Abstract
BACKGROUND: The objective of current study was to develop and validate a nomogram to predict overall survival in pancreatic neuroendocrine tumors (PNETs). METHODS: The Surveillance, Epidemiology, and End Results (SEER) database was queried for patients with PNETs between 2004 and 2015. Patients were randomly separated into the training set and the validation set. Cox regression model was used in training set to obtain independent prognostic factors to develop a nomogram for predicting overall survival (OS). The discrimination and calibration plots were used to evaluate the predictive accuracy of the nomogram. RESULTS: A total of 3142 patients with PNETs were collected from the SEER database. Sex, age, marital status, primary site, TNM stage, tumor grade, and therapy were associated with OS in the multivariate models. A nomogram was constructed based on these variables. The nomogram for predicting OS displayed better discrimination power than the Tumor-Node-Metastasis (TNM) stage systems 7th edition in the training set and validation set. The calibration curve indicated that the nomogram was able to accurately predict 3- and 5-year OS. CONCLUSIONS: The nomogram which could predict 3- and 5-year OS were established in this study. Our nomogram showed a good performance, suggesting that it could be served as an effective tool for prognostic evaluation of patients with PNETs.
Introduction
Pancreatic neuroendocrine neoplasms (PNETs) comprises a heterogeneous collective of malignant tumors arising from the islets of Langerhans and accounting for approximately 1% to 3% of all pancreatic neoplasms [1], [2]. Although PNETs is a relatively rare malignancy, its incidence and mortality have been increasing over the last decades, due to the improved medical technology of detection. The annual incidence of all PNETs in the United States is 8/1000,000 [3]. They are broadly categorized as functioning and non-functioning PNETs. The majority (60%) of PNETs are non-functional and are more aggressive compared with the functional PNETs. Although they are generally considered to be indolent, PNETs are highly heterogenous neoplasms and some subgroups can be highly malignant [4], [5]. As the majority of PNETs do not secrete hormones that cause clinical symptoms, patients are predominantly diagnosed with disseminated disease for whom curation is not possible [1], [6]. Due to this heterogeneous nature of PNETs, identifying reliable prognostic features have been a challenge.
Currently, the American Joint Commission on Cancer (AJCC) TNM stage systems 8th edition [7], which is widely used for prognostic evaluation of PNETs, only takes tumor size and histological metastasis into account. However, many other important variables such as age, gender, race, tumor size, tumor site, and tumor differentiation can also influence the survival of individual patients. In addition, the TNM 8th edition is still deficiently formulated for the prognostic prediction. In this sense, the traditional TNM staging system still needs further validation and improvement. Therefore, there is an urgent need to develop a staging system which is technically feasible and clinically easy-accessible to stratify the prognosis of patients with PNETs.
Nomogram, as a simple statistical predictive tool, has been widely used in clinical practice to predict prognosis [8], [9], [10]. Construction of a nomogram not only considers the prognostic weight of each factor when calculating the probability of an outcome, but also combines multiple independent factors to draw the best conclusion. Compared to the AJCC TNM staging system, nomograms can more accurately estimate survival for individual patients by integrating important prognostic variables [11], [12]. However, to our knowledge, a nomogram for patients with PNETs on the basis of population-based data has not been reported. Therefore, the current study sought to develop and validate a nomogram for predicting OS based on population-based data from the Surveillance, Epidemiology, and End Results (SEER) database.
Materials and Methods
Patients
The SEER program of the US National Cancer Institute provides data on cancer incidence and survival in the United States and covers approximately 30% of the US population across several geographic regions [13]. For this research, data about patients with a diagnosis of PNETs were extracted from the SEER Program (2004–2015), using the SEER*Stat software version 8.3.5. The study cohort consisted of patients with the following International Classification of Diseases for Oncology, Third Edition (ICD-O-3), histology codes: 8150, 8151, 8152, 8153, 8155, 8156, 8157, 8240, 8241, 8242, 8243, 8246 and 8249; and the ICD-O-3 site codes: C25.0-C25.9. Patients were excluded if the number of months survived was unknown, if they had more than 1 primary cancer and the PNETs was not the first, or if they had incomplete clinicopathological information (TNM stage and therapy). In addition, patients registered at the time of autopsy or death certificate only were excluded. To establish and validate the nomogram, all patients were randomly allocated to a training set and a validation set. Institutional review board approval was not required in the current study because SEER research data is publicly available and we received permission from SEER to access the research data (accession number: 10165-Nov 2017).
Variables
Demographic and clinical variables were extracted from the SEER database, including age, sex, race, marital status, primary site, tumor size, functional status, histological differentiation, T, N, and M stage, TNM stage, follow-up information and cause of death. Age and tumor size as continuous variables, were transformed into categorical variable on the basis of recognized cutoff values. The primary endpoint was OS. OS was defined as the time from diagnosis of PNETs to death or last follow-up, with no restriction on the cause of death.
Statistical Analyses
Construction of the Nomogram
Categorical data were shown as frequencies and proportions and compared with chi-square test and Fisher's exact test. Survival curves were generated using the Kaplan–Meier method and the log-rank test was used to compare the difference between the groups. Univariate and multivariate Cox regression analyses were performed to identify independent prognostic variables for predicting OS. A nomogram was formulated based on the results of the multivariate analyses.
Validation of the Nomogram
The nomogram was validated by measuring discrimination and calibration curves both internally (training set) and externally (validation set). Discrimination between observed and predicted outcome was assessed using the concordance Index (C-index) [14]. A higher C-index indicates a better ability to separate patients with different survival outcomes. Comparison between the nomogram and the AJCC TNM staging system 7th edition was performed with the rcorrp.cens package in Hmisc in R and were evaluated by the C-index. The calibration curves were used to compare the predicted probability with the cohort observed in the study. All statistical analyses were conducted using SPSS software (SPSS Inc., Chicago, USA, version 23) and the R software version 3.13 (Institute for Statistics and Mathematics, Vienna, Austria; www.r-project.org). A P-value of <0.05 was considered statistically significant.
Results
Patient Characteristics
A total of 3142 eligible patients with PNETs diagnosed between 2004 and 2015 were included in the study. Of those, 1555 patients were in the training set and 1587 were in the validation set. In the whole study set, the median age was 60 years (11–94 years). Among the eligible patients, 1721(54.8) were male and 1421 (45.2) were female. The majority of patients in both sets were married (62.2%) and white (76.6%). The most common tumor site was the pancreatic tail (34.3%), followed by pancreatic head (30.6%) and other sites. As to tumor size, ≥ 4 cm (36.1%) was the most common, followed by 2–4 cm (33.1%) and < 2 cm (29.3%). Well differentiation (53.7%) was most common tumor grade. In both sets, most patients received surgery, and had T1 stage (36.4%).
The median follow-up time was 20 months (range: 0–71 months) in both sets. By the end of follow up, 307 (19.7%) patients in the training set had died, including 268 (17.2%) who died from PNETs and 590 (2.5%) who died from other causes. The demographic features and clinicopathological characteristics are summarized in Table 1.
Table 1.
Patient Demographics and Pathological Characteristics
| Variables | All Patients (n = 3142) |
Training Set (n = 1555) |
Validation Set (n = 1587) |
|---|---|---|---|
| N (%) | N (%) | N (%) | |
| Sex | |||
| Male | 1721(54.8) | 841 (54.1) | 880 (55.5) |
| Female | 1421 (45.2) | 714 (45.9) | 707 (44.5) |
| Age | |||
| < 30 | 72 (2.3) | 32 (2.1) | 40 (2.5) |
| 30–60 | 1553 (49.4) | 772 (49.6) | 781 (49.2) |
| > 60 | 1517 (48.3) | 751 (48.3) | 766 (48.3) |
| Race | |||
| White | 2406 (76.6) | 1204 (77.4) | 1202 (75.7) |
| Black | 391 (12.4) | 186 (12.0) | 205 (12.9) |
| Other* | 345 (11.0) | 165 (10.6) | 180 (11.3) |
| Marital status | |||
| Married | 1953 (62.2) | 951 (61.2) | 1002 (63.1) |
| Unmarried | 1032 (32.8) | 520 (33.4) | 512 (32.3) |
| Unknown | 157 (5.0) | 84 (5.4) | 73 (4.6) |
| Primary site | |||
| Head | 960 (30.6) | 452 (29.1) | 508 (32.0) |
| Body | 492 (15.7) | 243 (15.6) | 249 (15.7) |
| Tail | 1077 (34.3) | 546 (35.1) | 531 (33.5) |
| Other | 613 (19.5) | 314 (20.2) | 299 (18.8) |
| Tumor Size (cm) | |||
| <2 | 920 (29.3) | 451 (29.0) | 469 (29.6) |
| 2–4 | 1039 (33.1) | 509 (32.7) | 530 (33.4) |
| ≥4 | 1135 (36.1) | 576 (37.0) | 559 (35.2) |
| Unknown | 48 (1.5) | 19 (1.2) | 29 (1.8) |
| Functional status | |||
| Functional | 191 (6.1) | 84 (5.4) | 107 (6.7) |
| Nonfunctional | 2951 (93.9) | 1471 (94.6) | 1480 (93.3) |
| AJCC TNM stage | |||
| I | 1452 (46.2) | 717 (46.1) | 735 (46.3) |
| II | 686 (21.8) | 340 (21.9) | 346 (21.8) |
| III | 92 (2.9) | 45 (2.9) | 47 (3.0) |
| IV | 912 (29.0) | 453 (29.1) | 459 (28.9) |
| Grade | |||
| I | 1687 (53.7) | 836 (53.8) | 851 (53.6) |
| II | 458 (14.6) | 231 (14.9) | 227 (14.3) |
| III | 160 (5.1) | 85 (5.5) | 75 (4.7) |
| IV | 58 (1.8) | 32 (2.1) | 26 (1.6) |
| Unknown | 779 (24.8) | 371 (23.9) | 408 (25.7) |
| Surgery | |||
| Performed | 2035 (64.8) | 1022 (65.7) | 1013 (63.8) |
| None | 1107 (35.2) | 533 (34.3) | 574 (36.2) |
Nomogram Construction
Data on the patients' sex, age, race, marital status, primary site, tumor size, functional status, TNM stage, tumor grade, and therapy were collected in the training set. In the univariate analysis, sex, age, marital status, primary site, tumor size, functional status, tumor grade, TNM stage, and therapy were significantly associated with OS, while race was not significantly related to OS (P > .05) (Table 2). After adjusting for other risk factors, the multivariate analysis showed that 7 variables were independent predictive factors, including age, sex, marital status, primary site, TNM stage, tumor grade, and therapy.
Table 2.
Univariate and Multivariate Analyses of Overall Survival in the Training Set
| Univariate Analysis |
Multivariate Analysis |
||
|---|---|---|---|
| Variable | P Value | HR(95%CI) | P Value |
| Sex | 0.003 | ||
| Male | Reference | ||
| Female | 0.762 (0.598–0.971) | 0.028 | |
| Age | < 0.001 | ||
| <30 | Reference | ||
| 30–60 | 1.347 (0.490–3.703) | 0.563 | |
| >60 | 2.120 (0.774–5.809) | 0.044 | |
| Race | 0.468 | ||
| White | |||
| Black | |||
| Other | |||
| Marital status | 0.029 | ||
| Married | Reference | ||
| Unmarried | 1.425 (1.111–1.828) | 0.005 | |
| Unknown | 1.472 (0.928–2.334) | 0.100 | |
| Primary site | < 0.001 | ||
| Head | Reference | ||
| Body | 0.794 (0.550–1.147) | 0.219 | |
| Tail | 0.589 (0.439–0.790) | < 0.001 | |
| Other | 0.720 (0.530–0.978) | 0.035 | |
| Tumor Size (cm) | < 0.001 | ||
| < 2 | Reference | ||
| 2–4 | 1.166 (0.715–1.904) | 0.538 | |
| ≥ 4 | 1.065 (0.650–1.745) | 0.804 | |
| Unknown | 1.106 (0.499–2.450) | 0.804 | |
| Functional status | 0.017 | ||
| Functional | Reference | ||
| Nonfunctional | 1.696 (0.895–3.213) | 0.105 | |
| AJCC TNM stage | < 0.001 | ||
| I | Reference | ||
| II | 3.322 (1.972–5.596) | < 0.001 | |
| III | 4.941 (2.487–9.816) | < 0.001 | |
| IV | 6.999 (4.297–11.400) | < 0.001 | |
| Grade | < 0.001 | ||
| I | Reference | ||
| II | 1.103 (0.708–1.718) | 0.664 | |
| III | 3.541 (2.360–5.313) | < 0.001 | |
| IV | 6.091 (3.631–10.218) | < 0.001 | |
| Unknown | 1.665 (1.190–2.330) | 0.003 | |
| Surgery | < 0.001 | ||
| Performed | Reference | ||
| None | 3.180 (2.284–4.429) | < 0.001 | |
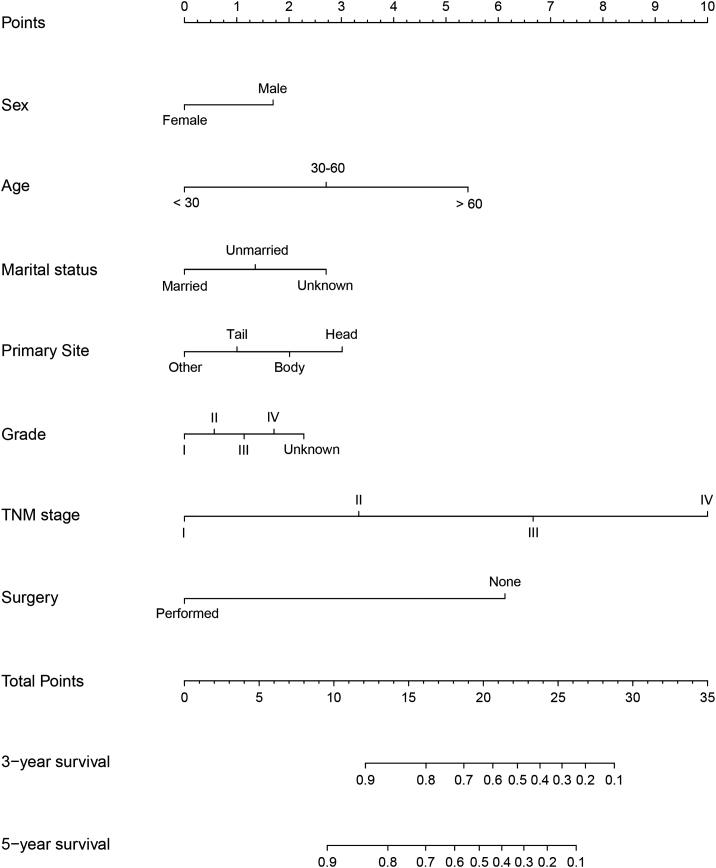
All the independent predictors in the whole study cohort were integrated into the nomogram. A nomogram for predicting 3- and 5-year OS was constructed based on the independent variables in the training set (Figure 1). This model revealed that TNM stage contributed most to prognosis, followed by the therapy, age, tumor site, marital status, tumor grade, and sex. By adding the scores of each selected variable, the likelihood of survival of the individual patient can be easily calculated.
Figure 1.
Nomogram for predicting 3- and 5-year overall survival.
Nomogram Validation
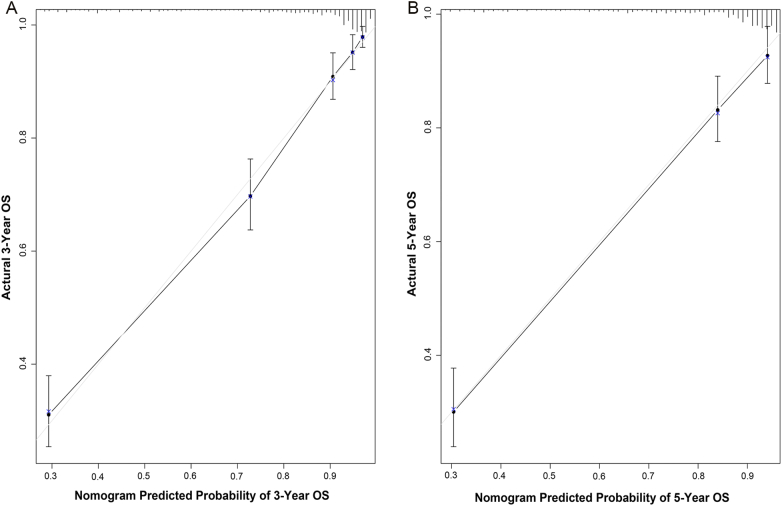
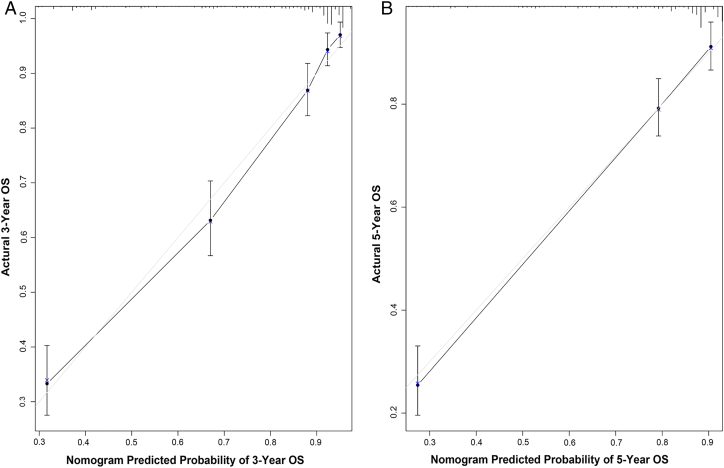
Internal validation via the training set indicated that the C-index for the nomogram to predict OS were 0.840 (95% CI, 0.818–0.862), which was in excellent agreement with actual OS. External validation using the validation set, the C-index for the nomogram to predict OS was 0.808 (95% CI, 0.784–0.832). The internal and external calibration plots of OS nomogram revealed a good correlation between prediction by the nomogram and actual observation (Figures 2 and 3). In addition, we compared the discrimination of the nomogram with that of the AJCC TNM staging system 7th edition in the training set. The nomogram discrimination for OS prediction was superior to that of the TNM 7th edition stage systems (C-index = 0.840, 95% CI, 0.818–0.862 vs 0.777, 95% CI, 0.754–0.800, P < .001). Moreover, the nomogram established in this study also displayed more powerful efficiency of discrimination for OS prediction in the validation set compared with the TNM staging (C-index = 0.808, 95% CI, 0.784–0.832 vs 0.784, 95% CI, 0.759–0.809, P < .001).
Figure 2.
Internal calibration plot. (A) 3-year and (B) 5-year overall survival nomogram calibration curves.
Figure 3.
External calibration plot. (A) 3-year and (B) 5-year overall survival nomogram calibration curves.
Discussion
The annual incidence of all PNETs is steadily on the rise [3]. What's more, PNETs are highly heterogeneous neoplasms presenting a spectrum of biologic behavior [15], [16]. It was imprecise to use traditional staging systems solely to evaluate prognosis. It was essential to establish an efficient prognostic system that could be used to estimate the prognosis for these patients. Thus, we sought to develop and validate a new prognostic nomogram for PNETs based on a large population from the SEER database. A total of 3142 patients with PNETs were analyzed. The nomogram showed good discrimination in both internal and external validation. Moreover, the calibration plots indicated that OS corresponded to actual outcome closely. Superior to the existing AJCC TNM staging system, the proposed nomogram was easily used clinical tools which would facilitate the popularization of patient counseling and personalized treatment.
In the present study, seven clinicopathological variables were proved to be independent prognostic factors, including age, sex, marital status, primary site, TNM stage, tumor grade, and therapy. Age was found to be an important prognostic variable on OS in several studies [17], [18], [19]. In the training set, the HR of OS increased with age, patients who were older than 60 years suffered worst survival than younger patients. This conclusion was consistent with a previous study [20]. Sex is an important variable related with different prognosis in malignancies [21], [22]. The present study showed male patients suffered a worse prognosis compared with female patients in PNETs. This result was similar with previous study [20]. Recent years, the impact of mental health on human body health is getting more and more attention [20], [23]. For example, the marital status significantly affected OS in our study. Married patients tend to obtain more social support and heart comfort, which may indicate a better prognosis.
Nomograms are an important component of modern medical decision-making [24]. The nomogram is a graphical presentation of a statistical prediction model that provides survival probability of a specific outcome [25], [26]. Thus, the parameters to consider should be easily available and measurable. Accumulating evidence has shown that the nomogram shows a better predictive ability than the classic AJCC TNM staging system in multiple malignancies, and thus they have been identified as an alternative or even a new standard [9], [27], [28]. Compared to the widely used TNM staging, our model is not only easy to use, but it also provides a quantitative prognosis for individual patients. In addition, nomograms are particularly suitable to help clinicians dealing with complex conditions where no firm clinical guidelines exist. Using of nomograms to predict patients' survival is simple. For example, consider two hypothetical stage III PNETs patients: case A) a 65-year-old married man patient, with tumor location of pancreatic head, grade IV, and case B) a 55-year-old unmarried woman patient, with tumor location of pancreatic tail, grade III. After using our nomogram, the 5-year OS probabilities of patients in case A and B are 58% and 82%, respectively. In contrast, both patients would be considered to be stage III according to the traditional TNM staging system, which indicates identical outcomes.
Our study has several advantages. The clinicopathological information of PTENs patients that we collected from SEER database was rich and detailed, thus ensuring the establishment of accurate prognostic nomogram. Our nomogram displays better discrimination power in predicting OS compared with TNM 7th edition stage systems. The presentation and validity of the nomogram were also confirmed by calibration. Moreover, the present study utilized 7 easily accessible clinical variables that are widely available in clinical practice, which bring convenience for using of the nomograms.
This study has several limitations that should be noted. First, the nomogram was constructed using retrospective data from the SEER database and this may lead to the risk of potential selection bias. Second, some important clinicopathological parameters associated with prognosis, such as surgical margin status and vascular invasion, was unavailable in SEER dataset. That will be a major part in our future research. Third, although the 8th edition of the AJCC TNM staging system has been released recently, the TNM stage data of SEER database were not updated timely. Therefore, we felt sorry that we were unable to use the newest TNM staging system. Finally, as a user-friendly tool for doctors to make decisions, nomogram didn't include all prognostic factors and couldn't always provide precise prognosis in clinical practice.
In conclusion, for patients with PNETs, we established a nomogram to predict 3- and 5-year OS based on the large, population-based cohort for the first time. Our model showed a good performance in both training and validation sets, suggesting that it could be served as an effective tool for prognostic evaluation of patients with PNETs. However, further external validation is still needed.
Acknowledgments
Acknowledgments
The authors would like to thank SEER program for providing open access to the database.
Author Contributions
Conception and design: Wei Song, Dong-liu Miao, Lei Chen.
Collection and assembly of data: Wei Song, Jun Qian, Zhi-gang Zhu, Qiong Wu, Chang-guang Lv.
Data analysis and interpretation: Wei Song, Dong-liu Miao, Jun Qian, Zhi-gang Zhu, Chang-guang Lv.
Manuscript writing: All authors.
Final approval of manuscript: All authors.
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest.
Sources of Funding: The present study was funded by the Special projects for diagnosis and treatment of clinical special diseases in Suzhou (no. LCZX201713).
References
- 1.Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors: pancreatic endocrine tumors. Gastroenterology. 2008;135:1469–1492. doi: 10.1053/j.gastro.2008.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halfdanarson TR, Rabe KG, Rubin J, Petersen GM. Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2008;19:1727–1733. doi: 10.1093/annonc/mdn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, Shih T, Yao JC. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3:1335–1342. doi: 10.1001/jamaoncol.2017.0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frilling A, Akerstrom G, Falconi M, Pavel M, Ramos J, Kidd M, Modlin IM. Neuroendocrine tumor disease: an evolving landscape. Endocr Relat Cancer. 2012;19:R163–R185. doi: 10.1530/ERC-12-0024. [DOI] [PubMed] [Google Scholar]
- 5.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–959. doi: 10.1002/cncr.11105. [DOI] [PubMed] [Google Scholar]
- 6.Kloppel G. Tumour biology and histopathology of neuroendocrine tumours. Best Pract Res Clin Endocrinol Metab. 2007;21:15–31. doi: 10.1016/j.beem.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Li X, Gou S, Liu Z, Ye Z, Wang C. Assessment of the American Joint Commission on Cancer 8th Edition Staging System for Patients with Pancreatic Neuroendocrine Tumors: A Surveillance, Epidemiology, and End Results analysis. Cancer Med. 2018;7:626–634. doi: 10.1002/cam4.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Touijer K, Scardino PT. Nomograms for staging, prognosis, and predicting treatment outcomes. Cancer. 2009;115:3107–3111. doi: 10.1002/cncr.24352. [DOI] [PubMed] [Google Scholar]
- 9.Fang C, Wang W, Feng X, Sun J, Zhang Y, Zeng Y, Wang J, Chen H, Cai M, Lin J. Nomogram individually predicts the overall survival of patients with gastroenteropancreatic neuroendocrine neoplasms. Br J Cancer. 2017;117:1544–1550. doi: 10.1038/bjc.2017.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou H, Zhang Y, Qiu Z, Chen G, Hong S, Chen X, Zhang Z, Huang Y, Zhang L. Nomogram to predict cause-specific mortality in patients with surgically resected Stage I non–small-cell lung cancer: A competing risk analysis. Clin Lung Cancer. 2017;2:195–203. doi: 10.1016/j.cllc.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 11.Liu J, Geng Q, Liu Z, Chen S, Guo J, Kong P, Chen Y, Li W, Zhou Z, Sun X. Development and external validation of a prognostic nomogram for gastric cancer using the national cancer registry. Oncotarget. 2016;7:35853–35864. doi: 10.18632/oncotarget.8221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang ZY, Luo QF, Yin XW, Dai ZL, Basnet S, Ge HY. Nomograms to predict survival after colorectal cancer resection without preoperative therapy. BMC Cancer. 2016;16:658. doi: 10.1186/s12885-016-2684-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cronin KA, Ries LA, Edwards BK. The Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute. Cancer. 2014;120(Suppl. 23):3755–3757. doi: 10.1002/cncr.29049. [DOI] [PubMed] [Google Scholar]
- 14.Wolbers M, Koller MT, Witteman JC, Steyerberg EW. Prognostic models with competing risks: methods and application to coronary risk prediction. Epidemiology. 2009;20:555–561. doi: 10.1097/EDE.0b013e3181a39056. [DOI] [PubMed] [Google Scholar]
- 15.Singh S, Dey C, Kennecke H, Kocha W, Maroun J, Metrakos P, Mukhtar T, Pasieka J, Rayson D, Rowsell C. Consensus recommendations for the diagnosis and management of pancreatic neuroendocrine tumors: guidelines from a Canadian National Expert Group. Ann Surg Oncol. 2015;22:2685–2699. doi: 10.1245/s10434-014-4145-0. [DOI] [PubMed] [Google Scholar]
- 16.Wiedenmann B, Pavel M, Kos-Kudla B. From targets to treatments: a review of molecular targets in pancreatic neuroendocrine tumors. Neuroendocrinology. 2011;94:177–190. doi: 10.1159/000329386. [DOI] [PubMed] [Google Scholar]
- 17.Shen W, Sakamoto N, Yang L. Cancer-specific mortality and competing mortality in patients with head and neck squamous cell carcinoma: a competing risk analysis. Ann Surg Oncol. 2015;22:264–271. doi: 10.1245/s10434-014-3951-8. [DOI] [PubMed] [Google Scholar]
- 18.Skillington SA, Kallogjeri D, Lewis JS, Jr., Piccirillo JF. Prognostic importance of comorbidity and the association between comorbidity and p16 in oropharyngeal squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg. 2016;142:568–575. doi: 10.1001/jamaoto.2016.0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wray CJ, Phatak UR, Robinson EK, Wiatek RL, Rieber AG, Gonzalez A, Ko TC, Kao LS. The effect of age on race-related breast cancer survival disparities. Ann Surg Oncol. 2013;20:2541–2547. doi: 10.1245/s10434-013-2913-x. [DOI] [PubMed] [Google Scholar]
- 20.Zhou H, Zhang Y, Song Y, Tan W, Qiu Z, Li S, Chen Q, Gao S. Marital status is an independent prognostic factor for pancreatic neuroendocrine tumors patients: An analysis of the Surveillance, Epidemiology, and End Results (SEER) database. Clin Res Hepatol Gastroenterol. 2017;4:476–486. doi: 10.1016/j.clinre.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Yang X, Sun F, Chen L, Shi M, Shi Y, Lin Z, Feng M, Zhan C, Jiang W, Wang Q. Prognostic value of visceral pleural invasion in non-small cell lung cancer: A propensity score matching study based on the SEER registry. J Surg Oncol. 2017;3:398–406. doi: 10.1002/jso.24677. [DOI] [PubMed] [Google Scholar]
- 22.Oweira H, Petrausch U, Helbling D, Schmidt J, Mannhart M, Mehrabi A, Schob O, Giryes A, Decker M, Abdel-Rahman O. Prognostic value of site-specific metastases in pancreatic adenocarcinoma: A Surveillance Epidemiology and End Results database analysis. World journal of gastroenterology: WJG. 2017;23:1872–1880. doi: 10.3748/wjg.v23.i10.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez ME, Anderson K, Murphy JD, Hurley S, Canchola AJ, Keegan TH, Cheng I, Clarke CA, Glaser SL, Gomez SL. Differences in marital status and mortality by race/ethnicity and nativity among California cancer patients. Cancer. 2016;122:1570–1578. doi: 10.1002/cncr.29886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16:e173–e180. doi: 10.1016/S1470-2045(14)71116-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kattan MW, Leung DH, Brennan MF. Postoperative nomogram for 12-year sarcoma-specific death. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20:791–796. doi: 10.1200/JCO.2002.20.3.791. [DOI] [PubMed] [Google Scholar]
- 26.Bochner BH, Kattan MW, Vora KC. Postoperative nomogram predicting risk of recurrence after radical cystectomy for bladder cancer. Journal of Clinical Oncology: official journal of the American Society of Clinical Oncology. 2006;24:3967–3972. doi: 10.1200/JCO.2005.05.3884. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Li J, Xia Y, Gong R, Wang K, Yan Z, Wan X, Liu G, Wu D, Shi L. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2013;31:1188–1195. doi: 10.1200/JCO.2012.41.5984. [DOI] [PubMed] [Google Scholar]
- 28.Cao J, Yuan P, Wang L, Wang Y, Ma H, Yuan X, Lv W, Hu J. Clinical nomogram for predicting survival of esophageal cancer patients after esophagectomy. Sci Rep. 2016;6 doi: 10.1038/srep26684. [DOI] [PMC free article] [PubMed] [Google Scholar]