Abstract
It is well established that in Escherichia coli, the histone‐like nucleoid structuring (H‐NS) protein also functions as negative regulator of rcsA transcription. However, the exact mode of regulation of rcsA transcription by H‐NS has not been studied extensively. Here, we report the multicopy effect of dominant‐negative hns alleles on the transcription of rcsA based on expression of cps‐lac transcriptional fusion in ∆lon, ∆lon rpoB12, ∆lon rpoB77 and lon + strains. Our results indicate that H‐NS defective in recognizing curved DNA fails to repress rcsA transcription significantly, while nonoligomeric H‐NS molecules still retain the repressor activity to an appreciable extent. Together with bioinformatics analysis, our study envisages a critical role for the putative curved DNA region present upstream of rcsA promoter in the transcriptional regulation of rcsA by H‐NS.
Keywords: curved DNA, H‐NS, rcsA
Abbreviations
- Ces
capsule expression suppression
- Cps
capsular polysaccharide
- H‐NS
histone‐like nucleoid structuring
- LB medium
Luria–Bertani medium
- OD
optical density
- RcsA
regulator of capsule synthesis A
- rpoB
RNA polymerase beta subunit
In Escherichia coli, the genetic material is organized in the form of nucleoid and the DNA‐binding proteins such as histone‐like proteins serve as a dynamic scaffold for nucleoid organization 1, 2, 3, 4. The histone‐like nucleoid structuring (H‐NS, previously denoted as H1) protein of E. coli is one of the major components of the nucleoid. hns gene was identified by Pon et al. 5, and it maps at 27 min of E. coli chromosome. H‐NS protein comprises 137 amino acids with 15.5 kDa molecular weight. Although initial studies suggested that H‐NS is involved only in the organization of chromosome, the identification that H‐NS has higher propensity to bind to DNA, especially the AT‐rich sequences, clearly indicated the regulatory function associated with H‐NS 6, 7, 8. H‐NS was found to affect gene expression in a number of different ways, and it has been reported that expression of over 5% of the E. coli genes is affected in hns mutant 9, 10, 11.
H‐NS binding does not seem to occur with any obvious sequence specificity 12, 13. Different mechanisms for transcriptional regulation by H‐NS have been proposed; the most accepted models are as follows: H‐NS might indirectly regulate initiation by binding to region distal from the promoter which causes change in supercoiling that in turn affects the supercoiling‐sensitive promoters; and H‐NS can also directly inhibit transcription by preferential binding to the promoter region. Many of the preferred H‐NS binding sites contain an A/T‐rich region, suggesting that a sequence‐induced curvature is causing the preferential binding 14, 15, 16, 17, 18. Studies on structural aspects of H‐NS revealed that H‐NS is comprised of a C‐terminal DNA‐binding domain and a coiled‐coil N‐terminal domain that mediates oligomerization, forming higher‐order homomeric or heteromeric complexes. At least two dimerization sites have been identified that allow H‐NS to form higher‐order oligomers 19, 20, 21, 22. The oligomerization and DNA‐binding domains are joined via a flexible linker.
H‐NS itself acts as a repressor for its own promoter, and apart from H‐NS, StpA, Fis and CspA also play a role in the regulation of H‐NS expression 23, 24. There is also a post‐transcriptional negative regulatory mechanism which involves a small RNA called DsrA and an RNA‐binding chaperon protein called Hfq 25, 26. The expression of H‐NS is also increased by an unknown mechanism during growth at elevated hydrostatic pressure. H‐NS and other nucleoid‐associated proteins can recognize horizontally acquired DNA and transcriptionally silence it through xenogeneic silencing under environmental conditions that do not require expression of horizontally acquired genes 27, 28. In addition to its role in nucleoid architecture, H‐NS plays a pleiotropic role in bacterial response to environmental stimuli such as starvation and changes in pH, temperature and osmolarity 29, 30, 31.
Very recently, we have reported the suppression of overexpression of genes implicated in colanic acid capsular polysaccharide (Cps) synthesis in ∆lon mutant of E. coli by two novel rpoB mutations, namely rpoB12 and rpoB77. Genetic and molecular analyses clearly showed that downregulation of rcsA transcription is the primary reason for the elicitation of this capsule expression suppression (Ces) phenotype by these two rif alleles. Furthermore, our study clearly indicated that the presence of functional H‐NS is mandatory for both the rpoB mutations to function as capsule expression suppressors in the ∆lon strain of E. coli 32. Sledjesky and Gottesman 25 have shown that H‐NS functions as a repressor for rcsA transcription, and their study also revealed the involvement of a small RNA, namely DsrA located downstream of rcsA, in the regulation of rcsA by H‐NS. DsrA binds to H‐NS and thereby inhibits the action of H‐NS on rcsA transcription. However, the mode of binding and the exact binding region for H‐NS in the promoter region of rcsA have not been reported so far. They have suggested that the upstream region of rcsA promoter might possess bending/curved DNA region 25. In our earlier study, we have provided evidence for the occurrence of bendable DNA region upstream of rcsA promoter through bioinformatics analyses 32. In this study, perhaps for the first time we have given the genetic evidence that supports the presence of putative curved DNA region upstream of rcsA promoter. Furthermore, we have shown that the H‐NS molecule which is defective in the formation of higher‐order oligomers can still function as a repressor at the rcsA promoter. The bioinformatics analyses show that the region around 400 bp upstream of rcsA promoter might serve as H‐NS binding site.
Materials and methods
Media composition, chemicals, fine chemicals and genetic and molecular techniques used in this study
The media (conventional LB and minimal media) composition used in this entire study is essentially as described in Ref. 33. Materials used for media, buffer, solutions, most of the antibiotics and other fine chemicals were purchased from HiMedia, India. Streptomycin was purchased from Sarabhai Chemicals, India, and the final concentration of each of them is quoted wherever appropriate. All the genetic techniques were according to Ref. 33 (with minor modifications), and molecular techniques employed in this study were as per Ref. 34.
Bacterial strains and phages used in this study
Table 1 gives the list of bacterial strains, phages and plasmids used in this study. All the bacterial strains are the derivatives of E. coli K‐12, and the genetic nomenclature is according to Refs 35 and 36.
Table 1.
List of bacterial strains/phages and plasmids used in this study
| Strain | Relevant genotype | Source/reference/construction |
|---|---|---|
| SG20780 | F– Δ(argF‐lac)169 lon510 cpsB10::lac rpsL150 | S Gottesman, NIH, USA |
| SG20781 | F– Δ(argF‐lac)169 lon + cpsB10::lac rpsL150 | S Gottesman, NIH, USA |
| MG1655 | F– rph‐1 | Laboratory collection |
| HR318 | F– λ– rph‐1 btuB::Tn10 rpoB8 | R. Harinarayanan, CDFD, India. |
| MGBT10 | The same as MG1655, but has btuB::Tn10 | This study, MG1655 X P1/(HR318) |
| MMRT6 | The same as SG20780, but has btuB::Tn10 rpoB12 | This study |
| MMRT23 | The same as SG20780, but has btuB::Tn10 rpoB77 | This study |
| Phage | Relevant genotype | Source/reference |
|---|---|---|
| P1 | Vir | Laboratory collection, originally obtained from N Willets, UK |
| Name of the plasmid carrying hns variant alleles | Base change(s)/amino acid change present/functional defect(s) reported/reference | Source |
|---|---|---|
| pLGhns‐P116S |
CCA to TCA Change of proline to serine at 116th amino acid position Shown to be defective in the recognition of curved DNA region, but retains nonspecific DNA binding [19, Ueguchi et al., 1996] |
J Gowrishankar, CDFD, Hyderabad, India |
| pLGhns‐∆64 |
ATG to TGC (deletion of A results in a frameshift leading to formation of in‐frame Cys codon and a stop codon). Produces truncated H‐NS protein bearing only first 64 amino acids. Shown to be defective in DNA binding and higher‐order oligomerization [19, Ueguchi et al., 1996] |
J Gowrishankar, CDFD, Hyderabad, India |
| pLGhns‐T55P |
ACT to CCT Change of threonine to proline at 55th amino acid position Shown to be defective in higher‐order oligomerization [19, Ueguchi et al., 1996] |
J Gowrishankar, CDFD, Hyderabad, India |
| pLGhns‐L26P |
CTG to CCG Change of leucine to proline at 26th amino acid position Shown to be defective in higher‐order oligomerization [19, Ueguchi et al., 1996] |
J Gowrishankar, CDFD, Hyderabad, India |
| pLGhns + |
Wild‐type DNA‐binding and oligomerization functions are normal (reviewed by Refs 8, 13) |
J Gowrishankar, CDFD, Hyderabad, India |
All the above‐mentioned plasmids are derivatives of pLG339 (pSC101 replicon, KanR).
Plasmid isolation, transformation and construction of strains bearing clones of dominant‐negative alleles of hns
The strains bearing the plasmids harbouring the dominant‐negative alleles of hns, namely pLGhns‐∆64, pLGhns‐P116S, pLGhns‐T55P, pLGhns‐L26P and pLGhns +, were procured from J Gowrishankar, CDFD, Hyderabad, India. The plasmids were isolated from relevant strains using the alkaline lysis method 37, and the presence of insert was confirmed through restriction digestion analyses. The strains, namely SG20780 (∆lon cps‐lac), SG201781 (lon + cps‐lac), MMRT6 (∆lon cps‐lac rpoB12) and MMRT23 (∆lon cps‐lac rpoB77), were transformed with the relevant clones. The CaCl2‐mediated transformation technique was followed. As the vector backbone (pLG339) bears kanamycin as selection marker, the transformants were selected on LB plates containing kanamycin. Representative transformants from each case were selected and purified for further use.
Beta‐galactosidase assay
0.1 mL of overnight cultures of each strain (carrying cps‐lac fusion) was subcultured into 5 mL of M9 minimal medium containing glucose as carbon source and grown at 30 °C. The cultures were allowed to attain mid‐log phase, and then, the optical density of the cultures was recorded at 600 nm wavelength. The beta‐galactosidase expressed from cps‐lac fusion was assayed as described in Ref. 33 with minor modifications.
Bioinformatics analyses
The DNA sequence of the coding region of rcsA including the upstream region of rcsA promoter (till ‐600) was retrieved from Ecocyc.org, and the structure of the DNA sequence was elucidated using the software model.it (http://hydra.icgeb.trieste.it/dna/index.php). Further analyses/manipulations of the structure were carried out using pymol (http://pymol.org/edu/?q=educational).
Results
H‐NS which is defective in recognizing curved DNA fails to repress rcsA transcription
In our earlier study pertaining to the isolation and characterization of novel rpoB mutations capable of suppressing the overproduction of colanic acid Cps in lon mutant of E. coli, we have substantiated the role of functional H‐NS in the elicitation of Ces phenotype by the two rpoB mutations, namely rpoB12 (C1576 to T1576; His526 to Tyr526) and rpoB77 (C1535 to T1535; Ser512 to Tyr512) 32. As a continuation to this aspect, the effect of dominant‐negative alleles of hns in Ces strains (∆lon rpoB12 and ∆lon rpoB77) and in parental strains (∆lon and lon +) was studied (strains bearing the dominant‐negative hns alleles were procured from Gowrishankar, CDFD, India). For information of different dominant‐negative alleles of hns used in this study (Table 2). All the hns alleles, namely hnsP116S, hns∆64, hnsT55P and hnsL26P, have been cloned into a vector with its native promoter. The clone bearing hns + (pLG‐hns +) was also used, as in this case the result could be presumed and it can be used for better comparison. All the clones were transformed into the relevant strains, namely SG20780 (∆lon cps‐lac), SG20781 (lon + cps‐lac), MMRT6 (∆lon cps‐lac rpoB12) and MMRT23 (∆lon cps‐lac rpoB77). In each case, a transformant was purified, cells were grown overnight in minimal glucose medium containing kanamycin till mid‐log phase and β‐galactosidase assay was carried out as described in Materials and methods. As was expected, introduction of the hns + clone reduced the expression of cps‐lac fusion to an appreciable degree in all the strains (Fig. 1). These results once again signify the role of H‐NS as a repressor of rcsA transcription. As shown in Fig. 1, introduction of clones bearing variant alleles of hns, namely pLGhns‐P116S and pLGhns‐∆64, into the relevant strains revealed the following: with reference to the mutant H‐NS (with P116S amino acid substitution) which is defective in binding to curved DNA, the expression level of cps‐lac has gone up to appreciable levels in all the above‐mentioned strains. Comparative analyses of the multicopy effect of hns + and hnsP116S alleles on the expression of cps‐lac fusion in the relevant strains clearly show the inability of the mutant form of H‐NSP116S molecules to exert complete repressor activity like the wild‐type although it is reported to retain nonspecific DNA‐binding activity (for the comparative values, see Table 2). These results strongly support the view that the upstream region of rcsA promoter is likely to contain a bendable/curved region and the inability of mutant form of H‐NS (H‐NSP116S) to bind to such putative bendable/curved region of rcsA promoter could be the cause for higher level of cps‐lac expression in the relevant strains. Introduction of pLG‐hns∆64 increased the cps‐lac expression to some extent that clearly implies that the H‐NS molecules bearing only the N‐terminal region are not completely defective in repression at rcsA promoter.
Table 2.
Summary of the multicopy effect of different dominant‐negative hns alleles on the level of expression of cps‐lac transcriptional fusion in the relevant strains and its implications on rcsA transcription
| Strain/plasmid harbouring hns variant alleles and the levels of expression of β‐galactosidase from cps‐lac fusion (in Miller units) in the indicated strains. Values are average of seven different experiments | Inference on rcsA transcription based on cps‐lac expression | |
|---|---|---|
| SG20780/pLGhns‐P116S | 431 |
Introduction of pLGhns‐P116S significantly increased the cps‐lac expression in all the four strains. It is very clear that in the strains bearing pLGhns‐P116S, the expression level of cps‐lac is increased to an appreciable degree when compared to that of the strains bearing pLG‐hns
+ and relevant strains without any plasmid. These results clearly indicate that the mutant H‐NSP116S molecules could no longer serve as repressors for rcsA transcription. As H‐NSP116S molecules are reported to be defective in recognizing curved DNA region (although it retains nonspecific DNA‐binding activity), this observation leads to the inference that the upstream region of rcsA promoter should bear curved DNA region |
| SG20781/pLGhns‐P116S | 200 | |
| MMRT6/pLGhns‐P116S | 382 | |
| MMRT23/pLGhns‐P116S | 272 | |
| SG20780/pLGhns‐∆64 | 335 |
In the presence of pLGhns‐∆64, there is little increase in β‐galactosidase activity from cps‐lac fusion when compared to the cps‐lac expression in the respective strains bearing no plasmid. Although C‐terminally deleted H‐NS molecules are shown to have deficiency in DNA binding, they are reported to have more binding affinity towards chromosomally encoded wild‐type H‐NS molecules. As pLGhns‐∆64 did not result in significant level of increase in cps‐lac expression, it suggests that binding of H‐NS∆64 with chromosomally encoded wild‐type H‐NS might probably help in retaining the repressor activity to some extent. However, when compared with the strains bearing pLGhns +, there was a considerable elevation in the level of cps‐lac expression in all strains. These results signify the fact that although H‐NS∆64‐H‐NS+ hetero‐oligomers retain repressor activity, it perhaps cannot be equated to the activity of H‐NS+‐H‐NS+ homo‐oligomers |
| SG20781/pLGhns‐∆64 | 77 | |
| MMRT6/pLGhns‐∆64 | 212 | |
| MMRT23/pLGhns‐∆64 | 113 | |
| SG20780/pLGhns‐T55P | 92 | Introduction of pLGhns‐T55P unexpectedly decreased the β‐galactosidase activity from cps‐lac fusion in all the strains to an appreciable degree. Comparison of the levels of expression of cps‐lac in the strains bearing pLGhns‐T55P with those of the relevant strains without plasmid clearly indicates the drastic reduction in the expression level of cps‐lac due to multicopy pLGhns‐T55P. It is possible when the oligomerization‐defective H‐NST55P molecules can still repress rcsA transcription, and it is perhaps due to nonspecific binding of the H‐NST55P molecules along the rcsA promoter region |
| SG20781/pLGhns‐T55P | 24 | |
| MMRT6/pLGhns‐T55P | 77 | |
| MMRT23/pLGhns‐T55P | 58 | |
| SG20780/pLGhns‐L26P | 139 | In a similar fashion to pLGhns‐T55P, introduction of pLGhns‐L26P also decreased the β‐galactosidase activity from cps‐lac fusion in the relevant strains. The inference and explanations could be the same as above (as in the case of pLGhns‐T55P) |
| SG20781/pLGhns‐L26P | 26 | |
| MMRT6/pLGhns‐L26P | 104 | |
| MMRT23/pLGhns‐L26P | 75 | |
| SG20780/pLGhns + | 88 | Overexpression of the wild‐type functional H‐NS molecules represses the rcsA transcription much better as was expected |
| SG20781/pLGhns + | 9 | |
| MMRT6/pLGhns + | 20 | |
| MMRT23/pLGhns + | 14 | |
| SG20780 | 323 | As was expected in the absence of any clone, in the ∆lon strain a higher level of expression of cps‐lac was seen. However, in ∆lon rpoB12 and ∆lon rpoB77 mutants due to elicitation of Ces phenotype, the cps‐lac expression was less as was expected. In the lon + strain, in accordance with expectation, a very low level of expression of cps‐lac was seen due to RcsA degradation |
| SG20781 | 9 | |
| MMRT6 | 149 | |
| MMRT23 | 84 | |
Figure 1.
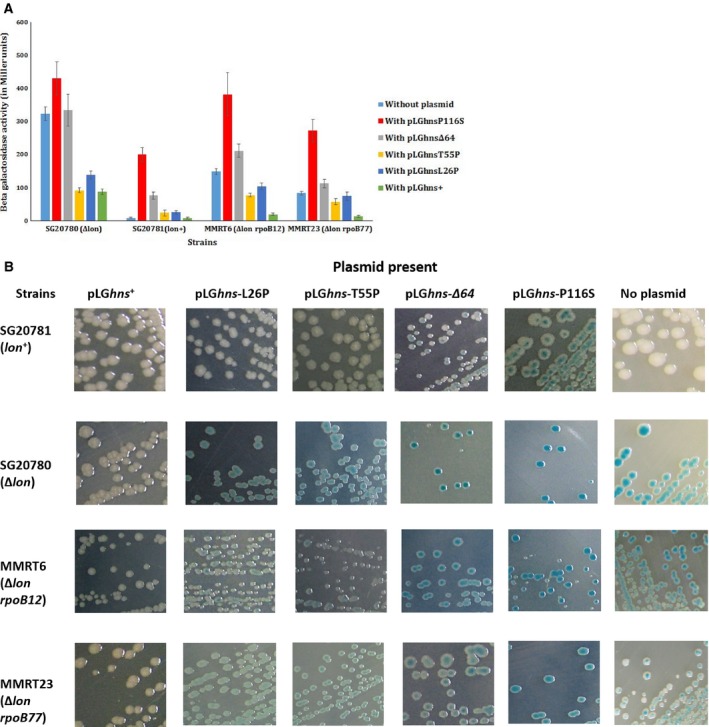
(A) Graphical representation of the expression pattern of cps‐lac fusion in relevant strains bearing clones harbouring dominant‐negative hns variant alleles, namely hnsP116S, hnsL26P, hnsT55P and hns +. The β‐galactosidase experiments were performed seven times to minimize the error. The average ± SEM of values obtained from seven independent experiments is shown. (B) Pictures of sections of LB agar plates containing X‐gal (30 μg·mL−1) showing the Cps‐Lac phenotype of the relevant strains. All the strains were streaked and incubated at 30 °C for ~ 32 h. It is clear from the picture that the colonies of ∆lon cps‐lac strain SG20780 are in blue (Cps‐Lac+) and the colonies of lon + cps‐lac strain SG20781 are in white (Cps‐Lac−). For more details on expression pattern of Cps‐Lac fusion in relevant strains, refer to (A), and for actual values, refer to Table 2.
Oligomerization‐defective H‐NS can still function as a repressor at rcsA promoter
Structural analyses have revealed that the N‐terminal (amino acids 1–46) region of H‐NS is involved in the oligomerization 8. The clones, namely pLGhnsL26P and pLGhnsT55P, when introduced into the strains, namely SG20780 (∆lon cps‐lac), SG20781 (lon + cps‐lac), MMRT6 (∆lon cps‐lac rpoB12) and MMRT23 (∆lon cps‐lac rpoB77), surprisingly decreased the cps‐lac expression remarkably. The hns alleles cloned into these vectors result in the amino acid substitution at N‐terminal region (at amino acid positions T55P and L26P), which is expected to affect the oligomerization property of H‐NS molecules. This indirectly but strongly supports the view that even the oligomerization‐defective H‐NS could interfere with cps‐lac expression perhaps by repressing rcsA transcription (Fig. 1).
Bioinformatics analyses reveal that H‐NS binding region could be present ~ 400 bp upstream of rcsA promoter
H‐NS has been shown to bind to the curved DNA region preferentially 14, 15, 16. In our earlier report, we have given evidence for the involvement of functional H‐NS in the elicitation of Ces phenotype by rpoB12 and rpoB77 mutations, and we have predicted the presence of bendable DNA sequence upstream of the rcsA promoter 32. Here, we show that the DNA sequence around 400 bp upstream of the rcsA promoter is probably bendable in nature. Using the software model.it, we have modelled the DNA region present upstream of rcsA, and the image was further manipulated by pymol software. Figure 2A,B clearly shows that the region ‐130 to ‐400 does exist as a putative curved DNA. Therefore, the sequence underlined in Fig. 2C could be the most probable region where the H‐NS might bind to and repress rcsA transcription.
Figure 2.
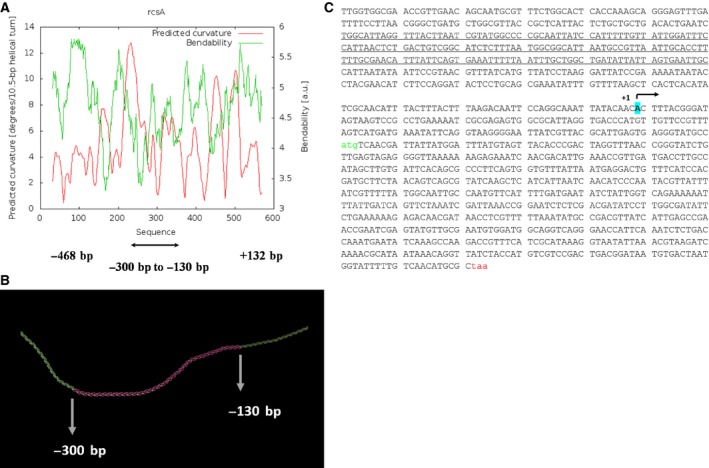
Bioinformatics analyses to show the bending nature of the DNA region present upstream of rcsA promoter. (A) Graphical representation to show the bending/curving nature of the upstream region of rcsA promoter. The software used for this analysis is bend.it, available at http://hydra.icgeb.trieste.it/dna/index.php. The base pair coordinate taken for this analysis is given below the figure. Shown in red is the bending ability, while green indicates the curving ability. (B) Using the software model.it, the curving/bending nature of the DNA region upstream of rcsA promoter is predicted. The region shown in red indicates the bending region corresponding to the base pair coordinate −130 to −300. Further analyses of the structure were carried out using PyMOL. (C) DNA sequence of rcsA gene was retrieved from Ecocyc.org, and the probable region for H‐NS binding has been predicted and is underlined.
Discussion
Overproduction of colanic acid Cps and extreme sensitivity to DNA‐damaging agents are considered as the iconic phenotypes of a lon mutant of E. coli. Detailed study on these aspects revealed that stabilization of two Lon substrates, namely RcsA (the positive regulator of cps transcription) and SulA (cell division inhibitor that gets induced upon DNA damage), is the main reason for the elicitation of the above‐mentioned phenotypes, respectively 38, 39, 40. Previous studies pertaining to isolation of suppressor(s) for these two hallmark phenotypes implicated a vital role of mutation in ssrA and a novel allele of dnaJ (faa) 41, 42, 43, 44. Earlier using an unorthodox, wee bit strategy, we sought for rif (rpoB) mutations capable of suppressing either one or both of the phenotypes of lon mutant. In such an attempt, we were indeed successful in isolating two such novel rif alleles (rpoB12 and rpoB77) that could suppress only the overproduction of capsule synthesis. Detailed analyses showed that the elicitation of this Ces phenotype by these rif mutations primarily stems from the downregulation of rcsA transcription. Our study also revealed the requirement of functional H‐NS in the elicitation of Ces phenotype 32. Although the role of H‐NS in the transcriptional regulation of rcsA has been reported, the exact mode of regulation of rcsA transcription by H‐NS has not been reported to date.
Much of the information about the properties of different domains of H‐NS came from the analyses of effect of different mutations on the functionality of H‐NS. Systematic mutational analyses with H‐NS revealed that C‐terminal region is crucial for DNA binding and the central and N‐terminal regions are involved in the formation of oligomer/higher‐order oligomerization 8. During the course of such analyses, dominant‐negative variants of hns have been isolated 19, 20. In this study, the effect of different clones bearing dominant‐negative alleles of hns such as hnsP116S, hnsT55P, hnsL26P, hns∆64 and hns +, in phenotypically Ces strains such as MMRT6 (∆lon rpoB12) and MMRT23 (∆lon rpoB77) and also in ∆lon and lon + strains has clearly revealed that the hnsP116S allele that codes for H‐NS but is defective in recognizing curved DNA almost completely abolished the elicitation of Ces phenotype in both ∆lon rpoB12 and ∆lon rpoB77 strains. This effect was seen even in ∆lon and lon + strains. This indirectly implies that the region upstream of rcsA promoter might possess putative curvature which might play an important role in the regulation of rcsA transcription by H‐NS.
Further, the clones bearing hns alleles coding for the amino acid substitutions, namely T55P and L26P (which are defective in the formation of higher‐order oligomers), significantly reduced the cps‐lac expression not only in the ∆lon rpoB12 and ∆lon rpoB77 strains but also in the ∆lon and lon + strains; that is, the effect is albeit closer to that of wild‐type H‐NS. This was totally unexpected as we imagined that the mutant forms of H‐NS cannot form higher‐order oligomers and therefore will not be able to repress rcsA transcription. But the fact that we have made such an observation compelled us to make a model that in a nonoligomeric state and even without forming higher‐order oligomers, these mutant forms of H‐NS perhaps might be able to bind to DNA and function as repressors for rcsA transcription. Williams et al. 19 have reported that the introduction of clone(s) bearing oligomerization‐defective hns alleles, namely L26P and T55P, drastically decreased the expression of semisynthetic 5A6Agal promoter. Similar analyses with one other H‐NS‐regulated gene, namely proU, indicate that the expression of its promoter was not found to be identical to that of 5A6Agal promoter. These observations signify the fact that the regulatory function of H‐NS depends on the sequence features of the promoters also. In similar analyses, it was also found that introduction of clone bearing hns allele (P116S) elevated the expression of 5A6Agal promoter to an appreciable degree, while the same was once again not found to be true with proU promoter. It has been reported that the upstream region of 5A6Agal promoter bears a curvature 45. However, in the case of proU, the presence of any curved DNA region has not been reported and notably the repression by H‐NS essentially needs extensive nucleoprotein formation at the proU promoter 45, 46, which perhaps gives a clue about H‐NS binding‐induced DNA bending at the proU promoter region.
The expression pattern of 5A6Agal promoter and rcsA promoter is found to be similar in the presence of different dominant‐negative hns alleles. These observations clearly favour the notion that the upstream region of rcsA might possess curved DNA which would serve as binding region for H‐NS, thus aiding H‐NS to transcriptionally regulate rcsA expression.
Author contributions
SM designed the study, performed experiments, analysed the results and wrote the manuscript. MK performed the experiments. MHM analysed the results, wrote the manuscript and provided resources for the study.
Acknowledgements
We thank Prof. R Jayaraman for his continued encouragement and valuable advice; Dr. S Gottesman, NCI, NIH, USA, for the support and concern in our laboratory progress and also for certain strains used in this study; and Dr. J Gowrishankar, CDFD, Hyderabad, India, for providing the clones bearing dominant‐negative alleles of hns. We also thank the past and present chairpersons of the School of Biological Sciences, especially Prof. P Gunasekaran, Prof. G Marimuthu, Prof. G S Selvam, Prof. Sripati Kandula and Prof. Sudhakar Swamy, for their kind support and also for permitting to use the common facilities of the SBS. We are thankful to Mr. J Kumaresan, Technical Officer, Department of Molecular Biology, for his valuable help; Dr. B Singaravelan, Dr. T Ponmani, Mr. N Arul Muthu Kumaran, Dr. Shanmugapriya Vinod, S Vinodha, M Karthik, S Ashwin Sri Bala, I Madhumathi, T Nagarajan, G Sutharsan (Hebrew University, Israel), Vivek Raj (UK), Dr. T Rajesh and Ms. R Aathmaja (Germany) for their help and support; and S Poovalingam and P Jagadeesh for laboratory errands. Finally, we thank the common programmes, namely UGC‐CAS and DBT‐IPLS given to SBS and DST‐PURSE given to Madurai Kamaraj University (MKU), for the financial assistance. Shanmugaraja Meenakshi thanks the Council of Scientific and Industrial Research, India, for providing Junior and Senior Research Fellowships.
References
- 1. Drlica K and Rouviere‐Yaniv J (1987) Histone like proteins of bacteria. Microbiol Rev 51, 301–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pettijohn DE (1996) The nucleoid In Escherichia coli and Salmonella, Vol. 1 (Neidhardt FC, ed.), pp. 158–166. American Society for Microbiology Press, Washington, DC. [Google Scholar]
- 3. Dillon SC and Dorman CJ (2010) Bacterial nucleoid‐associated proteins, nucleoid structure and gene expression. Nat Rev Microbiol 8, 185–195. [DOI] [PubMed] [Google Scholar]
- 4. Wang W, Li GW, Chen C, Xie XS and Zhuang X (2011) Chromosome organization by a nucleoid‐associated protein in live bacteria. Science 9, 1445–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pon CL, Calogaro RA and Gualerzi CO (1988) Identification, cloning, nucleotide sequence and chromosomal map location of hns, the structural gene for Escherichia coli DNA binding protein H‐NS. Mol Gen Genet 212, 199–202. [DOI] [PubMed] [Google Scholar]
- 6. Higgins CF, Hinton JC, Hulton CS, Owen HT, Pavitt GD and Seirafi A (1990) Protein H1: a role for chromatin structure in the regulation of bacterial gene expression and virulence?. Mol Microbiol 4, 2007–2012. [DOI] [PubMed] [Google Scholar]
- 7. Ussery DW, Hinton JC, Jordi BJ, Granum PE, Seirafi A, Stephen RJ, Tupper AE, Berridge G, Sidebotham JM and Higgins CF (1994) The chromatin associated protein H‐NS. Biochimie 76, 968–980. [DOI] [PubMed] [Google Scholar]
- 8. Dorman CJ (2004) H‐NS: a universal regulator for a dynamic genome. Nat Rev Microbiol 2, 391–400. [DOI] [PubMed] [Google Scholar]
- 9. Hommais F, Krin E, Laurent‐Winter C, Soutourina O, Malpertuy A, Le Caer JP, Danchin A and Bertin P (2001) Large scale monitoring of pleiotropic regulation of gene expression by the prokaryotic nucleoid‐associated protein, H‐NS. Mol Microbiol 40, 20–36. [DOI] [PubMed] [Google Scholar]
- 10. Yoshida T, Ueguchi C, Yamada H and Mizuno T (1993) Function of the Escherichia coli nucleoid protein, H‐NS: molecular analysis of a subset of proteins whose expression is enhanced in an hns deletion mutant. Mol Gen Genet 237 :113–122 [DOI] [PubMed] [Google Scholar]
- 11. Rimsky S and Travers A (2011) Pervasive regulation of nucleoid structure and function by nucleoid‐associated proteins. Curr Opin Microbiol 14, 136–141. [DOI] [PubMed] [Google Scholar]
- 12. Hayat MA and Mancarella DA (1995) Nucleoid proteins. Micron 26, 461–480. [DOI] [PubMed] [Google Scholar]
- 13. Winardhi RS, Yan J and Kenney LJ (2015) H‐NS regulates gene expression and compacts the nucleoid: insights from single‐molecule experiments. Biophys J 109, 1321–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yamada H, Yoshida T, Tanaka K, Sasakawa C and Mizuno T (1991) Molecular analysis of the Escherichia coli hns gene encoding a DNA binding protein, which preferentially recognizes curved DNA sequences. Mol Gen Genet 230, 332–336. [DOI] [PubMed] [Google Scholar]
- 15. Owen‐Hughes T, Pavitt GD, Santos DS, Sidebotham JM, Hulton CS, Hinton JC and Higgins CF (1992) The chromatin‐associated protein H‐NS interacts with curved DNA to influence DNA topology and gene expression. Cell 71, 255–265. [DOI] [PubMed] [Google Scholar]
- 16. Rimsky S and Spassky A (1990) Sequence determinants for H1 binding on Escherichia coli lac and gal promoters. Biochemistry 29, 3765–3771. [DOI] [PubMed] [Google Scholar]
- 17. Lang B, Blot N, Bouffartigues E, Buckle M, Geertz M, Gualerzi CO, Mavathur R, Muskhelishvili G, Pon CL, Rimsky S et al (2007) High‐affinity DNA binding sites for H‐NS provide a molecular basis for selective silencing within proteobacterial genomes. Nucleic Acids Res 35, 6330–6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bouffartigues E, Buckle M, Badaut C, Travers A and Rimsky S (2007) H‐NS cooperative binding to high‐affinity sites in a regulatory element results in transcriptional silencing. Nat Struct Mol Biol 14, 441–448. [DOI] [PubMed] [Google Scholar]
- 19. Williams RM, Rimsky S and Buc H (1996) Probing the structure, function and interactions of the Escherichia coli H‐NS and StpA proteins by using dominant negative derivatives. J Bacteriol 178, 4335–4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ueguchi C, Suzuki T, Yoshida T, Tanaka K and Mizuno T (1996) Systematic mutational analysis revealing the functional domain organization of Escherichia coli nucleoid protein H‐NS. J Mol Biol 263, 149–162. [DOI] [PubMed] [Google Scholar]
- 21. Spurio R, Falconi M, Brandi A, Pon CL and Gualerzi CO (1997) The oligomeric structure of nucleoid protein H‐NS is necessary for recognition of intrinsically curved DNA and for DNA bending. EMBO J 16, 1795–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schroder O and Wagner R (2002) The bacterial regulatory protein H‐NS – a versatile modulator of nucleic acid structures. Biol Chem 383, 945–960. [DOI] [PubMed] [Google Scholar]
- 23. Falconi M, Higgins NP, Spurio R, Pon CL and Gualerzi CO (1993) Expression of the gene encoding the major bacterial nucleotide protein H‐NS is subject to transcriptional auto‐repression. Mol Microbiol 10, 273–282. [PubMed] [Google Scholar]
- 24. Brandi A, Pon CL and Gualerzi CO (1994) Interaction of the main cold shock protein CS7.4 (CspA) of Escherichia coli with the promoter region of hns . Biochimie 76, 1090–1098. [DOI] [PubMed] [Google Scholar]
- 25. Sledjesky DD and Gottesman S (1995) A small RNA acts as an anti‐silencer of the H‐NS‐silenced rcsA gene of Escherichia coli . Proc Natl Acad Sci U S A 92:2003–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shi X and Bennett GN (1994) Plasmids bearing hfq and the hns‐like gene stpA complement hns mutants in modulating arginine decarboxylase gene expression in Escherichia coli . J Bacteriol 176, 6769–6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gordon BR, Li Y, Cote A, Weirauch MT, Ding P, Hughes TR, Navarre WW, Xia B and Liu J (2011) Structural basis for recognition of AT‐rich DNA by unrelated xenogeneic silencing proteins. Proc Natl Acad Sci U S A 108, 10690–10695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huttener M, Paytubi S and Juarez A (2015) Success in incorporating horizontally transferred genes: the H‐NS protein. Trends Microbiol 23, 67–69. [DOI] [PubMed] [Google Scholar]
- 29. Atlung T and Ingmer H (1997) H‐NS: a modulator of environmentally regulated gene expression. Mol Microbiol 24, 7–17. [DOI] [PubMed] [Google Scholar]
- 30. McLeod SM and Johnson RC (2001) Control of transcription by nucleoid proteins. Curr Opin Microbiol 4, 152–159. [DOI] [PubMed] [Google Scholar]
- 31. Williams RM and Rimsky S (1997) Molecular aspects of the E. coli nucleoid protein, H‐NS: a central controller of gene regulatory networks. FEMS Microbiol Lett 156, 175–185. [DOI] [PubMed] [Google Scholar]
- 32. Meenakshi S and Munavar MH (2015) Suppression of capsule expression in Δlon Strain of Escherichia coli by two novel rpoB mutations in concert with H‐NS: possible role for DNA bending at rcsA promoter. Microbiol Open 4, 712–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Miller JH (1992) A Short Course in Bacterial Genetics: A Laboratory Manual and Handbook for Escherichia coli and Related Bacteria. Cold Spring Harbor Laboratory Press, Plainview, NY. [Google Scholar]
- 34. Sambrook J and Russel DW (2001) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. [Google Scholar]
- 35. Demerec M, Adelberg EA, Clark AJ and Hartman PE (1966) A proposal for a uniform nomenclature in bacterial genetics. Genetics 54, 61–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Berlyn MK (1998) Linkage map of Escherichia coli K‐12, edition 10: the traditional map. Microbiol Mol Biol Rev 62, 814–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Birnboim HC and Doly J (1979) A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res 7, 1513–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gottesman S and Maurizi MR (1992) Regulation by proteolysis: energy‐dependent proteases and their targets. Microbiol Rev 56, 592–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gottesman S (1996) Proteases and their targets in Escherichia coli . Annu Rev Genet 30, 465–506. [DOI] [PubMed] [Google Scholar]
- 40. Majdalani N and Gottesman S (2005) The Rcs phosphorelay: the complex signal transduction system. Annu Rev Microbiol 59, 379–405. [DOI] [PubMed] [Google Scholar]
- 41. Trempy JE and Gottesman S (1989) Alp, a suppressor of Lon protease mutants in Escherichia coli . J Bacteriol 171, 3348–3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Trempy JE, Kirby JE and Gottesman S (1994) Alp suppression of Lon: dependence on the slpA gene. J Bacteriol 176, 2061–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kirby JE, Trempy JE and Gottesman S (1994) Excision of a P4‐like cryptic prophage leads to Alp protease expression in Escherichia coli . J Bacteriol 176, 2068–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Munavar MH, Zhou Y and Gottesman S (2005) Analysis of the Escherichia coli Alp phenotype: heat shock induction in ssrA mutants. J Bacteriol 187, 4739–4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zuber F, Kotlarz D, Rimsky S and Buc H (1994) Modulated expression of promoter containing upstream curved DNA sequences by the Escherichia coli nucleoid protein H‐NS. Mol Microbiol 12, 231–240. [DOI] [PubMed] [Google Scholar]
- 46. Lucht JM, Dersch P, Kempf B and Bremer E (1994) Interactions of the nucleoid‐associated DNA‐binding protein H‐NS with the regulatory region of the osmotically controlled proU operon of Escherichia coli . J Biol Chem 269, 6578–6586. [PubMed] [Google Scholar]


