Staphylococcus aureus is a human and animal pathogen that can cause biofilm-associated infections. PSMs have multiple functions in biofilm development and virulence in staphylococcal pathogenesis. This study has revealed that MgrA can negatively regulate psm expression by binding directly to the promoter regions of psm operons. Furthermore, our results show that MgrA can modulate biofilm structuring and development by repressing the production of PSMs in S. aureus. Our findings provide novel insights into the regulatory mechanisms of S. aureus psm gene expression, biofilm development, and pathogenesis.
KEYWORDS: Staphylococcus aureus, phenol-soluble modulins, MgrA, biofilm
ABSTRACT
Phenol-soluble modulins (PSMs) are amphipathic peptides that are produced by staphylococci and play important roles in Staphylococcus aureus biofilm formation and dissemination. Although the multiple functions of PSMs have been recognized, the regulatory mechanisms controlling the expression of psm operons remain largely unknown. In this study, we identified MgrA in a DNA pulldown assay and further demonstrated, by electrophoretic mobility shift assays and DNase I footprinting assays, that MgrA could bind specifically to the promoter regions of psm operons. We then constructed an isogenic mgrA deletion strain and compared biofilm formation and detachment in the wild-type and isogenic mgrA deletion strains. Our results indicated that biofilm formation and detachment were significantly increased in the mgrA mutant strain. Real-time quantitative reverse transcription-PCR data indicated that MgrA repressed the transcription of psm operons in cultures and biofilms, suggesting that MgrA is a negative regulator of psm expression. Furthermore, we analyzed biofilm formation by the psm mutant strains, and we found that PSMs promoted biofilm structuring and development in the mgrA mutant strain. These findings reveal that MgrA negatively regulates biofilm formation and detachment by repressing the expression of psm operons through direct binding to the psm promoter regions.
IMPORTANCE Staphylococcus aureus is a human and animal pathogen that can cause biofilm-associated infections. PSMs have multiple functions in biofilm development and virulence in staphylococcal pathogenesis. This study has revealed that MgrA can negatively regulate psm expression by binding directly to the promoter regions of psm operons. Furthermore, our results show that MgrA can modulate biofilm structuring and development by repressing the production of PSMs in S. aureus. Our findings provide novel insights into the regulatory mechanisms of S. aureus psm gene expression, biofilm development, and pathogenesis.
INTRODUCTION
Staphylococcus aureus is a human and animal pathogen that can cause various bacterial infections, including relatively benign and fatal systemic diseases (1, 2). S. aureus can develop biofilms on host tissues and medical devices and can cause chronic infections when S. aureus cells accumulate to form biofilms at the infection sites (3). Biofilms represent a protected environment that is essential for bacteria to resist host immune responses (4) and chemotherapies (5), making therapeutic intervention extremely difficult. Moreover, biofilm dissemination enables bacteria to spread, leading to infections in the body (6–8).
Biofilms are known as multicellular structures encased in a matrix of proteins, polysaccharides, extracellular DNA (eDNA), and other environmental factors (9). Biofilm formation is modulated by various regulatory systems, including the agr quorum-sensing system (10), the LuxS/AI-2 quorum-sensing system (11), the ArlR/S two-component system (12), and transcriptional regulators, such as MgrA (13), Rbf (14), Sar-family proteins (15–17), and IcaR (18). These regulators modulate biofilm formation by regulating the production of biofilm-formation-associated factors, including surface proteins, polysaccharide intercellular adhesin (PIA), eDNA, and other extracellular components in biofilms. For instance, MgrA can repress biofilm formation by controlling the release of eDNA and the expression of surface proteins and extracellular proteases in S. aureus (13, 19). The agr quorum-sensing system controls biofilm detachment by regulating biofilm-detachment-associated factors, including extracellular proteases, nucleases, and phenol-soluble modulins (PSMs) (10, 20). Biofilms are complex, however, and the regulatory mechanisms controlling biofilm development in S. aureus have not been thoroughly elucidated.
PSMs are known as biofilm-structuring-associated and biofilm-dissemination-associated factors involved in S. aureus biofilm-associated infections (8, 21–23). PSMs are amphipathic and surfactant-like peptides containing five α-peptides (PSMα1 to -4 and δ-toxin) and two β-peptides (PSMβ1 and -2) (24). PSMαs are encoded by the psmα operon, PSMβs are encoded by the psmβ operon, and the δ-toxin (also called δ-hemolysin [Hld]) is encoded within RNAIII, regulatory RNA encoded from the agr operon (24–26). With the given surfactant-like characteristics, PSMs promote biofilm structuring to form channels that make nutrition available to bacteria and facilitate biofilm detachment to free bacteria (21). Although the functions of PSMs have been studied extensively, the detailed regulatory mechanisms controlling the production of PSMs in S. aureus are poorly defined.
In this study, we found that MgrA could specifically bind to the promoter regions of psm operons and negatively regulate the expression of psm genes in S. aureus NCTC8325. Our findings reveal a regulatory mechanism through which MgrA regulates the production of PSMs to modulate biofilm formation and detachment in S. aureus.
RESULTS
Proteins binding the psmα promoter are identified.
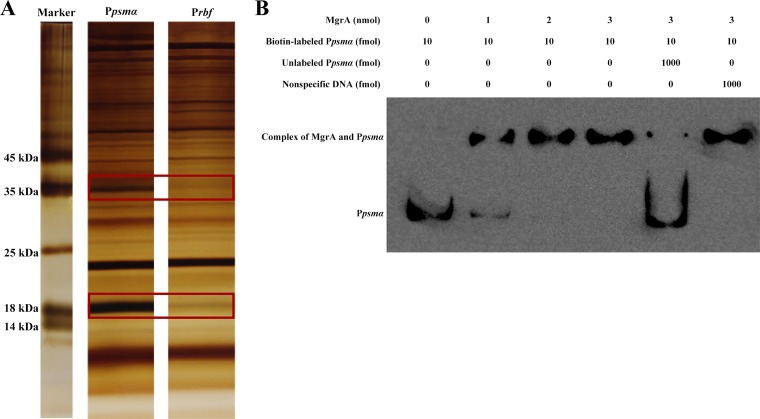
Previous studies have shown that PSMs are involved in the biofilm development and virulence of S. aureus (20, 21). AgrA is a positive regulator of psm operons and strictly regulates psm transcription in S. aureus (20). However, regulators other than AgrA that can directly regulate psm expression in S. aureus have not been reported. To identify other transcriptional factors that can directly regulate the expression of psm genes, we performed DNA affinity pulldown assays to screen for DNA-binding proteins in S. aureus. A biotin-labeled DNA fragment containing the promoter region of the psmα operon was amplified with primers Ppsmα-F and Ppsmα-biotin-R and then used for DNA pulldown assays. The pulled-down proteins were subjected to SDS-PAGE and liquid chromatography-tandem mass spectrometry (LC-MS/MS) analyses, as described in Materials and Methods. Potential DNA-binding proteins were separated by SDS-PAGE and stained with silver. The proteins that bound specifically to the promoter region of the psmα operon were identified by LC-MS/MS. The DNA-binding proteins are listed in Table 1, and proteins that did not contain DNA-binding domains are listed in Table S1 in the supplemental material. MgrA (∼17 kDa) is a helix-turn-helix (HTH)-type transcriptional regulator involved in autolytic activity (27), multidrug resistance (28), and virulence (29), suggesting that MgrA could be a regulator of the psmα operon. The hypothetical protein SAOUHSC_03049 (∼32 kDa) is an uncharacterized protein that contains a DNA-binding domain and belongs to the ParB family, which is involved in chromosome partitioning and cell division processes (30). It has been reported that SAOUHSC_03049 is an essential gene and its main function is similar to that of yyaA (also called noc), affecting nucleoid occlusion by binding nonspecifically to DNA in Bacillus subtilis (30, 31). Considering the function of the hypothetical protein SAOUHSC_03049 in chromosome partitioning, we did not choose it for further study, although it could be a transcriptional regulator in S. aureus. Other proteins, including PolA, TopA, RuvA, GyrA, and RpoC, are general DNA-binding proteins related with DNA duplication or repair. Two bands (∼18 kDa and 35 kDa) were specific to the promoter region of the psmα operon, compared with the control (Fig. 1A). Based on Table 1 and Table S1 in the supplemental material, the apparent 18-kDa protein band was most likely MgrA. The apparent 35-kDa protein band seemed to contain several proteins (Table 2). Among those proteins, only the hypothetical protein SAOUHSC_03049 (∼32 kDa) contained a DNA-binding domain, as described above (Tables 1 and 2). In this study, we aimed to identify the main transcriptional factors that regulate the expression of psm genes by binding directly to the promoter region of psm operons. Thus, only MgrA, among those pulled-down proteins, was considered a regulator of the psmα operon for further study.
TABLE 1.
Proteins with DNA-binding domains identified by LC-MS/MS
| Gene | Protein | Molecular function |
|---|---|---|
| SAOUHSC_00694 | MgrA | HTH-type transcriptional regulator MgrA; DNA binding transcription factor activity; transcription regulatory region DNA binding |
| SAOUHSC_03049 | Hypothetical protein SAOUHSC_03049 | Uncharacterized protein; DNA binding; similar to ParB, probably involved in chromosome partitioning and cell division processes |
| SAOUHSC_01797 | PolA | DNA polymerase I; 3′-5′ exonuclease activity; 5′-3′ exonuclease activity; DNA binding; DNA-directed DNA polymerase activity |
| SAOUHSC_01222 | TopA | DNA topoisomerase 1; DNA binding; DNA topoisomerase type I activity; metal ion binding |
| SAOUHSC_01751 | RuvA | Holliday junction ATP-dependent DNA helicase; ATP binding; DNA binding; four-way junction helicase activity |
| SAOUHSC_00006 | GyrA | DNA gyrase subunit A; ATP binding; DNA binding; DNA topoisomerase type II (ATP-hydrolyzing) activity |
| SAOUHSC_00525 | RpoC | DNA-directed RNA polymerase subunit β′; DNA binding; DNA-directed 5′-3′ RNA polymerase activity |
FIG 1.
Identification of the psmα promoter-specific binding protein. (A) SDS-PAGE analysis of proteins binding to the psmα promoter region or the rbf promoter region. The gel was stained with silver, and the specific bands boxed in red were analyzed by LC-MS/MS. (B) EMSA of the purified MgrA with a 336-bp psmα promoter fragment labeled with biotin. Increasing concentrations of the purified MgrA protein and 10 fmol of the Ppsmα-biotin probe were used in the reactions, and the reaction mixtures were incubated at 25°C for 1 h. The specific competition probe was the unlabeled psmα promoter fragment, and a pta gene fragment was used as a nonspecific competition probe. Ppsmα, psmα promoter fragment; Ppsmα-biotin, biotin-labeled Ppsmα; Prbf, rbf promoter fragment.
TABLE 2.
Proteins with estimated molecular masses of 32 to 36 kDa identified by LC-MS/MS
| Gene | Protein | Molecular mass (Da) | Molecular function |
|---|---|---|---|
| SAOUHSC_03049 | Hypothetical protein SAOUHSC_03049 | 32,197 | Uncharacterized protein; DNA binding; similar to ParB, probably involved in chromosome partitioning and cell division processes |
| SAOUHSC_01041 | Pyruvate dehydrogenase complex, E1 component subunit β | 35,246 | Pyruvate dehydrogenase complex, putative E1 component β subunit; pyruvate dehydrogenase (acetyl group-transferring) activity |
| SAOUHSC_00472 | RPPK | 35,284 | Ribose phosphate pyrophosphokinase; ATP binding; kinase activity; magnesium ion binding; ribose phosphate diphosphokinase activity |
| SAOUHSC_00499 | PdxS | 31,993 | Pyridoxal 5′-phosphate synthase subunit PdxS; pyridoxal 5′-phosphate synthase (glutamine-hydrolyzing) activity |
| SAOUHSC_00206 | L-LDH-1 | 34583 | l-Lactate dehydrogenase 1; l-lactate dehydrogenase activity |
MgrA specifically binds to the promoter region of the psmα operon.
MgrA (also called Rat or NorR), a homolog of MarR and SarA, contains a DNA-binding HTH motif and regulates certain target genes in S. aureus by binding directly to their promoter regions (16, 17, 19, 29). To confirm its ability and specificity to bind to the promoter region of the psmα operon in vitro, we purified a 6His-tagged MgrA protein and employed the same 336-bp psmα operon promoter fragment used in the DNA affinity pulldown assay to perform electrophoretic mobility shift assays (EMSAs). DNA fragments (10 fmol per reaction mixture) were end-labeled with biotin and used in EMSAs with various amounts of purified MgrA. MgrA was able to bind to the psmα promoter region (Fig. 1B). This binding could be outcompeted with a 100-fold excess of an identical unlabeled psmα promoter DNA fragment, while a 100-fold excess of a nonspecific probe was not able to compete for MgrA binding. This result further suggested that MgrA could specifically bind to the promoter region of the psmα operon.
The recognition region of MgrA on the psmα promoter overlaps the −35 and −10 regions.
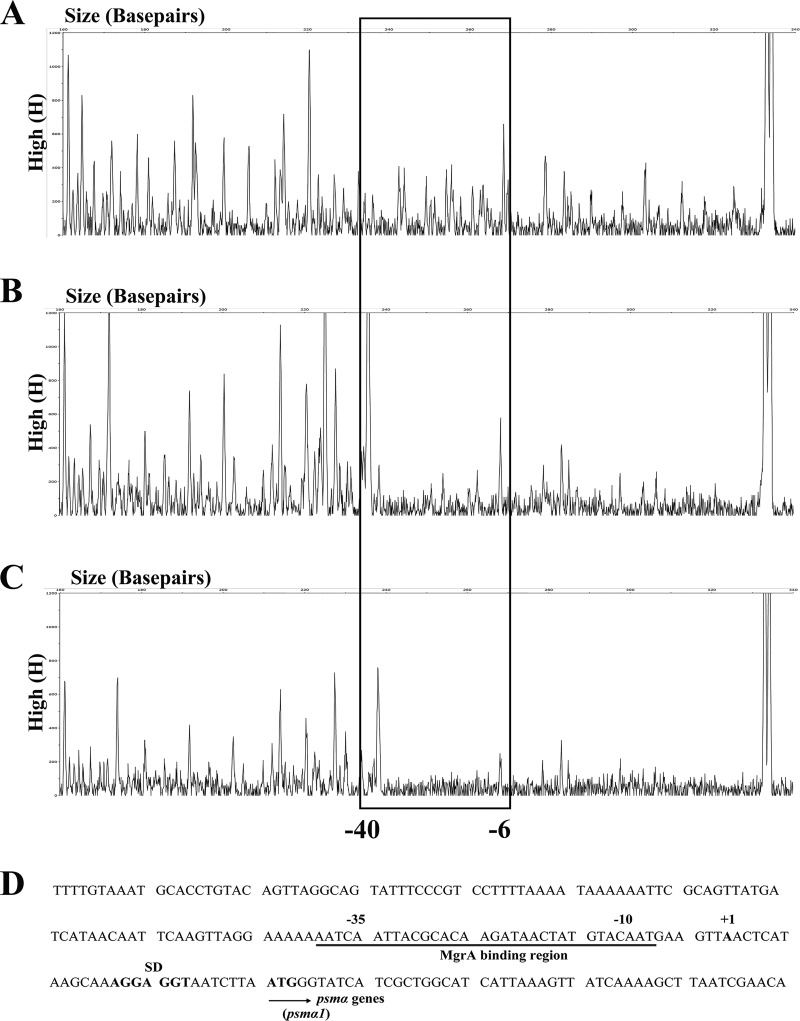
Since MgrA could bind directly to the promoter region of the psmα operon, we were interested in the MgrA recognition sites on the psmα operon promoter. We performed DNase I protection footprinting assays with the same 336-bp psmα operon promoter fragment labeled with 6-carboxylfluorescein (6-FAM) (Fig. 2). The 6-FAM-labeled DNA fragment was evenly digested by DNase I when MgrA protein was not added, as reflected by the even distribution of the 6-FAM signals (Fig. 2A). The region from −6 to −40 bp, relative to the transcription start site of the psmα operon (20), was protected, as indicated by the disappearing nucleotide peaks in Fig. 2A, compared to those in Fig. 2B and C. These data indicated that the MgrA recognition site may lie in the 34-bp region (AAATCAATTACGCACAAGATAACTATGTACAATG) of the psmα promoter overlapping the −35 to −10 transcriptional boxes of the psmα operon (Fig. 2D).
FIG 2.
(A to C) Mapping of the MgrA recognition site in the psmα promoter by DNase I footprinting. The 336-bp psmα promoter fragment was labeled with 6-FAM, and probes (1 pmol/100 μl) were incubated for 1 h at 25°C with MgrA at 0 μg/100 μl (A), 2 μg/100 μl (B), or 4 μg/100 μl (C) and then digested for 1 min at 37°C with DNase I at 0.1 U/100 μl. The protected region of MgrA is boxed in black. (D) MgrA binding sequence in the psmα promoter region. The MgrA binding region, based on the DNase footprinting analyses, is underlined in black. SD, Shine-Dalgarno sequence.
MgrA is a negative regulator of psm operons.
We constructed the isogenic mgrA deletion and mgrA-complemented strains and determined the transcriptional levels of the psmα operon in the wild-type (WT), mgrA mutant, and mgrA-complemented strains. First, we determined the growth curves of these strains and found that the mgrA mutant strain exhibited a nongrowth state at the 6- to 8-h time point, compared with the WT strain (Fig. 3A). Previous studies demonstrated that the autolysis of the mgrA mutant strain was increased at the mid-exponential phase (27), and the similar result was observed in our study (Fig. 3B). Real-time quantitative reverse transcription-PCR (qRT-PCR) was performed to determine the transcriptional levels of the psmα operon. The transcriptional level of psmα was significantly increased in the mgrA mutant strain, compared with the WT strain, and the change could be reversed by introducing plimgrA into the mgrA mutant strain (Fig. 3C). These data further indicated that MgrA could act as a negative regulator to modulate the expression of the psmα operon in S. aureus.
FIG 3.
MgrA as a negative regulator of psm operons. (A) Growth curves of NCTC8325-pli50, ΔmgrA-pli50, and ΔmgrA-plimgrA strains. Bacteria were grown at 37°C in TSB containing 15 μg/ml Cm, with shaking. OD600 values were measured every 1 h. Values are from three biological replicates (mean ± standard error of the mean [SEM]). Statistical values were determined with the Student t test and the F test to compare variances. ***, P < 0.001. (B) Autolysis induced by Triton X-100. Bacteria were grown at 37°C in TSB, with shaking, and cultures were collected at an OD600 of 3. Triton X-100 (0.1%) in Tris buffer (pH 7.5) was used to induce bacterial autolysis. Values are from three biological replicates (mean ± SEM). Statistical values were determined with the Student t test and the F test to compare variances. ***, P < 0.001. (C) qRT-PCR of psmα in NCTC8325-pli50, ΔmgrA-pli50, and ΔmgrA-plimgrA strains. Bacteria were grown at 37°C in TSB, with shaking. The cDNA samples used were prepared from RNA isolated from cells grown to the indicated growth phases (10 h). Probes were designed to align with part of the psmα operon. Values are from three biological replicates (mean ± SEM). Statistical values were determined by one-way analysis of variance (ANOVA). **, P < 0.01. (D) EMSA of purified MgrA with a 243-bp psmβ promoter fragment labeled with biotin. Increasing concentrations of the purified MgrA protein and 20 fmol of the Ppsmβ-biotin probe were used in the reactions, and the reaction mixtures were incubated at 25°C for 1 h. The specific competition probe was the unlabeled psmβ promoter fragment, and a pta gene fragment was used as a nonspecific competition probe. (E) Putative MgrA-binding regions (underlined in black), based on the sequence analysis. (F) qRT-PCR of psmβ in NCTC8325-pli50, ΔmgrA-pli50, and ΔmgrA-plimgrA strains. The cDNA samples used were prepared from RNA isolated from cells grown to the indicated growth phase (10 h). Probes were designed to align with part of the psmβ operon. Values are from three biological replicates (mean ± SEM). Statistical values were determined by one-way ANOVA. ***, P < 0.001. NCTC8325-pli50, NCTC8325 WT strain carrying plasmid pli50; ΔmgrA-pli50, mgrA mutant strain carrying plasmid pli50; ΔmgrA-plimgrA, mgrA mutant strain carrying plasmid plimgrA; Ppsmβ, psmβ promoter fragment; Ppsmβ-biotin, biotin-labeled Ppsmβ; SD, Shine-Dalgarno sequence.
Unlike PSMα, PSMβ is expressed from the psmβ operon (24). We further determined whether MgrA could regulate the expression of the psmβ operon in S. aureus. To determine whether MgrA could bind to the promoter region of the psmβ operon, we employed a 243-bp psmβ promoter fragment upstream of the initiation codon (20) to perform an EMSA. MgrA was able to bind to the promoter region of the psmβ operon; this binding could be outcompeted with a 50-fold excess of an identical unlabeled psmβ promoter DNA fragment, and a 50-fold excess of a nonspecific probe was not able to outcompete MgrA binding (Fig. 3D). The DNase I footprinting assay was performed with the same 243-bp promoter fragment of the psmβ operon labeled with 6-FAM, but it failed to identify the MgrA-binding region. Then, we analyzed the promoter sequence of the psmβ operon and found that several putative MgrA-binding regions may exist (Fig. 3E). Furthermore, qRT-PCR was performed to determine the transcriptional levels of psmβ in the WT, mgrA mutant, and mgrA-complemented strains. The transcriptional level of psmβ was significantly increased in the mgrA mutant strain, and the change could be reversed by introducing plimgrA into the mgrA mutant strain (Fig. 3F). These data indicated that MgrA could repress the expression of the psmβ operon by binding directly to the promoter region of the psmβ operon.
The amounts of PSMs in cultures can be determined by reverse-phase (RP)-high-performance liquid chromatography (HPLC) (24). Since MgrA can repress the expression of the psmα and psmβ operons, we analyzed the production of PSMs by RP-ultra-performance liquid chromatography (UPLC) to determine the changes of PSMs in the mgrA mutant strain. Overnight cultures were collected, and the culture filtrate samples were analyzed by RP-UPLC. The signals of PSMs were significantly enhanced in the mgrA mutant strain, compared with the WT strain (Fig. S1). These results suggested that MgrA could repress the transcription of psm operons and the repression led to the alteration of PSM production.
MgrA regulates biofilm development by repressing the expression of psm operons.
Previous studies showed that PSMs are involved in biofilm formation and detachment (21, 32), and MgrA represses biofilm formation in S. aureus (13). To demonstrate that MgrA can regulate biofilm development by repressing the expression of psm operons in S. aureus, we detected the biofilm formation in the WT, mgrA mutant, and mgrA-complemented strains and examined the transcriptional levels of psm genes in biofilms. Biofilm formation was significantly increased in the mgrA mutant strain, compared with the WT strain, after 12 h and 24 h of incubation, but biofilms of the mgrA mutant strain were much weaker than those of the WT strain after 36 h, 48 h, 60 h, and 72 h of incubation, and these changes could be reversed by introducing plimgrA into the mgrA mutant strain (Fig. 4A). Similar results were observed when the stained biofilms were solubilized with 30% glacial acetic acid and quantitated by reading the optical density at 560 nm (OD560) (Fig. 4B). These results suggested that MgrA repressed biofilm formation at the early biofilm development stage (before 24 h of incubation) and repressed biofilm disassembly at the late biofilm development stage (after 36 h of incubation).
FIG 4.
MgrA modulation of biofilm development by repression of psm gene expression. (A) Biofilm formation by NCTC8325-pli50, ΔmgrA-pli50, and ΔmgrA-plimgrA strains. Static biofilms were grown in 96-well plates at 37°C. At the indicated time points, biofilms were washed, stained with 0.5% crystal violet, and visualized with a camera. (B) Biofilm quantitation. Stained biofilms (A) were solubilized with 30% glacial acetic acid, and biofilm biomass was quantitated by measuring the OD560. Values are from three biological replicates (mean ± SEM), and statistical values were determined by two-way ANOVA. ns, P > 0.05; *, P < 0.05; **, P < 0.01; ***, P < 0.001. (C and D) qRT-PCR of psmα (C) and psmβ (D) in biofilms. Static biofilms were grown in 12-well plates for 12 h at 37°C. The cDNA samples used were prepared from RNA isolated from cells grown in biofilms. Values are from three biological replicates (mean ± SEM). Statistical values were determined by one-way ANOVA. ***, P < 0.001. (E) Biofilm formation by NCTC8325 WT, Δα/β, ΔmgrA, and ΔmgrA/α/β strains. Static biofilms were grown in 96-well plates at 37°C. At the indicated time points, biofilms were washed, stained with 0.5% crystal violet, and visualized with a camera. (F) Biofilm quantitation. Stained biofilms (E) were solubilized with 30% glacial acetic acid, and biofilm biomass was quantitated by measuring the OD560. Values are from three biological replicates (mean ± SEM), and statistical values were determined by two-way ANOVA. ns, P > 0.05; *, P < 0.05; **, P < 0.01; ***, P < 0.001. NCTC8325-pli50, NCTC8325 WT strain carrying plasmid pli50; ΔmgrA-pli50, mgrA mutant strain carrying plasmid pli50; ΔmgrA-plimgrA, mgrA mutant strain carrying plasmid plimgrA; NCTC8325 WT, NCTC8325 WT strain; Δα/β, psmα psmβ double mutant strain; ΔmgrA, mgrA mutant strain; ΔmgrA/α/β, mgrA psmα psmβ triple mutant strain.
To determine whether MgrA could regulate the expression of psm operons in biofilms, the bacterial cells in biofilms were collected after 12 h of incubation and RNAs were extracted to quantitate the transcriptional levels of psm genes. The transcriptional levels of psmα and psmβ were significantly increased in the mgrA mutant strain, compared with the WT strain, and the changes could be reversed by introducing plimgrA into the mgrA mutant strain (Fig. 4C and D). These findings indicated that MgrA could repress the expression of psm operons in cultures and biofilms. Thus, we speculated that MgrA could modulate biofilm formation by repressing the production of PSMs in S. aureus.
To test our speculation, we constructed the isogenic psm deletion strains and detected biofilm formation (Fig. 4E and F). Biofilm formation was significantly increased in the psmα psmβ double mutant strain, compared with the WT strain, which was consistent with a previous report (21). However, biofilm formation was decreased in the mgrA psmα psmβ triple mutant strain, compared with the mgrA mutant strain, after 18 h of incubation (Fig. 4E and F). These data suggested that the overexpression of psm genes could promote biofilm formation at the early biofilm development stage in the mgrA mutant strain. After 36 h of incubation, more biofilm was detected in the mgrA psmα psmβ triple mutant strain, compared with the mgrA mutant strain (Fig. 4E and F), suggesting that the overexpression of psm genes promoted biofilm disassembly quickly at the late biofilm development stage in the mgrA mutant stain. After 72 h of incubation, less biofilm was detected and no difference between the mgrA mutant and mgrA psmα psmβ triple mutant strains was observed, suggesting that biofilms detached totally in the mgrA mutant and mgrA psmα psmβ triple mutant strains (Fig. 4E and F). Collectively, these results indicated that MgrA could modulate biofilm development by repressing the expression of psm operons in S. aureus.
MgrA modulates biofilm structuring at the early biofilm development stage by repressing the expression of psm operons.
Previous studies proved that PSMs are involved in biofilm structuring in the premier biofilm-forming pathogen S. aureus (21). To determine the changes in biofilm structuring in the mgrA mutant strain, we constructed a plasmid containing a green fluorescent protein (GFP)-coding gene, transformed it into the WT, psmα psmβ double mutant, mgrA mutant, and mgrA psmα psmβ triple mutant strains, and assessed biofilm samples with confocal laser scanning microscopy (CLSM) after 12 h of incubation (Fig. 5A). In static biofilms, total biofilm volume and mean thickness values were significantly greater in these mutants, compared with the WT strain, after 12 h of incubation (Fig. 5B and C), suggesting that both MgrA and PSMs can repress biofilm formation in the WT strain. Additionally, total biofilm volume was decreased in the mgrA psmα psmβ triple mutant strain, compared with the mgrA mutant strain, after 12 h of incubation (Fig. 5B), suggesting that PSMs can promote biofilm formation in the mgrA mutant strain. Furthermore, average biofilm volume values (indicating the degree of channel formation) were higher and total biofilm voxel values were lower in the mgrA psmα psmβ triple mutant strain, compared with the mgrA mutant strain (Fig. 5D and E), indicating that the mgrA mutant strain formed less compact and rougher biofilms on the surface and had more prominent biofilm channels. These results suggested that PSMs promoted biofilm structuring under static conditions in the mgrA mutant strain. Collectively, these findings indicated that MgrA can modulate biofilm structuring by repressing the production of PSMs in S. aureus.
FIG 5.
Static biofilm formation by S. aureus. Static biofilms were grown in chambered cover glass plates at 37°C for 12 h. Z-stacks were obtained with CLSM at 2-μm intervals, reconstructed with Zeiss ZEN 2012 software, and analyzed with Imaris 7.0 software. All confocal parameters were set with the settings for NCTC8325 pGFP biofilm as standard settings for comparing the biofilms produced by ΔmgrA pGFP, Δα/β pGFP, and ΔmgrA/α/β pGFP strains. (A) Biofilm images of S. aureus strains. Biofilm images were obtained in at least 10 randomly chosen fields of the same biofilm. Extensions and scales are the same in all images (total extension on the x axis, 170 μm; total extension on the y axis, 170 μm). (B to E) Biofilm quantitation. Biofilm parameters were measured at 12 h in at least 15 randomly chosen biofilm CLSM images of the same extension. Horizontal bars depict the means. Statistical analysis was performed with t tests. ns, P > 0.05; ***, P < 0.001; ****, P < 0.0001. NCTC8325 pGFP, NCTC8325 WT strain carrying plasmid pGFP; ΔmgrA pGFP, mgrA mutant strain carrying plasmid pGFP; Δα/β pGFP, psmα psmβ double mutant strain carrying plasmid pGFP; ΔmgrA/α/β pGFP, mgrA psmα psmβ triple mutant strain carrying plasmid pGFP.
The mgrA mutant strain exhibits increased dynamic biofilm formation and detachment.
Biofilm formation is commonly analyzed under static or dynamic (flow cell-grown) conditions, and these conditions may be strongly divergent. Therefore, we measured biofilm development under dynamic conditions using the flow cell system. First, we compared the biofilm formation of the WT, psmα psmβ double mutant, mgrA mutant, and mgrA psmα psmβ triple mutant strains at the early biofilm development stage. Biofilm formation was increased in all of these mutant strains, compared with the WT strain, after 24 h of incubation (Fig. 6A, D, and E). More biofilm was detected in the psmα psmβ double mutant strain than in the WT strain, and the mgrA mutant strain formed a little less biofilm than did the mgrA psmα psmβ triple mutant strain (Fig. 6D). These data indicated that MgrA and PSMs could repress biofilm formation in the WT strain and PSMs could slightly repress biofilm formation under dynamic conditions at the early biofilm development stage in the mgrA mutant strain. To further investigate what changes would occur as the incubation time was extended, we assessed biofilm development successively and found that the mgrA mutant and mgrA psmα psmβ triple mutant strains exhibited fast biofilm detachment, while the WT and psmα psmβ double mutant strains kept stable biofilm growth (Fig. 6B and C). Moreover, compared with the mgrA psmα psmβ triple mutant strain, the biofilms of the mgrA mutant strain disassembled more quickly and had less biofilm volume after 48 h (Fig. 6B and H) and 72 h (Fig. 6C and I) of incubation. These results suggested that MgrA repressed biofilm detachment by controlling the production of PSMs in biofilms under dynamic conditions. We also measured other biofilm parameters of 24-h biofilms (Fig. 6F and G). Average biofilm volume values and total biofilm voxel values were higher in the psmα psmβ double mutant strain, compared with the WT strain, indicating that the psmα psmβ double mutant strain formed more compact biofilms on the surface and had more prominent biofilm channels. Average biofilm volume values were lower and total biofilm voxel values were higher in the mgrA mutant strain, compared with the WT strain, indicating that the mgrA mutant strain formed less compact biofilms on the surface and had more prominent biofilm channels. Moreover, average biofilm volume values were a little higher in the mgrA psmα psmβ triple mutant strain than in the mgrA mutant strain, indicating that the mgrA mutant strain formed slightly less compact biofilms on the surface. These results allowed us to conclude that PSMs promoted biofilm structuring, under dynamic conditions, at the early biofilm development stage in the WT and mgrA mutant strains. Collectively, these results suggested that MgrA repressed biofilm formation and structuring at the early biofilm development stage and weakened biofilm detachment at the late biofilm development stage by modulating the production of PSMs in biofilms.
FIG 6.
Dynamic S. aureus biofilm formation and detachment. (A to C) Biofilm images. Dynamic biofilms were grown at 37°C in the flow cell system. At the indicated time points, z-stacks were obtained with CLSM at 1-μm intervals, reconstructed with Zeiss ZEN 2012 software, and analyzed with Imaris 7.0 software. All confocal parameters were set with the settings for NCTC8325 pGFP biofilm as standard settings for comparing the biofilms produced by ΔmgrA pGFP, Δα/β pGFP, and ΔmgrA/α/β pGFP strains. Biofilm images were obtained at 24 h (A), 48 h (B), and 72 h (C) in at least 10 randomly chosen fields of the same biofilm. Extensions and scales are the same in all images (total extension on the x axis, 210 μm; total extension on the y axis, 210 μm). (D to G) Biofilm quantitation. Biofilm parameters were measured at 24 h of incubation in at least 10 randomly chosen biofilm CLSM images of the same extension. (H and I) Intensity sum of GFP for the 48-h biofilms (H) and the 72-h biofilms (I). Horizontal bars depict the means. Statistical analysis was performed with t tests. ns, P > 0.05; *, P < 0.05; ***, P < 0.001; ****, P < 0.0001. NCTC8325 pGFP, NCTC8325 WT strain carrying plasmid pGFP; ΔmgrA pGFP, mgrA mutant strain carrying plasmid pGFP; Δα/β pGFP, psmα psmβ double mutant strain carrying plasmid pGFP; ΔmgrA/α/β pGFP, mgrA psmα psmβ triple mutant strain carrying plasmid pGFP.
DISCUSSION
PSMs are amphipathic and surfactant-like peptides that play multiple roles in biofilm formation and virulence in S. aureus. Despite the multiple functions of PSMs in S. aureus, the regulatory mechanism controlling the expression of psm operons has not been thoroughly elucidated. AgrA, a regulator of the agr quorum-sensing system, is a positive regulator of psm operons and strictly regulates psm expression by binding directly to the promoter regions of psm operons (20). No other regulators that could directly modulate psm expression in S. aureus have been reported. In this study, we have demonstrated that MgrA can negatively regulate the expression of psm genes by binding directly to the promoter regions of psm operons. Thus, the functions of MgrA and AgrA in the regulation of psm genes in S. aureus seem to be opposite. Since no PSMs are produced in agr-negative strains (20), we tried to restore the production of PSMs by inactivating MgrA in agr-negative strains. However, the deletion of mgrA could not restore the production of PSMs in agr-negative strains (see Fig. S2 in the supplemental material). Previous studies suggested that MgrA and the agr system can regulate each other in S. aureus. For instance, MgrA can regulate the expression of the agr system and virulence genes (29), and RNAIII, which is encoded by the agr system, can modulate the production of MgrA (33). It seems that AgrA and MgrA play roles in several common regulatory pathways and AgrA is a positive and indispensable regulator of PSMs in S. aureus. To our surprise, other regulators, such as AgrA, were not identified in our pulldown assay. However, we could not exclude the possibility that other transcriptional regulators are involved in the modulation of psm expression.
MgrA regulates biofilm- and virulence-associated genes by binding directly to the promoter regions of target genes in S. aureus (16, 17, 19). MgrA activates sarX transcription by binding upstream of the −35 box region on the promoter of sarX (16), represses sarV expression by binding upstream of the −35 box region on the promoter of sarV (17), and represses the expression of surface proteins by binding to the −10 and −35 promoter regions of those genes (19). In this study, we have demonstrated that MgrA can repress psm expression by binding directly to the −10 and −35 promoter regions of psm operons, which is consistent with previous studies. However, the microarray analysis by Crosby et al. failed to identify psm operons as members of the MgrA regulon (19). It has been reported that the transcription of psm operons is strictly controlled by AgrA (20). As we know, the expression of psm genes is initiated when the agr quorum-sensing system is activated at a certain threshold level of bacterial cell density in the postexponential phase (24). Crosby et al. performed RNA sequencing to identify genes regulated by mgrA in S. aureus strain LAC (19). On the basis of the RNA sequencing data, they failed to identify psm operons as members of the MgrA regulon, most likely due to the threshold for microarray data. In their study, S. aureus strain LAC and the isogeneic mgrA mutant were grown in rich medium to an OD600 of 1.5, a point at which the bacterial cell density is not enough to activate the agr quorum-sensing system, leading to a low level of psm operon transcription and the failure of RNA sequencing to identify psm operons as members of the MgrA regulon.
MgrA modulates biofilm formation by controlling surface protein expression, protease production, and eDNA release in S. aureus (13, 19). Our data indicated that the mgrA mutant developed increased biofilm formation at the early biofilm development stage (before 24 h of incubation). This result is consistent with the report by Trotonda et al. (13). However, our findings showed decreased biofilm formation at the late biofilm development stage in the mgrA mutant strain (Fig. 4 and 6). These data are not completely in agreement with previous studies, which showed that MgrA repressed biofilm formation in S. aureus (13, 15, 19). This difference can be explained by the finding that MgrA can repress the expression of psm operons in our strain. On one hand, the development of biofilms contains three stages, including attachment, maturation, and detachment (9). Thus, the biofilms begin to disassemble at the late biofilm development stage (after 36 h of incubation). On the other hand, PSMs are surfactant-like peptides and can promote biofilm disassembly in S. aureus (21). At the late biofilm development stage, large amounts of PSMs were produced, which can promote biofilm disassembly in the mgrA mutant strain. As a result, the mgrA mutant strain had less biofilm than did the WT strain at the late biofilm development stage. In fact, our findings are not totally opposed to and can be taken as a supplement to previous studies.
Although PSMs are associated with biofilm structuring, biofilms are also significantly reduced by PSMs due to the surfactant-like properties of PSMs (21). However, the mgrA mutant strain developed enhanced biofilm formation along with increased expression of psm genes at the early biofilm development stage. We considered that other biofilm-associated factors regulated by MgrA, such as the increased eDNA release in the mgrA mutant strain, might contribute to the enhancement of biofilm formation (Fig. S3). Indeed, autolysis of the mgrA mutants is increased, and the release of eDNA is increased in biofilms of the mgrA mutant strain (13, 29, 34). Several studies have shown that eDNA is a component of biofilms and can promote biofilm formation in S. aureus (35–38). Moreover, MgrA can regulate the production of nucleases (39), proteases (13), and surface proteins (19) to modulate biofilm formation.
Additionally, it has been reported that PSMs are involved in the pathogenesis of S. aureus (8, 24) and that MgrA can modulate the expression of virulence-associated genes in S. aureus (16, 19, 29). In this study, we found that the ability to lyse blood cells and epithelial cells was decreased in the mgrA psmα psmβ triple mutant strain in comparison with the mgrA mutant strain and was increased in the mgrA psmα psmβ triple mutant strain in comparison with the psmα psmβ double mutant strain (Fig. S4). These results suggested that MgrA may modulate the pathogenesis of S. aureus by repressing the production of PSMs in vivo.
In conclusion, our findings have revealed that MgrA is a negative regulator of psm genes and represses the production of PSMs by binding directly to the promoter regions of the psm operons. Our findings provide novel insights into the regulatory mechanisms of psm gene expression, biofilm development, and S. aureus pathogenesis.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 3. S. aureus cells were grown at 37°C, with aeration, in tryptic soy broth (TSB; BD) supplemented with antibiotics when necessary. Luria-Bertani (LB) medium (Oxoid) was used for cultivation of Escherichia coli. Antibiotics were used at the following concentrations: for S. aureus, chloramphenicol (Cm) at 15 μg/ml; for E. coli, ampicillin at 150 μg/ml and kanamycin at 50 μg/ml.
TABLE 3.
Strains and plasmids used in this study
| Strain or plasmid | Descriptiona | Source or reference |
|---|---|---|
| Strains | ||
| Escherichia coli | ||
| Trans-T1 | Cloning strain | TransGen |
| BL21(DE3) | Expression strain | TransGen |
| Staphylococcus aureus | ||
| NCTC8325 WT | NCTC8325 WT strain | NARSAb |
| RN4220 | 8325-4; restriction modification deficient | NARSA |
| ΔmgrA | NCTC8325 mgrA mutant strain | This study |
| Δα/β | NCTC8325 psmα psmβ double mutant strain | This study |
| ΔmgrA/α/β | NCTC8325 mgrA psmα psmβ triple mutant strain | This study |
| Δagr | NCTC8325 agr mutant strain | This study |
| Δagr/mgrA | NCTC8325 agr mgrA double mutant strain | This study |
| N315 WT | N315 (agr-negative) WT strain | NARSA |
| N315ΔmgrA | N315 mgrA mutant strain | This study |
| NCTC8325-pli50 | NCTC8325 carrying plasmid pli50 | This study |
| ΔmgrA-pli50 | ΔmgrA carrying plasmid pli50 | This study |
| ΔmgrA-plimgrA | ΔmgrA carrying plasmid plimgrA | This study |
| Δα/β-pli50 | Δα/β carrying plasmid pli50 | This study |
| ΔmgrA/α/β-pli50 | ΔmgrA/α/β carrying plasmid pli50 | This study |
| NCTC8325 pGFP | NCTC8325 carrying plasmid pGFP | This study |
| Δα/β pGFP | Δα/β carrying plasmid pGFP | This study |
| ΔmgrA pGFP | ΔmgrA carrying plasmid pGFP | This study |
| ΔmgrA/α/β pGFP | ΔmgrA/α/β carrying plasmid pGFP | This study |
| Plasmids | ||
| pBTs | E. coli-S. aureus shuttle vector; temperature sensitive, Ampr, Cmr | 43 |
| pBTs-mgrA | pBTs plasmid containing mutant allele for mgrA deletion; Ampr, Cmr | This study |
| pBTs-psmα | pBTs plasmid containing mutant allele for psmα deletion; Ampr, Cmr | This study |
| pBTs-psmβ | pBTs plasmid containing mutant allele for psmβ deletion; Ampr, Cmr | This study |
| pli50 | E. coli-S. aureus shuttle vector; Ampr, Cmr | Addgene |
| plimgrA | Complete mgrA gene under control of native promoter in pli50; Ampr, Cmr | This study |
| pGFP | gfp expression with promoter of S10 ribosomal gene; Ampr, Cmr | 49 |
Ampr, ampicillin resistant; Cmr, chloramphenicol resistant.
NARSA, Network on Antimicrobial Resistance in Staphylococcus aureus.
Genetic manipulation in E. coli and S. aureus.
Construction of recombinant plasmids was performed in E. coli Trans-T1 with standard molecular biology and recombinant DNA techniques. Genomic DNA of S. aureus was prepared by using a standard protocol for Gram-positive bacteria (40). Plasmid DNA was extracted with a plasmid purification kit (Promega), according to the manufacturer's instructions. All plasmids transformed into the indicated S. aureus strains were introduced first into S. aureus RN4220 for modification by electroporation, as described previously (41).
To construct the isogenic mgrA deletion strain, two fragments flanking upstream and downstream of mgrA were amplified from S. aureus NCTC8325 genomic DNA with the primer pairs mgrA-up-F/mgrA-up-R and mgrA-down-F/mgrA-down-R (Table 4). The two PCR products had a 20-base complementary region to facilitate ligation followed by the seamless ligation cloning extract (SLiCE) method, as described previously (42). Outside primers mgrA-up-F and mgrA-down-R were then used to amplify a single fragment from the ligated products, and the fusion product was purified, digested with KpnI and SalI, and cloned into the shuttle vector pBTs to generate pBTs-mgrA (Table 3). The plasmid pBTs-mgrA was transformed first into RN4220 at 30°C on tryptic soy agar (TSA) containing 15 μg/ml Cm (TSA Cm plates) for modification and then into the WT and agr mutant strains by electroporation. Allelic exchange in the absence of the selection marker was performed as described previously (43). Briefly, individual colonies were streaked on TSA Cm plates and incubated at 30°C. Single colonies were grown in TSB at 42°C and diluted 1:200 in fresh medium for 3 successive days before being diluted to 10−3 and plated on TSA Cm plates to select for integration into the chromosome. Single colonies were grown in TSB at 30°C and diluted 1:200 in fresh medium for 2 successive days before being diluted to 10−6 and plated on TSA plates containing 0.1 μg/ml anhydrotetracycline to select for loss of the plasmid. Colonies were screened for resistance to Cm, and Cm-sensitive colonies were screened for deletion of mgrA by PCR. The mgrA mutants were verified by PCR and sequencing. A similar strategy was used to construct S. aureus agr and psm deletion mutant strains. All primers used in this study are listed in Table 4.
TABLE 4.
Sequences of primers used in this study
| Primer name | Sequence (5′ to 3′)a | Modification |
|---|---|---|
| Ppsmα-F | CCGGAATTCTCTGTTCAATTCATCTTCATA | |
| Ppsmα-biotin-R | CGCGGATCCCCGCCAGCGATGATACCCATTAAG | 5′-Biotin |
| Prbf-F | CCGGAATTCCAGGTGTACTTGCCTTTCTA | |
| Prbf-biotin-R | CGCGGATCCCCCAAGCATGATTTTGCCATAAC | 5′-Biotin |
| Ppsmα-F | CCGGAATTCTCTGTTCAATTCATCTTCATA | |
| Ppsmα-R | CGCGGATCCCCGCCAGCGATGATACCCATTAAG | |
| Ppsmβ-F | ACTTAAATACGAATTCAGGCAACT | |
| Ppsmβ-R | AACCTTCCATTGAAAACACTCC | |
| Ppsmβ-biotin-R | AACCTTCCATTGAAAACACTCC | 5′-Biotin |
| pta-F | AAAGCGCCAGGTGCTAAATT | |
| pta-R | CTGGACCAACTGCATCATAT | |
| Ppsmα-FAM-F | TCTGTTCAATTCATCTTCATA | 5′-6-FAM |
| Ppsmβ-FAM-F | ACTTAAATACGAATTCAGGCAACT | 5′-6-FAM |
| mgrA-up-F | GCGggtaccATGTCACTTAGTTTCAAC | |
| mgrA-up-R | TTACCTAATAAGCGATTAAGTGCTGTTCTTTTAAATTATG | |
| mgrA-down-F | ACTTAATCGCTTATTAGGTAA | |
| mgrA-down-R | GCGgtcgacCAGGGTTATATCAATTAGATAG | |
| psmα-up-F | GCGGAATTCGAGCTCggtaccAATGTAATACCCCAGCAGAGTGCC | |
| psmα-up-R | GGACGGGAAATACTGCCTAACTGT | |
| psmα-down-F | ACAGTTAGGCAGTATTTCCCGTCCTCTCAGGCCACTATACCAATAGGG | |
| psmα-down-R | CTTGCATGCCTGCAGGTCGACCCAGAATATGGCGATCGTCA | |
| psmβ-up-F | GCGGAATTCGAGCTCggtaccCCAGAATATGGCGATCGTCA | |
| psmβ-up-R | CAATTAGTTTGTTTATCCGCACA | |
| psmβ-down-F | TGTGCGGATAAACAAACTAATTGCAATTAGTTTGTTTATCCGCACA | |
| psmβ-down-R | CTTGCATGCCTGCAGGTCGACGTGCTGTCTTTCATCCTCACCA | |
| mgrA-c-F | GCGggatccTCATCATTTTTTAATAAT | |
| mgrA-c-R | GCGgtcgacCAATTACTAGCTAATCAAGG | |
| mgrA-RT-F | GACAAGTTAATCGCTACTAC | |
| mgrA-RT-R | GAGTGCTAATTCAGTTACG | |
| psmα-RT-F | GTATCATCGCTGGCATCA | |
| psmα-RT-R | AAGACCTCCTTTGTTTGTTATG | |
| psmβ-RT-F | TGGACTAGCAGAAGCAATC | |
| psmβ-RT-R | TAGTAAACCCACACCGTTAG | |
| hld-RT-F | GTGAATTTGTTCACTGTGTCGA | |
| hld-RT-R | GGAGTGATTTCAATGGCACAAG | |
| hu-RT-F | AAAAAGAAGCTGGTTCAGCAGTAG | |
| hu-RT-R | TTTACGTGCAGCACGTTCAC |
The sequences in lowercase letters refer to restriction endonuclease recognition sites.
Complementation plasmids were created as follows. The complete mgrA gene with its native promoter was amplified by PCR with primers mgrA-c-F and mgrA-c-R (Table 4). The resulting product was digested with BamHI and SalI and ligated with pli50, which had been digested with the same enzymes, to generate plimgrA. The complementing plasmids were transformed into S. aureus RN4220 and then into the mgrA mutant strain. The WT and mgrA mutant strains were also transformed with the pli50 plasmid, which was used as the control.
DNA pulldown assay.
The DNA pulldown assay was performed as described previously (40, 44), with minor modifications. Briefly, the biotin-labeled DNA fragment containing the promoter region was amplified from S. aureus NCTC8325 genomic DNA using primers Ppsmα-F and Ppsmα-biotin-R (Table 4). The control DNA fragment of the rbf promoter sequence was amplified with primers Prbf-F and Prbf-biotin-R (45). S. aureus cultures at different growth phases were collected and were resuspended in lysis buffer (10 mM HEPES [pH 7.0], 200 mM NaCl, 1% Triton X-100, 10 mM MgCl2, 1 mM dithiothreitol) containing protease inhibitor cocktail (Sangon), 1 U/ml DNase I (Promega), and 40 U/ml lysostaphin (AMBI). To lyse the cells completely, the suspension was incubated at 37°C with shaking until the cells were thoroughly lysed. The lysate was centrifuged at 12,000 × g for 30 min at 4°C to remove insoluble debris, and the supernatants were then collected. The total protein was stored temporarily at 4°C for later use. At the same time, streptavidin-MagneSphere paramagnetic particles (SA-PMPs) (Promega) were rinsed twice with 0.5× standard saline citrate (SSC) (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) and then rinsed twice with lysis buffer. The DNA sample (200 μl; 200 to 400 ng/μl) was incubated with the prepared SA-PMPs at 25°C for 1 h on a rotating shaker; total protein was then added, and the mixture was incubated at 4°C for 1.5 h on a rotating shaker. The SA-PMPs was then washed three times with fresh lysis buffer. The proteins captured were incubated at 95°C for 10 min, separated by SDS-PAGE, and then stained with silver or brilliant blue R-250. The gel bands were excised and digested with trypsin (0.6 mg), and the tryptic peptides were subjected to LC-MS/MS analysis with a linear trap quadrupole (LTQ) mass spectrometer (ProteomeX-LTQ; Thermo Fisher Scientific). Sequence and peptide fingerprint data were analyzed using the NCBI database.
MgrA expression and purification.
The 444-bp DNA fragment containing the complete mgrA gene (SAOUHSC_00694 of S. aureus NCTC8325) was amplified from S. aureus NCTC8325 genomic DNA using primers containing flanking restriction sites (NdeI and XhoI) to facilitate in-frame cloning into the expression vector pET28a(+) to obtain pETmgrA. The recombinant plasmid containing the mgrA coding region was confirmed by restriction digestion analysis and DNA sequencing and was subsequently transformed into E. coli BL21(DE3). The expression and purification of the recombinant His6-MgrA protein were performed as described previously (17). The purity of the purified His6-MgrA fusion protein was determined by SDS-PAGE, with brilliant blue R-250 staining. The concentration of the purified MgrA protein was determined with the Bradford protein assay (Bio-Rad, Hercules, CA), using bovine serum albumin as the standard.
EMSA.
The 5′-biotin-labeled DNA fragments containing the promoter region were amplified from S. aureus NCTC8325 genomic DNA and incubated at 25°C for 1 h with various amounts of MgrA in 8 μl of incubation buffer (50 mM Tris-HCl [pH 8.0], 200 mM NaCl). After incubation, 2 μl of gel loading buffer was added to the mixtures, and then the mixtures were electrophoresed in a 5% native polyacrylamide gel in 1× Tris-borate-EDTA buffer. The band shifts were detected and analyzed with the chemiluminescent nucleic acid detection module (Pierce), according to the manufacturer's instructions. Images were obtained using an ImageQuant LAS 4000 mini imager (GE, Piscataway, NJ). Unlabeled promoter fragments in 100-fold (or 50-fold) excess were added as specific competitors. An unlabeled DNA fragment of the housekeeping gene pta in 100-fold (or 50-fold) excess was added as a nonspecific competitor.
DNase I footprinting assay.
Footprinting assays with the psmα promoter region and DNase I were performed as described previously (46). The forward primer was synthesized and 5′ labeled with 6-FAM. The labeled DNA fragment was amplified from S. aureus NCTC8325 genomic DNA by PCR. The binding reactions were carried out for 1 h at 25°C in a 100-μl reaction volume containing 20 mM Tris-HCl (pH 8.0), 100 mM NaCl, 5 mM MgCl2, 1 mM CaCl2, 2 mM dithiothreitol, 1 pmol 5′-6-FAM-labeled template DNA, and various amounts of purified MgrA. DNase I (0.1 U in 100 μl; Promega) was added to the reaction mixture, the mixture was incubated for 1 min at 37°C, and then DNA was extracted with phenol-chloroform, precipitated with 95% ethanol, washed with 75% ethanol, dried, and dissolved in double-distilled water. DNA samples were detected by short tandem repeat sequencing. The protected region of MgrA was derived by comparing the sequencing results with versus without MgrA, using Peak Scanner software (v1.0; Applied Biosystems).
Total RNA isolation, cDNA generation, and qRT-PCR.
Total RNA was prepared with a TRIzol isolation kit (TaKaRa) and a reciprocating shaker, as described previously (40). Briefly, overnight cultures of S. aureus were diluted 1:200 in fresh TSB containing 15 μg/ml Cm, grown at 37°C with shaking (220 rpm), and then grown to the indicated OD600 before being collected. The collected cells were processed with 1 ml of RNAiso Plus reagent (TaKaRa) in combination with 0.1-mm-diameter silica beads in a FastPrep-24 automated system (MP Biomedicals, Solon, OH), and the residual DNA was removed with RNase-free DNase I (TaKaRa). The concentration of total RNA was determined by measuring the absorbance at 260 nm, and the concentration of total RNA was adjusted to 200 ng/μl. For reverse transcription, cDNA templates were synthesized from 200 ng of total RNA with the PrimeScript first-strand cDNA synthesis kit (TaKaRa). qRT-PCR was performed with SYBR Premix Ex Taq reagent (TaKaRa), using the StepOne real-time PCR system (Applied Biosystems) and following the MIQE guidelines. The housekeeping gene hu was used as an endogenous control, and the quantity of cDNA measured through qRT-PCR was normalized to the abundance of hu cDNA, as described previously (47). The qRT-PCR specificity was confirmed using a melting curve of the products. To assess DNA contamination, each RNA sample was subjected to qRT-PCR using SYBR Premix Ex Taq reagent (TaKaRa). To determine the reaction efficiency of qRT-PCR, a series of diluted cDNA samples of the WT strain were subjected to qRT-PCR using SYBR Premix Ex Taq reagent (TaKaRa). Relative expression levels were determined by the comparative threshold cycle (ΔΔCT) method. qRT-PCR was repeated three times in triplicate parallel experiments.
Biofilm formation and analysis.
Biofilm formation under static conditions was determined by microtiter plate assays, based on the method described previously (13). Briefly, overnight cultures were diluted 1:200 in fresh TSB and inoculated (200 μl in each well) in flat-bottom 96-well polystyrene plates (Costar), in triplicate. The plates were incubated at 37°C for different times, and the wells were washed gently three times with water (to remove nonadherent cells), stained with 0.5% crystal violet for 5 min, and then again washed three times with water. To visualize biofilms, photographs of the inverted plate were taken with a camera. To quantitate the biofilms, the crystal violet stain was solubilized with 30% glacial acetic acid for 15 min, and the relative biofilm formation was determined by reading OD560 values using an enzyme-linked immunosorbent assay reader (Bio-Tek).
For CLSM, static biofilms were grown in chambered cover glass plates, as described previously (21), and analyzed with a Zeiss LSM 700 confocal microscope after gentle washing. Dynamic biofilms were grown in a flow cell system (Stovall Life Science, Greensboro, NC), which was assembled and prepared according to the manufacturer's instructions. Dynamic biofilm formation and CLSM were performed as described previously (48). Overnight cultures of different strains were adjusted to an OD600 of 5 and diluted 1:200 in fresh 0.2% TSB containing 15 μg/ml Cm and 0.2% glucose. The flow cells were inoculated with 5 ml of the culture dilutions and incubated at 37°C for 1 h. After this incubation period, the culture medium was continually perfused over the flow cell system, using a high-precision tubing pump with a planetary drive (ISMATEC, Switzerland), at a rate of 0.5 ml/min. Biofilms of different strains, which were transformed with the GFP plasmid for fluorescence detection, were cultivated at 37°C in three individual channels in 0.2% TSB containing 15 μg/ml Cm and 0.2% glucose. CLSM was performed with a Zeiss LSM710 system (Carl Zeiss, Jena, Germany) with a 20× apochromatic objective (numerical aperture of 0.8), fluorescence was excited with an argon laser at 488 nm, an emission band-pass filter of 515 ± 15 nm was used, and z-stacks were collected at 1-μm intervals. Confocal parameters set for detection of the WT biofilm were taken as the standard settings. Selected confocal images represented different areas of the biofilms and were selected from at least 10 different microscopic fields. Confocal images were acquired with the Zeiss ZEN 2010 software package (Carl Zeiss). Three-dimensional biofilm images were reconstructed with Zeiss ZEN 2012 software (Carl Zeiss) and analyzed with Imaris 7.0 software (Bitplane, Zurich, Switzerland).
Supplementary Material
ACKNOWLEDGMENTS
We thank the Network on Antimicrobial Resistance in Staphylococcus aureus for providing the bacterial strains.
This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (grant XDPB03).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.01008-18.
REFERENCES
- 1.Panizzi P, Nahrendorf M, Figueiredo JL, Panizzi J, Marinelli B, Iwamoto Y, Keliher E, Maddur AA, Waterman P, Kroh HK, Leuschner F, Aikawa E, Swirski FK, Pittet MJ, Hackeng TM, Fuentes-Prior P, Schneewind O, Bock PE, Weissleder R. 2011. In vivo detection of Staphylococcus aureus endocarditis by targeting pathogen-specific prothrombin activation. Nat Med 17:1142–1146. doi: 10.1038/nm.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bubeck Wardenburg J, Bae T, Otto M, Deleo FR, Schneewind O. 2007. Poring over pores: α-hemolysin and Panton-Valentine leukocidin in Staphylococcus aureus pneumonia. Nat Med 13:1405–1406. doi: 10.1038/nm1207-1405. [DOI] [PubMed] [Google Scholar]
- 3.Toba FA, Akashi H, Arrecubieta C, Lowy FD. 2011. Role of biofilm in Staphylococcus aureus and Staphylococcus epidermidis ventricular assist device driveline infections. J Thorac Cardiovasc Surg 141:1259–1264. doi: 10.1016/j.jtcvs.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fedtke I, Gotz F, Peschel A. 2004. Bacterial evasion of innate host defenses: the Staphylococcus aureus lesson. Int J Med Microbiol 294:189–194. doi: 10.1016/j.ijmm.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 5.Jones SM, Morgan M, Humphrey TJ, Lappin-Scott H. 2001. Effect of vancomycin and rifampicin on meticillin-resistant Staphylococcus aureus biofilms. Lancet 357:40–41. doi: 10.1016/S0140-6736(00)03572-8. [DOI] [PubMed] [Google Scholar]
- 6.Adair CG, Gorman SP, Feron BM, Byers LM, Jones DS, Goldsmith CE, Moore JE, Kerr JR, Curran MD, Hogg G, Webb CH, McCarthy GJ, Milligan KR. 1999. Implications of endotracheal tube biofilm for ventilator-associated pneumonia. Intensive Care Med 25:1072–1076. doi: 10.1007/s001340051014. [DOI] [PubMed] [Google Scholar]
- 7.Feldman C, Kassel M, Cantrell J, Kaka S, Morar R, Goolam Mahomed A, Philips JI. 1999. The presence and sequence of endotracheal tube colonization in patients undergoing mechanical ventilation. Eur Respir J 13:546–551. doi: 10.1183/09031936.99.13354699. [DOI] [PubMed] [Google Scholar]
- 8.Wang R, Khan BA, Cheung GY, Bach TH, Jameson-Lee M, Kong KF, Queck SY, Otto M. 2011. Staphylococcus epidermidis surfactant peptides promote biofilm maturation and dissemination of biofilm-associated infection in mice. J Clin Invest 121:238–248. doi: 10.1172/JCI42520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Otto M. 2008. Staphylococcal biofilms. Curr Top Microbiol Immunol 322:207–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boles BR, Horswill AR. 2008. Agr-mediated dispersal of Staphylococcus aureus biofilms. PLoS Pathog 4:e1000052. doi: 10.1371/journal.ppat.1000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu D, Zhao L, Xue T, Sun B. 2012. Staphylococcus aureus autoinducer-2 quorum sensing decreases biofilm formation in an icaR-dependent manner. BMC Microbiol 12:288. doi: 10.1186/1471-2180-12-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toledo-Arana A, Merino N, Vergara-Irigaray M, Debarbouille M, Penades JR, Lasa I. 2005. Staphylococcus aureus develops an alternative, ica-independent biofilm in the absence of the arlRS two-component system. J Bacteriol 187:5318–5329. doi: 10.1128/JB.187.15.5318-5329.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trotonda MP, Tamber S, Memmi G, Cheung AL. 2008. MgrA represses biofilm formation in Staphylococcus aureus. Infect Immun 76:5645–5654. doi: 10.1128/IAI.00735-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim Y, Jana M, Luong TT, Lee CY. 2004. Control of glucose- and NaCl-induced biofilm formation by rbf in Staphylococcus aureus. J Bacteriol 186:722–729. doi: 10.1128/JB.186.3.722-729.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ballal A, Ray B, Manna AC. 2009. sarZ, a sarA family gene, is transcriptionally activated by MgrA and is involved in the regulation of genes encoding exoproteins in Staphylococcus aureus. J Bacteriol 191:1656–1665. doi: 10.1128/JB.01555-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manna AC, Cheung AL. 2006. Expression of SarX, a negative regulator of agr and exoprotein synthesis, is activated by MgrA in Staphylococcus aureus. J Bacteriol 188:4288–4299. doi: 10.1128/JB.00297-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manna AC, Ingavale SS, Maloney M, van Wamel W, Cheung AL. 2004. Identification of sarV (SA2062), a new transcriptional regulator, is repressed by SarA and MgrA (SA0641) and involved in the regulation of autolysis in Staphylococcus aureus. J Bacteriol 186:5267–5280. doi: 10.1128/JB.186.16.5267-5280.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jefferson KK, Cramton SE, Gotz F, Pier GB. 2003. Identification of a 5-nucleotide sequence that controls expression of the ica locus in Staphylococcus aureus and characterization of the DNA-binding properties of IcaR. Mol Microbiol 48:889–899. doi: 10.1046/j.1365-2958.2003.03482.x. [DOI] [PubMed] [Google Scholar]
- 19.Crosby HA, Schlievert PM, Merriman JA, King JM, Salgado-Pabon W, Horswill AR. 2016. The Staphylococcus aureus global regulator MgrA modulates clumping and virulence by controlling surface protein expression. PLoS Pathog 12:e1005604. doi: 10.1371/journal.ppat.1005604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Queck SY, Jameson-Lee M, Villaruz AE, Bach TH, Khan BA, Sturdevant DE, Ricklefs SM, Li M, Otto M. 2008. RNAIII-independent target gene control by the agr quorum-sensing system: insight into the evolution of virulence regulation in Staphylococcus aureus. Mol Cell 32:150–158. doi: 10.1016/j.molcel.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Periasamy S, Joo HS, Duong AC, Bach TH, Tan VY, Chatterjee SS, Cheung GY, Otto M. 2012. How Staphylococcus aureus biofilms develop their characteristic structure. Proc Natl Acad Sci U S A 109:1281–1286. doi: 10.1073/pnas.1115006109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao Y, Sturdevant DE, Otto M. 2005. Genomewide analysis of gene expression in Staphylococcus epidermidis biofilms: insights into the pathophysiology of S. epidermidis biofilms and the role of phenol-soluble modulins in formation of biofilms. J Infect Dis 191:289–298. doi: 10.1086/426945. [DOI] [PubMed] [Google Scholar]
- 23.Zheng Y, Joo HS, Nair V, Le KY, Otto M. 2017. Do amyloid structures formed by Staphylococcus aureus phenol-soluble modulins have a biological function? Int J Med Microbiol doi: 10.1016/j.ijmm.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang R, Braughton KR, Kretschmer D, Bach TH, Queck SY, Li M, Kennedy AD, Dorward DW, Klebanoff SJ, Peschel A, DeLeo FR, Otto M. 2007. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med 13:1510–1514. doi: 10.1038/nm1656. [DOI] [PubMed] [Google Scholar]
- 25.Janzon L, Lofdahl S, Arvidson S. 1989. Identification and nucleotide sequence of the delta-lysin gene, hld, adjacent to the accessory gene regulator (agr) of Staphylococcus aureus. Mol Gen Genet 219:480–485. doi: 10.1007/BF00259623. [DOI] [PubMed] [Google Scholar]
- 26.Mehlin C, Headley CM, Klebanoff SJ. 1999. An inflammatory polypeptide complex from Staphylococcus epidermidis: isolation and characterization. J Exp Med 189:907–918. doi: 10.1084/jem.189.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ingavale SS, Van Wamel W, Cheung AL. 2003. Characterization of RAT, an autolysis regulator in Staphylococcus aureus. Mol Microbiol 48:1451–1466. doi: 10.1046/j.1365-2958.2003.03503.x. [DOI] [PubMed] [Google Scholar]
- 28.Truong-Bolduc QC, Dunman PM, Strahilevitz J, Projan SJ, Hooper DC. 2005. MgrA is a multiple regulator of two new efflux pumps in Staphylococcus aureus. J Bacteriol 187:2395–2405. doi: 10.1128/JB.187.7.2395-2405.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ingavale S, van Wamel W, Luong TT, Lee CY, Cheung AL. 2005. Rat/MgrA, a regulator of autolysis, is a regulator of virulence genes in Staphylococcus aureus. Infect Immun 73:1423–1431. doi: 10.1128/IAI.73.3.1423-1431.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaudhuri RR, Allen AG, Owen PJ, Shalom G, Stone K, Harrison M, Burgis TA, Lockyer M, Garcia-Lara J, Foster SJ, Pleasance SJ, Peters SE, Maskell DJ, Charles IG. 2009. Comprehensive identification of essential Staphylococcus aureus genes using transposon-mediated differential hybridisation (TMDH). BMC Genomics 10:291. doi: 10.1186/1471-2164-10-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu LJ, Errington J. 2004. Coordination of cell division and chromosome segregation by a nucleoid occlusion protein in Bacillus subtilis. Cell 117:915–925. doi: 10.1016/j.cell.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz K, Syed AK, Stephenson RE, Rickard AH, Boles BR. 2012. Functional amyloids composed of phenol soluble modulins stabilize Staphylococcus aureus biofilms. PLoS Pathog 8:e1002744. doi: 10.1371/journal.ppat.1002744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta RK, Luong TT, Lee CY. 2015. RNAIII of the Staphylococcus aureus agr system activates global regulator MgrA by stabilizing mRNA. Proc Natl Acad Sci U S A 112:14036–14041. doi: 10.1073/pnas.1509251112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trotonda MP, Xiong YQ, Memmi G, Bayer AS, Cheung AL. 2009. Role of mgrA and sarA in methicillin-resistant Staphylococcus aureus autolysis and resistance to cell wall-active antibiotics. J Infect Dis 199:209–218. doi: 10.1086/595740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fournier B, Hooper DC. 2000. A new two-component regulatory system involved in adhesion, autolysis, and extracellular proteolytic activity of Staphylococcus aureus. J Bacteriol 182:3955–3964. doi: 10.1128/JB.182.14.3955-3964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rice KC, Mann EE, Endres JL, Weiss EC, Cassat JE, Smeltzer MS, Bayles KW. 2007. The cidA murein hydrolase regulator contributes to DNA release and biofilm development in Staphylococcus aureus. Proc Natl Acad Sci U S A 104:8113–8118. doi: 10.1073/pnas.0610226104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bao Y, Zhang X, Jiang Q, Xue T, Sun B. 2015. Pfs promotes autolysis-dependent release of eDNA and biofilm formation in Staphylococcus aureus. Med Microbiol Immunol 204:215–226. doi: 10.1007/s00430-014-0357-y. [DOI] [PubMed] [Google Scholar]
- 38.Schwartz K, Ganesan M, Payne DE, Solomon MJ, Boles BR. 2016. Extracellular DNA facilitates the formation of functional amyloids in Staphylococcus aureus biofilms. Mol Microbiol 99:123–134. doi: 10.1111/mmi.13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luong TT, Dunman PM, Murphy E, Projan SJ, Lee CY. 2006. Transcription profiling of the mgrA regulon in Staphylococcus aureus. J Bacteriol 188:1899–1910. doi: 10.1128/JB.188.5.1899-1910.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang S, Ma R, Liu X, Zhang X, Sun B. 2015. Modulation of ccrAB expression and SCCmec excision by an inverted repeat element and SarS in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 59:6223–6232. doi: 10.1128/AAC.01041-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schenk S, Laddaga RA. 1992. Improved method for electroporation of Staphylococcus aureus. FEMS Microbiol Lett 73:133–138. doi: 10.1111/j.1574-6968.1992.tb05302.x. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y, Werling U, Edelmann W. 2012. SLiCE: a novel bacterial cell extract-based DNA cloning method. Nucleic Acids Res 40:e55. doi: 10.1093/nar/gkr1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu JF, Zhang X, Liu XY, Chen C, Sun BL. 2015. Mechanism of reduced vancomycin susceptibility conferred by walK mutation in community-acquired methicillin-resistant Staphylococcus aureus strain MW2. Antimicrob Agents Chemother 59:1352–1355. doi: 10.1128/AAC.04290-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jutras BL, Verma A, Stevenson B. 2012. Identification of novel DNA-binding proteins using DNA-affinity chromatography/pull down. Curr Protoc Microbiol Chapter 1:Unit 1F.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma R, Qiu S, Jiang Q, Sun H, Xue T, Cai G, Sun B. 2017. AI-2 quorum sensing negatively regulates rbf expression and biofilm formation in Staphylococcus aureus. Int J Med Microbiol 307:257–267. doi: 10.1016/j.ijmm.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 46.Xue T, You Y, Hong D, Sun H, Sun B. 2011. The Staphylococcus aureus KdpDE two-component system couples extracellular K+ sensing and Agr signaling to infection programming. Infect Immun 79:2154–2167. doi: 10.1128/IAI.01180-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valihrach L, Demnerova K. 2012. Impact of normalization method on experimental outcome using RT-qPCR in Staphylococcus aureus. J Microbiol Methods 90:214–216. doi: 10.1016/j.mimet.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 48.You Y, Xue T, Cao L, Zhao L, Sun H, Sun B. 2014. Staphylococcus aureus glucose-induced biofilm accessory proteins, GbaAB, influence biofilm formation in a PIA-dependent manner. Int J Med Microbiol 304:603–612. doi: 10.1016/j.ijmm.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 49.Bao Y, Li Y, Jiang Q, Zhao L, Xue T, Hu B, Sun B. 2013. Methylthioadenosine/S-adenosylhomocysteine nucleosidase (Pfs) of Staphylococcus aureus is essential for the virulence independent of LuxS/AI-2 system. Int J Med Microbiol 303:190–200. doi: 10.1016/j.ijmm.2013.03.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.