] [Google Scholar]
- 16.Kudela P, Paukner S, Mayr UB, Cholujova D, Schwarczova Z, Sedlak J, Bizik J, Lubitz W. Bacterial ghosts as novel efficient targeting vehicles for DNA delivery to the human monocyte-derived dendritic cells. J Immunother. 2005;28:136–143. doi: 10.1097/01.cji.0000154246.89630.6f. [DOI] [PubMed] [Google Scholar]
- 17.Langemann T, Koller VJ, Muhammad A, Kudela P, Mayr UB, Lubitz W. The bacterial ghost platform system: production and applications. Bioeng Bugs. 2010;1:326–336. doi: 10.4161/bbug.1.5.12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin FY, Vo AH, Phan VB, Nguyen TT, Bryla D, Tran CT, Ha BK, Dang DT, Robbins JB. The epidemiology of typhoid fever in the Dong Thap Province, Mekong Delta region of Vietnam. Am J Trop Med Hyg. 2000;62:644–648. doi: 10.4269/ajtmh.2000.62.644. [DOI] [PubMed] [Google Scholar]
- 19.Lutz MB, Kukutsch N, Ogilvie AL, Rössner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 20.Nielsen KN, Steffensen MA, Christensen JP, Thomsen AR. Priming of CD8 T cells by adenoviral vectors is critically dependent on B7 and dendritic cells but only partially dependent on CD28 ligation on CD8 T cells. J Immunol. 2014;193:1223–1232. doi: 10.4049/jimmunol.1400197. [DOI] [PubMed] [Google Scholar]
- 21.Overbergh L, Valckx D, Waer M, Mathieu C. Quantification of murine cytokine mRNAs using real time quantitative reverse transcriptase PCR. Cytokine. 1999;11:305–312. doi: 10.1006/cyto.1998.0426. [DOI] [PubMed] [Google Scholar]
- 22.Park SJ, Nakagawa T, Kitamura H, Atsumi T, Kamon H, Sawa S, Kamimura D, Ueda N, Iwakura Y, Ishihara K, Murakami M, Hirano T. IL-6 regulates in vivo dendritic cell differentiation through STAT3 activation. J Immunol. 2004;173:3844–3854. doi: 10.4049/jimmunol.173.6.3844. [DOI] [PubMed] [Google Scholar]
- 23.Sanders CJ, Yu Y, Moore DA, 3rd, Williams IR, Gewirtz AT. Humoral immune response to flagellin requires T cells and activation of innate immunity. J Immunol. 2006;177:2810–2818. doi: 10.4049/jimmunol.177.5.2810. [DOI] [PubMed] [Google Scholar]
- 24.Seder RA, Hill AV. Vaccines against intracellular infections requiring cellular immunity. Nature. 2000;406:793–798. doi: 10.1038/35021239. [DOI] [PubMed] [Google Scholar]
- 25.Sojka DK, Bruniquel D, Schwartz RH, Singh NJ. IL-2 secretion by CD4+ T cells in vivo is rapid, transient, and influenced by TCR-specific competition. J Immunol. 2004;172:6136–6143. doi: 10.4049/jimmunol.172.10.6136. [DOI] [PubMed] [Google Scholar]
- 26.Srikanth CV, Mercado-Lubo R, Hallstrom K, McCormick BA. Salmonella effector proteins and host-cell responses. Cell Mol Life Sci. 2011;68:3687–3697. doi: 10.1007/s00018-011-0841-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol. 2006;311:17–58. doi: 10.1007/3-540-32636-7_2. [DOI] [PubMed] [Google Scholar]
- 28.Steinman RM, Pope M. Exploiting dendritic cells to improve vaccine efficacy. J Clin Invest. 2002;109:1519–1526. doi: 10.1172/JCI15962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thoma-Uszynski S, Kiertscher SM, Ochoa MT, Bouis DA, Norgard MV, Miyake K, Godowski PJ, Roth MD, Modlin RL. Activation of toll-like receptor 2 on human dendritic cells triggers induction of IL-12, but not IL-10. J Immunol. 2000;165:3804–3810. doi: 10.4049/jimmunol.165.7.3804. [DOI] [PubMed] [Google Scholar]
- 30.Wick MJ. Living in the danger zone: innate immunity to Salmonella. Curr Opin Microbiol. 2004;7:51–57. doi: 10.1016/j.mib.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 31.Winzler C, Rovere P, Rescigno M, Granucci F, Penna G, Adorini L, Zimmermann VS, Davoust J, Ricciardi-Castagnoli P. Maturation stages of mouse dendritic cells in growth factor-dependent long-term cultures. J Exp Med. 1997;185:317–328. doi: 10.1084/jem.185.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]