Abstract
Background
An urgent need exists for faster-acting pharmacological treatments in major depressive disorder (MDD). The glutamatergic modulator ketamine has been shown to have rapid antidepressant effects, but much remains unknown about its mechanism of action. Functional MRI (fMRI) can be used to investigate how ketamine impacts brain activity during cognitive and emotional processing.
Methods
This double-blind, placebo-controlled, crossover study of 33 unmedicated participants with MDD and 26 healthy controls (HCs) examined how ketamine affected fMRI activation during an attentional bias dot probe task with emotional face stimuli across multiple time points. A whole brain analysis was conducted to find regions with differential activation associated with group, drug session, or dot probe task-specific factors (emotional valence and congruency of stimuli).
Results
A drug session by group interaction was observed in several brain regions, such that ketamine had opposite effects on brain activation in MDD versus HC participants. Additionally, there was a similar finding related to emotional valence (a drug session by group by emotion interaction) in a large cluster in the anterior cingulate and medial frontal cortex.
Conclusions
The findings show a pattern of brain activity in MDD participants following ketamine infusion that is similar to activity observed in HCs after placebo. This suggests that ketamine may act as an antidepressant by normalizing brain function during emotionally valenced attentional processing.
Clinical trial
NCT#00088699: https://www.clinicaltrials.gov/ct2/show/NCT00088699
Keywords: Depression, Functional magnetic resonance imaging (fMRI), Ketamine, Emotion, Attentional processing, Dot probe
Highlights
-
•
The effects of ketamine versus placebo on brain activation were studied using fMRI.
-
•
MDD and healthy participants were tested on an fMRI emotion-based attentional task.
-
•
Ketamine had opposite effects on brain activity in MDD versus healthy participants.
-
•
In MDD, brain activity post-ketamine was similar to healthy controls post-placebo.
-
•
These findings suggest that ketamine may act by normalizing brain function.
1. Introduction
Currently available FDA-approved pharmacological treatments for major depressive disorder (MDD) take several weeks to achieve their full antidepressant effects, thus significantly impacting patient function and well-being and underscoring the urgent need for faster-acting medications to treat this disorder. The glutamatergic modulator ketamine has rapid antidepressant effects (Berman et al., 2000), even in treatment-resistant MDD (Zarate Jr. et al., 2006). However, much remains unknown about ketamine's precise mechanism of action in the brain, including its effects on neurobiology and specific depressive symptomatology.
In this regard, it would be valuable to examine the effects of ketamine on cognitive and affective processing domains, with particular interest in those previously found to differ in MDD, such as emotion processing. Specifically, numerous behavioral studies have suggested that, compared to healthy controls (HCs), individuals with MDD demonstrate a cognitive bias towards negative emotional information (Dalgleish and Watts, 1990; Mathews and Macleod, 1994). For instance, studies have reported biases for stimuli including depression- and anxiety-related words (Mogg et al., 1995), socially threatening words (Mathews et al., 1996), and sad faces (Gotlib et al., 2004; Joormann and Gotlib, 2007). However, some studies have also noted the absence of a behavioral bias in depression, such as with angry, sad, happy (Mogg et al., 2000), or fearful (Amico et al., 2012) facial expressions.
Dot probe attentional bias tasks have been used with emotion-related word and face stimuli to assess cognitive biases in depressed participants (Peckham et al., 2010). Generally in a dot probe task, two different stimuli are presented for a short time, followed by a dot in the same location as one of the prior stimuli. The participant responds to indicate the location of the dot, and reaction time is measured to analyze attentional bias towards a specific type of stimulus. Thus, the task can span both attentional and emotional processing domains. Notably, the administration of dot probe tasks during functional magnetic resonance imaging (fMRI) can identify brain regions associated with attentional bias towards or away from certain emotional stimuli. In general, neuroimaging findings may also be more stable (based on test-retest reliability) and sensitive than behavioral measures of attentional bias using this type of task (White et al., 2016). For example, fMRI results associated with a dot probe task showed activation differences between MDD participants and HCs associated with fearful stimuli in the absence of behavioral differences that would be consistent with an attentional bias; specifically, MDD participants had less activation in the left middle cingulum and left insula (Amico et al., 2012). Other fMRI research using dot probe tasks showed increased activation in temporo-parietal and occipito-parietal regions in response to fearful versus happy faces in HCs (Pourtois et al., 2006). In addition, decreased anterior cingulate cortex activation was found during incongruent trials across healthy and anxious youth participants (Price et al., 2014).
In addition to probing baseline differences in emotional processing, dot probe tasks can also be used to investigate neuroimaging changes associated with the influence of treatment. For instance, multiple dot probe studies have demonstrated the effects of anxiolytic treatments on attentional biases and related changes in the brain (Britton et al., 2015; Ironside et al., 2016); to date, however, research into treatment approaches for depression using dot probe tasks has been limited.
Due to its rapid onset and short duration of action, ketamine is uniquely suited to studying antidepressant response using cognitive tasks and fMRI. Indeed, recent research has examined ketamine's effects on cognitive and emotional processing using other cognitive tasks, though no studies have yet examined dot probe tasks specifically. Ketamine would also be expected to affect individuals with treatment-resistant MDD, given its demonstrated effects as a glutamatergic modulator and the potential dysfunctions of the glutamate system associated with depression (Niciu et al., 2014; Zarate Jr. et al., 2010). In an emotion perception task, participants with treatment-resistant MDD showed increased activation in response to positive emotion in the right caudate after ketamine treatment (Murrough et al., 2015), but this study included no placebo condition for comparison. In a working memory task with emotional stimuli in HCs, ketamine decreased activity in the left and right insula and right dorsolateral prefrontal cortex (DLPFC) specifically during negative emotion conditions (Scheidegger et al., 2016a). In HCs who received a single ketamine infusion, reduced activation was observed in an amygdalo-hippocampal area during emotional processing (Scheidegger et al., 2016b), as was greater deactivation in the anterior cingulate cortex (ACC) in response to negative stimuli (Lehmann et al., 2016). While these studies investigated ketamine's effects using fMRI, only limited conclusions can be drawn, given that they did not involve simultaneous longitudinal assessments of both MDD and HC participants, and most lacked a placebo control condition.
The current study is the first to use a dot probe task to investigate fMRI activation during emotion-related attentional bias in treatment-resistant participants with MDD and HCs across multiple time points. Participants were studied first at baseline and then at about two days and eleven days following ketamine and placebo infusions. We hypothesized that ketamine, in contrast to placebo, would alter brain activity in regions associated with emotional processing and depression. This activity could potentially vary in MDD participants versus healthy individuals, given that this has not yet been well-studied in ketamine fMRI research.
2. Method and materials
2.1. Participants
Participants in this study included 33 individuals with treatment-resistant MDD (12 M/21 F, mean age = 36.1 ± 9.7 years) and 26 HCs (10 M/16 F, mean age = 33.9 ± 10.4 years), ages 18 to 65. Diagnoses were made using the Structured Clinical Interview for DSM-IV-TR (SCID-P for MDD participants and SCID-NP for HCs) (First et al., 2002). Inclusion criteria for participants with MDD included an age of onset of <40 years, a current depressive episode lasting at least four weeks, an initial score of at least 20 on the Montgomery-Åsberg Depression Rating Scale (MADRS), and a past failure to respond to at least one adequate trial of an antidepressant during a depressive episode; on average, MDD participants had failed to respond to six antidepressant trials. All participants had no serious, unstable illnesses, as assessed via a medical screening by a clinician and by laboratory tests that included blood labs and urine drug screens; negative drug screens were also required throughout the study. Exclusion criteria included a history of drug or alcohol dependency/abuse within the past three months for MDD patients or any such diagnosis for HCs, psychotic symptoms, a medical illness likely to affect brain structure or physiology, any contraindications for MRI, and anatomical brain abnormalities found on a clinical MRI. MDD participants with comorbid Axis I disorders or personality disorders were not excluded. For HCs, exclusion criteria included a prior Axis I diagnosis or any psychiatric disorder in a first-degree relative.
Prior to the study, MDD participants were tapered off medications, followed by a drug-free period lasting at least two weeks before study procedures began. Participants were studied as inpatients at the National Institutes of Health (NIH) Clinical Research Center. All participants gave written informed consent to participate in the study, which was approved by the NIH Combined Neuroscience Institutional Review Board. Data drawn from other studies using the same participants have been previously published (Nugent et al., n.d.; Evans et al., 2018).
2.2. Study design
This study was part of a randomized, double-blind, placebo-controlled, crossover protocol (Nugent et al., n.d.); the study design is shown in Fig. 1. Participants first took part in a baseline fMRI scan and were subsequently randomized to receive either a ketamine (subanesthetic dose, 0.5 mg/kg over 40 min) or placebo (saline solution) infusion. Two weeks later, participants crossed over to receive the other treatment condition. MDD participants were required to have a MADRS score of at least 20 to cross over to the second treatment condition.
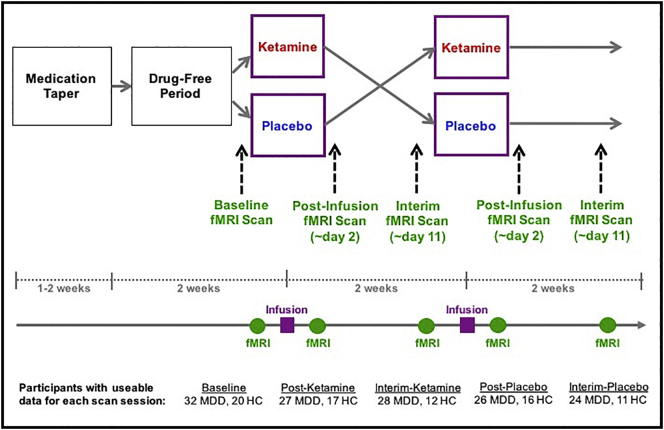
Fig. 1.
Graphic representation of the study design. This was a randomized, placebo-controlled, crossover study. It included five fMRI scans per participant; the timepoints at which these were conducted are noted in the figure. Information about the number of participants with usable data for each scan session is also provided.
In order to examine drug effects in each treatment phase (post-ketamine and post-placebo), fMRI scans took place one to three days after each infusion; 95% of scans took place two days post-infusion. Additional interim fMRI scans were performed nine to 13 days after each infusion (interim-ketamine and interim-placebo); 85% of scans took place 11 days post-infusion. There was no between-group difference in the number of scans that took place on days other than the two-day and 11-day post-infusion time points. Thus, the five scan sessions in the study were baseline, post-ketamine, interim-ketamine, post-placebo, and interim placebo. Given that our main hypothesis centered around differences in fMRI activation between the post-ketamine and post-placebo time points, we focused on contrasts specific to these scan sessions, referred to hereafter as drug sessions. Throughout the study, the severity of depressive symptoms was assessed via the MADRS. MADRS scores were compared between pre-infusion and the day two post-infusion time point using paired t-tests and between groups at each of these time points using paired t-tests.
It should be noted here that not all participants had usable data for all five scan sessions, due to either exclusion for imaging data quality (excessive motion or poor alignment), exclusion for low accuracy on behavioral data, technical problems with acquiring task output data, or because some did not complete every scan. The final set of scans used in the analysis included 52 baseline (32 MDD; 20 HC), 44 post-ketamine (27 MDD; 17 HC), 40 interim-ketamine (28 MDD; 12 HC), 42 post-placebo (26 MDD; 16 HC), and 35 interim-placebo (24 MDD; 11 HC). While we did not have usable data for all five scans for all of the 59 included participants, the analysis models used (see Analysis sections, below) allowed for missing data and enabled us to include each participant's usable scans. Thus, rather than participants with data for fewer than all five sessions being excluded from analyses, only the specific sessions with missing data were left out for such participants.
2.3. Experimental task
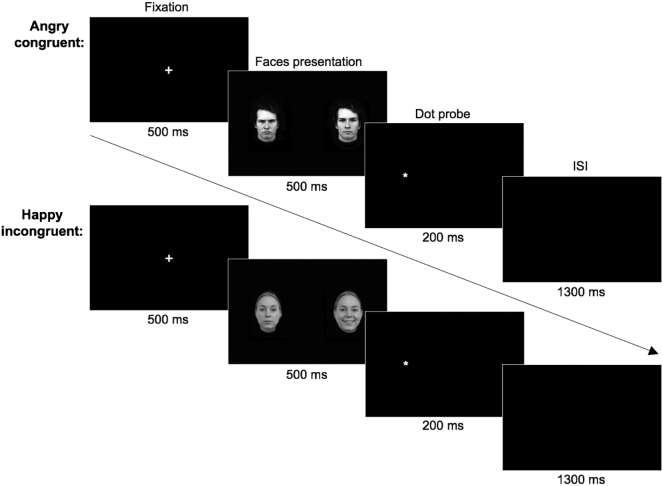
To examine the brain correlates underlying attentional bias, a dot probe task with emotional face stimuli was administered during fMRI scanning using E-Prime presentation software (Psychology Software Tools, Pittsburgh, PA). This task used a mixed block/event-related design. In each trial (Fig. 2), a fixation cross was presented for 500 ms in the center of the screen, where the participant had been instructed to focus. This was followed by two faces presented side by side for 500 ms; one displayed an angry, happy, or neutral expression and the other was always neutral. After the faces were presented, a single dot was presented on one side for 200 ms, to which the participant responded with a button press to indicate whether the dot probe was on the left or right. Trials in which the dot replaced the emotional face were considered congruent, according to the expectation of attention being biased more towards an emotional than a neutral face. Trials in which the dot replaced the neutral face were considered incongruent. Trials were randomized and counterbalanced for emotion, gender of face, side of emotional face, and side of probe. There was then an interstimulus interval in which a blank screen appeared for 1300 ms. Jitter was randomly added in as additional trials in which only a fixation cross appeared in place of the faces and probe. Trials were grouped into blocks: angry blocks comprised trials with angry and neutral faces or two neutral faces, and happy blocks comprised trials with happy and neutral faces or two neutral faces. The task comprised two runs, and each run included one angry block and one happy block. For each scanning session, the task used a unique set of facial stimuli that were counterbalanced between the ketamine and placebo arms of the study according to the randomized crossover study design.
Fig. 2.
Schematic representation of the dot probe task. In this task, a fixation cross was presented for 500 ms, followed by the presentation of faces for 500 ms, then the dot probe on the left or right for 200 ms, and then a 1300 ms interstimulus interval. The trial types in the analysis included angry congruent (example shown on top), angry incongruent, happy congruent, and happy incongruent (example shown on bottom).
2.4. Imaging acquisition and analysis
Participants were scanned using a 3 Tesla General Electric HDx scanner (GE Signa, Milwaukee, WI) to measure blood oxygen-level-dependent (BOLD) signal using echo-planar imaging with a T2* weighted sequence (echo time (TE) = 23 ms; repetition time (TR) = 2500 ms; voxel = 3.75 × 3.75 × 3.5 mm, flip angle = 90 degrees, matrix 64 × 64, 45 sagittal slices, phase encode direction: anterior-posterior, interleaved acquisition) and a high resolution anatomical scan. Imaging data were collected in two runs of 8.75 min each. The first four repetition times (TRs) acquired before each run were discarded for time series stabilization. Data were preprocessed using standard steps in AFNI (Cox, 1996), including despiking, slice timing correction, realignment to the third volume, aligning the anatomical images to the echo-planar images with an affine transform using the AFNI LPC cost-function (or LPA cost-function for a few scans for better alignment), normalizing to standard Talairach space using nonlinear warp, blurring to 6 mm, and motion regressing and censoring. Outlying time points and any time points occurring during periods of high motion were censored; any scan that had >15% of all time points censored was excluded. Individual subject regressors were created to model: 1) emotion blocks (angry and happy blocks), 2) each type of stimulus event (angry congruent, angry incongruent, happy congruent, and happy incongruent trials), and 3) instruction screens presented during the task; fixation was used as the baseline. Both the blocks and the individual stimulus events were modeled because this task had a mixed block/event-related design, and different results could potentially be found for activity associated with the blocks versus the individual trials (Scheibe et al., 2006; Visscher et al., 2003). However, given the small number of each block type presented (two angry and two happy blocks per session) and the fact that brain activity averaged across entire blocks was not specifically of interest in this study, event-related effects pertaining to the individual trial types were only analyzed in the group analysis model.
For the group analysis, a linear mixed-effect model with emotion, congruency, scan session (all five sessions), and diagnostic group as factors was performed using the data from each trial type from the individual participant analyses. For main and interaction effect tests, we initially thresholded images at a voxel-level threshold of p < .001. At the cluster-level threshold, we then used a family wise error (FWE) corrected p < .05, calculated using 3dClustSim with the ACF (auto-correlation function) method (Cox et al., 2017) (resulting in a significant cluster size of 10 voxels or greater). The same thresholds were used for general linear tests within the main linear mixed-effect model in AFNI in order to examine specific contrasts. For significant effects involving session, we focused on the contrast between post-ketamine and post-placebo (drug sessions). Such contrasts used the same level of voxel- and cluster-wise thresholding, with FWE-correction for significant clusters at the whole-brain level.
As a follow-up analysis, we explored the association between brain activity post-ketamine and change in MADRS score in MDD participants. For this analysis, a linear mixed-effect model was used with emotion, congruency, scan session (only post-ketamine and post-placebo—referred to as drug session), and a quantitative variable for percent change in MADRS score from pre-infusion to the day of the post-infusion scan. The same threshold of p < .001 at the voxel level and pFWE < 0.05 for significant clusters was used. For both main and follow-up analyses, activation values represent BOLD percent signal change.
2.5. Behavioral data analysis
Accuracy on the dot probe task was analyzed using IBM SPSS, Version 23.0 (Armonk, NY) to ensure adequate compliance with task procedures. Participants who demonstrated <75% accuracy for a session were excluded from all analyses for that session. Reaction times included in the analyses were all between 200 and 1500 ms. A mixed model was used for the reaction time analysis with the same factors as in the imaging analysis (emotion, congruency, session, and diagnostic group), with a significance level of p < .05. Attentional bias scores were calculated for each emotion as the difference in reaction time between incongruent and congruent trials (calculated as the mean of this difference for trials with the dot probe on the right and the difference for trials with the dot probe on the left, as in previous research (Gotlib et al., 2004)). Attentional bias scores were also analyzed in a mixed model with factors of emotion, session, and group. Only correct trials were included in the reaction time and attention bias score analyses.
3. Results
3.1. Participants
No significant difference in age or gender was observed between the participant groups (Table 1). Mean MADRS scores—which were collected for each participant group 60 min prior to each infusion and on the day of each post-infusion scan—differed between groups at all time points (p < .001; Table 1). In the MDD participants, MADRS scores decreased significantly from the pre- to post-infusion time point in response to ketamine (p < .001) but not in response to placebo. In HCs, MADRS scores did not significantly differ between these time points for ketamine but did for placebo (p = .029); specifically, HCs had slightly lower scores post-placebo infusion, although the mean difference (1.12) was not clinically significant. MADRS scores also differed between the post-ketamine and post-placebo scan days in MDD participants, with lower scores post-ketamine (p = .009).
Table 1.
Demographic characteristics.
| MDD Participants | HC Participants | p-value | |
|---|---|---|---|
| Gender | 12 M; 21 F | 10 M; 16 F | n.s. |
| Age | 36.06 (+/− 9.74) | 33.88 (+/− 10.42) | n.s. |
| MADRS: Before ketamine | 33.96 (+/− 4.64) | 1.00 (+/− 1.32) | <0.001 |
| MADRS: Ketamine scan day | 24.78 (+/− 10.04) | 1.71 (+/− 4.09) | <0.001 |
| MADRS: Before placebo | 32.69 (+/− 5.20) | 1.50 (+/− 1.83) | <0.001 |
| MADRS: Placebo scan day | 30.96 (+/− 5.79) | 0.38 (+/− 1.03) | <0.001 |
MADRS: Montgomery-Asberg Depression Rating Scale; MDD = major depressive disorder; HC = healthy control.
3.2. Neuroimaging results
3.2.1. Main effects: Group, emotion, congruency, and session
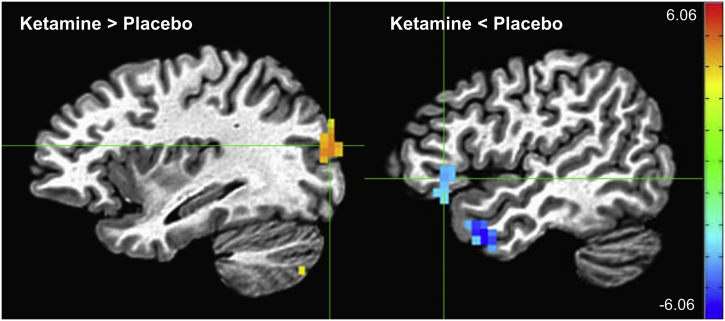
No significant main effect of group was observed across sessions and task conditions. Across groups and sessions, the dot probe task elicited a robust main effect of emotion, with participants showing greater activation to the presentation of angry stimuli than happy stimuli (Fig. 3, Table 2). Significant clusters (pFWE < 0.001) were found encompassing regions of bilateral dorsal anterior cingulate cortex (dACC), precentral and medial frontal gyri, fusiform and middle temporal gyri, subcortical areas including putamen and thalamus, and posterior cingulate. A significant main effect was also noted for session (pFWE < 0.05). Significant regions based on our F-test were selected, and activity was plotted for each session across group and task conditions (Supplementary Fig. S1). Prominent differences were seen between the baseline session and the subsequent scanning sessions, potentially accounted for by the novelty of the task and by the scanning environment. With specific regard to the effects of ketamine versus placebo, we observed significantly greater activation post-ketamine compared to post-placebo in the left middle occipital gyrus across groups (pFWE < 0.001) (Table 3, Supplementary Fig. S2). In contrast, significantly reduced activation post-ketamine compared to post-placebo was observed in the left temporal and inferior frontal cortices (pFWE < 0.002).
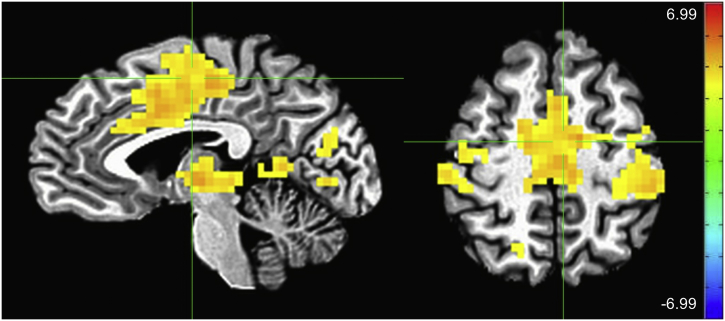
Fig. 3.
Main effect of emotion (angry > happy). Crosshairs are located at coordinates [−4–10 49], the center of mass of the largest cluster, which extends into the bilateral cingulate, medial frontal, and precentral gyri (scale: Z-score; left = right).
Table 2.
Main effect of emotion.
| Angry > Happy | |||||
|---|---|---|---|---|---|
| Region | X | Y | Z | # Voxels | Cluster p-value |
| Bilateral cingulate/medial frontal/precentral gyri | −4 | −10 | 49 | 1205 | <0.001 |
| Bilateral lingual/fusiform/middle temporal gyri | 15 | −63 | −2 | 771 | <0.001 |
| Right lentiform nucleus/insula | 30 | 1 | 4 | 397 | <0.001 |
| Left lentiform nucleus/thalamus | −17 | −5 | 4 | 270 | <0.001 |
| Left middle occipital/middle temporal gyri | −36 | −73 | 13 | 202 | <0.001 |
| Right inferior parietal lobule | 49 | −26 | 28 | 179 | <0.001 |
| Left superior temporal gyrus | −40 | −30 | 13 | 58 | <0.001 |
| Left superior parietal lobule | −26 | −54 | 56 | 56 | <0.001 |
| Right precentral gyrus | 55 | −1 | 33 | 46 | <0.001 |
| Bilateral posterior cingulate | 1 | −47 | 10 | 35 | <0.001 |
| Left inferior frontal gyrus | −44 | 0 | 25 | 18 | <0.002 |
| Right inferior frontal gyrus | 37 | 24 | −16 | 17 | <0.003 |
| Right cingulate gyrus | 18 | 24 | 26 | 14 | <0.008 |
| Right superior parietal lobule | 17 | −61 | 53 | 14 | <0.008 |
| Right insula | 36 | 18 | −2 | 13 | <0.02 |
| Right cuneus | 29 | −84 | 33 | 13 | <0.02 |
| Right middle temporal gyrus | 51 | −10 | −13 | 12 | <0.02 |
| Right cerebellum | 9 | −43 | −37 | 10 | <0.05 |
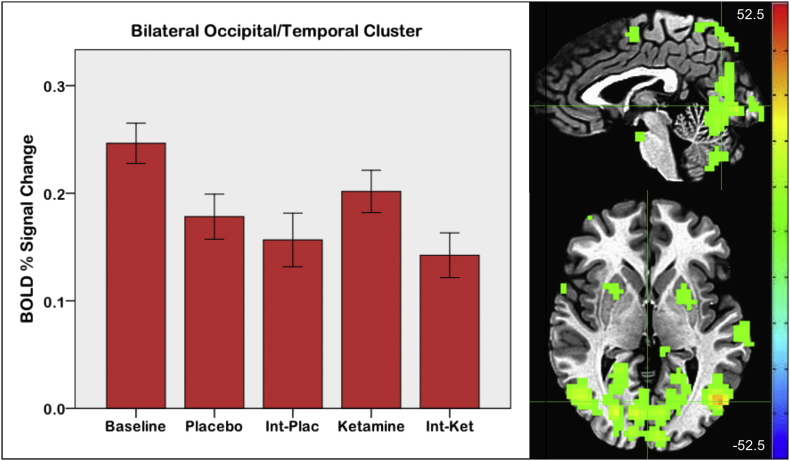
Supplementary Fig. S1.
Main effect of session. Crosshairs are located at coordinates [2 -67 2], the center of mass of the large occipital/temporal cluster. Error bars on the graph indicate +/- 1 standard error (Int = interim scan; scale: F value; left = right).
Table 3.
Effect of drug session.
| Ketamine > Placebo | |||||
|---|---|---|---|---|---|
| Region | X | Y | Z | # Voxels | Cluster p-value |
| Left middle occipital gyrus | −33 | −84 | 17 | 45 | <0.001 |
| Left cerebellum | −14 | −72 | −31 | 41 | <0.001 |
| Right cerebellum | 34 | −62 | −41 | 15 | <0.006 |
| Left cerebellum | −28 | −72 | −41 | 10 | <0.05 |
| Ketamine < Placebo: | |||||
|---|---|---|---|---|---|
| Region | X | Y | Z | # Voxels | Cluster p-value |
| Left middle temporal gyrus | −46 | 8 | −26 | 29 | <0.001 |
| Left inferior frontal gyrus | −46 | 24 | −1 | 19 | <0.002 |
| Left superior temporal gyrus | −59 | −21 | 1 | 11 | <0.03 |
| Left supramarginal gyrus | −56 | −51 | 30 | 10 | <0.05 |
Supplementary Fig. S2.
Effect of drug session (ketamine > placebo on left in yellow; ketamine < placebo on right in blue). Crosshairs on the left are located at coordinates [-33 -84 17], the center of mass of the left middle occipital gyrus cluster. Crosshairs on the right are located at coordinates [-46 24 -1], the center of mass of the left inferior frontal gyrus cluster (scale: Z-score).
3.2.2. Two-way interaction effects
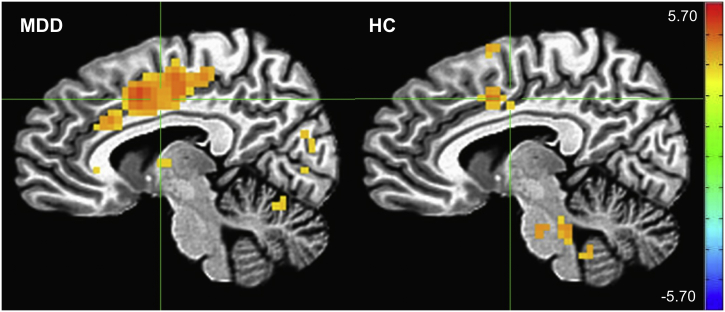
A significant diagnosis by emotion interaction was observed. A post-hoc t-test of this interaction was significant in small clusters in the periphery of the brain and brainstem (pFWE < 0.05). When post-hoc tests comparing angry versus happy faces in the HC and MDD groups were conducted separately, the effects of emotion were more prominent overall in the MDD group but consistent across both groups (Fig. 4).
Fig. 4.
Effect of emotion (angry > happy) in the major depressive disorder (MDD) and healthy control (HC) groups, displayed separately for each group. Crosshairs for both images are located at coordinates [7–4 38], the center of mass of the cingulate gyrus cluster in the MDD group (scale: Z-score).
A significant session by emotion interaction was also observed across both groups, most notably in the bilateral orbital cortex and ACC (pFWE < 0.05) (Supplementary Fig. S3). A contrast comparing differences in activation across the happy versus angry conditions between the ketamine and placebo sessions revealed only small focal clusters in white matter and in the periphery of the brain that were significant (pFWE < 0.05) (Supplementary Table S1).
Supplementary Fig. S3.
Session by emotion interaction effect. Crosshairs are located at coordinates [0 36 1], the center of mass of the bilateral anterior cingulate cluster (scale: F value, left = right).
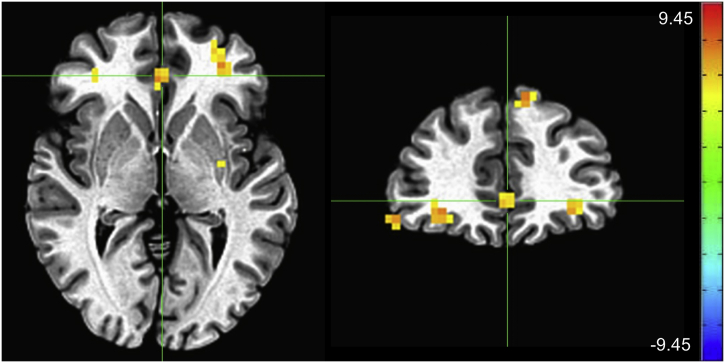
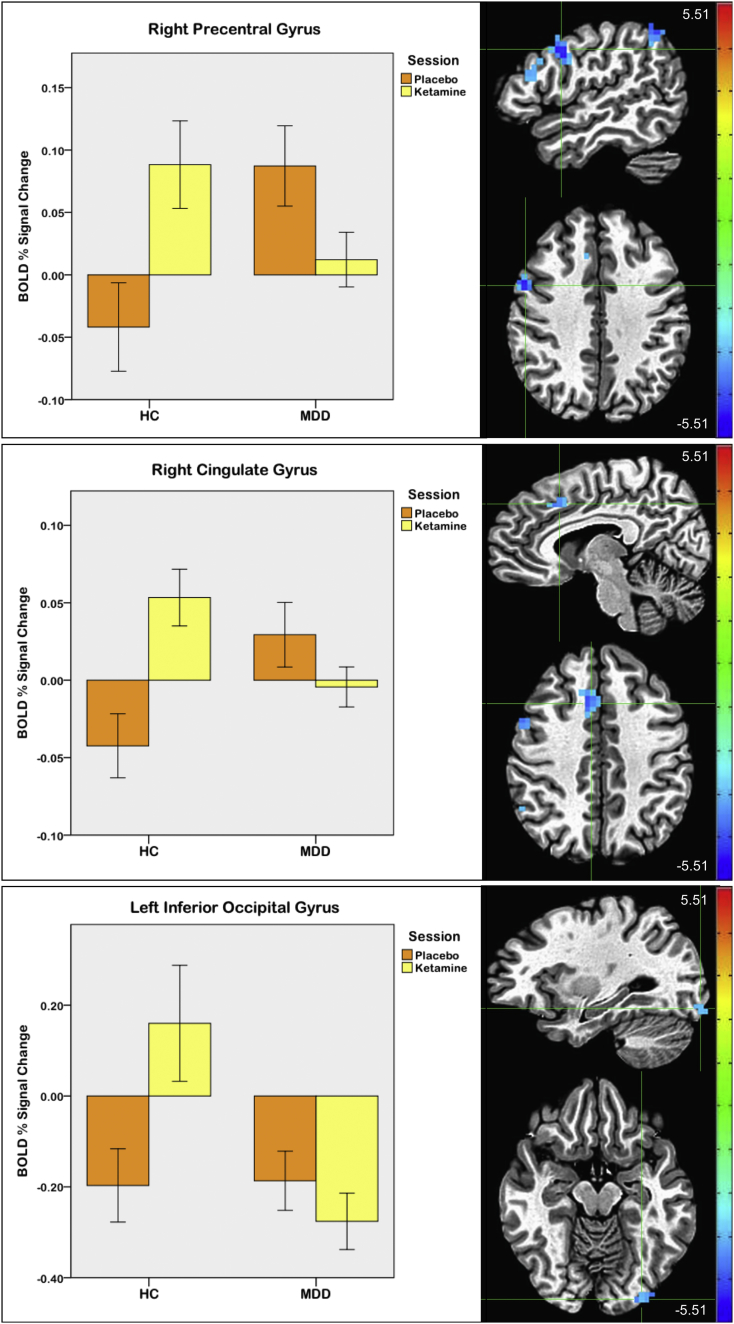
A significant group by session interaction was also observed. In post-hoc tests, between-group differences were primarily driven by reduced activity in MDD participants compared to controls at baseline, with MDD participants exhibiting less activation in clusters including bilateral thalamus and caudate and right middle/inferior temporal gyri (pFWE < 0.001) (Supplementary Fig. S4). These group differences were not observed post-placebo infusion, nor at the interim scans, again likely due to the novelty of the task and/or the scanning environment. When the ketamine versus placebo effect between groups was investigated, a pattern of significantly increased activation post-ketamine versus post-placebo was seen in HCs, and decreased activation post-ketamine versus post-placebo was seen in MDD participants, most notably in the right frontal cortex, dACC, and left inferior occipital gyrus (pFWE < 0.05) (Fig. 5, Table 4).
Supplementary Fig. S4.
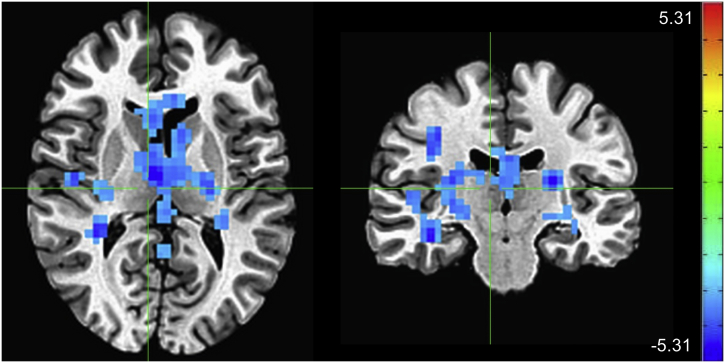
Effect of group (major depressive disorder (MDD) vs. healthy control (HC)) at baseline. Crosshairs are located at coordinates [8 -17 10], the center of the mass of the large cluster extending from the bilateral thalamus into the caudate and posterior cingulate (scale: Z-score; left = right).
Fig. 5.
Drug session (post-ketamine versus post-placebo) by group interaction. Crosshairs are located at the center of mass of each cluster: [50 4 35] for right precentral gyrus; [7 18 39] for right cingulate gyrus; and [−29–89-12] for left inferior occipital gyrus. Error bars on graphs indicate +/−1 standard error (scale: Z-score; left = right).
Table 4.
Drug session by group interaction effect.
| Region | X | Y | Z | # Voxels | Cluster p-value |
|---|---|---|---|---|---|
| Right precentral gyrus | 50 | 4 | 35 | 27 | <0.001 |
| Right cingulate gyrus | 7 | 18 | 39 | 21 | <0.001 |
| Left inferior temporal gyrus | −53 | −54 | −5 | 18 | <0.002 |
| Right middle frontal gyrus | 48 | 20 | 22 | 16 | <0.004 |
| Right inferior parietal lobule | 51 | −52 | 43 | 14 | <0.008 |
| Right superior parietal lobule | 28 | −53 | 61 | 14 | <0.008 |
| Left uncus | −37 | −20 | −34 | 12 | <0.02 |
| Left inferior occipital gyrus | −29 | −89 | −12 | 12 | <0.02 |
| Left cerebellum | −19 | −78 | −25 | 11 | <0.03 |
| Right precentral gyrus | 40 | −9 | 50 | 10 | <0.05 |
No significant emotion by congruency, group by congruency, or session by congruency interactions were observed.
3.2.3. Three- and four-way interaction effects
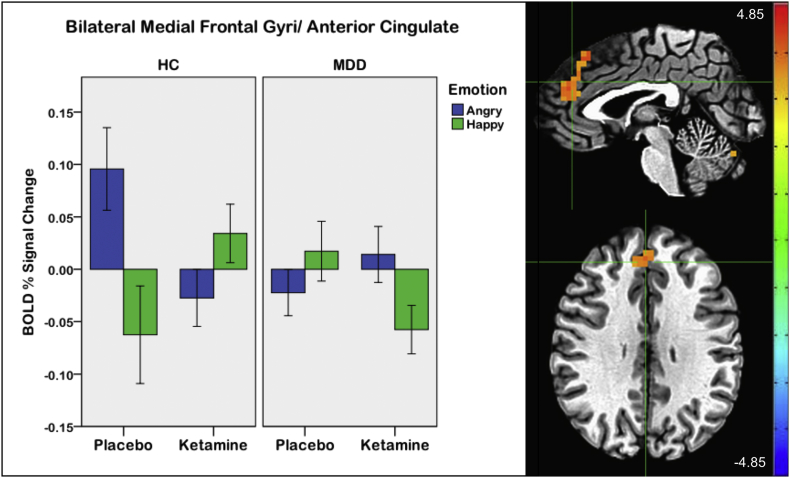
A significant three-way session by emotion by group interaction was observed. To specifically examine the group by emotion interaction in the post-ketamine versus post-placebo sessions, this contrast showed a large medial prefrontal and anterior cingulate cluster (pFWE < 0.001) (Fig. 6; Table 5). Extracted beta values showed that in HCs during placebo, this region showed activation during angry trials and deactivation during happy trials, which reversed after ketamine. The opposite pattern was found in MDD participants; deactivation to angry trials and activation to happy trials occurred during placebo, a pattern that was reversed after ketamine. No other significant three- or four-way interactions were observed, including no interactions with the congruency factor.
Fig. 6.
Drug session (post-ketamine versus post-placebo) by emotion by group interaction. Crosshairs are located at coordinates [2 39 29], the center of mass of the bilateral medial frontal gyri/anterior cingulate cluster. Error bars on the graph indicate +/−1 standard error (scale: Z-score; left = right).
Table 5.
Drug session by emotion by group interaction effect.
| Region | X | Y | Z | # Voxels | Cluster p-value |
|---|---|---|---|---|---|
| Bilateral medial frontal gyri/anterior cingulate | 2 | 39 | 29 | 68 | <0.001 |
| Right cerebellum | 6 | −69 | −20 | 10 | <0.05 |
3.2.4. Association with MADRS in MDD participants
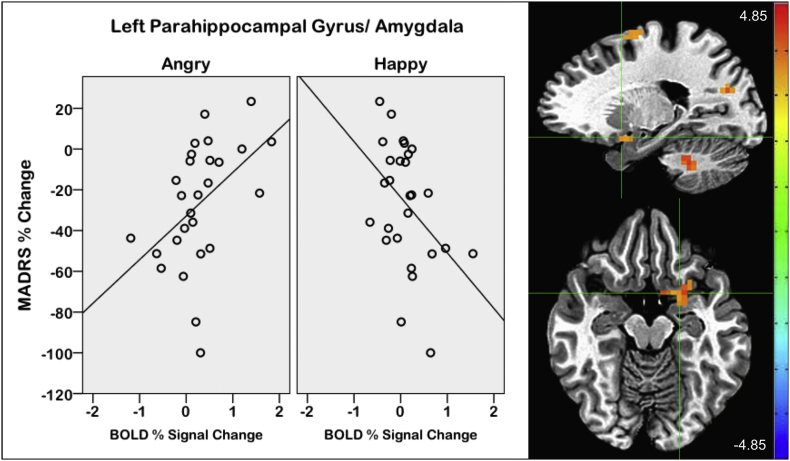
Percent change in MADRS score was significantly associated with the magnitude of activation, showing primarily positive associations post-ketamine and mainly negative associations post-placebo (Supplementary Tables S2 and S3). For the ketamine condition specifically, an emotion by MADRS interaction on activation was also found in several brain regions. This included a cluster in the left parahippocampal gyrus and amygdala (pFWE < 0.002), in which decreased MADRS score (showing symptom improvement) was associated with less activation to angry trials and greater activation to happy trials (Fig. 7). The same pattern was also found in other regions, including bilateral cingulate gyri, precuneus, and left medial and middle frontal gyri (pFWE < 0.002). In the placebo scans, an emotion by MADRS interaction was observed only in the left frontal gyrus (pFWE < 0.001).
Fig. 7.
Interaction between emotion and percent change in Montgomery-Asberg Depression Rating Scale (MADRS) score on blood oxygen-level-dependent (BOLD) signal during post-ketamine scans in participants with major depressive disorder (MDD). Crosshairs located at [−19 6–13], the center of mass of the left parahippocampal gyrus/amygdala cluster (scale: Z-score, left = right).
3.3. Behavioral results
3.3.1. Reaction times
For behavioral analyses, only correct trials were included. Accuracy was similar across groups and sessions and was very high overall, with an average of 97%. The mean reaction time across trials was 516.56 ms (SD = 82.06). The reaction time analysis showed a significant main effect of emotion (F = 10.935; p = .001), in which responses were faster to angry than to happy trials. A main effect was also seen for session (F = 11.335; p < .001), but the post-hoc test for the ketamine versus placebo sessions was not significant. There was a trend for the main effect of congruency (F = 3.275; p = .071), in which responses to congruent trials were slightly faster than to incongruent trials. There was no significant effect of group nor any interaction effects.
3.3.2. Attention bias scores
The mean angry and happy bias scores were 3.39 ± 19.75 and 6.18 ± 26.25, respectively. For the attention bias score analysis, no main effects were observed for emotion, session, or group, nor were any interaction effects found.
4. Discussion
This study is the first to use a dot probe task to investigate fMRI activation during emotion-related attentional bias in treatment-resistant MDD participants and HCs across multiple time points. We found that, compared to placebo, the glutamatergic modulator ketamine affected fMRI BOLD signal during emotionally valenced attentional processing. Specifically, significant differences in brain activation were observed between trials featuring angry and happy faces, as well as between ketamine and placebo conditions. Interaction effects were also observed; in particular, the drug session (post-ketamine versus post-placebo) by group and the drug session by emotion by group interactions demonstrated that ketamine had opposite effects in MDD participants than in HCs in certain brain regions. In general, brain activity in MDD participants post-ketamine infusion resembled brain activity in HCs post-placebo, suggesting a normalization of function.
In the drug session by group interaction, several brain areas showed similar activity patterns, in which HCs had greater general task activation post-ketamine compared to post-placebo but MDD participants showed the opposite pattern. Thus, post-ketamine, MDD participants tended to “normalize” and more closely resembled HCs following the placebo infusion. In this sense, normalization refers to BOLD activation at a system or network level, given that fMRI does not allow us to make conclusions at a more precise (cellular or neurotransmitter) level. The effects found were seen in regions involved in the executive control network, including frontal and parietal regions (Seeley et al., 2007), specifically the right middle frontal gyrus and right inferior and superior parietal lobules. Our findings, in which the MDD participants showed greater activity than the HCs during the placebo condition, are consistent with published findings of regions associated with emotion regulation in depression, specifically related to automatic attentional control (Rive et al., 2013). We also found a drug session by group interaction in the occipital gyrus, which is particularly intriguing in light of studies showing that middle occipital activity predicts antidepressant response to scopolamine, a muscarinic antagonist that also modulates glutamate (Furey et al., 2013; Furey et al., 2015).
A cluster within the ACC, extending bilaterally into the medial frontal gyri, exhibited a significant drug session by emotion by group interaction. In this three-way interaction, opposite patterns of activation were observed in HC and MDD participants, which differed by both drug session and emotion. The HCs exhibited greater activation in response to angry versus happy trials post-placebo, and greater activation in response to happy versus angry trials post-ketamine. MDD participants displayed an opposite pattern, with slightly more activation in response to happy versus angry trials post-placebo, but greater activation in response to angry versus happy trials post-ketamine. Here, again, the pattern of activity in MDD participants post-ketamine was similar to the pattern of activity in the HCs post-placebo, potentially suggesting normalization of aberrant brain function. The brain area involved in this interaction, the ACC, has been implicated in attentional and emotional abnormalities in depression (Groenewold et al., 2013; Hamilton et al., 2012).
A recent magnetoencephalography (MEG) study that used data from the same participants as the current study found that gamma power in MDD participants post-ketamine infusion was similar to that in HCs post-placebo. The finding, which occurred across several brain regions, suggested that homeostatic dysregulation in MDD was improved by ketamine. In that recent study, HCs were also found to have transient but significant increases in MADRS scores post-ketamine (Nugent et al., n.d.). Though the increase in depressive symptoms did not persist through the day of the fMRI scan, subtle changes in neural activity may nevertheless persist longer than more global mood changes. This could potentially be linked to the present findings, suggesting that post-ketamine, HCs had similar activation patterns to MDD participants post-placebo.
Interestingly, while ours is the first study to use the dot probe task to investigate emotional processing post-ketamine, our findings nevertheless echo previous studies conducted in HCs. For instance, decreased activation during negative emotional stimuli was found in a region of the ACC (Lehmann et al., 2016) as well as the right dorsolateral prefrontal cortex (DLPFC) (Scheidegger et al., 2016a) post-ketamine in HCs. Similarly, the present study found deactivation to negative stimuli in an anterior cingulate and frontal region in HCs post-ketamine; however, this finding differed from the results obtained in MDD participants, suggesting that ketamine may act differently in MDD participants than in HCs, and underscoring the importance of including both participant groups in such research studies. While we did not find activation differences in the right caudate associated with ketamine as reported by a previous study (Murrough et al., 2015), this could have been because our task differed considerably from the emotion perception task used in that study.
It is also worth noting that in the follow-up analysis of activation associated with percent change in MADRS score, an emotion by MADRS interaction effect was observed in the MDD participants with regard to activation during the ketamine scan. This was found across multiple brain areas, including a cluster in the left parahippocampal gyrus and amygdala. Activation associated with this interaction showed that change in MADRS scores was associated in opposite directions for angry and happy trials. As depressive symptoms decreased, BOLD signal decreased during angry trials and increased during happy trials. This finding suggests that ketamine's antidepressant effects may reduce brain response to negative stimuli and increase response to positive stimuli in emotional processing regions.
The present study has several strengths. First, the study included both MDD and HC participants, and all were assessed longitudinally at multiple time points. Second, placebo infusions were used as a control condition. Third, participants took part in the study as inpatients, which allowed potential confounding variables—such as the use of other medications—to be well-controlled. Despite the intriguing nature of the results, the study is also associated with several limitations. First, MDD participants displayed no behavioral bias towards the angry face stimuli, as would be expected in accordance with a negative cognitive bias (Dalgleish and Watts, 1990; Mathews and Macleod, 1994), suggesting that such a bias is more likely to be associated with specific types of negative stimuli (e.g., faces with sad expressions (Gotlib et al., 2004; Joormann and Gotlib, 2007) as opposed to perhaps angry faces). However, consistent with the negative behavioral findings seen here, previous research on attention bias also found neuroimaging differences without behavioral findings (Amico et al., 2012), which may be related to fMRI's potentially greater sensitivity to detect significant effects, as compared to behavioral measurements. Similarly, neural findings may be more stable than behavioral ones (White et al., 2016). Second, although our sample size included 59 participants across all scan time points, not all participants had usable data for the post-ketamine and post-placebo scans on which we focused, thereby reducing the sample sizes for these sessions. A third potential weakness of the study may be the complexity of the study design, as the several factors involved in the analysis resulted in numerous contrast variations to examine.
4.1. Conclusions
This study found that ketamine's effects on brain activity differed from those of placebo in several brain regions, and that the effects in MDD participants were often opposite to those seen in HCs. This variation was also specific to emotion in a large fronto-cingulate area of the brain. The interaction effects demonstrated that the activation pattern in MDD participants post-ketamine resembled the activation pattern in HCs post-placebo, suggesting that ketamine may have a normalizing effect on brain function during attentional and emotional processing. Notably, these findings improve our understanding of ketamine's mechanism of action in the brain.
The following are the supplementary data related to this article.
Supplementary material
Declaration of interest
Dr. Zarate is listed as a co-inventor on a patent for the use of ketamine in major depression and suicidal ideation; as a co-inventor on a patent for the use of (2R,6R)-hydroxynorketamine, (S)-dehydronorketamine, and other stereoisomeric dehydro and hydroxylated metabolites of (R,S)-ketamine metabolites in the treatment of depression and neuropathic pain; and as a co-inventor on a patent application for the use of (2R,6R)-hydroxynorketamine and (2S,6S)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation, and post-traumatic stress disorders. He has assigned his patent rights to the U.S. government but will share a percentage of any royalties that may be received by the government. Dr. Furey is identified as a coinventor on a patent in the US and in Europe for the use of scopolamine as an antidepressant agent. She has assigned her patent rights to the U.S. government but will share a percentage of any royalties that may be received by the government. Dr. Furey is currently an employee of Janssen Pharmaceuticals of Johnson & Johnson, Inc. All other authors have no conflict of interest to disclose, financial or otherwise.
Acknowledgements and role of funding source
Funding for this work was supported by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH; NCT00088699/04-M-0222/ZIA MH002857), by a NARSAD Independent Investigator Award to Dr. Zarate, and by a Brain and Behavior Mood Disorders Research Award to Dr. Zarate. These organizations had no further role in study design; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
The authors thank the NIH 7SE research unit and staff for their support. Ioline Henter (NIMH) provided invaluable editorial assistance.
Contributor Information
Jessica L. Reed, Email: jessica.reed2@nih.gov.
Allison C. Nugent, Email: nugenta@mail.nih.gov.
Maura L. Furey, Email: mfurey1@its.jnj.com.
Joanna E. Szczepanik, Email: szczepaj@mail.nih.gov.
Jennifer W. Evans, Email: jennifer.evans@nih.gov.
Carlos A. Zarate, Jr, Email: zaratec@mail.nih.gov.
References
- Amico F. Functional anomalies in healthy individuals with a first degree family history of major depressive disorder. Biol. Mood Anxiety Disord. 2012;2(1) doi: 10.1186/2045-5380-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman R.M. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry. 2000;47(4):351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Britton J.C. Neural changes with attention bias modification for anxiety: a randomized trial. Soc. Cogn. Affect. Neurosci. 2015;10(7):913–920. doi: 10.1093/scan/nsu141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cox R.W. FMRI clustering in AFNI: false-positive rates redux. Brain Connect. 2017;7(3):152–171. doi: 10.1089/brain.2016.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgleish T., Watts F.N. Biases of attention and memory in disorders of anxiety and depression. Clin. Psychol. Rev. 1990;10(5):589–604. [Google Scholar]
- Evans J.W. Default mode connectivity in major depressive disorder measured up to 10 days after ketamine administration. Biol. Psychiatry. 2018 Feb 15 doi: 10.1016/j.biopsych.2018.01.027. (pii: S0006-3223(18)30085-4. [Epub ahead of print]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M.B., Spitzer R.L., Gibbon M., Williams J.B. New York State Psychiatric Institute: Biometrics Research; New York: 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) [Google Scholar]
- Furey M.L. Potential of pretreatment neural activity in the visual cortex during emotional processing to predict treatment response to scopolamine in major depressive disorder. JAMA Psychiatr. 2013;70(3):280–290. doi: 10.1001/2013.jamapsychiatry.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furey M.L. Pretreatment differences in BOLD response to emotional faces correlate with antidepressant response to scopolamine. Int. J. Neuropsychopharmacol. 2015;18(8) doi: 10.1093/ijnp/pyv028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib I.H. Attentional biases for negative interpersonal stimuli in clinical depression. J. Abnorm. Psychol. 2004;113(1):121–135. doi: 10.1037/0021-843X.113.1.121. [DOI] [PubMed] [Google Scholar]
- Groenewold N.A. Emotional valence modulates brain functional abnormalities in depression: evidence from a meta-analysis of fMRI studies. Neurosci. Biobehav. Rev. 2013;37(2):152–163. doi: 10.1016/j.neubiorev.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Hamilton J.P. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. Am. J. Psychiatry. 2012;169(7):693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ironside M. Frontal cortex stimulation reduces vigilance to threat: implications for the treatment of depression and anxiety. Biol. Psychiatry. 2016;79(10):823–830. doi: 10.1016/j.biopsych.2015.06.012. [DOI] [PubMed] [Google Scholar]
- Joormann J., Gotlib I.H. Selective attention to emotional faces following recovery from depression. J. Abnorm. Psychol. 2007;116(1):80–85. doi: 10.1037/0021-843X.116.1.80. [DOI] [PubMed] [Google Scholar]
- Lehmann M. Differential effects of rumination and distraction on ketamine induced modulation of resting state functional connectivity and reactivity of regions within the default-mode network. Soc. Cogn. Affect. Neurosci. 2016;11(8):1227–1235. doi: 10.1093/scan/nsw034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews A., Macleod C. Cognitive approaches to emotion and emotional disorders. Annu. Rev. Psychol. 1994;45:25–50. doi: 10.1146/annurev.ps.45.020194.000325. [DOI] [PubMed] [Google Scholar]
- Mathews A., Ridgeway V., Williamson D.A. Evidence for attention to threatening stimuli in depression. Behav. Res. Ther. 1996;34(9):695–705. doi: 10.1016/0005-7967(96)00046-0. [DOI] [PubMed] [Google Scholar]
- Mogg K., Bradley B.P., Williams R. Attentional bias in anxiety and depression: the role of awareness. Br J Clin Psychol. 1995;34(Pt 1):17–36. doi: 10.1111/j.2044-8260.1995.tb01434.x. [DOI] [PubMed] [Google Scholar]
- Mogg K., Millar N., Bradley B.P. Biases in eye movements to threatening facial expressions in generalized anxiety disorder and depressive disorder. J. Abnorm. Psychol. 2000;109(4):695–704. doi: 10.1037//0021-843x.109.4.695. [DOI] [PubMed] [Google Scholar]
- Murrough J.W. Regulation of neural responses to emotion perception by ketamine in individuals with treatment-resistant major depressive disorder. Transl. Psychiatry. 2015;5:e509. doi: 10.1038/tp.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niciu M.J. Glutamate and its receptors in the pathophysiology and treatment of major depressive disorder. J. Neural Transm. (Vienna) 2014;121(8):907–924. doi: 10.1007/s00702-013-1130-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent, A.C., et al., Ketamine has distinct electrophysiological and behavioral effects in depressed and healthy subjects. Mol. Psychiatry, (in press). [DOI] [PMC free article] [PubMed]
- Peckham A.D., McHugh R.K., Otto M.W. A meta-analysis of the magnitude of biased attention in depression. Depress. Anxiety. 2010;27(12):1135–1142. doi: 10.1002/da.20755. [DOI] [PubMed] [Google Scholar]
- Pourtois G. Neural systems for orienting attention to the location of threat signals: an event-related fMRI study. NeuroImage. 2006;31(2):920–933. doi: 10.1016/j.neuroimage.2005.12.034. [DOI] [PubMed] [Google Scholar]
- Price R.B. Looking under the hood of the dot-probe task: an fMRI study in anxious youth. Depress. Anxiety. 2014;31(3):178–187. doi: 10.1002/da.22255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rive M.M. Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies. Neurosci. Biobehav. Rev. 2013;37(10):2529–2553. doi: 10.1016/j.neubiorev.2013.07.018. Pt 2. [DOI] [PubMed] [Google Scholar]
- Scheibe C. Neural correlates of the interaction between transient and sustained processes: a mixed blocked/event-related fMRI study. Hum. Brain Mapp. 2006;27(7):545–551. doi: 10.1002/hbm.20199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidegger M. Effects of ketamine on cognition-emotion interaction in the brain. NeuroImage. 2016;(124):8–15. doi: 10.1016/j.neuroimage.2015.08.070. Pt A. [DOI] [PubMed] [Google Scholar]
- Scheidegger M. Ketamine administration reduces amygdalo-hippocampal reactivity to emotional stimulation. Hum. Brain Mapp. 2016;37(5):1941–1952. doi: 10.1002/hbm.23148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visscher K.M. Mixed blocked/event-related designs separate transient and sustained activity in fMRI. NeuroImage. 2003;19(4):1694–1708. doi: 10.1016/s1053-8119(03)00178-2. [DOI] [PubMed] [Google Scholar]
- White L.K. Behavioral and neural stability of attention bias to threat in healthy adolescents. NeuroImage. 2016;136:84–93. doi: 10.1016/j.neuroimage.2016.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate C.A., Jr. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry. 2006;63(8):856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- Zarate C., Jr. Glutamatergic modulators: the future of treating mood disorders? Harv. Rev. Psychiatr. 2010;18(5):293–303. doi: 10.3109/10673229.2010.511059. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material