Abstract
This review summarizes data that assign morphological, biochemical and functional characteristics of two types of structures that are associated with centrioles: distal appendages and subdistal appendages. The description of centriole subdistal appendages is often a matter of confusion, both due to the numerous names used to describe these structures and because of their variability among species and cell types. Thus, we have summarized our current knowledge in this review. We conclude that distal appendages and subdistal appendages are fundamentally different in composition and function in the cell. While in centrioles there are always nine distal appendages, the number of subdistal appendages can vary depending on the type of cells and their functional state.
Keywords: centriole, distal appendages, subdistal appendages
1. Introduction
The fact that the centrosome is a complex organelle consisting of many components is today generally accepted [1–4]. The ultrastructural features of these components were described in detail in the middle of the last century. At the beginning of the cell cycle, the centrosome contains two centrioles surrounded by a pericentriolar material, the protein composition of which is now intensively studied [5]. The two centrioles in the centrosome differ in age, functional activity in the cell and the presence of additional structures associated with their surface. The older centriole, which is called the mother, arose at least one cell cycle earlier than the second, which is called the daughter centriole. Centrioles are cylindrical polar structures built of microtubules (MT). At the proximal end of the centriolar cylinder is the minus and at the distal end is the MT plus-end. The distal end of the mother centriole can also grow a primary cilium. In the process of centriole duplication, which begins in the G1-phase of the cell cycle [6,7], the formation of new young centrioles—procentrioles occurs on both pre-existing centrioles near their proximal ends. Further, the distal surfaces of mother centrioles are associated with two types of outgrowths, the distal and subdistal appendages. This review focuses on subdistal appendages.
In the past, many cellular components have been simultaneously described by several researchers and given, respectively, different names. In addition, the same component sometimes has intrinsic variations in different cells, which may also lead to terminological confusion. Over time, most of the terms were unambiguously defined. But even now, at the beginning of the twenty-first century, there are terminological discrepancies that complicate the interpretation of the results obtained by modern imaging methods.
It is generally believed that the morphological components of the centrosome are well described and the discrepancies in their definition have long since been eliminated. However, it turns out that in fact it is not so. Different researchers dealing with biology of the centrosome have different ideas about what a subdistal appendage is. Numerous discrepancies found in modern literature motivated us to write this review, the purpose of which is to clarify numerous terms proposed in the literature to describe two types of outgrowths associated centriole cylinders: distal and subdistal appendages.
2. ‘Massules’, ‘subdistal appendage’, ‘distal appendage’, ‘pericentriolar satellite’—who is who on the centriole wall?
To give a definitive answer to the question what a subdistal appendage eventually is, it is necessary to make a short digression into the history of the centriole studies, and to define the terms such as ‘appendages’, ‘distal appendages’, ‘subdistal appendages’, ‘satellites’ and ‘pericentriolar satellites’.
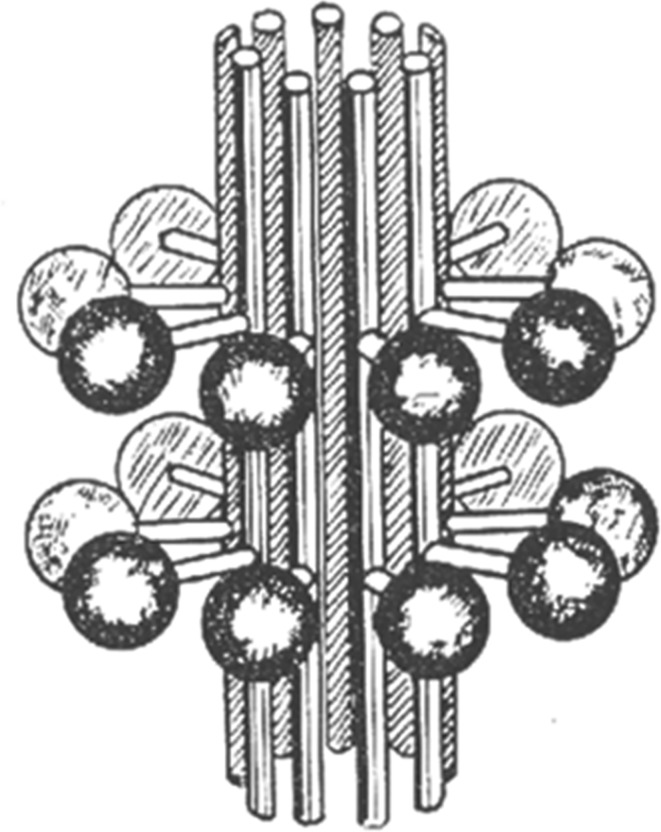
The first volumetric model of the centriole, on which the outgrowth appeared on the surface of a centriolar cylinder, was published by Bessis & Breton-Gorius in 1958 [8] (figure 1). This was not a 3D reconstruction, but a drawing made by the authors on the basis of their own observations. The authors depicted a centriole which it contained two groups of nine appendages connected with centriole surface, which the authors called ‘massules’.
Figure 1.
Bessis & Breton-Gorius [8] discovered that the surface of leucocyte centrioles was often covered with growths, consisting of a stem with a round cap, which these authors called ‘massules’.
In this figure, both groups of appendages are equidistant from the ends of the centriolar cylinder (it was not yet known that centrioles have proximal-distal polarity). Later, one of these groups, which is located at the distal end of the centriole, was renamed distal appendages, or appendages (figure 2). There are also other terms in literature that were used to refer to similar structures on basal bodies. For example, the distal appendages were called satellites by Szöllösi [10], alar sheets by Anderson [11] and transitional fibres by O'Hara [12]. However, in this paper we will mainly talk about the structures associated with centrioles of the centrosome, but not basal bodies.
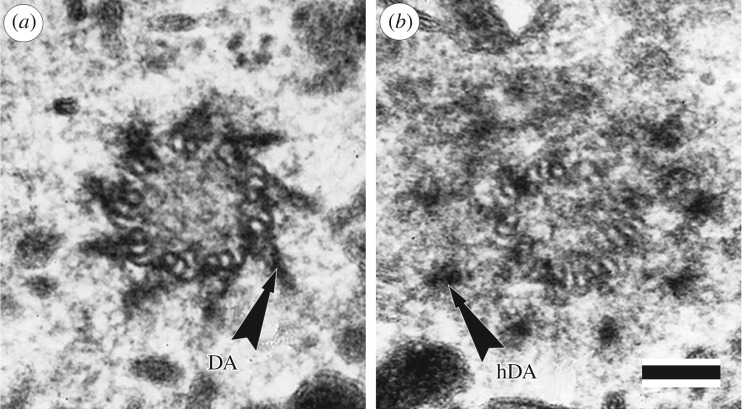
Figure 2.
Two consecutive ultrathin sections through the distal part of mother centriole in the cell of porcine kidney embryo cell line. (a) Distal appendages (DA) are always present in an amount of 9—one for each triplet (doublet) of MT of the mother centriole. (b) Heads of the distal appendages (hDA) are located more distally in comparison with points of attachment of the distal appendages to the surface of the centriolar cylinder and therefore are detected on the next ultrathin section. In the view from the proximal end of the centriole, the MT triplets are twisted counterclockwise, distal appendages are twisted in the opposite direction (clockwise). Scale bar, 100 nm. From [9].
The second group of outgrowths on the surface of the centriole, which are located closer to the central part of the centriolar cylinder, were originally named pericentriolar satellites [13], satellites [14], radial arms [15] and, finally, subdistal appendages [16]. The term subdistal appendages [16,17] seems to us less successful, since by the time of its appearance it was already known that subdistal appendages can be located along the entire length of the centriolar cylinders [18]. However, since at present this term is the most common, we will use it in our review.
Although only a hypothetical scheme, the ideas proposed by Bessis and Breton-Gorius in 1958 [8] (figure 1) proved to be tenacious and are regularly reproduced in the diagrams given in modern articles and reports at scientific conferences and unfortunately even in textbooks (for example, Alberts et al.'s figs 16–48 [19]). According to these schemes, the centriole is depicted with two rows of outgrowths (nine outgrowths in each); while in each row both subdistal appendages and distal appendages are located on the same level. Sometimes even specialists draw diagrams, where these structures look almost identical [20].
One particular paper played a decisive role in spreading this perception. In 1992, Journal of Structural Biology published the work with stunningly beautiful photos of centrioles [16], several images of which the publishers put on the cover. In one of the photos, the number of subdistal appendages was 9, although even in this photo it can be seen that part of the subdistal appendages have their base on two triplets of the centriole (figure 3c). Later this photo was included in several centrosome reviews [3,17,21–23].
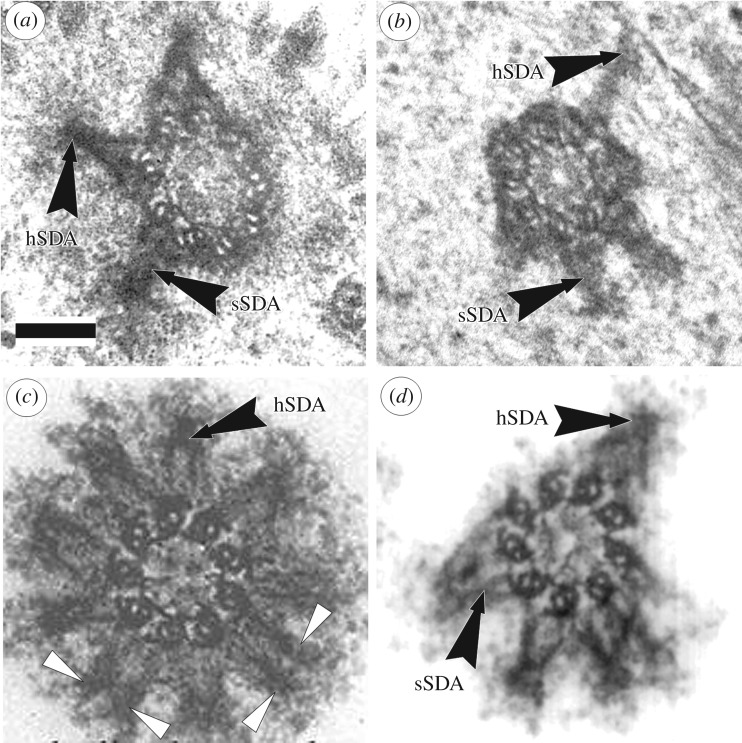
Figure 3.
Ultrathin sections through the subdistal part of mother centriole in different types of cells. (a) Cell with three subdistal appendages from epithelial pig kidney (PK) embryo cell [9], conical form of subdistal appendages stem (sSDA) with spherical heads (hSDA) are visible. Each subdistal appendage based on 2 or 3 centriolar MT triplets. (b) Cell with four subdistal appendages from pig oviduct cell [4]; (c,d) cells with nine and six subdistal appendages from KE-37 human cell line [16]. Arrowheads show cylindrical subdistal appendages without visible heads and based on 1 MT triplet of centriolar cylinder. In (a,c,d) the triplets are twisted clockwise, hence the view from the distal of the centriole, for photo ‘b’ the triplets are twisted counterclockwise, hence the view from the proximal end of the centriole. Scale bar, 100 nm.
In their article, the authors explicitly state: ‘The number, thickness, and distribution of the subdistal appendages can vary significantly from one centrosome to the other in the same preparation’ [16]. Indeed, we can see photographs of centrioles with 4, 6, 8, 9 subdistal appendages (for example figure 3c,d) in this article. One should also take into account the fact that isolated centrioles were studied in the work, whose morphology changes under the influence of experimental procedures used to extract centrosomes from cells—use of inhibitors of cytoskeleton components (Nocodazole, Cytochalasin), low temperatures and ultracentrifugation. In particular, in a number of cases, the heads of subdistal appendages are lost, which leads to the loss of their conical shape due to the divergence of parts of the stem associated with each of the triplets (figure 3c).
In conclusion, the confusion with the names of the initially identified centriole structures, characteristic for the initial period of electron microscope research, was gradually eliminated and common terms were agreed on. However, from all the terminological diversity in modern literature there is one controversial term—subdistal appendages (pericentriolar satellites)—which is still treated differently by different researchers.
The most common misconception in the field is that each of the subdistal appendages is associated with one triplet of a centriolar cylinder and that; hence the number of subdistal appendages is always nine. Such an opinion is reflected in numerous publications on which, like Bessis & Breton-Gorius [8], the authors depict or describe nine subdistal appendages [16,19,21,23–29]. However, for the sake of justice, it should be noted that in Paintrand et al. [16], along with the assertion that subdistal appendages in KE37 cells are always exactly nine, there is a reservation that the number of these structures may be different in other types of cells. The earlier work of the same laboratory [15] demonstrated that the number of subdistal appendages (radial arms in this paper) never exceeded six and they were not located symmetrically upon the centriole.
We fully subscribe to this statement and in this review we present data that distal appendages and subdistal appendages (they are also called ‘pericentriolar satellites’ in other publications) are morphologically different structures having different protein composition and functional purpose.
3. Morphological differences between distal appendages and subdistal appendages
The centriole structure proposed by Bessis & Breton-Gorius [8] (figure 1) assumes that both groups of structures connected to the centriole have identical morphology—they have a thin stem and a large round head. However, at that time the methods of sample preparation for electron microscopic analysis were imperfect. The improvement in both specimen fixation and staining methods for electron microscopy [30–32] as well as the more recent development of cryo-electron microscopy [33] have made it possible to refine the description of the ultrastructure of centrioles and basal bodies, including distal and subdistal appendages (figures 2 and 3).
Distal appendages (figure 2) in electron microscopic images look like ‘blades of a turbine’. They are always located centrally symmetrically at the distal end of the cylinder of the mother centriole, their number is constant—they are always nine per centriole. Each distal appendage is associated with only one of the centriole triplets and departs from the centriole surface at an angle of about 50 degrees. Distal appendages are twisted in the opposite direction to the twist of the centriole triplets, that is, counterclockwise, when viewed from the distal end of the centriole. Distal appendages are always present on centrioles in a more or less pronounced form, they are found both on interphase centrioles and on mitotic ones [34]. Distal appendages appear on the centriole during second cell cycle after its ‘birth’, when this centriole becomes the mother and acquires the ability to form the primary cilium.
Subdistal appendages with their base are associated with 2–3 triplets of centrioles and move away from its surface at a right angle (figure 3). Subdistal appendages in the classical form represent a two-component formation, consisting of a rounded head and a conical, sometimes striated stem (figure 3). Figures 2a and 3a show distal appendages and subdistal appendages in cells of the same PK cell line. Their morphology excludes the possibility of confusing them for the same structure, and further study of their behaviour in the cell only confirms this fact.
Unlike the distal appendages, which are constant in number and structure, subdistal appendages can change morphologically after some influences we will mention below, and are distinguished by variability of form in specialized cells of some organisms. For example, the stem striation can practically disappear, and the stem itself can change shape in the axolotl blood cells [18]. The study of endotheliocytes in intact zones, as well as in fibrous and atheromatous areas of the artery obtained from autopsy material of adults, showed that the form of subdistal appendages was different: in four of the five types of cells identified by the authors, subdistal appendages had a conical shape, and to their heads MT fit, and in cells of one group of the body, subdistal appendages were a complex of fibrils of constant thickness [35,36].
The location of the subdistal appendages on the centriole wall is not tied to its distal end and can be very different—several subdistal appendages may be located on the same level (figure 3) or can be spaced along the length of the centriolar cylinder (figure 4).
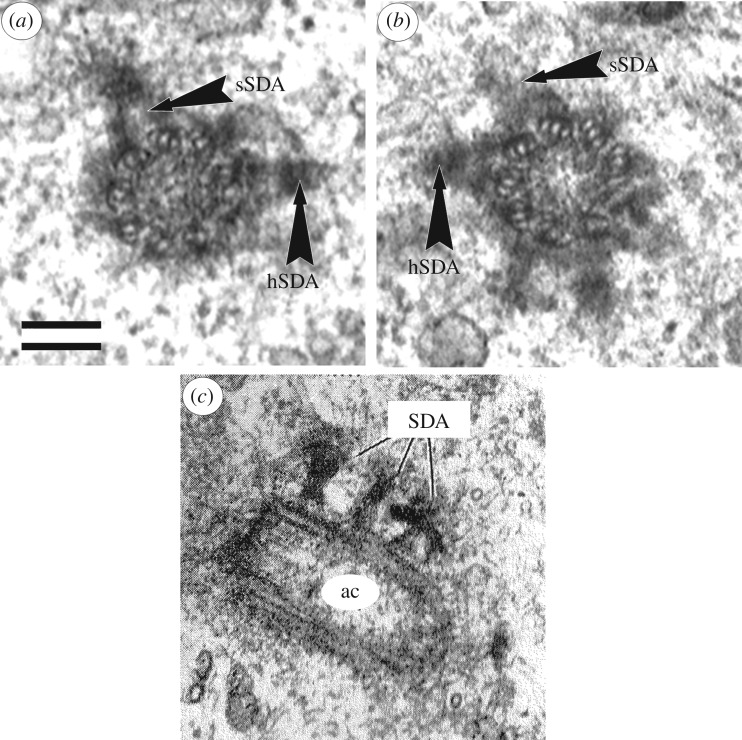
Figure 4.
Subdistal appendages can be on the different distance from distal end of centriole. (a,b) Two consecutives serial cross sections of distal part of mother centriole in the cell of BHK-21 (baby hamster kidney cell line), five subdistal appendages with well visible stem (sSDA) and head (hSDA) parts placed in ‘two flours’ (from [4]). (c) The view from the proximal end of the centriole (the MT triplets are twisted counterclockwise); near longitudinal section of active (mother) centriole (ac) from axolotl cell, subdistal appendages (SDA) placed in ‘three flours’ (from [18]). Scale bar, 100 nm.
De Harven & Dustin in 1960 [37] first noted that subdistal appendages are detected only on interphase centrioles and never on mitotic ones. A detailed ultrastructural study showed that subdistal appendages (in the author's terminology—pericentriolar satellites) disappear during the pre-mitotic G2 phase of the cell cycle and reappear on centrosomes at the beginning of the post-mitotic G1 phase of the cell cycle [34].
4. How many distal appendages and subdistal appendages can be located on one centriole?
Unlike distal appendages, the number of subdistal appendages is variable; it depends on a number of different factors—the type of cell, its functional state, external influences (including hormonal ones) and even the age of the cell. Subdistal appendages may be completely absent in some types of cells [38–43] or there may be even more than nine per centriole [18,35,36].
A change in the functional activity of the centrosome (for example, activation of the centrosome's MT-nucleating capacity by certain metabolic inhibitors) leads simultaneously to an increase in the average number of subdistal appendages [44,45]. In endothelial cells, the number of subdistal appendages on the mother centrioles of human artery cells is very rapidly increased after thrombin treatment [46]. If normal to the centriole of endotheliocytes is present from one to four subdistal appendages (three subdistal appendages for 60% cells), then after 5 min of exposure to thrombin, the number of subdistal appendages grows to 3–7 (four and more subdistal appendages for 70% cells), with no centrioles having less than three subdistal appendages. However, when thrombin was exposed to human vein endotheliocytes, this effect was not observed and the number of subdistal appendages remained the same. In both untreated and treated with thrombin cells, the number of subdistal appendages varied from 3 to 6 (in majority of cells 4 or 5) [46].
In human endotheliocytes, the mother centriole is able to carry an extremely variable number of subdistal appendages from 2 to even 12 per centriole [35,36].
In most cell types, subdistal appendages are only found on a mature mother centriole [47]. However, the presence of these structures on both centrioles is described in embryonic fibroblasts [9] and neutrophils [48], but even in these cases, the number of subdistal appendages on the mother centrioles is always greater than on the daughter centrioles [48].
No one now doubts the correctness of the hypothesis of Henneguy [49] and Lenhossék [50] about the homology of the cilia and flagella basal bodies and centrioles. Subdistal appendages of centrioles are, likewise, homologous structures of the basal feet found on basal bodies. There is one or two basal feet per each basal body [51] and they determine the direction of the ciliary beating [52]. In many cell cultures, the number of subdistal appendages is also 1 or 2. In the case of distal appendages, they are always exactly 9, and they are called the same called the same for both the centriole and the basal body [12]. However, for some objects, the distal appendages all still have their own names, for example, in the basal bodies of Chlamydomonas they were called [53] and continue to be called transitional fibres [54].
5. Functional and immunochemical differences subdistal appendages and distal appendages
Distal and subdistal appendages are not only morphologically different, but also functionally diverse. The first attaches the centriole to the cell membrane during the formation of the cilium, while the latter represent miniature centres of microtubule nucleation—MT are actively nucleated from their heads (and they are attached for a while) [34,45].
Proteins of the distal and subdistal appendages have been actively studied since the beginning of this century, immunochemical studies have allowed us to compile a unique map of protein localization in various centrosomal components, including subdistal appendages [4,55]. γ-tubulin—the protein that directly participates in the nucleation of MT on centrosome—is present in the heads of subdistal appendages [56]. Proteins involved in the process of stabilization and anchorage of MT into subdistal appendages and in the regulation of the maturation of centrioles were found in subdistal appendages too [57,58]. Later, several other proteins were also classified as subdistal appendages proteins: ε-tubulin [59], CEP110 = centriolin [60,61], CEP170 [62], CC2D2A [63]. Their interactions were described and the sequence of their appearance on the centriole was established. It turned out that ninein binds to the centriole at its C-terminus and is capable of retaining the γ-TURC-containing microtubule-nucleating complex (gamma-TURC) at the N-terminus [56]. The localization of ODF2 is described as being closest to the surface of the centriolar cylinder and critical for the formation of subdistal appendages. Originally, it has been proposed that ODF2 participates in the formation of both distal and subdistal appendages [64], but this has been disputed for distal appendages by Tanos et al. in 2013 [65].
Summarizing the accumulated data, Huang et al. in 2017 [29] described two new subdomain appendages of both CCDC120 and CCDC6. They further dissected a hierarchical assembly scheme for subdistal appendages. According to this scheme, ODF2 acts upstream to initiate the assembly [26,64]; TCHP mediates the interaction between ODF2 and ninein [66]; CEP170 can be recruited by ninein [67]. CCDC120 is recruited to subdistal appendages by ODF2 and recruits both CEP170 and ninein to subdistal appendages through two distinct domains [29], CCDC68 competes with CCDC120 in recruiting CEP170 [29].
In the past decade, the proteins of the distal appendages of the CEP family—CEP164 [68], CEP89 [69], CEP83, and the proteins SCLT1 and FBF1 [65] have been characterized. It has been shown that suppression of CEP164 protein expression leads to blockage of ciliogenesis [68]. The suppression of each of the proteins CEP89, CEP83, SCLT1 and FBF1 blocked the appearance of distal appendages on the surface of the centrioles and ciliogenesis [65]. Based on their experimental data on the differential reduction of various proteins, the authors constructed a hierarchical scheme in which CEP83 is the first protein appearing on the centriole surface, the presence of CEP89 and SCLT1 proteins depends on the presence of CEP83 on the centriole. From the appearance of SCLT1 depends on the accumulation on the centrioles of two more proteins of the distal appendages CEP164 and FBF1 [65].
Depletion of CEP83 did not affect the localization of ODF2, which the authors interpreted as the independence of the regulation of assembly of the distal and subdistal appendages [65]. We note that for electron microscopists who observed the distal and the subdistal appendages both components of the centrosome in cells at different stages of the cell cycle and in different experimental situations, this conclusion was obvious in advance.
6. Conclusion
The important take-home message of this review is that distal and subdistal appendages differ quantitatively, morphologically, biochemically and functionally. Common to these structures is their location on the older mother centriole; although in cells with very high MT nucleation activity subdistal appendages may develop on younger centrioles, as well in leucocytes of axolotl [18] and granulocytes of human blood [48].
The differences between these structures are significant: (i) the distal appendages are located centrally symmetrically (symmetry of the 9th order), while the subdistal (as a rule) are not symmetrical; (ii) the distal appendages are rigidly attached to the distal end of the centriole at the same level while the subdistal appendages can be located along the entire length of the centriole down to its proximal end; (iii) the number of distal appendages is always 9 (one for each triplet of the centriolar cylinder), but the number of subdistal appendages can vary from 0 to 14 depending on the type of cells and their functional state; (iv) two types of appendages are composed of different sets of proteins; and (v) two types of appendages are functionally different (figure 5).
Figure 5.
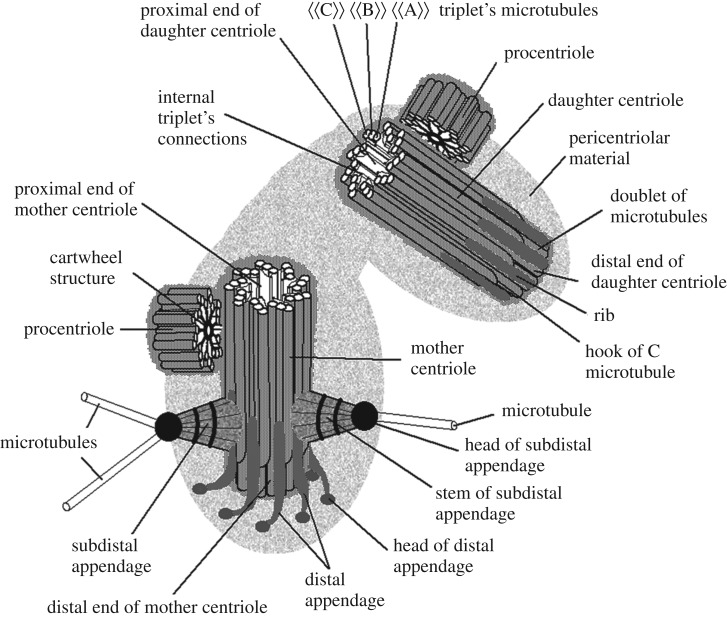
A scheme of centrosome in S-phase of cell cycle. Two subdistal appendages are shown here what is typical for epithelial pig kidney (PK) embryo cells. Serial sections cells of this cell line were used for model construction. Near the distal pole the MT ‘C’ terminates earlier than MT ‘A’ and MT ‘B’. In the region of triplet to doublet transition MT ‘C’ is uncomplete; this structure is called as ‘hook’. There are no distal appendages on the daughter centriole, but electronically dense plats (called ‘ribs’) are there on each triplet. Other terms are explained in the text. From [70,71] with small modifications.
Acknowledgements
We thank Michael Jennings, Jadranka Loncarek, Anthony Hyman and Greenfield Sluder for critical reading, helpful comments and English language editing.
Data accessibility
This article has no additional data.
Authors' contributions
Both authors R.U. and I.A. wrote and edited the article.
Competing interests
We have no competing interests.
Funding
Our data were obtained with the assistance of the IBISA Electron Microscopy Facility of Tours University and the University hospital of Tours.
References
- 1.Stubblefield E. 1968. Centriole replication in a mammalian cell. In The proliferation and spread of neoplastic cells (ed. MD Anderson), pp. 175–193. Baltimore, MD: Williams and Wilkins Co.
- 2.Wheatley DN. 1982. The centriole: a central enigma of cell biology. New York, NY: Elsevier Biomedical Press. [Google Scholar]
- 3.Bornens M. 2002. Centrosome composition and microtubule anchoring mechanisms. Curr. Opin. Cell Biol. 14, 25–34. ( 10.1016/S0955-0674(01)00290-3) [DOI] [PubMed] [Google Scholar]
- 4.Uzbekov RE, Alieva IB. 2013. Centrosome: history of investigations and new discoveries; from cytoplasmic granule to the centre of intracellular regulation. Nadezhdina ES (ed). Moscow, Russia: Moscow University Press.
- 5.Woodruff JB, Wueseke O, Hyman AA. 2014. Pericentriolar material structure and dynamics. Phil. Trans. R. Soc. B 369, 20130459 ( 10.1098/rstb.2013.0459) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uzbekov RE. 2007. Centriole duplication in PE (SPEV) cells starts before beginning of DNA replication. Biochem. (Mosc.) Suppl. Ser. A Membr. Cell Biol. 1, 206–211. ( 10.1134/S1990747807030026) [DOI] [Google Scholar]
- 7.Uzbekov RE, Alieva IB. 2010. Centriole duplication or DNA replication—what starts earlier? In Cytoskeleton: cell movement, cytokinesis and organelles organization (eds Lansing S, Rousseau T), pp. 127–137. Hauppauge, NY: Nova Science Publishers, Inc. [Google Scholar]
- 8.Bessis M, Breton-Gorius J. 1958. Sur une structure inframicroscopique péricentriolaire. Etude au microscope électronique sur des leucocytes Mammifères. Comp. Rend Acad Sc 246, 1289–1291. [PubMed] [Google Scholar]
- 9.Alieva IB, Vorobjev IA. 2004. The vertebrate primary cilium is a sensory part of centrosomal complex in tissue cells but it is ‘Sleeping Beauty’ in cultured cells. Cell Biol. Int. 28, 139–150. ( 10.1016/j.cellbi.2003.11.013) [DOI] [PubMed] [Google Scholar]
- 10.Szöllösi D. 1964. The structure and function of centrioles and their satellites in the jellyfish Phialidium gregarium. J. Cell Biol. 21, 465–479. ( 10.1083/jcb.21.3.465) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson RG. 1972. The three-dimensional structure of the basal body from the rhesus monkey oviduct. J. Cell Biol. 54, 246–265. ( 10.1083/jcb.54.2.246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Hara PT. 1970. Spiral tilt of triplet fibers in human leukocyte centrioles. J. Ultrastruct. Res. 31, 195–198. ( 10.1016/S0022-5320(70)90154-1) [DOI] [PubMed] [Google Scholar]
- 13.Bernhard W, de Harven E. 1960. L'ultrastructure du centriole et d'autres éléments de l'appareil achromatique. In Vierter internationaler kongress fur elektronenmikroskopie, 1958, vol. 2 (eds Bargmann W, Mollenstedt G, Niehrs H, Peters D, Ruska E, Wolpers C), pp. 217–227. Berlin, Germany: Springer. [Google Scholar]
- 14.De Harven E. 1968. The centriole and the mitotic spindle. In The nucleus, vol. 3 (eds Dalton AJ, Haguenau F), pp. 197–227. New York, NY: Academic Press. [Google Scholar]
- 15.Komesli S, Tournier F, Paintrand M, Margolis RL, Job D, Bornens M. 1989. Mass isolation of calf thymus centrosomes: identification of a specific configuration. J. Cell Biol. 109, 2869–2878. ( 10.1083/jcb.109.6.2869) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paintrand M, Moudjou M, Delacroix H, Bornens M. 1992. Centrosome organization and centriole architecture: their sensitivity to divalent cations. J. Struct. Biol. 108, 107–128. ( 10.1016/1047-8477(92)90011-X) [DOI] [PubMed] [Google Scholar]
- 17.Tournier F, Bornens M. 1994. Cell cycle regulation of centrosome function. In Microtubules (ed. Hyams J.), pp. 303–324. New York, NY: Wiley-Liss. [Google Scholar]
- 18.Vorobjev IA, Chentsov YS. 1977. The ultrastructure of the centrosome in the cells of hematopoietic tissues of axolotl. Tsitologiia 19, 598–603. [Google Scholar]
- 19.Alberts B, Johnson A, Lewis J, Morgan D, Raff M, Roberts K, Walter P. 2016. Molecular biology of the cell, 6th edn New York, NY: Garland Science. [Google Scholar]
- 20.Chavali PL, Pϋtz M, Gergely F. 2014. Small organelle, big responsibility: the role of centrosomes in development and disease. Phil. Trans. R. Soc. B 369, 20130468 ( 10.1098/rstb.2013.0468) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andersen SSL. 1999. Molecular characteristics of the centrosome. Int. Rev. Cytol. 187, 51–109. ( 10.1016/S0074-7696(08)62416-X) [DOI] [PubMed] [Google Scholar]
- 22.Nigg E. 2002. Centrosome aberrations: cause or consequence of cancer progression? Nat. Rev. 2, 1–11. ( 10.1038/nrc924) [DOI] [PubMed] [Google Scholar]
- 23.Azimzadeh J, Bornens M. 2007. Structure and duplication of the centrosome. J. Cell Sci. 120, 2139–2142. ( 10.1242/jcs.005231) [DOI] [PubMed] [Google Scholar]
- 24.Chretien D, Buendia B, Fuller SD, Karsenti E. 1997. Reconstruction of the centrosome cycle from cryoelectron micrographs. J. Struct. Biol. 120, 117–133. ( 10.1006/jsbi.1997.3928) [DOI] [PubMed] [Google Scholar]
- 25.Ibrahim R, Messaoudi C, Chichon FJ, Celati C, Marco S. 2009. Electron tomography study of isolated human centrioles. Microsc. Res. Tech. 72, 42–48. ( 10.1002/jemt.20637) [DOI] [PubMed] [Google Scholar]
- 26.Tateishi K, Yamazaki Y, Nishida T, Watanabe S, Kunimoto K, Ishikawa H, Tsukita S. 2013. Two appendages homologous between basal bodies and centrioles are formed using distinct Odf2 domains. J. Cell Biol. 203, 417–425. ( 10.1083/jcb.201303071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hung HF, Hehnly H, Doxsey S. 2016. The mother centriole appendage protein cenexin modulates lumen formation through spindle orientation. Curr. Biol. 26, 793–801. ( 10.1016/j.cub.2016.01.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mazo G, Soplop N, Wang WJ, Uryu K, Tsou MB. 2016. Spatial control of primary ciliogenesis by subdistal appendages alters sensation-associated properties of cilia. Dev. Cell 39, 424–437. ( 10.1016/j.devcel.2016.10.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang N, Xia Y, Zhang D, Wang S, Bao Y, He R, Teng J, Chen J. 2017. Hierarchical assembly of centriole subdistal appendages via centrosome binding proteins CCDC120 and CCDC68. Nat. Commun. 8, 15057 ( 10.1038/ncomms15057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watson ML. 1958. Staining of tissue sections for electron microscopy with heavy metals. J. Biophys. Biochem. Cytol. 4, 475–478. ( 10.1083/jcb.4.4.475) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sabatini DD, Bensch KG, Barnett RJ. 1963. Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J. Cell Biol. 17, 19–58. ( 10.1083/jcb.17.1.19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reynolds ES. 1963. The use of lead citrate at high pH as electron-opaque stain in electron microscopy. J. Cell Biol. 17, 208 ( 10.1083/jcb.17.1.208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guichard P, Chrétien D, Marco S, Tassin AM. 2010. Procentriole assembly revealed by cryo-electron tomography. EMBO J 29, 1565–1572. ( 10.1038/emboj.2010.45) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vorobjev IA, Chentsov Yus. 1982. Centrioles in the cell cycle. I epithelial cells. J. Cell Biol. 98, 938–949. ( 10.1083/jcb.93.3.938) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bystrevskaya VB, Lichkun VV, Antonov AS, Perov NA. 1988. An ultrastructural study of centriolar complexes in adult and embryonic human aortic endothelial cells. Tissue Cell 20, 493–503. ( 10.1016/0040-8166(88)90052-3) [DOI] [PubMed] [Google Scholar]
- 36.Bystrevskaya VB, Lichkun VV, Krushinsky AV, Smirnov VN. 1992. Centriole modification in human aortic endothelial cells. J. Struct. Biol. 109, 1–12. ( 10.1016/1047-8477(92)90061-E) [DOI] [PubMed] [Google Scholar]
- 37.De Harven E, Dustin P Jr. 1960. Etude au microscope électronique de la stathmocinése chez le rat. Colloq. Intern. Centre Natl. Rech. Sci. (Paris) 88, 189–197. [Google Scholar]
- 38.Stubblefield E, Brinkley BR. 1967. Architecture and function of the mammalian centriole. In Formation and fate of cell organelles (ed. Warren KB.), pp. 175–218. New York, NY: Academic Press Inc. [Google Scholar]
- 39.Wheatley DN. 1969. Cilia in cell-cultured fibroblasts. I. On their occurrence and relative frequencies in primary cultures and established cell lines. J. Anat. 105, 351–362. [PMC free article] [PubMed] [Google Scholar]
- 40.Wheatley DN. 1974. Pericentriolar virus-like particles in Chinese hamster ovary cells. J. Gen. Virol. 24, 395–399. ( 10.1099/0022-1317-24-2-395) [DOI] [PubMed] [Google Scholar]
- 41.Onishchenko GE, Chentsov Ius. 1986. Structure of the centriolar complex in the hepatocytes of intact and regenerating mouse live. Tsitologiia. 28, 403–408. [PubMed] [Google Scholar]
- 42.Uzbekov RE, Maurel DB, Aveline PC, Pallu S, Benhamou CL, Rochefort GY. 2012. Fine ultrastructure of the centrosome and morphological aspects of in vivo realization of mechanotransduction primary cilia function in rat osteocytes. Microsc. Microanal. 18, 1430–1441. ( 10.1017/S1431927612013281) [DOI] [PubMed] [Google Scholar]
- 43.Uzbekov RE, Benhamou CL. 2014. Cilia-like structure, primary cilium and mechano-transduction in the osteocyte. Osteoporos. Int. 25(Suppl 3), S490–S495. [Google Scholar]
- 44.Alieva IB, Vorobjev IA. 1994. Centrosome behavior under the action of a mitochondrial uncoupler and the effect of disruption of cytoskeleton elements on the uncoupler-induced alterations. J. Struct. Biol. 113, 217–224. ( 10.1006/jsbi.1994.1056) [DOI] [PubMed] [Google Scholar]
- 45.Alieva IB, Vorobjev IA. 1995. Centrosome behaviour and orientation of centrioles under the action of energy transfer inhibitors. Cell Biol. Int. 19, 103–112. ( 10.1006/cbir.1995.1050) [DOI] [PubMed] [Google Scholar]
- 46.Vinogradova TM, Balashova EE, Smirnov VN, Bystrevskaya VB. 2005. Detection of the centriole tyr- or acet-tubulin changes in endothelial cells treated with thrombin using microscopic immunocytochemistry. Cell Motil. Cytoskeleton 62, 1–12. ( 10.1002/cm.20079) [DOI] [PubMed] [Google Scholar]
- 47.Vorobjev IA, Nadezhdina ES. 1987. The centrosome and its role in the organization of microtubules. Int. Rev. Cytol. 106, 227–293. ( 10.1016/S0074-7696(08)61714-3) [DOI] [PubMed] [Google Scholar]
- 48.Gudima GO, Vorob'ev IA, Chentsov Yus. 1984. Cell center of macrophages, granulocytes and lymphocytes during in vitro cell spreading, polarization and movement. Tsitologiia 26, 1002–1007. [PubMed] [Google Scholar]
- 49.Henneguy LF. 1898. Sur les rapports des cils vibratiles avec les centrosomes. Arch. Microsc. Morph. Exp. 1, 481–496. [Google Scholar]
- 50.Von Lenhossék M. 1898. Uber Flimmerzellen. Verh. anat. Ges. Kiel. 12, 106–128. [Google Scholar]
- 51.Wolfe J. 1972. Basal body fine structure and chemistry. Adv. Cell Mol. Biol. 2, 151–192. [Google Scholar]
- 52.Gibbons IR. 1961. The relationship between the fine structure and direction of beat in gill cilia of a lamellibranch mollusc. J. Biophys. Biochem. Cytol. 11, 179–205. ( 10.1083/jcb.11.1.179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ringo DL. 1967. Flagellar motion and fine structure of the flagellar apparatus in Chlamydomonas. J. Cell Biol. 33, 543–571. ( 10.1083/jcb.33.3.543) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O'Toole ET, Dutcher SK. 2014. Site-specific basal body duplication in Chlamydomonas. Cytoskeleton (Hoboken) 71, 108–118. ( 10.1002/cm.21155) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alieva IB, Uzbekov RE. 2008. Centrosome is a polyfunctional multiprotein cell complex. Biochemistry (Moscow) 73, 626–643. ( 10.1134/S0006297908060023) [DOI] [PubMed] [Google Scholar]
- 56.Delgehyr N, Sillibourne J, Bornens M. 2005. Microtubule nucleation and anchoring at the centrosome are independent processes linked by ninein function. J. Cell Sci. 118, 1565–1575. ( 10.1242/jcs.02302) [DOI] [PubMed] [Google Scholar]
- 57.Mogensen MM, Malik A, Piel M, Bouckson Castang V, Bornens M. 2000. Microtubule minus-end anchorage at centrosomal and non-centrosomal sites: the role of ninein. J. Cell Sci. 113, 3013–3023. [DOI] [PubMed] [Google Scholar]
- 58.Nakagawa Y, Yamane Y, Okanoue T, Tsukita S, Tsukita S. 2001. Outer dense fiber 2 is a widespread centrosome scaffold component preferentially associated with mother centrioles: its identification from isolated centrosomes. Mol. Biol. Cell 12, 1687–1697. ( 10.1091/mbc.12.6.1687) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chang P, Giddings TH, Winey M, Stearns T. 2003. Epsilon-tubulin is required for centriole duplication and microtubule organization. Nat. Cell Biol. 5, 71–76. ( 10.1038/ncb900) [DOI] [PubMed] [Google Scholar]
- 60.Ou YY, Mack GJ, Zhang M, Rattner JB. 2002. CEP110 and ninein are located in a specific domain of the centrosome associated with centrosome maturation. J. Cell Sci. 115, 1825–1835. [DOI] [PubMed] [Google Scholar]
- 61.Gromley A. 2003. A novel human protein of the maternal centriole is required for the final stages of cytokinesis and entry into S phase. J. Cell Biol. 161, 535–545. ( 10.1083/jcb.200301105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guarguaglini G. 2005. The forkhead-associated domain protein Cep170 interacts with Polo-like kinase 1 and serves as a marker for mature centrioles. Mol. Biol. Cell 16, 1095–1107. ( 10.1091/mbc.e04-10-0939) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Veleri S, et al. 2014. Ciliopathy-associated gene Cc2d2a promotes assembly of subdistal appendages on the mother centriole during cilia biogenesis. Nat. Commun. 5, 4207 ( 10.1038/ncomms5207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ishikawa H, Kubo A, Tsukita S. 2005. Odf2-deficient mother centrioles lack distal/subdistal appendages and the ability to generate primary cilia. Nat. Cell Biol. 7, 517–524. ( 10.1038/ncb1251) [DOI] [PubMed] [Google Scholar]
- 65.Tanos BE, Yang HJ, Soni R, Wang WJ, Macaluso FP, Asara JM, Tsou MF. 2013. Centriole distal appendages promote membrane docking, leading to cilia initiation. Genes Dev. 27, 163–168. ( 10.1101/gad.207043.112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ibi M, et al. 2011. Trichoplein controls microtubule anchoring at the centrosome by binding to Odf2 and ninein. J. Cell Sci. 124, 857–864. ( 10.1242/jcs.075705) [DOI] [PubMed] [Google Scholar]
- 67.Lamla S. 2009. Functional Characterisation of the Centrosomal Protein Cep170. PhD thesis, Ludwig-Maximilians-University, Munich, Germany. [Google Scholar]
- 68.Graser S, Stierhof Y-D, Lavoie SB, Gassner OS, Lamla S, Le Clech M, Nigg EA. 2007. Cep164, a novel centriole appendage protein required for primary cilium formation. J. Cell Biol. 179, 321–330. ( 10.1083/jcb.200707181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sillibourne JE, Specht CG, Izeddin I, Hurbain I, Tran P, Triller A, Darzacq X, Dahan M, Bornens M. 2011. Assessing the localization of centrosomal proteins by PALM/STORM nanoscopy. Cytoskeleton (Hoboken) 68, 619–627. ( 10.1002/cm.20536) [DOI] [PubMed] [Google Scholar]
- 70.Uzbekov R, Prigent C. 2007. Clockwise or anticlockwise? Turning the centriole triplets in the right direction! FEBS Lett. 581, 1251–1254. ( 10.1016/j.febslet.2007.02.069) [DOI] [PubMed] [Google Scholar]
- 71.Uzbekov RE, Alieva IB. 2008. The centrosome—a riddle of the ‘cell processor’. Tsitologiia 50, 91–112. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.