Abstract
PURPOSE: Performance of anatomical metrics of Response Evaluation Criteria in Solid Tumors (RECIST1.1) versus Positron Emission Tomography Response Criteria in Solid Tumors (PERCIST1.0) for neoadjuvant chemoradiation (nCR) of pancreatic adenocarcinoma was evaluated based on the pathological treatment response (PTR) data. METHODS AND MATERIALS: The pre- and post-nCR CT and PET data for 14 patients with resectable or borderline resectable pancreatic head adenocarcinoma treated with nCR followed by surgery were retrospectively analyzed. These data were compared with the PTR which were graded according to tumor cell destruction (cellularity), with Grade 0, 1, 2 or 3 (G0, G1, G2 or G3) for complete, moderate, minimal and poor responses, respectively. Maximum standardized uptake value (SUVmax) was defined using body-weight (SUVbw). PERCIST1.0 was defined using lean-body mass normalized SUV (SUVlb or SUL). RECIST1.1 was defined by contouring the whole pancreas head on the CT image. Pre- and post-SUL-peak and SUVmax, RECIST1.1 and PETRECIST1.0 were correlated with PTR using Pearson’s correlation coefficient test. RESULTS: The average mean and SD in SUL-peak for all patients analyzed were lower in post-nCR (3.63±1.06) compared to those at pre-nCR (4.29±0.89). Using PERCIST1.0, 62% of patients showed stable metabolic disease (SMD), 23% partial metabolic response (PMR), and 15% progressive metabolic disease (PMD). Using RECIST1.1, 85% of patients showed stable disease (SD), 8% partial response (PR), and 7% progressive diseases (PD). A poor insignificant correlation was established between PRT and PERECIST1.0 (r=0.121), whereas no correlation was seen with RECIST1.1. CONCLUSIONS: PERCIST1.0 appears to increase the chance of detecting patients with progressive disease compared to the conventional anatomical-based assessment of RECIST1.1. The integration of these additional radiographic metrics in assessing treatment response to nCR for pancreatic adenocarcinoma may provide a promising strategy to better select patients that are most suitable for therapeutic intensification.
Introduction
Despite the continuous progress in diagnosis and treatment, the prognosis of pancreatic cancer remains very poor [1]. In the USA, the number of estimated new pancreatic cancer patients in 2018 is 55,440, whereas the number of pancreatic cancer estimated death in 2018 are 44,330. This indicates the number of patients with pancreatic cancer is almost equal to the number of pancreatic related deaths [2]. Treatment regimens of this disease are determined on the basis of the extent and resectability of pancreatic cancer that has primarily been evaluated by contrast-enhanced abdominal computer tomography (abdominal CE-CT) [3], [4], [5]. Moreover, evaluation of neoadjuvant treatment effect/response and prediction of resectability were [6], [7] also assessed solely based on anatomical changes using computer tomography (CT) or CE-CT images, following guidelines of Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 [8]. Anatomical-based changes criteria typically refer to tumor shrinkage or increase in size that has been defined commonly as complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) [8]. However, the wide range spanned by the current cutoff values of 30% decrease considered for PR, 20% increase considered for PD and neither sufficient 30% shrinkage nor 20% increase considered for SD using RECIST1.1 may delay detection of disease progression and regression [9] In addition, a potential limitation of using CT imaging to evaluate response in pancreatic cancer is attributed to the nature of sporadically scattered cancer cells in vigorously growth fibrosis and connective tissue background [10], eliminating the possibility of identifying tumor lesions. In contrast, apparent diffusion coefficient (ADC) parameter map obtained from diffusion weighted (DW) magnetic resonance imaging (MRI) shows promising in predicting and assessing neoadjuvant therapy in pancreatic cancers [11], [12], [13]. Further, 18Fluro-deoxy-glucose positron emission tomography (FDG-PET) has several theoretical advantages over conventional CT, partially because FDG capacity reflects tumor cell viability. FDG-PET appears to be superior to the anatomical-base tumor response criteria in lymphoma, lung cancer, mesothelioma [12], pancreas cancer [10], [14], [15] and esophageal cancer [16]. Furthermore, FDG-PET efficacy in determining treatment response in pancreatic cancer suggested reducing unnecessary operations [17], [18]. Considering the previous facts, an updated PET criterion, known as Positron Emission Tomography Response Criteria in Solid Tumors (PERCIST1.0), has been proposed for consideration in clinical trials and possibly in clinical practice [19]. PERCIST1.0 criteria established slightly different cutoff values with at least 30% decrease in SUL-peak considered for partial metabolic response (PMR), at least 30% increase in SUL-peak considered for progressive metabolic disease (PMD) and stable metabolic disease (SMD) classified if any of the PMR or PMD were not met. The aim of this exploratory study is to investigate the potentials of PERCIST1.0 versus RECIST1.1 in predicting more accurate tumor treatment effect/response that is validated with pathology outcomes.
Materials and Methods
Imaging and pathological data from a total of 15 randomly-selected patients with histologic confirmation of pancreatic adenocarcinoma were retrospectively reviewed. All these patients had tumor in their pancreatic heads and underwent surgery after completing neoadjuvant chemoradiation (nCR) therapy. By our institutional protocol, resectable and borderline resectable pancreatic cancers are treated with chemotherapy (Gemcitabine or Xeloda and FOLFIRINOX or Gemcitabine-Abraxane) and radiation therapy (RT) dose of 50.4 cGy delivered in 28 fractions using intensity modulated radiation therapy (IMRT). All 15 patients had a pre- and –post nCR FDG-PET/CT scans. All patients underwent surgery in a period ranging from 29 to 50 days after completing the nCR treatment, whereas PET/CT scan was usually one week prior to the surgery. Patient characteristics are shown in Table 1.
Table 1.
Patient Characteristics
| Factors | Groups |
|---|---|
| Total Sample Size | 15 |
| Treatment Effect Grading | |
| G1 | 2 (%14) |
| G2 | 9 (%57) |
| G3 | 4 (%29) |
| Tumor status | |
| Resectable | 2 (%13) |
| Borderline | 13 (%87) |
PET/CT Imaging
Free-breathing PET studies were performed using a PET/CT system (Discover Loadstone; GE). Patients were instructed to fast for at least 6 hours before acquisition of the PET study to eliminate FDG uptake in normal background organs. For imaging of the pancreatic mass, approximately 10-19 mCi (370-703 MBq) of FDG was injected intravenously. Images from the base of the skull through the mid-thigh level were acquired after a delay period of 45-60 minutes using a whole-body protocol. Non-contrast-enhanced axial CT was obtained over the same geometric prescription for attenuation correction of the PET images. All PET images were reconstructed using the ordered subset expectation maximization (OSEM) algorithm to an average slice thickness of 3.25 mm and pixel spacing of 3.6× 3.6 mm2. CT images were acquired with 3.75 mm slice thickness, and 1.4 mm pixel size. The PET/CT images were transferred offline and post processed using MIM system (version 6.4.5; Cleveland, OH, USA).
Pathological Treatment Response
After pancreatectomy, specimens were fixed in formalin overnight and gross examination was performed. The area of tumor and surrounding fibrosis in the head of the pancreas was serially sectioned. Hematoxylin and eosin sections were prepared and treatment effect was evaluated microscopically. The College of American Pathologist (CAP) grading system [20] was used to evaluate the extent of residual tumor as follows: Grade 0 (G0) for complete response (no viable cancer cells), Grade1 (G1) for moderate response (single cells or small groups of residual cancer cells in extensive fibrosis), Grade 2 (G2) for minimal response (residual cancer outgrown by fibrosis), and Grade 3 (G3) for poor response (extensive residual cancer).
PERCIST1.0 vs. RECIST1.1 Criteria
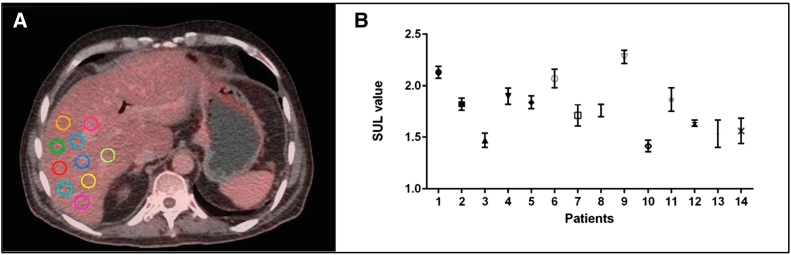
PERCIST1.0 necessitates a consistent and reliable measure of tumor activity, hence it recommends using a lean body mass normalize standard uptake value (SUVlb or SUL), which is a less weight dependent approach compared to the standard body weight normalized standard uptake value (SUVbw). Herein the native PET images were converted to SUL on a voxel wise-basis using the built-in algorithm in MIM. Moreover, PERCIST1.0 criterion enforces quantifying the background uptake level by calculating the SUL in one of the two recommended body reference organs; in case of healthy liver, the right lobe of the liver is used, otherwise the descending aorta. The background uptake in the reference organ is determined from both the mean SUL (denoted SULmean) and the SUL standard deviation (denoted SULSD) in 10 spherical volumes, each with 1 cm3 spherical region of interest (ROI) located at the right liver lobe, Figure 1A. The SUL in the reference organ can then be calculated following Eqs. (1) and (2) and used to threshold the tumor lesion to create a volume of interest (VOI). The uptake in the reference organs should not vary more than 0.3 SUL units between the different time points of measurements i.e., pre- and post nCR PET/CT scans. The key parameter used in the PERCIST1.0 analysis is the SUL-peak (reportable value) that is defined as the largest possible mean value in a 1 cm3 spherical ROI positioned within a tumor [14]. A built-in algorithm in MIM (MIM Software Inc.) was used to calculate the SUL-peak. The reader is refereed to [21] for the mathematical detailed aspect of PERCIST1.0.
Figure 1.
(A) 10 spherical volumes each with 1 cm3 spherical region of interest (ROI) located at the right liver lobe to determine the mean and SD of post-SUL value in liver of patient 1#. (B) significant post-SUL values in the liver between individuals (P < .05) based on Mann Whitney test.
SUL in the right lobe of liver is calculated as:
| (1) |
SUL in the descending aorta is calculates as:
| (2) |
Treatment Response Assessment
Treatment response was assessed over two time points, pre- and post nCR, for all 15 patients who underwent PET/CT scan and surgery after completing the nCR therapy. RECIST 1.1 was assessed by measuring the maximum diameter of the whole pancreas head on the CT image from the PET/CT. First, the whole pancreas head was manually contoured on the CT image followed by MIM built-in algorithm of RECIST1.1 to determine the maximum diameter (in cm). For the PETRECIST1.0, the calculated SUL value in the liver was used to threshold the PET image to identify the lesion on the pancreas tumor. With tumor lesion being identified on the PET images, MIM built-in algorithm of PERCIST1.0 was used to determine the SUL-peak. After determining the increase and decrease in tumor size (in cm) and in the SUL-peaks, PERCIST1.0 and RECIST1.1 were correlated with pathological treatment response (PTR) cellularity. This analysis was conducted only on patients whose SUL-peak variation between the two time-points was <0.3 SUL units. In addition, the maximum standard uptake value (SUVmax) was measured in pre- and post nCR PET/CT scans for all 15 patients. For this part, the native PET images were converted to SUVbw on a voxel bases using the MIM build-in algorithm. A ROI circle that is large enough to include all lightens up voxels within the suspected region i.e. pancreas head, would provide enough statistics to measure the SUVmax within the ROI.
To study the correlation between PERCIST1.0 and PTR, a loose criterion was followed with PTR Grade 1 considered a PMR, Grade 2 a SMD, and Grade 3 a PMD.
Statistical Analysis
The pre- and post SUL-peaks and SUVmax, PTR, PERCIST1.0, and RECIST1.1 for the patients studied were transferred to Prism (version 6; GraphPad Software, La Jolla, CA) for statistical analysis. A Mann Whitney test was used to determine whether significant differences existed between pre- and post SUL-peaks and SUVmax, and between PERCIST1.0, RESICT1.1 and PTR. A Person’s correlation coefficient test was used to determine the correlation between RECIST1.1 and PERCIST1.0. For all statistical tests, P ≤ .05 was used for significance.
Results
SUL Values
The SUL value in the liver was calculated for the two time-points, pre- and –post nCR, for all 15 patients. The SUL variations between the two time-points were <0.3 SUL units except for one patient, leading to exclude that particular patient from the study. SUL values were significantly different between individuals (P < .05) based on Mann Whitney test, Figure 1B, meaning each patient had a different thresholding cutoff value depending on their physiology and general health condition. The mean and range of SULs (i.e., the cutoff values) for pre- and post nCR was 3.04 (2.51-4.33), and 2.96 (2.35-3.73) for all 14 patients, respectively.
SUL-Peak Data
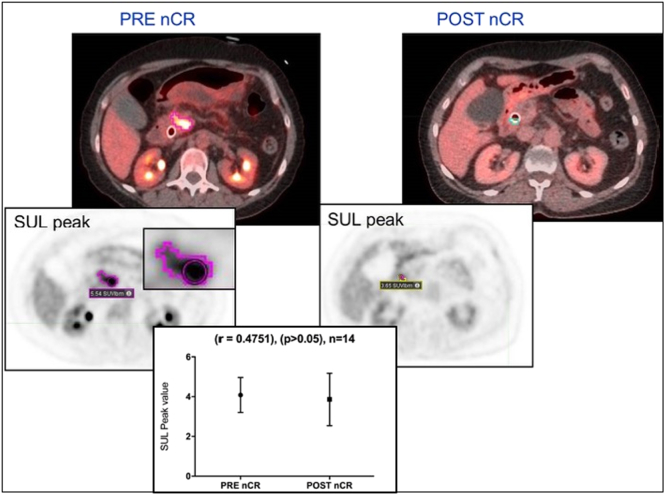
Pre- and post nCR SUL-peak values were statistically correlated (r = 0.4751) using Person’s test for all 14 patients. There was no statistical difference (P < .05) between the pre- and post nCR SUL-peak values based on Mann Whitney test. A lower mean average and SD SUL-peaks were seen in post-nCR (3.63±1.06) compared to those in pre-nCR (4.29±0.89) (Figure 2). Pre- and post nCR SUVmax were statistically correlated (r=0.4848) using Person’s test for all 14 patients. The differences were statistically significant (P < .05) based on Mann Whitney test. A lower mean average and SD SUVmax were seen in post-nCR (4.31±1.35) compared to those at pre-nCR (7.37±1.96).
Figure 2.
Delineation of pancreatic adenocarcinoma defined by applying an individualized threshold value based on individuals SUL value in the liver for patient #4, resulting in an insignificant statistical difference between pre-and-post nCR SUL-peak.
Pathological Treatment Response
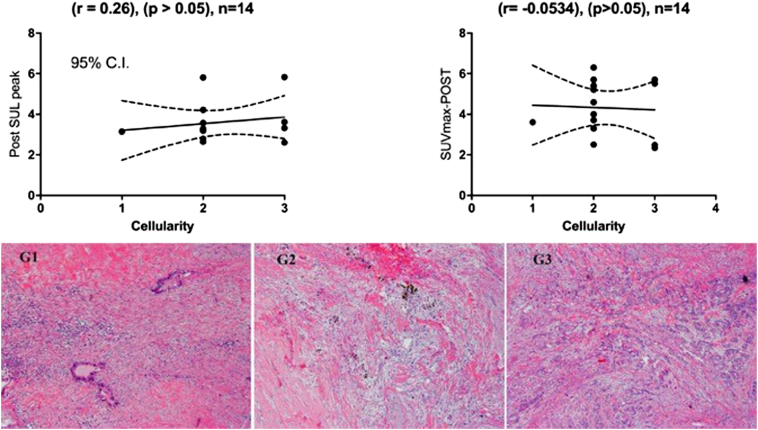
Post nCR SUL-peaks were correlated with PTR cellularity, using the grading system of Grade 0, 1, 2 or 3 (G0, G1, G2 or G3) for complete, moderate, minimal and poor responses, respectively. A moderate positive insignificant correlation was obtained using SUL-peak (r=0.26), (P < .05) for all 14 patients, whereas a negative weak insignificant correlation was obtained correlating post SUVmax (i.e. calculated using SUVbw) and PTR (r = -0.0534), (P < .05), Figure 3.
Figure 3.
Moderate correlation with post-SUL peak versus poor correlation with post-SUVmax (top) of pathological response (cellularity) and the stained pictures (bottom) of all 3 categories, with G1 shown single cells or small clusters of residual cancer cells in extensive fibrosis, G2 residual cancer outgrown by fibrosis, and G3 extensive residual cancer.
A poor insignificant correlation was established between PRT (cellularity) and PERCIST1.0 (r=0.121) while no correlation was seen with RECIST1.1.
PERCIST1.0 vs. RECIST1.1
Using PERCIST1.0, 62% of the patients showed stable SMD, 23% PMR and 15% PMD. Using RECIST1.1, 85% of patients showed SD, 8% PR, and 7% PD.
Discussion
In this study, the tumor treatment response after neoadjuvant chemoradiotherapy for resectable and borderline resectable pancreatic cancer was assessed according to PERCIST1.0 and RECIST guidelines. PERCIST1.0 was determined by measuring the SUL-peaks whereas RECIST1.1 was determined by measuring the longest diameter of the measurable lesion i.e. pancreas head using the CT images from an abdominal PET/CT.
Anatomical tumor response evaluation metrics for individuals to certain treatment was found unsatisfactory [9]. It is well known that tumor prognosis is associated with biological aggressiveness of the tumor rather than residual tumor volume after therapy [22]. Typically, large residual masses may only be containing inflammation of fibrotic tissue, whereas smaller residual may contain high resistant and aggressive cell clusters that may lead to relapse or tumor recurrence [2]. Thus, determining the lack of tumor progression by relying solely on tumor size would require more regular and systematic assessments of tumor burden [23], raising the need to develop newer metrics such as PET that permits visualizing tumor heterogeneity and allows more accurate differentiation between treatment induced fibrosis/necrosis [24], [25].
In advanced non-small cell lung cancer (NSCLC) Birchard et al. [26] reported no correlation between pathological response outcome and tumor shrinkage or increase in size metrics following neoadjuvant treatment, which could in part, be related to the one dimensional (1D) measurement of the longest tumor axis on an axial slice [27] and to the poor predictive ability of CT to trace cell viability [28]. On the other hand, tumor metabolic response metrics were found to be superior to the anatomical based metric in locally advanced pancreatic tumors after chemoradiation therapy [15]. The multivariate study analysis by Birchard et al [26] found that PERCIST1.0 was the only independent prognostic factor that was associated with overall survival in advanced NCSLC, suggesting that metabolic response to neoadjuvant therapy in advanced NCSLC maybe a better predictor for overall survival than RECIST1.1. A similar finding was confirmed and reported by Ding et al [29]. Recently, the metabolic metrics of FDG-PET was found to increase the chance of detecting local tumor progression in locally advance pancreatic cancer following stereotactic body radiation therapy (SBRT) [30], [31].
Our study revealed a poor insignificant correlation when cross validating PERECIST1.0 with pathological treatment response outcome (cellularity), while no correlation was established with RECIST1.1. This observation is in line with results discussed previously. On the other hand, a phase II treatment response study of an advanced esophageal and gastroesophageal carcinoma suggested that PERCIST1.0 response in combination with RECIST1.1 may better assess clinical outcome compared to RECIST1.1 response alone [28]. van Veldhuisen et al. [32] also recommended the need for serum CA19-9 response in addition to RECIST criteria for a more accurate selection of exploratory laparotomy patients. These studies showed that a single metrics may not be enough to accurately assess tumor response to treatment.
Based on the findings obtained in this pilot study, SUL-peak values correlates better with pathology treatment response (cellularity) comparing to the SUVmax values, with (r=0.2392) verses (r=0.0534), respectively. Post nCR SUL-peak and SUVmax values were lower comparing to pre nCT SUL-peak and SUVmax values. PERCIST1.0 criteria allows for individual PET thresholding cutoff values that is determined by patient physiology and overall health condition. Using PERCIST1.0, 15% of the patients were found with progressive disease comparing to only 7% using RECIST1.1.
A major limitation in our study is the small patient number. Furthermore, the 3D free breathing PET/CT may underestimate SUL-peak and SUvmax values due to motion of pancreas [33], demanding thorough investigation perhaps with a 4D PET/CT. For the future work, in addition to increasing number of patients, we will also try to combine PERCIST1.0 response and RECIST1.1 response for improving the correlation between imaging treatment response data and pathological treatment response data.
Conclusion
FDG-PET treatment response metric may be more informative than the CT-based assessment with an enhanced predicative ability related to cell viability information, providing a promising strategy to better select patients that are most suitable for therapeutic intensification.
Footnotes
This work is partially supported by MCW Cancer Center Meiner Foundation.
Conflicts of Interest Notification: Do not exist.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.ACS American Cancer Society . American Cancer Society; Atlanta, Ga: 2018. Cancer Facts & Figures; p. 2018. [Google Scholar]
- 3.Lee ES, Lee JM. Imaging diagnosis of pancreatic cancer: A state-of-the-art review. World J Gastroenterol. 2014;28:7864–7877. doi: 10.3748/wjg.v20.i24.7864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tummala P, Junaidi O, Agarwal B. Imaging of pancreatic cancer: an overview. J Gastrointest Oncol. 2011;2:168–174. doi: 10.3978/j.issn.2078-6891.2011.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Somers I, Bipat S. Contrast-enhanced CT in determining resectability in patients with pancreatic carcinoma: a meta-analysis of the positive predictive values of CT. Eur Radiol. 2017;27:3408–3435. doi: 10.1007/s00330-016-4708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cassinotoo C, Mouries A, Lafourcade J, Terrebonne E, Belleannee G, Blanc J, Lapuyade B, Venderly V, Laurent C, Chiche L. Locally advanced pancreatic adenocarcinoma: reassessment of response with CT after neoadjuvant chemotherapy and radiation therapy. Radiology. 2017;273:108–116. doi: 10.1148/radiol.14132914. [DOI] [PubMed] [Google Scholar]
- 7.Cassinotto C, Sa-Cunha A, Trillaud H. Radiological evaluation of response to neoadjucant treatment in pancreatic cancer. Diagn Interv Imaging. 2016;97:1225–1232. doi: 10.1016/j.diii.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 8.O J H, Lodge MA, Wahl RL. Practical PERCIST: a simplified guide to PET response criteria in solid tumors 1.0. Radiology. 2016;280:576–584. doi: 10.1148/radiol.2016142043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao B, Schwartz LH, Larson SM. Imaging surrogates of tumor response to therapy: anatomic and functional biomarkers. J Nucl Med. 2009;50:239–249. doi: 10.2967/jnumed.108.056655. [DOI] [PubMed] [Google Scholar]
- 10.Bang S, Chung HW, Park SW, Chung JB, Yun M, Lee JD, Song SY. The clinical usefulness of 18-fluorodeoxyglucose positron emission tomography in the different diagnosis, staging, and response evaluation after concurrent chemoradiatiotherapy for pancreatic cancer. J Clin Gastroenterol. 2006;40:923–929. doi: 10.1097/01.mcg.0000225672.68852.05. [DOI] [PubMed] [Google Scholar]
- 11.Cuneo KC, Chenevert TL, Ben-Josef E, Feng MU, Greenson JK, Hussain HK, Simeone DM, Schipper MJ, Anderson MA, Zalupski MM. A pilot study of diffusion-weighted MRI in patients undergoing neoadjuvant chemoradiation for pancreatic cancer. Transl Oncol. 2014;7:644–649. doi: 10.1016/j.tranon.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trajkovic-Arsic M, Heid I, Steiger K, Gupta A, Fingerle A, Worner C, Teichmann N, Sengkwawoh-Lueong S, Wenzel P, Beer AJ. Apparent diffusion coefficient (ADC) predicts therapy response in pancreatic ductal adenocarcinoma. Sci Rep. 2017;7:1–9. doi: 10.1038/s41598-017-16826-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalah E, Erickson B, Oshima K, Schott D, Hall WA, Paulson E, Tai A, Knechtges P, Li XA. Correlation of ADC with pathological treatment response for radiation therapy of pancreatic cancer. 2018;11:391–398. doi: 10.1016/j.tranon.2018.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuwatani M, Kawakami H, Eto K, Haba S, Shinga T, Tamaki N, Asaka M. Modalities for evaluating chemotherapeutic efficacy and survival time in patients with advanced pancreatic cancer: comparison between FDG-PET, CT and serum tumor markers. Int Med. 2009;48:867–875. doi: 10.2169/internalmedicine.48.2009. [DOI] [PubMed] [Google Scholar]
- 15.Grassetto G, Rubello D. Role of FDG-PET/CT in diagnosis, staging, response to treatment, and prognosis of pancreatic cancer. 2011;34:111–114. doi: 10.1097/COC.0b013e3181d275a0. [DOI] [PubMed] [Google Scholar]
- 16.Lordick F, Ott K, Krause BJ, Weber WA, Becker K, Stein HJ, Lorenzen S, Schuster T, Wieder H, Herrmann K. PET to assess early metabolic response and to guide treatment of adenocarcinoma of the esophagogastric junction: the MUNICON phase II trial. Lancet Oncol. 2007;8:797–805. doi: 10.1016/S1470-2045(07)70244-9. [DOI] [PubMed] [Google Scholar]
- 17.Yoshioka M, Sato T, Furuya T, Shibata S, Andoh H, Asanuma Y, Hatazawa J, Shimosegawa E, Koyama K, Yamamoto Y. Role of positron emission tomography with 2-deoxy-2[18F]fluoro-D-glucose in evaluating the effects of arterial infusion chemotherapy and radiotherapy on pancreatic cancer. J Gastroenterol. 2004;39:50–55. doi: 10.1007/s00535-003-1244-2. [DOI] [PubMed] [Google Scholar]
- 18.Orlando LA, Kulasingam SL, Matchar BD. Meta-analysis: the detection of pancreatic malignancy with positron emission tomography. Aliment Pharmacol Ther. 2004;20:1063–1070. doi: 10.1111/j.1365-2036.2004.02266.x. [DOI] [PubMed] [Google Scholar]
- 19.Wahl RL, Jacene H, Kasamon Y, Lodge M. From RECIST to PERCISIT: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50:112S–150S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Washington K, Berlin J, Branton P, Burgart LJ, Carter DK, Compton CC, Fitzgibbons P, Frankel W, Jessup J, Kakar S. PancreasExocrine 3.2.0.1. 2013. Protocol for the examination of specimens from patients with carcinoma of the exocrine pancreas. [Google Scholar]
- 21.Ghosh P., Kelly M. Expanding the power of PET with PERCIST. White paper Siemens. 2010 [Google Scholar]
- 22.Farnebo J, Gryback P, Harmenberg U, Laurell A, Wersall P, Blomqvist L, Ullen A, Sandstrom P. Volumetric FDG-PET predicts overall and prognosis-free survival after 14 days of targeted therapy in metastatic renal cell carcinoma. BMC Cancer. 2014;14:408. doi: 10.1186/1471-2407-14-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petullo B, Wei L, Yereb M, Neal A, Rose J, Bekaii-Saab T, Wu C. A phase II study biweekly pralatrexate and docetaxel in patients with advanced esophageal and gastroesophageal carcinoma that have failed first-line platinum-based therapy. J Gastrointest Oncol. 2015;6:336–340. doi: 10.3978/j.issn.2078-6891.2015.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scialpi M, Reginelli A, D’Andrea A, Gravante S, Falcone G, Baccari P, Manganaro L, Palumbo B, Cappabianca S. Pancreatic tumors imaging: an update. Int J Surg. 2016;28:S142–S155. doi: 10.1016/j.ijsu.2015.12.053. [DOI] [PubMed] [Google Scholar]; Toesca DA, Pollom EL, Poulloe PD, Flynt L, Cui Y, Quon A, von Eyben R, Koong AC, Chang DT. Assessing local progression after stereotactic body radiation therapy for unresectable pancreatic adenocarcinoma: CT versus PET. Pract Radiat Oncol. 2017;7:120–125. doi: 10.1016/j.prro.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Birchard KR, Hoang JK, Herndon JE, Patz EF. Early changes in tumor size in patients treated for advanced stage nonsmall cell lung cancer do not correlate with survival. Cancer. 2009;115:581–586. doi: 10.1002/cncr.24060. [DOI] [PubMed] [Google Scholar]
- 27.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larson SM, Schwartz LH. 18F-FDG PET as a candidate for “qualified biomarker”: functional assessment of treatment response in oncology. J Nucl Med. 2006;47:901–903. [PubMed] [Google Scholar]
- 29.Ding Q, Cheng X, Yang L, Zhang Q, Chen J, Li T, Shi H. PET/CT evaluation of response to chemotherapy in non-small cell lung cancer: PET response criteria in solid tumors (PERCIST) versus response evaluation criteria in solid tumors (RECIST) J Thorac Dis. 2014;6:677–683. doi: 10.3978/j.issn.2072-1439.2014.05.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asagi A, Ohta K, Nasu J, Tanada M, Nadano S, Nishimura R, Teramoto N, Yamamto K, Inoue T, Iguchi H. Utility of contrast-enhanced FDG-PET/CT in the clinical management of pancreatic cancer. Pancreas. 2013;42:11–19. doi: 10.1097/MPA.0b013e3182550d77. [DOI] [PubMed] [Google Scholar]
- 31.Toesca DA, Pollom EL, Poulloe PD, Flynt L, Cui Y, Quon A, von Eyben R, Koong AC, Chang DT. Assessing local progression after stereotactic body radiation therapy for unresectable pancreatic adenocarcinoma: CT versus PET. Pract Radiat Oncol. 2017;7:120–125. doi: 10.1016/j.prro.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 32.van Veldhuisen E, Vogel JA, Klompmaker S, Busch OR, van Laarhoven H, van Lienden KP, Wilmink JW, Marsman HA, Besselink MG. Added value of CA 19-9 response in predicting resectability of locally advanced pancreatic cancer following induction chemotherapy. HPB. 2018 doi: 10.1016/j.hpb.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 33.Harteela M, Hirvi H, Mäkipää A, Teuho J, Koivumäki T, Mäkelä MM, Teräs M. Comparison of end-expiratory respiratory gating methods for PET/CT. Acta Oncol. 2014;53(8):1079–1085. doi: 10.3109/0284186X.2014.926028. [DOI] [PubMed] [Google Scholar]