Abstract
Cells constantly encounter mechanical stimuli in their environment, such as dynamic forces and mechanical features of the extracellular matrix. These mechanical cues are transduced into biochemical signals, and integrated with genetic and chemical signals to modulate diverse physiological processes. Cells also actively generate forces to internally transport cargo, to explore the physical properties of their environment and to spatially position themselves and other cells during development. Mechanical forces are therefore central to development, homeostasis, and repair. Several molecular and biophysical strategies are utilized by cells for detecting and generating mechanical forces. Here we discuss an important class of molecules involved in sensing and transducing mechanical forces – mechanically-activated ion channels. We focus primarily on the Piezo1 ion channel, and examine its relationship with the cellular cytoskeleton.
Keywords: Piezo1, Mechanically-activated ion channels, Cytoskeleton, Traction forces, Mechanotransduction, Calcium signaling
1. Introduction
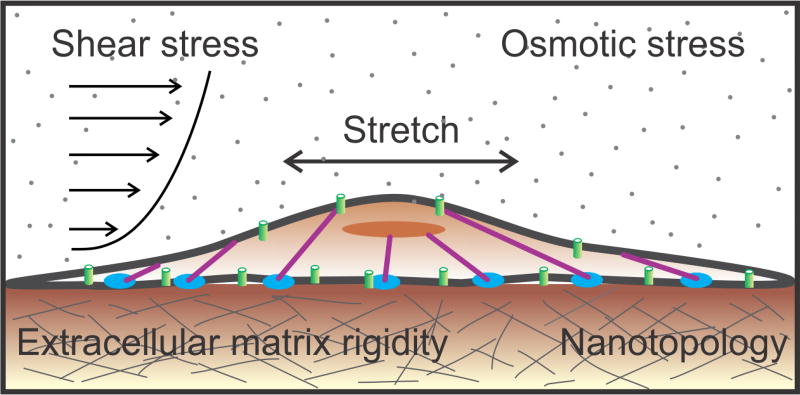
Cells are dynamic physical structures governed by the laws of Newtonian mechanics, with an exquisite ability to experience and exert mechanical forces. They integrate mechanical information with genetic and chemical cues, and change behavior in response to a variety of external mechanical signals such as stretching, shear stress, matrix stiffness, osmotic stress, and substrate nanotopology (texture) (Fig. 1). In addition, cells are able to actively generate mechanical forces at the expense of ATP. These cell-generated forces serve as a means to explore the mechanical landscape of their environment, move cargo from one part of the cell to another, determine cell shape, and segregate chromosomes during cell division. At a macroscopic scale, the interplay of forces between cells and tissues governs organismal growth, dynamics and homeostasis. Thus, life is an intensely mechanical process, and yet we have only a limited understanding of how mechanical forces shape biological processes.
Fig. 1.
Mechanical stimuli encountered by cells. Cells are subject to a variety of dynamic mechanical stimuli in the environment such as shear forces, osmotic stress, and stretch. They also sense mechanical cues in the matrix such as substrate rigidity and nanotopology (texture).
Mechanical forces are detected by specialized molecules in cells and transduced into biochemical signals that shape molecular and genetic events. A variety of bottlenecks have limited our understanding of how mechanical forces shape cellular and organismal physiology, most notably the following: (i) unknown identities of key molecular players, (ii) an inability to measure molecular- and cellular-scale mechanical forces in situ, and (iii) an inability to manipulate mechanical forces in situ. A concerted, multi-disciplinary effort over the last decade is clearing these obstacles, ushering in a new age of Mechanobiology [1]. Arguably, one of the most exciting developments in this area has been the discovery of the Piezo channels by the Patapoutian group [2], which opened up new areas of mechanotransduction research. This review places these new developments in the context of cell biology and argues that a new approach, combining the techniques and ideas from ion channel biophysics with cell biological principles, is required for understanding the role of this important class of molecules in cellular and organismal physiology. We focus primarily on the Piezo1 ion channel, but the basic principles discussed are broadly applicable to other mechanically-activated ion channels.
2. Force transduction: a focus on ion channels
Mechanical force must be transduced to intracellular signaling pathways in order to influence cell behavior. A cell utilizes a variety of mechanisms for the transmission and transduction of force [3]. For instance, external mechanical forces can be transmitted and detected at focal adhesion zones (FAZs), cytoskeletal structures that connect the extracellular matrix to the intracellular actin cytoskeleton. The filamentous actin network can relay the forces over long distances within cells, with some actin filaments running from focal adhesion zones in the plasma membrane to Linker of Nucleoskeleton and Cytoskeleton (LINC) complexes in the nuclear membrane. LINC complexes are analogous to FAZs and can transduce mechanical force into the nucleus; thus, external mechanical force can modulate nuclear events, such as transcription. Transmission of forces between proteins can be mediated by so-called catch bonds, non-covalent bonds that increase in strength and lifetime under mechanical tension, serving as mechanical switches. Force-dependent conformational changes can reveal or conceal binding sites for other molecules, providing a means of translating mechanical force to biochemical events. Among this multiplicity of force transduction paradigms, force transduction by ion channels has a unique role in cellular mechanics.
Ion channels are proteinaceous pores embedded in the plasma membrane. Under resting conditions, cells maintain an electrical and chemical gradient across the plasma membrane through a combination of passive diffusion and active transport of ions across the membrane. When a channel opens, ions flow through the channel’s pore down their electrochemical gradient in an energetically favorable process that does not require ATP hydrolysis or transport of counter ions. The opening and closing of the channel pore can be regulated by either physical or chemical stimuli, such as voltage, temperature, chemical ligands – or, in the case of mechanically-activated ion channels – mechanical force. The physical, chemical and electrical properties of the inner pore lining determine the biophysical fingerprints of the pore. These include the type(s) of ions that can pass through the open channel (ion selectivity) and the flux of the permeating ions (conductance). Nonselective cation channels pass a combination of cations (e.g. Na+, Ca2+, K+) and their activity depolarizes the cell and also increases intracellular levels of Ca2+, an important second messenger.
Ion channel dynamics are traditionally measured by patch clamp electrophysiology, which involves making a tight electrical seal between a glass micropipette and the cell membrane. A patch clamp amplifier connected to the micropipette controls the electrical potential across the cell membrane and measures ionic currents passing through ion channels. For studying mechanically-activated ion channels, the technique is modified to allow for mechanical stimulation of the cell. In the whole-cell patch clamp configuration, a mechanical stimulus is usually imparted by indenting the cell with a fire-polished glass probe, while the electrical response is recorded from the surface of the entire cell. In the cell-attached patch clamp configuration, the patch clamp amplifier only records the electrical activity of ion channels in the microscopic patch of membrane within the micropipette, and mechanical stimulus is administered by suction pulses applied to the back of the patch pipette.
2.1. Dynamics of channel function
Several features of ion channels set them apart as sensors and transducers of mechanical force. Since their action can cause an influx of Ca2+ ions, which can diffuse over a larger cellular region to modulate a broad range of biochemical processes, channels have the ability to convert local mechanical events to global cellular events. Mechanical force directly gates the channel, inducing a conformational change from a resting-closed state to an activated-open state. This does not require second messenger cascades that typically occur on a millisecond time scale. The direct gating mechanism allows the channels to open on microsecond timescales, providing the fastest transduction of mechanical forces into biochemical signals. Consequently, mechanically-activated ion channels are likely first responders to mechanical stimuli.
Ion channels are tuned to a certain range of mechanical stimuli. For instance, the Msc family of bacterial and plant mechanosensitive ion channels has several members that differ in mechanical sensitivity [4]. These channels activate with increases in membrane tension induced by osmotic stress. Individual channels in the family have different operating ranges: e.g., bacterial MscM opens at low membrane tension (~1 mN/m), MscS opens at moderate tension (~5 mN/m) and MscL opens at just under lytic tension of 10–12 mN/m [5,6]. These differences in sensitivity are governed by differences in the primary, secondary and tertiary structures of the channels. Individual channels elicit somewhat different downstream effects, so that different ranges of osmotic pressures can elicit different cellular behavior, with MscL operating as the final safety valve that prevents lysis of the cell [5].
Activation of a channel involves a transition from a resting-closed state to an activated-open state that conducts an ionic current. Some mechanically-activated ion channels show a spontaneous reduction of currents after activation, even as the mechanical stimulus persists. This phenomenon can result from one of two distinct processes: adaptation or inactivation. In adaptation, the operating range of the channel resets towards a higher range, so that the channel retains the ability to respond to a subsequent mechanical stimulus of greater magnitude. Conversely, in the absence of a mechanical stimulus, the operating range shifts to a lower range, so that smaller stimuli can be detected. Adaptation serves to increase the overall dynamic range of an ion channel. For example, adaptation of mechanically-activated ion channels in inner ear hair cells underlies our ability to detect sounds that range in intensity from just above 0dB, corresponding to air pressure changes of less than a billionth of standard atmospheric pressure, to painfully loud noises of ~130 dB.
Inactivation, which produces an ionic current pattern similar to adaptation, is a mechanistically distinct process. From the conducting, activated-open state, the channel enters a non-conducting “inactivated” state which is non-responsive to mechanical stimuli. Recovery from inactivation is usually a slower process than activation gating, and happens after the mechanical stimulus is terminated. An easy way to differentiate between adaptation and inactivation is to apply a larger stimulus after the initial activating stimulus. If the second stimulus produces an ionic current, adaptation is at play; if not, inactivation is occurring. Adaptation and inactivation dynamically modulate channel biophysics to powerfully impact cellular physiology. These processes provide a temporal filtering of mechanical stimuli for cells, convert a persistent mechanical stimulus to a transient biochemical signal, and confer a memory of past mechanical events.
The activity of ion channels can be modulated by a variety of channel-intrinsic and channel-extrinsic regulatory mechanisms [7,8]. Specific residues on the protein may be phosphorylated or glycosylated to modulate channel gating, dynamics or localization. Moreover, alternative splicing, interacting proteins, accessory subunits, intracellular and extracellular pH, ionic composition, membrane lipid composition, and the action of antagonistic or synergistic channels may further modulate ion channel activity. These regulatory processes provide additional layers of control over mechanotransduction.
2.2. Molecular identification of the Piezo channels
The first evidence for the existence of mechanically-gated ion channels emerged from seminal recordings of vertebrate inner ear hair cells by Corey and Hudspeth almost 40 years ago [9]. Within the next decade, Guharay and Sachs discovered ion channels activated by membrane stretch in chick skeletal muscle cells [10], and Kung and colleagues reported a similar activity in bacterial spheroplasts [11]. The bacterial Msc channels were cloned soon after [12,13], but channels encoding eukaryotic stretch-activated channels proved harder to identify. Over the last decade, Trp channels (reviewed in [14]), two-pore potassium channels (reviewed in [15]) and TMC channels [16] have emerged as prominent players. However, the molecular identity of mechanically-activated ion channels in several cell types remained unknown.
A tour de force effort by Coste et al. uncovered a novel ion channel family responsible for excitatory mechanically-activated ionic currents [2]. Christened the Piezo family, its members – Piezo1 and Piezo2 – are emerging to be critically important molecules in normal physiology as well as pathological dysfunction. The biophysics and physiological roles of these channels have recently been reviewed in excellent articles elsewhere [17–21], including a comprehensive book devoted to the topic [22]. Hence, we focus on an emerging topic of interest to the readers of this special issue on Mechanosensing – the interplay between Piezo1 and the cytoskeleton. We apologize to colleagues whose work we are unable to cite or cover in depth due to constraints of space and scope.
3. Effects of the cytoskeleton on Piezo1 activation
Piezo1 has been shown to respond to a wide range of mechanical cues. These include cell indentation [2], membrane stretch [2], shear flow [23,24], substrate stiffness [25], substrate nanotopology [26], tissue compression [27], tissue distention [28], confinement [29], and osmotic stress [30]. The diverse nature of the activating stimuli naturally raises the question: what are the relevant physical and cellular parameters that gate the channel? Mechanically-activated ion channels can gate by two different paradigms: force transduction through cytoskeletal tethers (a.k.a. force-through-filaments) or force transduction through the membrane (a.k.a. force-through-lipid) [31–33]. The force-through-filament model postulates that an extracellular or intracellular tether (e.g. a cytoskeletal element) tugs on the channel, inducing a conformational change of the gate. In the force-through-lipid model, the physical stimulus that gates the channel is mechanical tension in the lipid bilayer. Bacterial Msc channels as well as mammalian two-pore potassium channels have been shown to be gated by membrane tension [4,34]. The mammalian haircell mechanotransduction channel [35] and touch receptor channel [36] require filamentous tethers to function, though it remains to be determined whether these channels are gated purely through the force-through-filament scheme, or by a combination of the two models. The C. elegans touch receptor has been proposed to be gated by a hybrid mechanism [37], while strong evidence supports a force-through-filament gating scheme for the Drosophila NOMPC channel of the TRP family [38]. A series of recent studies have investigated the gating mechanism of Piezo1, as described below.
3.1. Piezo1 gating and the cytoskeleton
Lewis and Grandl examined Piezo1 activation in HEK cells over-expressing Piezo1 [39]. They used cell-attached patch clamp electrophysiology to measure channel activity and simultaneously imaged the membrane patch. By Laplace’s law, the membrane tension (T) is given by T = R*P/2, where R is the radius of the patch and P is the pressure applied to the membrane patch. Therefore, by measuring the patch radius from images of the patch pipette, the tension in the membrane can be calculated for a given negative pressure applied. This approach revealed that the Piezo1 channel activates at membrane tension in the range of 1–5mN/m, with a half-maximal tension (T50) of 2.7 mN/m. It has been known for some time that the gigaseal connection between the glass pipette and membrane itself confers mechanical tension in the bilayer [40]. This resting tension, which occurs in the absence of an applied pressure, has a magnitude of 1–4 mN/m [40]. Lewis and Grandl found that the resting tension induced inactivation and affected the sensitivity of the channel: it was easier to open the channel if the resting tension in the patch was relieved by continuous application of a small positive pressure. Under these conditions, T50 for Piezo1 was reduced to 1.4 mN/m. Taken together, these data hint that, like the bacterial Msc channels, Piezo1 is gated through membrane tension.
Syeda and colleagues took a different approach to uncover the gating mechanism of Piezo1 [30]. They reconstituted purified channel protein into droplet lipid bilayers with a symmetrical lipid composition of the two leaflets, and recorded the channel’s electrical activity. They reasoned that mechanically-induced channel activation in this minimal, cell-free system would demonstrate inherent mechanosensitivity of the channel. They observed that channels were not active in baseline conditions, but could be opened by mechanical stimuli such as an osmotic gradient across the bilayer or solvent injection on one side of the bilayer. Unilateral solvent injection leading to channel activation was estimated to increase the membrane tension to 3.4 mN/m, in agreement with the activation range observed by Lewis and Grandl [39]. Channel activation induced by osmotic stress in this minimal system indicates that Piezo1 can gate in response to mechanical stress in the lipid bilayer.
These studies demonstrate that Piezo1 gates with lipid tension. But, is there a role for the cytoskeleton in gating or modulating channel activity? Indeed, many studies point toward interesting dynamics between Piezo1 and the cytoskeleton. In whole-cell patch mode, where the channel is stimulated by indenting the top surface of the cell with a glass probe, disrupting the actin filaments with cytochalasin D reduced current magnitude, suggesting that the actin cytoskeleton is partially responsible for transmitting mechanical stimuli from the indentation probe to the channels [41]. In this configuration, one can imagine that the mechanical stimulus is partly transmitted through the cell membrane and partly through the cytoskeleton.
Cox et al. systematically examined the role of the cytoskeleton on Piezo1 activation by comparing Piezo1 activation in cell-attached patches to that in membrane blebs formed by hypotonically stressing HEK cells that over-express Piezo1 [42]. Their rationale for making this comparison was that cell-attached patches retain connections between the membrane and cytoskeleton, while bleb-attached patches lack cytoskeleton. Therefore, comparing Piezo1 dynamics between the two conditions would reveal the contribution of the cytoskeleton to channel function. They found that in bleb-attached patches Piezo1 could be more easily activated by negative suction pulses. Moreover, the authors reported a 5-fold higher basal activity of Piezo1 in bleb-attached patches than in cell-attached patches. They estimated the gating tension of Piezo1 with two different methods: using Laplace’s law and by comparison with co-expressed bacterial MscL, an ion channel with known sensitivity. Their estimated T50 of 5.1 mN/m and 4.5 mN/m from the two approaches, respectively, agrees with estimates from Lewis and Grandl [39] and Syeda et al. [30]. Based on the differences in sensitivity seen between bleb-attached and cell-attached Piezo1 channels, the authors proposed that the cytoskeleton has a mechano-protective effect on Piezo1, whereby presence of the cytoskeleton makes it harder to open the channel. These findings are consistent with reports by Retailleau et al. wherein they found that knocking out filamin, a scaffold protein that connects the actin network to membrane proteins, made it easier to activate the channel in cell-attached patch clamp assays [43].
Mechanical cues are also known to direct differentiation outcomes of stem cells in a variety of lineages, including mesenchymal stems cells [44] and neural stem cells [45]. We previously reported that in human neural stem/progenitor cells, Piezo1 mediates mechanosensitive lineage choice [25]. Piezo1 detects matrix mechanical cues, influences nucleo-cytoskeletal localization of the transcriptional co-activator Yap, and affects neuronal versus astrocytic lineage specification. We observed spontaneous Piezo1 activity at the cell-substrate interface by imaging Ca2+ influx through the channel using total internal reflection fluorescence microscopy (TIRFM). The greater signal-to-noise ratio afforded by TIRFM allowed us to detect tiny signals arising from the activity of a small number of endogenously expressed channels at the cell-substrate interface. These spontaneous Ca2+ transients required extracellular Ca2+, and were inhibited by pharmacological inhibition of Piezo1 and by siRNA-mediated channel knockdown. Importantly, Piezo1 activity occurred in the absence of mechanical stimulation of the cells (i.e. no poking, stretching, shear flow, etc.), suggesting that cell-generated intracellular forces were at play. We hypothesized that traction forces – acto-myosin-based contractile forces generated by cells – which have previously been linked to mechanosensitive lineage specification of stem cells [44], may activate the channel. Consistent with this hypothesis, we observed that pharmacological inhibition of myosin 2, the actin-based molecular motor that generates traction forces, reversibly inhibited spontaneous Piezo1 activity. Moreover, we also found spontaneous Piezo1 activity to be regulated by matrix stiffness such that stiffer substrates – which are known to induce higher traction forces [46] – elicited greater Piezo1 activity. In sum, mechanical forces generated by the cell’s acto-myosin machinery have the ability to activate Piezo1. These findings are consistent with a previous proposal by Kobayashi and Sokabe that cells may use mechanically-activated ion channels to sense substrate rigidity [47].
3.2. The paradox of the cytoskeleton and Piezo1 activation
How can one reconcile these seemingly contradictory findings – where on the one hand the actin cytoskeleton makes it harder to open the channel [42,43], and on the other the channel is activated by the acto-myosin cytoskeleton [25]? The plasma membrane – the interface between the cell’s intra- and extra-cellular environments – is subject to mechanical forces from both, inside and outside the cell. When a cell encounters external forces, mechanical work is done on the cell, and the cell passively responds to this stimulus. This passive mode of mechanotranduction is also called outside-in mechanotransduction [48] (Fig. 2; Table 1). On the other hand, when a cell probes matrix stiffness with traction forces, it actively generates a mechanical force using motor proteins, and mechanical work is done by the cell. This active mode of mechanotransduction is also called inside-out mechanotransduction [48] (Fig. 3, Table 1). Piezo1 in the plasma membrane is situated to respond to outside-in as well as inside-out mechanical stimuli.
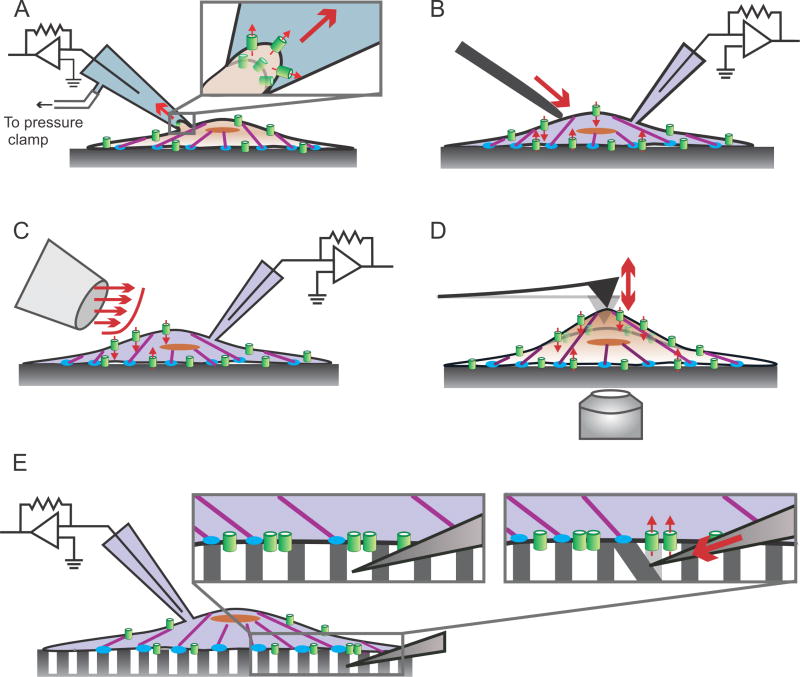
Fig. 2.
Piezo1 transduces outside-in mechanical forces. A variety of techniques have been developed to study transduction of outside-in mechanical forces by Piezo1. Some of these include: A) Membrane stretch elicited by suction pulses imparted by a high-speed pressure clamp in cell-attached patch clamp mode. B) Membrane stretch elicited by cell indentation with a glass probe controlled by a piezoelectric actuator in whole-cell patch clamp configuration. C) Shear stress induced by pulses of fluid flow from a perfusion pipette in whole-cell patch clamp configuration. D) Pulling or pushing on the cell surface by an AFM cantilever, while channel activity is measured by Ca2+ imaging on a confocal microscope. E) Cells are seeded on an array of microposts; a glass probe mounted on a piezoelectric actuator deflects a single micropost, mechanically stimulating a small number of channels in the vicinity of the micropost, while electrical activity is measured with whole-cell patch clamp. See Section 3 of the text for details on the techniques and results obtained. In all panels, actin filaments are shown in purple, focal adhesion zones in blue, Piezo1 molecules in green. Solid red arrows indicate force application; small broken arrows indicate ionic conduction through the channel.
Table 1.
Piezo1 activity in passive and active mechanotransduction.
| Passive mechanotransduction | Active mechanotransduction | |
|---|---|---|
| Piezo1 is… | active | active |
| The direction of force is… | outside-in | inside-out |
| Force is… | exerted on the cell | exerted by the cell |
| ATP hydrolysis is… | not required | required |
| The cytoskeleton has a(n)… | mechanoprotective effect | activating effect |
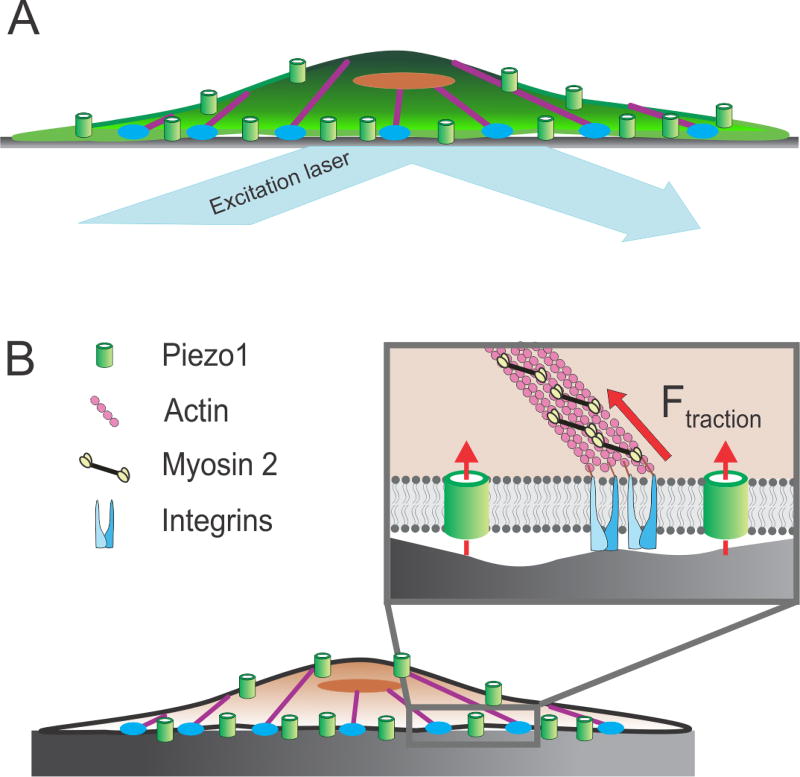
Fig. 3.
Piezo1 transduces inside-out mechanical forces. A) Activation of Piezo1 by inside-out forces is studied by imaging Ca2+ influx through the channel with TIRFM. Since forces are generated by the cell itself, no external force stimulus is applied to the cell. B) Piezo1 is activated by traction forces (solid red arrow) generated at integrin-rich focal adhesion zones (blue) by myosin 2 molecules (black and yellow) along the actin cytoskeleton (purple). Broken red arrows denote ionic conduction through the channel. See Section 3 for details.
Piezo1 activation by outside-in mechanical stimuli is well appreciated [2,23,24,30,41], while its response to inside-out mechanical stimuli has recently come to light [25]. Inside-out mechanotransduction underlies the spontaneous activity of Piezo1 observed in the absence of externally-applied mechanical forces [25]. The forces generated by molecular motors are transmitted along the actin and microtubule cytoskeleton. The cytoskeleton is thus pre-stressed, and the cell’s response to external mechanical forces will vary with its internal tension [49]. In a cell with an intact cytoskeleton, the membrane is mechanically supported by the cytoskeleton: the combination of the membrane and the cytoskeleton is stiffer, requiring a greater force to deform the membrane. Once the actin cytoskeleton is disrupted, the same mechanical stimulus will result in a greater deformation of the membrane, and therefore greater evoked Piezo1 activity. This idea is consistent with the findings described in Section 3.1 above, where disrupting the actin cytoskeleton yielded greater outside-in activity of Piezo1 in cell-attached patches [42,43].
Actively generated traction forces trigger channel activity, whereas disruption of these forces inhibits channel activity. This finding opens up a new set of questions: how are traction forces conveyed to the channel? Do other types of cell-generated forces also activate the channel? Is the actively-generated force transmitted to the channel directly through cytoskeletal tethers or indirectly through the membrane? Or a combination of the two? What is the interplay between Piezo1 response to outside-in and inside-out mechanical forces? For instance, Piezo1 may integrate outside-in and inside-out stimuli to determine the cellular response to mechanical forces. Another possibility is that one modulates the channel’s response to the other: e.g. activation of Piezo1 by inside-out mechanical forces may inactivate the channel, affecting the pool of channel molecules available to transduce outside-in mechanical stimuli. Future studies should shed light on molecular mechanisms underlying activation of Piezo1 by inside-out as well as outside-in mechanical forces.
3.3. Modulation of Piezo1 by scaffold proteins and ECM chemistry
While global disruption of the cell’s cytoskeleton can make it easier to activate the channel with outside-in stimulation, more nuanced manipulations of cellular architecture can yield the opposite results. Poole et al. found that knocking out Stomatin-like protein-3 (STOML3), a membrane-localized scaffold protein, made it harder to open the channel, as evidenced by the increases in the activation threshold, half-maximal stimulation as well as latency of evoked Piezo1 currents [50]. For these studies, the authors developed a novel stimulation paradigm for evoking Piezo1 activity specifically at the cell-substrate interface (Fig. 2E). They grew the cells on an array of polydimethyl-siloxane microposts and indented a single micropost with a fire-polished glass probe. This approach allowed precise stimulation of a small number of channels at the cell substrate interface. Electrical activity was measured in the whole-cell patch clamp configuration. Using this approach, they found that expression of STOML3 sensitized the channel to molecular scale stimuli in dorsal root ganglion neurons. Currents were observed with ~10nm pillar deflection, as compared to 100–1000nm deflections in the absence of STOML3. Subsequently, Qi et al. showed that STOML3-mediated sensitization of Piezo1 depends on cholesterol binding, and proposed that STOML3 influences membrane mechanics and facilitates force transfer to the channel protein [51].
Gaub and Muller developed a novel assay for evoked Piezo1 activity, using an Atomic Force Microscopy (AFM) cantilever to push or pull on the cell’s dorsal surface, and confocal Ca2+ imaging to measure Piezo1 activity [52] (Fig. 2D). They examined the effect of coating the AFM cantilever with different extracellular matrix (ECM) proteins on Piezo1 activation. The response mediated by pushing forces was unchanged by the cantilever coating, with ~200 nN pushing force eliciting Piezo1 activation. However, the response to pulling forces depended on the nature of ECM protein coating the AFM tip. No response was observed with pulling by uncoated tips or those coated by non-ECM adhesive protein concanvalin A, but robust Piezo1-mediated Ca2+ signals were observed with Matrigel- or Collagen IV-coated tips. Importantly, the force eliciting Piezo1 activation was ~6-fold lower for ECM-coated AFM pulling than for AFM pushing (33 nN for pulling as compared to 200 nN for pushing). The authors proposed that the channel functions in two distinct regimes – a high-threshold regime where it responds to membrane stretch alone in the absence of an ECM protein, and a low-threshold regime, in the presence of cytoskeletal tethers, where it is sensitized to lower mechanical forces. Whether the low-threshold regime depends on specific interactions between the channel and the ECM protein, or reflects more efficient stretching of the membrane mediated by adhesion of a “sticky” cantilever to the cell surface remains to be determined. Taken together, the results from the studies by Poole et al. [50], Qi et al. [51], and Gaub and Miller [52] suggest that the response of Piezo1 to outside-in mechanical forces can be modulated by cytoskeletal tethers and scaffold proteins.
In sum, emerging studies in the field summarized in Section 3 indicate that the cytoskeleton exerts complex effects on Piezo1 activity. Patch clamp electrophysiology has yielded valuable insights into channel biophysics, but it has a significant limitation for studying mechanically-activated ion channels: the measurements alter cellular structures and cytoskeletal organization, and mechanical stimulation paradigms accompanying patch clamp measurements do not faithfully recapitulate several mechanical stimuli experienced by cells under physiological conditions. Noninvasive imaging of channel activity, for instance of Ca2+ influx through the channel [25,52], provides an effective solution around this issue. Fully understanding the ways in which the cytoskeleton controls Piezo1 function will require following spatial and temporal dynamics of both Piezo1 and the cytoskeleton.
4. Effect of Piezo1 activity on the cytoskeleton
Piezo1 activity triggers a variety of intracellular processes, some of which involve the cytoskeleton. This raises the interesting possibility of a feedback mechanism where the cytoskeleton regulates Piezo1 activation as discussed in Section 3, and Piezo1 activity in turn modulates cytoskeletal dynamics. Emerging data in the field support the idea for such a feedback loop.
4.1. Piezo1 and focal adhesion zone dynamics
One of the first pieces of evidence for Piezo1 affecting cytoskeletal dynamics came to light before it was identified as a mechanically-activated ion channel. A screen designed to discover integrin activators turned up the Fam38A gene [53], and shortly thereafter, Fam38A was reported to be a mechanically-activated ion channel and re-christened Piezo1 [2].
Integrins are heterodimeric transmembrane receptors that bind ECM proteins at focal adhesion zones [54]. They regulate a broad range of physiological processes through their control of cell adhesion, traction force generation and cell migration. Integrin affinity to extracellular ligands is modulated by interactions between the intracellular tail domain of the integrin molecule and specific cytoplasmic proteins. To identify novel activators of integrin affinity, McHugh et al. performed a functional screen in Chinese Hamster Ovary (CHO) cells stably expressing integrins. Fam38A/Piezo1 was discovered as a positive hit [53]. In the same study, knockdown of endogenous Piezo1 in HeLa cells reduced integrin B activation and cell adhesion. Cells that remain attached had disorganized focal adhesion zones and lower spread area relative to control cells.
In studies to determine the mechanism underlying integrin activation by Fam38A/Piezo1, McHugh et al. inferred from bioinformatic analysis that Fam38A may be a Ca2+-permeable ion channel. This prompted an examination of calpains, Ca2+- dependent proteases that have previously been associated with integrin activation. Calpain 1 requires micromolar concentrations of Ca2+ for activation, whereas Calpain 2 requires millimolar levels of Ca2+ to be activated [55]. Calpain 2 is known to modulate FAZ dynamics through cleavage of FAZ proteins such as talin, vinculin, paxillin, and focal adhesion kinase (FAK) [56–60]. The authors found that siRNA knockdown of Fam38A/Piezo1 decreased Calpain activity. Further, they found that the integrin-activating mechanism of Piezo1 requires the FAZ protein talin. Talin cleavage by Calpain is known to increase integrin activation [61]. Thus, the model to emerge is that Piezo1 activity increases intracellular Ca2+, which activates Calpain; Calpain then cleaves talin to a form that is more efficient at binding and activating integrins.
McHugh et al. also reported that recruitment of the Ras Family Small GTP Binding Protein R-Ras to the ER is involved in integrin activation by Piezo1. A confusing aspect of the study is that the authors found Fam38A/Piezo1 to be localized to the ER, based on antibody staining and overexpression of reporter-tagged constructs – at odds with its now-established role as a plasma-membrane ion channel. Curiously, the construct used for over-expression studies lacked over 400 amino acids from the N-terminus of the full length protein, providing a possible explanation for the ER localization. Alternately, it is possible that some cells express a splice variant that lacks this region, and consequently, Piezo1 may reside predominantly in the ER in those cells. In either case, the missing amino acids could explain the lack of plasma membrane localization in this study. It would be valuable to re-examine the role of R-Ras in Piezo1-mediated activation of integrins with the full-length Piezo1 channel.
Modulation of the cytoskeleton also underlies Piezo1 function in vascular development. In normal development, shear stress directs endothelial cells lining blood vessels to align with the direction of blood flow. Blood flow begins at E8 in mice, and in homozygous knockout mice, morphological defects in blood vessels are visible starting at E8.5 and embryonic lethality occurs from E9.5 onwards [23,24]. In the absence of Piezo1 expression or activity, endothelial cells failed to reorient along the direction of shear flow in vivo as well as in vitro [23,24]. This reorientation requires disassembly and re-assembly of FAZs and a reorganization of the actin cytoskeleton. Therefore, in the absence of Piezo1 activity, actin stress fibers also did not reorient to the direction of shear flow and interestingly, they were thicker in morphology [23,24]. Unbiased proteomic analysis yielded proteins whose expression is altered by Piezo1 knockout under shear stress [23]. These proteins included proteins associated with cytoskeletal dynamics: Calpain 2 and Calpain substrates, such as several focal adhesion proteins. Consistent with the findings by McHugh et al., Piezo1 knockout or GsMTx-4-mediated inhibition of the channel significantly reduced Calpain activity in endothelial cells [23], suggesting that Calpain 2 activation by Piezo1 is central to the cytoskeletal changes required for normal vascular development.
4.2. Piezo1 and cell migration
Cell migration is a fundamental aspect of normal development and homeostasis and also underlies the pathophysiology of some disease states. FAZ disassembly and re-assembly are central to cell migration. Hence, the effects of Piezo1 on FAZ dynamics discussed in Section 4.1 suggest that it may play a role in cell migration. Indeed, in the developing vasculature, Piezo1 mediates the migration of endothelial cells towards Vascular Endothelial Growth Factor, a potent stimulator of angiogenesis [23]. Piezo1 activates Endothelial Nitric Oxide Synthase (eNOS) [23], which generates nitric oxide, a key regulator of cell migration during angiogenesis [62].
Hung et al. found that Piezo1 is also involved in confined migration [29]. Cells are induced to migrate in confined spaces through bioengineered fibronectin-coated microchannels or along 8 um- wide 1D fibronectin-printed lines. Reducing Piezo1 function with the Piezo1 inhibitor, GsMTx-4, or with Piezo1 knockdown inhibited the required Ca2+ increase that occurs when switching from unconfined to confined migration. In this study, migration through unconfined space did not require Piezo1 or an increase in Ca2+. Mechanistically, the authors found that Piezo1 increases Ca2+- dependent phosphodiesterase 1 activity, which causes a decrease cAMP-dependent Protein Kinase A activity in confined migration.
Cell migration is a defining feature of cancer metastasis, as cancerous cells invade surrounding tissue and generate tumors at distant foci. Results from studies on cancer cells suggest a complex role for Piezo1 in cell migration. Highly metastatic breast cancer cells exhibit high Piezo1 expression, which has been correlated with worse outcomes in one patient population [63]. Metastatic breast cancer cells, invasive melanoma cells and gastric cancer cells exhibited reduced migration when Piezol was knocked down or pharmacologically inhibited [29,63,64]. In these cell types, higher Piezo1 activity has been proposed to underlie greater migration associated with the metastatic state. However, in a metastatic small cell lung cancer line, low Piezo1 expression has been correlated with increased migration [65].
To reconcile these seemingly contradictory findings on Piezo1 in cancer cell migration, it is important to consider that cancer cells can switch from an integrin-dependent form of migration to an integrin-independent form also known as “amoeboid migration” [66]. Reduced Calpain activity has been shown to trigger this switch in some cell types [67,68]. Interestingly, Piezo1 knockdown in normal bronchial epithelial cells induced a switch to amoeboid migration; resulted in decreased Calpain activity, integrin affinity and cell adhesion; increased cell migration in 2D and in 3D; and induced a ring-like organization of the actin cytoskeleton [65].
Tensin proteins bind both actin and integrin, linking FAZ to the actin cytoskeleton. In amoeboid migration, Tensin 4 (TNS4) displaces other Tensin proteins by binding integrin. Since TNS4 lacks the actin binding homology sites, this isoform switch results in the disruption of actin stress fibers, decreased focal adhesions and increased migration [69]. TNS4 has been recognized as a protooncogene whose expression correlates with metastasis and is a marker of amoeboid migration [69,70]. Piezo1 knockdown in normal bronchial epithelial cells dramatically increased cytoplasmic staining for TNS4 [65], suggesting a mechanistic link between lower Piezo1 expression and metastatic potential.
The data clearly links Piezo1 to metastatic processes of cancer, but it remains to be determined why Piezo1 expression is upregulated in some metastatic cancers and downregulated in others, and how this affects outcomes of the disease. Overall, studies on Piezo1 and cell migration suggest that migration outcomes may rely on different downstream targets based on the cell type and physiological context.
4.3. Piezo1 and tissue mechanics
Mechanical forces that persist over longer time scales in cellular systems often induce tissue-scale remodeling. Recent studies suggest a role for Piezo1 in shaping mechanical responses at the tissue and organ scale.
An important aspect of organismal homeostasis is the optimization of cell number and barrier function in epithelial tissue. When cells are too sparse, their proliferation increases. When epithelial cells are too dense or the integrity of the barrier is threatened by apoptotic cells, cells are extruded from the epithelium by the coordinated action of neighboring cells [71]. Eisenhoffer et al. found that epithelial cell extrusion requires Piezo1, as knockdown of the channel prevented extrusion and induced formation of epithelial cell masses [72]. On the other hand, when mechanical stretch distends an epithelium the density of cells is effectively reduced, and Piezo1 activity induces proliferation of cells [73]. Gudipaty et al. [73] also found that stretch-dependent and Ca2+-dependent ERK1/2 phosphorylation and translocation to the nucleus drives Cyclin B1 mRNA expression. Treatment of stretched epithelia with Gd3+ ions prevented the activation of ERK1/2 and Cyclin B expression. However, Gd3+ is not a specific inhibitor for Piezo1; it also inhibits the activity of other mechanically-activated ion channels as well as that of voltage-gated Ca2+ channels [74]. Hence, demonstration that ERK1/2 and Cyclin B are regulated by Piezo1 will require more specific manipulation of Piezo1 activity and expression. However, strong evidence for Piezo1 in mechanically-induced epithelial cell division stems from the finding of reduced cell division in zebrafish fin epithelia of Piezo1 Crispr/Cas9 knockouts, which undergo mechanical stretch during fin development. The authors propose that Piezo1 is a homeostatic sensor that controls epithelial cell numbers, triggering cell extrusion in crowded conditions and cell division in sparse conditions.
Chronic high blood pressure results in remodeling of the arterial structure, which worsens the outcome of cardiovascular disease [75]. Such remodeling results in increased wall thickness and reduced lumen diameter of small diameter arteries. Retailleau et al. found that Piezo1 expression in arterial smooth muscle cells is solely responsible for mechanically-induced ionic currents in this cell type [43]. High blood pressure elicits Ca2+ influx through Piezo1, which induces arterial remodeling through tissue transglu-taminase 2. Tissue transglutaminase 2 is a Ca2+-dependent enzyme that underlies hypertension-dependent remodeling of small arteries [76], smooth muscle cell proliferation [77], and pulmonary artery hypertension [78]. Mice with smooth-muscle specific knock-out of Piezo1 displayed reduced arterial remodeling in response to high blood pressure [43]. Interestingly, deletion of the actin binding protein Filamin A in smooth muscles enhanced the activity of Piezo1-mediated stretch-activated currents, increased tissue trans-glutaminase 2 activity, and induced arterial remodeling even in the absence of hypertension [43].
Taken together, a recurring theme in the studies discussed in Section 4 is that Piezo1 activity can elicit changes in the cytoskeleton at multiple length- and time-scales. This connection between Piezo1 and the cytoskeleton is worth considering further in studies examining the mechanistic basis of the channel’s physiological functions.
5. Conclusions
The importance of Piezo1 and its paralog, Piezo2, in normal physiology, as well as in diseased conditions, have put these channels at the center-stage of the new field of Mechanobiology. Piezo1 is expressed in several cell types, where it can sense a variety of mechanical stimuli and has a heterogeneous array of molecular targets that trigger different functional outcomes depending on the cell type. How does a channel that is sensitive to such a diversity of stimuli distinguish between them to elicit different cellular outcomes? Furthermore, the channel is activated in submillisecond timescales, but its activity affects biological processes that occur over timescales of seconds, minutes and days. How are these time scales bridged? The answer to these questions likely lies in differential regulation of Piezo1 activity by different types of mechanical forces, cytoskeletal structures, cellular mechanics and biophysical mechanisms. New approaches are required, that combine theory and techniques from ion channel biophysics with cell biological principles. To make such approaches feasible, new tools are needed for visualizing and manipulating mechanical forces at molecular, cellular and organismal scales. Collaborative efforts between ion channel biophysicists, cell biologists and bioengineers to quantitatively track channel activity, cellular organization and mechanical forces will be key to answering these questions.
Acknowledgments
We are grateful to Juhi Gopal for help with illustrations, and to Kyle Ellefsen, Francesco Tombola, Steven Tan, and members of the Pathak lab for insightful comments on the manuscript. This work was supported in part by a grant from the National Institutes of Health (NS085628), and seed grants from the Sue and Bill Stem Cell Research Center and the School of Medicine at UC Irvine. Funding sources played no role in preparation of the article.
References
- 1.Iskratsch T, Wolfenson H, Sheetz MP. Appreciating force and shape—the rise of mechanotransduction in cell biology. Nat. Rev. Mol. Cell Biol. 2014;15:825–833. doi: 10.1038/nrm3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330:55–60. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yusko EC, Asbury CL. Force is a signal that cells cannot ignore. Mol. Biol. Cell. 2014;25:3717–3725. doi: 10.1091/mbc.E13-12-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kung C, Martinac B, Sukharev S. Mechanosensitive channels in microbes. Annu. Rev. Microbiol. 2010;64:313–329. doi: 10.1146/annurev.micro.112408.134106. [DOI] [PubMed] [Google Scholar]
- 5.Haswell ES, Phillips R, Rees DC. Mechanosensitive channels: what can they do and how do they do it? Structure. 2011;19:1356–1369. doi: 10.1016/j.str.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anishkin A, Loukin SH, Teng J, Kung C. Feeling the hidden mechanical forces in lipid bilayer is an original sense. Proc. Natl. Acad. Sci. U. S. A. 2014;111:7898–7905. doi: 10.1073/pnas.1313364111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hille B. Ion Channels of Excitable Membranes. 2001 http://www.esalq.usp.br/lepse/imgs/conteudothumb/Ion-Channels-of-Excitable-Membranes.pdf.
- 8.Zheng J, Trudeau M. The Handbook of Ion Channels. CRC Press; 2015. [Google Scholar]
- 9.Corey DP, Hudspeth AJ. Ionic basis of the receptor potential in a vertebrate hair cell. Nature. 1979;281:675–677. doi: 10.1038/281675a0. [DOI] [PubMed] [Google Scholar]
- 10.Guharay F, Sachs F. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. J. Physiol. 1984;352:685–701. doi: 10.1113/jphysiol.1984.sp015317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinac B, Buechner M, Delcour AH, Adler J, Kung C. Pressure-sensitive ion channel in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 1987;84:2297–2301. doi: 10.1073/pnas.84.8.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sukharev SI, Blount P, Martinac B, Blattner FR, Kung C. A large-conductance mechanosensitive channel in E. coli encoded by mscL alone. Nature. 1994;368:265–268. doi: 10.1038/368265a0. [DOI] [PubMed] [Google Scholar]
- 13.Levina N, Tötemeyer S, Stokes NR, Louis P, Jones MA, Booth IR. Protection of Escherichia coli cells against extreme turgor by activation of MscS and MscL mechanosensitive channels: identification of genes required for MscS activity. EMBO J. 1999;18:1730–1737. doi: 10.1093/emboj/18.7.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu C, Montell C. Forcing open TRP channels: mechanical gating as a unifying activation mechanism. Biochem. Biophys. Res. Commun. 2015;460:22–25. doi: 10.1016/j.bbrc.2015.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brohawn SG. How ion channels sense mechanical force: insights from mechanosensitive K2P channels TRAAK, TREK1 and TREK2. Ann. N. Y. Acad. Sci. 2015;1352:20–32. doi: 10.1111/nyas.12874. [DOI] [PubMed] [Google Scholar]
- 16.Pan B, Géléoc GS, Asai Y, Horwitz GC, Kurima K, Ishikawa K, Kawashima Y, Griffith AJ, Holt JR. TMC1 and TMC2 are components of the mechanotransduction channel in hair cells of the mammalian inner ear. Neuron. 2013;79:504–515. doi: 10.1016/j.neuron.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu J, Lewis AH, Grandl J. Touch, tension, and transduction—the function and regulation of Piezo ion channels. Trends Biochem. Sci. 2016;42(1):57–71. doi: 10.1016/j.tibs.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Honoré E, Martins JR, Penton D, Patel A, Demolombe S. The Piezo mechanosensitive ion channels: may the force be with you! Rev. Physiol. Biochem. Pharmacol. 2015;169:25–41. doi: 10.1007/112_2015_26. [DOI] [PubMed] [Google Scholar]
- 19.Ranade SS, Syeda R, Patapoutian A. Mechanically activated ion channels. Neuron. 2015;87:1162–1179. doi: 10.1016/j.neuron.2015.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walsh CM, Bautista DM, Lumpkin EA. Mammalian touch catches up. Curr. Opin. Neurobiol. 2015;34:133–139. doi: 10.1016/j.conb.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Volkers L, Mechioukhi Y, Coste B. Piezo channels: from structure to function. Pflug. Arch. 2015;467:95–99. doi: 10.1007/s00424-014-1578-z. [DOI] [PubMed] [Google Scholar]
- 22.Gottlieb P. Piezo Channels. Elsevier; 2017. [Google Scholar]
- 23.Li J, Hou B, Tumova S, Muraki K, Bruns A, Ludlow MJ, Sedo A, Hyman AJ, McKeown L, Young RS, Yuldasheva NY, Majeed Y, Wilson LA, Rode B, Bailey MA, Kim HR, Fu Z, Carter DAL, Bilton J, Imrie H, Ajuh P, Dear TN, Cubbon RM, Kearney MT, Prasad KR, Evans PC, Ainscough JFX, Beech DJ. Piezo1 integration of vascular architecture with physiological force. Nature. 2014;515:279–282. doi: 10.1038/nature13701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ranade SS, Qiu Z, Woo S-H, Hur SS, Murthy SE, Cahalan SM, Xu J, Mathur J, Bandell M, Coste B, Li Y-SJ, Chien S, Patapoutian A. Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc. Natl. Acad. Sci. U. S. A. 2014;111:10347–10352. doi: 10.1073/pnas.1409233111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pathak MM, Nourse JL, Tran T, Hwe J, Arulmoli J, Le DTT, Bernardis E, Flanagan LA, Tombola F. Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proc. Natl. Acad. Sci. U. S. A. 2014;111:16148–16153. doi: 10.1073/pnas.1409802111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blumenthal NR, Hermanson O, Heimrich B, Shastri VP. Stochastic nanoroughness modulates neuron-astrocyte interactions and function via mechanosensing cation channels. Proc. Natl. Acad. Sci. U. S. A. 2014;111:16124–16129. doi: 10.1073/pnas.1412740111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin Y, Li J, Wang Y, Ye R, Feng X, Jing Z, Zhao Z. Functional role of mechanosensitive ion channel Piezo1 in human periodontal ligament cells. Angle Orthod. 2015;85:87–94. doi: 10.2319/123113-955.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michishita M, Yano K, Tomita K-I, Matsuzaki O, Kasahara K-I. Piezo1 expression increases in rat bladder after partial bladder outlet obstruction. Life Sci. 2016;166:1–7. doi: 10.1016/j.lfs.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 29.Hung W-C, Yang JR, Yankaskas CL, Wong BS, Wu P-H, Pardo-Pastor C, Serra SA, Chiang M-J, Gu Z, Wirtz D, Valverde MA, Yang JT, Zhang J, Konstantopoulos K. Confinement sensing and signal optimization via Piezo1/PKA and myosin II pathways. Cell Rep. 2016;15:1430–1441. doi: 10.1016/j.celrep.2016.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Syeda R, Florendo MN, Cox CD, Kefauver JM, Santos JS, Martinac B, Patapoutian A. Piezo1 channels are inherently mechanosensitive. Cell Rep. 2016;17:1739–1746. doi: 10.1016/j.celrep.2016.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sukharev S, Corey DP. Mechanosensitive channels: multiplicity of families and gating paradigms. Sci. STKE. 2004;2004:re4. doi: 10.1126/stke.2192004re4. [DOI] [PubMed] [Google Scholar]
- 32.Katta S, Krieg M, Goodman MB. Feeling force: physical and physiological principles enabling sensory mechanotransduction. Annu. Rev. Cell Dev. Biol. 2015;31:347–371. doi: 10.1146/annurev-cellbio-100913-013426. [DOI] [PubMed] [Google Scholar]
- 33.Teng J, Loukin S, Anishkin A, Kung C. The force-from-lipid (FFL) principle of mechanosensitivity, at large and in elements. Pflug. Arch. 2015;467:27–37. doi: 10.1007/s00424-014-1530-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brohawn SG, Campbell EB, MacKinnon R. Physical mechanism for gating and mechanosensitivity of the human TRAAK K+ channel. Nature. 2014;516:126–130. doi: 10.1038/nature14013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Assad JA, Shepherd GM, Corey DP. Tip-link integrity and mechanical transduction in vertebrate hair cells. Neuron. 1991;7:985–994. doi: 10.1016/0896-6273(91)90343-x. [DOI] [PubMed] [Google Scholar]
- 36.Hu J, Chiang L-Y, Koch M, Lewin GR. Evidence for a protein tether involved in somatic touch. EMBO J. 2010;29:855–867. doi: 10.1038/emboj.2009.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cueva JG, Mulholland A, Goodman MB. Nanoscale organization of the MEC-4 DEG/ENaC sensory mechanotransduction channel in Caenorhabditis elegans touch receptor neurons. J. Neurosci. 2007;27:14089–14098. doi: 10.1523/JNEUROSCI.4179-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jin P, Bulkley D, Guo Y, Zhang W, Guo Z, Huynh W, Wu S, Meltzer S, Cheng T, Jan LY, Jan YN, Cheng Y. Electron cryo-microscopy structure of the mechanotransduction channel NOMPC. Nature. 2017;547(7661):118–122. doi: 10.1038/nature22981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lewis AH, Grandl J. Mechanical sensitivity of Piezo1 ion channels can be tuned by cellular membrane tension. Elife. 2015;4 doi: 10.7554/elife.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suchyna TM, Markin VS, Sachs F. Biophysics and structure of the patch and the gigaseal. Biophys. J. 2009;97:738–747. doi: 10.1016/j.bpj.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gottlieb PA, Bae C, Sachs F. Gating the mechanical channel Piezo1: a comparison between whole-cell and patch recording. Channels. 2012;6:282–289. doi: 10.4161/chan.21064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cox CD, Bae C, Ziegler L, Hartley S, Nikolova-Krstevski V, Rohde PR, Ng C-A, Sachs F, Gottlieb PA, Martinac B. Removal of the mechanoprotective influence of the cytoskeleton reveals PIEZO1 is gated by bilayer tension. Nat. Commun. 2016;7:10366. doi: 10.1038/ncomms10366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Retailleau K, Duprat F, Arhatte M, Ranade SS, Peyronnet R, Martins JR, Jodar M, Moro C, Offermanns S, Feng Y, Demolombe S, Patel A, Honoré E. Piezo1 in smooth muscle cells is involved in hypertension-dependent arterial remodeling. Cell Rep. 2015;13:1161–1171. doi: 10.1016/j.celrep.2015.09.072. [DOI] [PubMed] [Google Scholar]
- 44.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 45.Saha K, Keung AJ, Irwin EF, Li Y, Little L, Schaffer DV, Healy KE. Substrate modulus directs neural stem cell behavior. Biophys. J. 2008;95:4426–4438. doi: 10.1529/biophysj.108.132217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Discher DE, Janmey P, Wang Y-L. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 47.Kobayashi T, Sokabe M. Sensing substrate rigidity by mechanosensitive ion channels with stress fibers and focal adhesions. Curr. Opin. Cell Biol. 2010;22:669–676. doi: 10.1016/j.ceb.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 48.Holle AW, Engler AJ. More than a feeling: discovering, understanding, and influencing mechanosensing pathways. Curr. Opin. Biotechnol. 2011;22:648–654. doi: 10.1016/j.copbio.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stamenovic´ D. Cytoskeletal prestress as a determinant of deformability and rheology of adherent cells. In: Obradovic´ B, editor. Cell and Tissue Engineering. Springer; Berlin, Heidelberg: 2012. pp. 92–118. [Google Scholar]
- 50.Poole K, Herget R, Lapatsina L, Ngo H-D, Lewin GR. Tuning piezo ion channels to detect molecular-scale movements relevant for fine touch. Nat. Commun. 2014;5:3520. doi: 10.1038/ncomms4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qi Y, Andolfi L, Frattini F, Mayer F, Lazzarino M, Hu J. Membrane stiffening by STOML3 facilitates mechanosensation in sensory neurons. Nat. Commun. 2015;6:8512. doi: 10.1038/ncomms9512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gaub BM, Müller DJ. Mechanical stimulation of Piezo1 receptors depends on extracellular matrix proteins and directionality of force. Nano Lett. 2017;17:2064–2072. doi: 10.1021/acs.nanolett.7b00177. [DOI] [PubMed] [Google Scholar]
- 53.McHugh BJ, Buttery R, Lad Y, Banks S, Haslett C, Sethi T. Integrin activation by Fam38A uses a novel mechanism of R-Ras targeting to the endoplasmic reticulum. J. Cell Sci. 2010;123:51–61. doi: 10.1242/jcs.056424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat. Rev. Mol. Cell Biol. 2009;10:21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- 55.Campbell RL, Davies PL. Structure-function relationships in calpains. Biochem. J. 2012;447:335–351. doi: 10.1042/BJ20120921. [DOI] [PubMed] [Google Scholar]
- 56.Chan KT, Bennin DA, Huttenlocher A. Regulation of adhesion dynamics by calpain-mediated proteolysis of focal adhesion kinase (FAK) J. Biol. Chem. 2010;285:11418–11426. doi: 10.1074/jbc.M109.090746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Franco SJ, Rodgers MA, Perrin BJ, Han J, Bennin DA, Critchley DR, Huttenlocher A. Calpain-mediated proteolysis of talin regulates adhesion dynamics. Nat. Cell Biol. 2004;6:977–983. doi: 10.1038/ncb1175. [DOI] [PubMed] [Google Scholar]
- 58.Lebart M-C, Benyamin Y. Calpain involvement in the remodeling of cytoskeletal anchorage complexes. FEBS J. 2006;273:3415–3426. doi: 10.1111/j.1742-4658.2006.05350.x. [DOI] [PubMed] [Google Scholar]
- 59.Cortesio CL, Boateng LR, Piazza TM, Bennin DA, Huttenlocher A. Calpain-mediated proteolysis of paxillin negatively regulates focal adhesion dynamics and cell migration. J. Biol. Chem. 2011;286:9998–10006. doi: 10.1074/jbc.M110.187294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Serrano K, Devine DV. Vinculin is proteolyzed by calpain during platelet aggregation: 95 kDa cleavage fragment associates with the platelet cytoskeleton. Cell Motil. Cytoskelet. 2004;58:242–252. doi: 10.1002/cm.20011. [DOI] [PubMed] [Google Scholar]
- 61.Yan B, Calderwood DA, Yaspan B, Ginsberg MH. Calpain cleavage promotes talin binding to the !33 integrin cytoplasmic domain. J. Biol. Chem. 2001;276:28164–28170. doi: 10.1074/jbc.M104161200. [DOI] [PubMed] [Google Scholar]
- 62.Ziche M, Morbidelli L, Masini E, Amerini S, Granger HJ, Maggi CA, Geppetti P, Ledda F. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J. Clin. Invest. 1994;94:2036–2044. doi: 10.1172/JCI117557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li C, Rezania S, Kammerer S, Sokolowski A, Devaney T, Gorischek A, Jahn S, Hackl H, Groschner K, Windpassinger C, Malle E, Bauernhofer T, Schreibmayer W. Piezo1 forms mechanosensitive ion channels in the human MCF-7 breast cancer cell line. Sci. Rep. 2015;5:8364. doi: 10.1038/srep08364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang X-N, Lu Y-P, Liu J-J, Huang J-K, Liu Y-P, Xiao C-X, Jazag A, Ren J-L, Guleng B. Piezo1 is as a novel trefoil factor family 1 binding protein that promotes gastric cancer cell mobility in vitro. Dig. Dis. Sci. 2014;59:1428–1435. doi: 10.1007/s10620-014-3044-3. [DOI] [PubMed] [Google Scholar]
- 65.McHugh BJ, Murdoch A, Haslett C, Sethi T. Loss of the integrin-activating transmembrane protein Fam38A (Piezo1) promotes a switch to a reduced integrin-dependent mode of cell migration. PLoS One. 2012;7:e40346. doi: 10.1371/journal.pone.0040346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Friedl P. Prespecification and plasticity: shifting mechanisms of cell migration. Curr. Opin. Cell Biol. 2004;16:14–23. doi: 10.1016/j.ceb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 67.Carragher NO, Walker SM, Scott Carragher LA, Harris F, Sawyer TK, Brunton VG, Ozanne BW, Frame MC. Calpain 2 and Src dependence distinguishes mesenchymal and amoeboid modes of tumour cell invasion: a link to integrin function. Oncogene. 2006;25:5726–5740. doi: 10.1038/sj.onc.1209582. [DOI] [PubMed] [Google Scholar]
- 68.Storr SJ, Carragher NO, Frame MC, Parr T, Martin SG. The calpain system and cancer. Nat. Rev. Cancer. 2011;11:364–374. doi: 10.1038/nrc3050. [DOI] [PubMed] [Google Scholar]
- 69.Mouneimne G, Brugge JS. Tensins: a new switch in cell migration. Dev. Cell. 2007;13:317–319. doi: 10.1016/j.devcel.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 70.Katz M, Amit I, Citri A, Shay T, Carvalho S, Lavi S, Milanezi F, Lyass L, Amariglio N, Jacob-Hirsch J, Ben-Chetrit N, Tarcic G, Lindzen M, Avraham R, Liao Y-C, Trusk P, Lyass A, Rechavi G, Spector NL, Lo SH, Schmitt F, Bacus SS, Yarden Y. A reciprocal tensin-3-cten switch mediates EGF-driven mammary cell migration. Nat. Cell Biol. 2007;9:961–969. doi: 10.1038/ncb1622. [DOI] [PubMed] [Google Scholar]
- 71.Gudipaty SA, Rosenblatt J. Epithelial cell extrusion: pathways and pathologies. Semin. Cell Dev. Biol. 2016;67:132–140. doi: 10.1016/j.semcdb.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eisenhoffer GT, Loftus PD, Yoshigi M, Otsuna H, Chien C-B, Morcos PA, Rosenblatt J. Crowding induces live cell extrusion to maintain homeostatic cell numbers in epithelia. Nature. 2012;484:546–549. doi: 10.1038/nature10999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gudipaty SA, Lindblom J, Loftus PD, Redd MJ, Edes K, Davey CF, Krishnegowda V, Rosenblatt J. Mechanical stretch triggers rapid epithelial cell division through Piezo1. Nature. 2017;543:118–121. doi: 10.1038/nature21407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Caldwell RA, Clemo HF, Baumgarten CM. Using gadolinium to identify stretch-activated channels: technical considerations. Am. J. Physiol. 1998;275:C619–21. doi: 10.1152/ajpcell.1998.275.2.C619. [DOI] [PubMed] [Google Scholar]
- 75.Renna NF, de Las Heras N, Miatello RM. Pathophysiology of vascular remodeling in hypertension. Int. J. Hypertens. 2013;2013:808353. doi: 10.1155/2013/808353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bakker ENTP, Buus CL, Spaan JAE, Perree J, Ganga A, Rolf TM, Sorop O, Bramsen LH, Mulvany MJ, Vanbavel E. Small artery remodeling depends on tissue-type transglutaminase. Circ. Res. 2005;96:119–126. doi: 10.1161/01.RES.0000151333.56089.66. [DOI] [PubMed] [Google Scholar]
- 77.Penumatsa KC, Toksoz D, Warburton RR, Hilmer AJ, Liu T, Khosla C, Comhair SAA, Fanburg BL. Role of hypoxia-induced transglutaminase 2 in pulmonary artery smooth muscle cell proliferation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014;307:L576–85. doi: 10.1152/ajplung.00162.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.DiRaimondo TR, Klöck C, Warburton R, Herrera Z, Penumatsa K, Toksoz D, Hill N, Khosla C, Fanburg B. Elevated transglutaminase 2 activity is associated with hypoxia-induced experimental pulmonary hypertension in mice. ACS Chem. Biol. 2014;9:266–275. doi: 10.1021/cb4006408. [DOI] [PMC free article] [PubMed] [Google Scholar]