Highlights
-
•
The significance of human babesiosis, caused by the bovine pathogen Babesia divergens, a zoonotic, tickborne disease, can have a severe course with immunocompromised patients which usually ends up with death.
-
•
Falciparum malaria was falsely diagnosed due to the fulminant course of a disease, clinical manifestation and to the presence of small ring forms of the parasites inside the erythrocytes. The differential diagnosis is important as the specific cure is different for these two diseases.
-
•
The main morphological differences were pointed out between Babesia and Plasmodium using the original microphotograph.
Keywords: Protozoan parasites, Tick born disease, Human babesiosis, Babesia divergens, Diagnosis
Abstract
We report a fatal case of human babesiosis caused by bovine pathogen Babesia divergens in Russia. Falciparum malaria was falsely diagnosed due to the presence of small ring forms in the blood smear. Laboratory diagnosis can distinguish between babesiosis and malaria according to the examination of stained blood smears.
Introduction
Human babesiosis caused by the bovine pathogen Babesia divergens is a sporadic, zoonotic, tickborne disease. To date, about 50 cases of human babesiosis have been reported in Europe [1,2]. The significance of this disease can be a severe course in immunocompromised patients resembling complicated falciparum malaria, which can be end up with a fatal outcome.
B. divergens, a protozoan blood parasite (Apicomplexa: Babesiidae) is primarily specific to cattle and are widespread throughout the world within the vector - Ixodes ricinus. They have two different types of hosts, vertebrate and invertebrate. The invertebrate host's sexual cycle (gamogony, formation and fusion of gametes) occurs in the tick gut. Sporogony (the asexual reproduction of sporozoites) occurs within the salivary glands of a tick. There is transstadial and transovarial transmission of the parasites in the tick host [3,4]. Merogony (asexual reproduction) occurs in the vertebrate host inside the erythrocytes.
Parasites are transmitted to the vertebrate host when the infected tick takes a blood meal. The incubation period from the time of tick transmission of the organisms to the appearance of symptoms usually takes 1–3 weeks. As the tick feeds on a vertebrate host, sporozoites are inoculated into the host. These sporozoites invade the host's erythrocytes directly. The process of asexual reproduction (merogony) begins. Inside the host erythrocytes, sporozoites become trophozoites and divide asynchronously by binary fission. This process produces more merozoites, which lyse the host cells and continue to infect additional erythrocytes.
The disease manifestation of a vertebrate host is caused by the asexual stage of the organism proliferating in the erythrocytes. Rapid reproduction of the parasites can destroy a great number of the host cells and lead to severe hemolysis, massive hemoglobinuria and renal failure in the host particularly in the asplenic individual [5,6].
Case report
The patient, a 58-year old man, who lived in the Krasnodar Krai area of the North Caucasus region in south Russia and was a huntsman. Posttraumatic splenectomy had been performed 12 years ago. His illness was fulminant and took an acute course with severe fever (as high as 40 °C), hemoglobinuria, hemolytic anemia, jaundice, uremia, renal insufficiency, anuria. This patient was brought to the hospital extremely ill. The laboratory manifestations were: hemoglobinuria, high-level free hemoglobin (3 g/dl) and urea (49 mmol/l) in the serum, TBi (69,1 μmol/l), DBi (18,3 μmol/l), WBC (12.64 × 10³/μl), and Hb 72,3 g/dl. The diagnosis at admission was a renal failure of obscure etiology. Peritoneal dialysis was started and the patient was treated with prednisolone. On the fifth day of illness, the blood film was examined and the numerous intra-erythrocytic parasites were found. Falciparum malaria was falsely diagnosed due to the presence of small ring forms. Anti-malarial therapy with quinine and doxycycline was started, but it was ineffective. The patient remained quite ill.
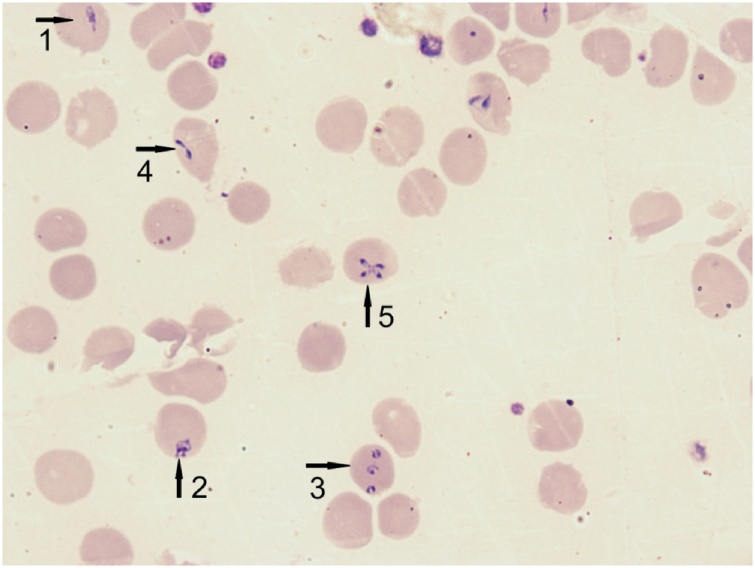
This patient was living in a temperate region where falciparum malaria is absent. He did not travel to an endemic area. The smear was re-examined in the reference laboratory. The numerous intra-erythrocytic inclusions were single or multiple, some rod-like, pear-shaped, often circular and vacuolated. The level of parasitemia was about 23%. Pigment depositions were absent inside cytoplasm of the parasites. The paired forms were diverged at a wide-angle (up to 180°) and were situated on the periphery of the erythrocyte (Fig. 1). Complex morphological characteristics (great variation in the forms seen, pear-shaped trophozoites, paired and tetrad forms, absence of hemozoin) was sufficient to speciated parasites as Babesia divergens.
Fig. 1.
Babesia divergens. Romanovsky-stained thin blood smear. Diversity forms of trophozoites: rod-like (1), pear-shaped (2), multiple invasion of the ring forms (3), paired form – figure «eight» (4), tetrad form «Maltese Cross» (5). (Original), (×1000).
Babesiosis was diagnosed on the seventh day of illness and therapy with clindamycin was started. Pathogenetic and symptomatic therapy was continued. The multisystem failure increased progressively and the patient died on day 10 of illness.
Discussion
Delayed diagnosis and confusion between human babesiosis and malaria have been reported [7,8]. The clinical manifestations and nonspecific laboratory signs are common to both diseases. The previous case of human babesiosis in the USSR has been falsely diagnosed as malaria as well. Babesiosis has been diagnosed post mortum only [9]. The differential diagnosis is essential to determine whether the causative agent of disease is Babesia or Plasmodium. This is important as the specific terapy is different for these two diseases.
Definitive diagnosis of babesiosis infection generally must be established by the microscopic identification of the organism on stained blood smears [2,8,10]. The main morphological differences exist between the asexual stages Babesia and Plasmodium.
-
1
Polymorphism is definitive of the erythrocytic stages of Babesia. There is a great variation in the morphological forms seen: rod-like, pear-shaped, or ring. The small ring forms B.divergens seen inside the erythrocytes can be wrongly identified as Plasmodium falciparum. Babesia parasites divide asynchronous by the binary fission forming «Figure 8s» and rare tetrad «Maltese Cross», whereas malarial parasites are divided synchronous by schizogony. P. falciparum has a tendency to appear in the peripheral blood at two points only in the whole course of its development in the human host: the early trophozoite stage – ring and the gametocyte stage.
-
2
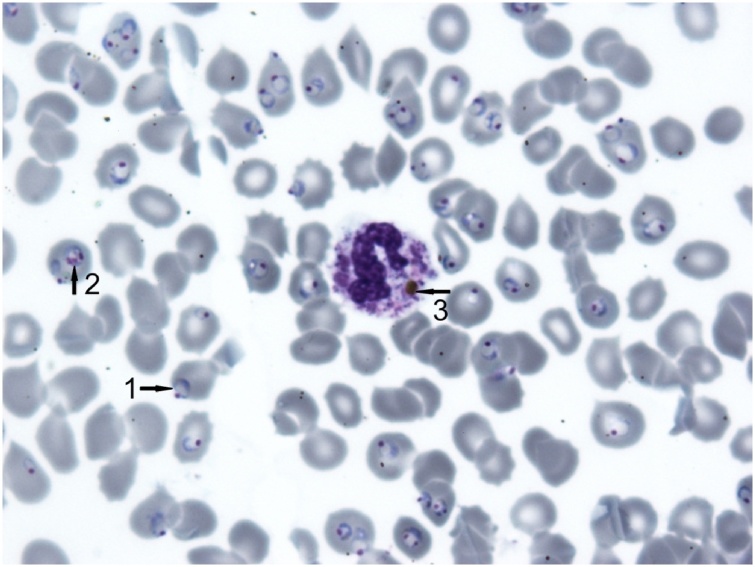
All asexual stages of Babesia do not contain hemozoin within the cytoplasm. There is no pigment in the early ring forms of P. falciparum as well. But phagocytosed masses of hemozoin may be seen inside the cytoplasm of leukocytes (neutrophils and monocytes) in the case of malarial infection (Fig. 2).
Fig. 2.
Plasmodium falciparum, malignant infection. Romanovsky-stained thin blood smear. There is no pigment in the cytoplasm of the numerous ring forms (1). The deposition of pigment is seen in the cytoplasm of the late trophozoite (2). The cytoplasm of neutrophil contains the phagocytosed mass of hemozoin (3). (Original), (×1000).
Conclusion
Animal babesiosis is widespread throughout the world. A transovarial and transstadial transmission can theoretically result in large numbers of infected ticks in the area where babesiosis is endemic. People working in the rural areas are frequently exposed to the bites of infected ticks. The immunocompromised patients (splenectomized, elderly, HIV infected) are in the vulnerable group of morbidity rate of babesiosis. Human babesiosis is probably underdiagnosed disease. The diagnosis of babesiosis is based on appropriate clinical manifestations, epidemiological and medical history, physical examination, and confirmatory laboratory detection parasites in the blood.
Competing interests
The authors declare that they have no competing interests.
Funding source
None.
Ethical approval
None.
References
- 1.Hildebrandt A., Hunfeld K.P. Human babesiosis – a rare but potentially dangerous zoonosis. Dtsch Med Wochenschr. 2014;139(May (18)):957–962. doi: 10.1055/s-0034-1369936. German. PubMed PMID:24760717. [DOI] [PubMed] [Google Scholar]
- 2.Rożej-Bielicka W., Stypułkowska-Misiurewicz H., Gołąb E. Human babesiosis. Przegl Epidemiol. 2015;69(3):489–494. Polish. PubMed PMID:26519845. [PubMed] [Google Scholar]
- 3.Vasilieva I.S., Ganushkina L.A. 2017. Ticks that harm human health. Rostov on Don: Phoenix; Russian. [Google Scholar]
- 4.Rabinovich S.A., Zelya O.P. Babesiosis. In: Sergiev V.P., Yushchuk N.D., Vengerov Y.Y., Zavoikin V.D., editors. Tropical diseases. Guide for doctors. BINOM; Moscow: 2015. pp. 432–435. Russian. [Google Scholar]
- 5.Gorenflot A., Moubri K., Preciqout E., Carcy B., Schetters T.P. Human babesiosis. Ann Trop Med Parasitol. 1998;92(June (4)):489–501. doi: 10.1080/00034989859465. PubMed PMID:9683900. [DOI] [PubMed] [Google Scholar]
- 6.Hunfeld K.P., Hildebrandt A., Gray J.S. Babesiosis: recent insights into an ancient disease. Int J Parasitol. 2008;38(September (11)):1219–1237. doi: 10.1016/j.ijpara.2008.03.001. PubMed PMID:18440005. [DOI] [PubMed] [Google Scholar]
- 7.Garnham P.C. Human babesiosis: European aspects. Trans R Soc Trop Med Hyg. 1980;74(2):153–155. doi: 10.1016/0035-9203(80)90232-1. PubMed PMID:6770500. [DOI] [PubMed] [Google Scholar]
- 8.Homer M.J., Aguilar-Delfin I., Telford S.R., 3rd, Krause P.J., Persing D.H. Babesiosis. Clin Microbiol Rev. 2000;13(July (3)):451–469. doi: 10.1128/cmr.13.3.451-469.2000. PubMed PMID: 10885987; PMCID: PMC88943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rabinovich S.A., Voronina Z.K., Stepanova N.I., Maruashvili G.M., Bakradze T.L. 1st detection of human babesiasis in the USSR and a short analysis of the cases described in literature. Med Parazitol (Mosk) 1978;47(May–June (3)):97–107. Russian. PubMed PMID:149906. [PubMed] [Google Scholar]
- 10.Hildebrandt A., Gray J.S., Hunfeld K.P. Human babesiosis in Europe: what clinicians need to know. Infection. 2013;41(December (6)):1057–1072. doi: 10.1007/s15010-013-0526-8. PubMed PMID:24104943. [DOI] [PubMed] [Google Scholar]