Picocyanobacteria are highly diverse and abundant in the ocean and display remarkable global biogeography and a vertical distribution pattern. However, how the diversity and distribution of picocyanobacteria affect those of the viruses that infect them remains largely unknown. Here we synchronously analyzed the community structures of cyanopodoviruses and picocyanobacteria at spatial and temporal scales. Both spatial and temporal variations of cyanopodoviral communities can be linked to those of picocyanobacteria. The coastal area, upper euphotic zone, and middle-to-lower euphotic zone of the open ocean have distinct cyanopodoviral communities, showing horizontal and vertical variation patterns closely related to those of picocyanobacteria. These findings emphasize the driving force of host community in shaping the biogeographic structure of viruses. Our work provides important information for future assessments of the ecological roles of viruses and hosts for each other.
KEYWORDS: DNA polymerase, community composition, cyanophages, cyanopodoviruses, picocyanobacteria
ABSTRACT
Picocyanobacteria Prochlorococcus and Synechococcus are abundant in the global oceans and subject to active viral infection. In this study, the genetic diversity of picocyanobacteria and the genetic diversity of cyanopodoviruses were synchronously investigated along water columns in the equatorial Indian Ocean and over a seasonal time course in the coastal Sanya Bay, South China Sea. Using the 16S-23S rRNA internal transcribed spacer (ITS)-based clone library and quantitative PCR (qPCR) analyses, the picocyanobacterial community composition and abundance were determined. Sanya Bay was dominated by clade II Synechococcus during all the seasons, and a typical population shift from high-light-adapted Prochlorococcus to low-light-adapted Prochlorococcus was found along the vertical profiles. Strikingly, the DNA polymerase gene sequences of cyanopodoviruses revealed a much greater genetic diversity than we expected. Nearly one-third of the phylogenetic groups were newly described here. No apparent seasonal pattern was observed for the Sanya Bay picocyanobacterial or cyanopodoviral communities. Different dominant cyanopodovirus lineages were identified for the coastal area, upper euphotic zone, and middle-to-lower euphotic zone of the open ocean. Diversity indices of both picocyanobacteria and cyanopodoviruses were highest in the middle euphotic zone and both were lower in the upper euphotic zone, reflecting a host-virus interaction. Cyanopodoviral communities differed significantly between the upper euphotic zone and the middle-to-lower euphotic zone, showing a vertical pattern similar to that of picocyanobacteria. However, in the surface waters of the open ocean, cyanopodoviruses exhibited no apparent biogeographic pattern, differing from picocyanobacteria. This study demonstrates correlated distribution patterns of picocyanobacteria and cyanopodoviruses, as well as the complex biogeography of cyanopodoviruses.
IMPORTANCE Picocyanobacteria are highly diverse and abundant in the ocean and display remarkable global biogeography and a vertical distribution pattern. However, how the diversity and distribution of picocyanobacteria affect those of the viruses that infect them remains largely unknown. Here we synchronously analyzed the community structures of cyanopodoviruses and picocyanobacteria at spatial and temporal scales. Both spatial and temporal variations of cyanopodoviral communities can be linked to those of picocyanobacteria. The coastal area, upper euphotic zone, and middle-to-lower euphotic zone of the open ocean have distinct cyanopodoviral communities, showing horizontal and vertical variation patterns closely related to those of picocyanobacteria. These findings emphasize the driving force of host community in shaping the biogeographic structure of viruses. Our work provides important information for future assessments of the ecological roles of viruses and hosts for each other.
INTRODUCTION
Viruses are extremely abundant in the ocean (1, 2). They play key roles in the marine ecosystem via modulating the abundance, diversity, and evolution of hosts, speeding the recycling of nutrients and consequently influencing the global biogeochemical cycling (1, 3). The global viral communities are of vast genetic diversity and exhibit biogeographic structures (4–6). However, our understanding of how viral communities change in response to the variation of the host community is limited. Cyanophages and picocyanobacteria have emerged to be a valuable system to investigate and demonstrate the ecologically antagonistic relationship of viruses and hosts in the ocean. Extensive studies have revealed the diversity breadth and community compositions of picocyanobacteria and have delineated explicit global biogeographic patterns of their genetic taxa. Therefore, the investigation on cooccurring cyanophage and picocyanobacterial communities at different spatial and temporal scales could deepen our understanding of the ecological interactions between viruses and hosts in the marine environment.
Marine unicellular picocyanobacteria of the genera Prochlorococcus and Synechococcus are major primary producers in the ocean. Both genera are divided into a few clades with genetic, physiological, and ecological features. Prochlorococcus clades display remarkable vertical depth distributions, referred to as high-light (HL)-adapted and low-light (LL)-adapted ecotypes, which are designated on the basis of their light optima (7–9). HL ecotypes also display horizontally latitudinal distributions, in response to temperature (10) or iron (a trace metal element) availability (11). HL Prochlorococcus is phylogenetically more cohesive, while LL Prochlorococcus is more divergent (12). Marine Synechococcus organisms comprise three discrete subclusters, 5.1, 5.2, and 5.3, among which subcluster 5.1 is the major one and encompasses many defined clades (13, 14). The biogeographic distribution patterns of a few Synechococcus clades are clear now. For instance, clades II and III are more likely distributed in warm oceanic waters, clades I and IV prefer high-latitude temperate waters, and clade CRD1 prefers upwelling iron-depleted waters (15–18).
Cyanophages are believed to affect their hosts in respect to their abundance, diversity, and evolution (19–23). They have been found in diverse marine environments and are predominantly lytic phages (24–31). All the known cyanophage isolates belong to one of the three tailed double-stranded DNA virus families, Myoviridae, Podoviridae, and Siphoviridae, on the basis of tail morphology. Cyanopodoviruses isolated thus far are genetically and morphologically similar to coliphage T7 (32–36). Metagenomic surveys revealed that T7-like cyanopodoviruses are abundant in the sea (5, 35, 37–39). The viral DNA polymerase gene (pol) has been explored as a molecular marker to investigate the diversity of cyanopodoviruses. Two major phylogenetic clusters, MPP-A and MPP-B, were identified via pol (27). The former is much less abundant than the latter (30, 35, 38, 40, 41), in general, and the latter contains numerous defined subclusters (30, 40, 41). However, only a few studies have delineated the diversity of wild cyanopodoviral communities. One of our previous studies showed that cyanopodoviral communities displayed a seasonal variation in Chesapeake Bay, a temperate estuarine ecosystem (41). Another survey showed that some ubiquitous phylogenetic groups of cyanopodoviruses were commonly detected across distant locations in the surface water, and the open ocean communities were less diverse than those in Chesapeake Bay (40).
Many studies have revealed that cyanophage titers covary with picocyanobacterial abundances (19, 24, 25, 28, 42–44). However, only a few studies synchronously investigated the genetic diversity of both picocyanobacteria and cyanophages, and these early studies mostly focused on cyanomyoviruses (42, 45). Picocyanobacterial ecotypes exhibit striking vertical distribution patterns and seasonal variations. Therefore, it is interesting to further answer whether and how the change of picocyanobacterial communities affects the distribution of podoviruses in the ocean over spatial and temporal scales.
In this study, we investigated the community structures of cyanopodoviruses and picocyanobacteria along vertical profiles located in the Indian Ocean and over a seasonal time course in Sanya Bay, South China Sea. Many phylogenetic groups of cyanopodoviruses were newly found here, suggesting a greater diversity of cyanobacterial podoviruses exists in the marine environment. A clear vertical pattern of cyanopodoviral community variation was observed, showing that the middle-to-lower euphotic communities are much more diverse than the upper euphotic communities. Such a pattern was correlated with that of picocyanobacteria.
RESULTS AND DISCUSSION
Sampling environments.
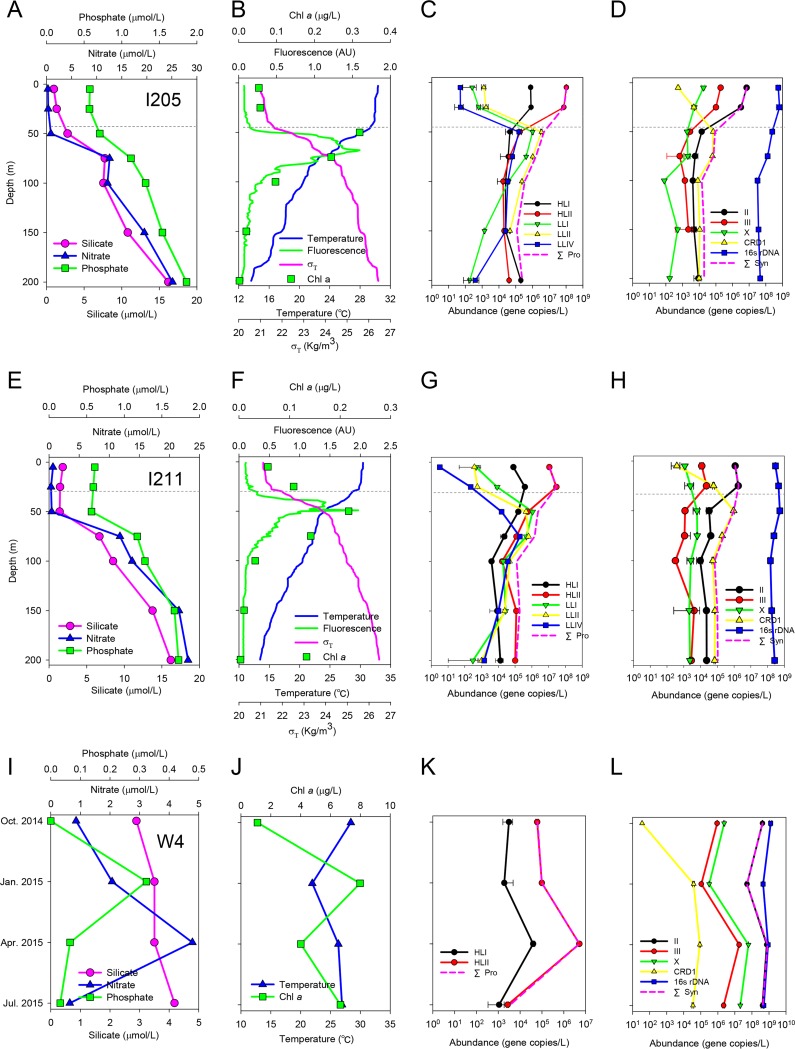
Water samples were collected at three sites. The vertical stations I205 and I211 are located in the equatorial Indian Ocean (∼780 km apart). Hydrologic parameters, including water temperature, density (σT), and concentrations of macronutrients (nitrate, phosphate, and silicate) and chlorophyll a, indicate that stations I205 and I211 represent stratified water columns of the open ocean (Fig. 1A, B, E, and F; see also Table S1 in the supplemental material). The mixed layer depths were at 50 m and 30 m, and the deep chlorophyll maximum (DCM) depths were at 75 m and 50 m for I205 and I211, respectively (Fig. 1B and F). Another coastal seasonal time serial station, W4, is located in Sanya Bay, South China Sea. This sampling site represents a tropical coastal water body without strong seasonal temperature variation (22 to 28°C) (Fig. 1I and J; Table S1).
FIG 1.
Hydrographic features (A, B, E, F, I, and J) and abundances of Prochlorococcus (C, G, and K) and Synechococcus (D, H, and L) ecotypes at Indian Ocean stations I205 (A to D) and I211 (E to H) and Sanya Bay station W4 (I to L). Hydrographic parameters include concentrations of macronutrients and chlorophyll a, fluorescence intensity, water temperature, and water density (σT). The mixing layer depth is indicated by gray dashed lines. Abundances of 14 Prochlorococcus and Synechococcus ecotypes and total bacteria (16S rRNA gene copies) were measured, but only the detectable ecotypes are shown. Pink dashed lines (C, D, G, H, K, and L) indicate summed ecotype abundance. Note that, in panel L, the black line representing Synechococcus clade II overlaps the pink line representing total Synechococcus.
Phylogenetic diversity and abundance of picocyanobacteria.
We built clone libraries based on the 16S-23S rRNA internal transcribed spacer (ITS) amplicons to investigate the genetic diversity of picocyanobacteria and used ITS-based quantitative PCR (qPCR) to quantify 14 different ecotypes of Prochlorococcus and Synechococcus. A total of 925 ITS environmental sequences were obtained from the 18 samples. The observed operational taxonomic unit (OTU) numbers dropped dramatically from using the OTU definition cutoff of 99% to using 95%, and leveled off below a cutoff of 95% (see Fig. S1A). Due to the highly divergent nature of ITS sequences compared with 16S rRNA sequences, we used a 90% DNA sequence similarity cutoff to cluster OTUs rather than the standard cutoff of 97% for 16S rRNA sequences. Rarefaction curves determined with the OTU definition at a cutoff of 90% (see Fig. S2A) show that the increase of OTUs reached a plateau for 10 samples, while the other 8 samples from the middle-to-lower euphotic zone (50 to 150 m) did not. This pattern suggests that, with such a definition of OTU, the communities in the low-light zone were not completely sampled. This is due to the extremely high genetic diversity of LL Prochlorococcus. Nevertheless, we still believe that the majority of Prochlorococcus and Synechococcus lineages have been sampled when the lineage is defined as the phylotype (see below).
We built phylogenetic trees separately for Synechococcus (see Fig. S3), HL Prochlorococcus (see Fig. S4), and LL Prochlorococcus (see Fig. S5). Environmental sequences were assigned to genetic lineages (i.e., phylotype) on the basis of the trees. At the equatorial Indian Ocean stations I205 and I211, the clade HLII Prochlorococcus clones dominated at 5 m and 25 m, accounting for nearly 100% of the libraries (Fig. 2A). In the middle-to-deep euphotic zone (50 to 150 m), the community became much more diverse, where sequences affiliated with LL Prochlorococcus clades and Synechococcus clade CRD1 were present in abundance. However, HLII Prochlorococcus sequences still constituted a large portion of the libraries at the lower euphotic layers (100 to 200 m) of I211 and at the bottom layer of I205 (200 m). This community composition variation matches the qPCR results of picocyanobacterial ecotypes very well (Fig. 1C, D, G, and H). Prochlorococcus ecotype HLII reached the maximum concentrations, 1.0 × 108 cells · liter−1 at I205 and 2.9 × 107 cells · liter−1 at I211, in the upper euphotic waters (5 m and 25 m) and then decreased dramatically to around 104 to 105 cells · liter−1 in the mid- to lower depths. The abundances of LL Prochlorococcus ecotypes peaked at 50 to 75 m and dropped faster than those of HL Prochlorococcus. This may explain why HL Prochlorococcus clones still dominated the libraries of deep euphotic samples. Overall, the pattern represents a typical stratified water column profile of Prochlorococcus ecotypes in the tropical open ocean (10, 46–48).
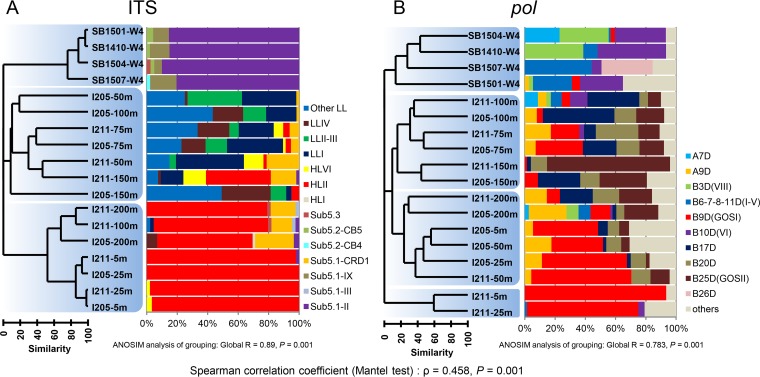
FIG 2.
Clustering of the samples on the basis of compositions of ITS (A) and pol sequences (B). OTU was defined at a cutoff of 90% DNA sequence similarity for both ITS and pol. ANOSIM was used to test the significance of the grouping, which is indicated by light blue shading in the clustering dendrograms. Phylotype-based community composition of each sample is shown to the right of the dendrograms. Spearman correlation coefficient (Mantel test) that reflects the relationship between picocyanobacterial and cyanopodoviral communities is shown below the chart. “Other LL” Prochlorococcus does not represent a real clade but is comprised of other sequences that cannot be assigned to any known LL lineages.
There was a large number of unassigned sequences (labeled “other LL” in Fig. 2A) in 50- to 150-m samples, constituting 2% to 49% (mean, 25%; n = 8) of sequences for one sample. These sequences formed many deeply branching nodes (Fig. S5). Previously reported environmental sequences that were assigned to LL Prochlorococcus clade NC1 (12) or LLVII (49) were scattered among these nodes (Fig. S5). This phylogenetic pattern suggests NC1 might be polyphyletic instead of forming a coherent clade. Our finding and previous knowledge (49) suggest that we do not know much about many extremely diverse LL-adapted Prochlorococcus organisms. These members could be a significant fraction and be missed during current qPCR quantification of Prochlorococcus ecotypes.
It is noteworthy that Synechococcus clades II and CRD1 exhibited depth-related distribution patterns (Fig. 1D and H) similar to those of HL and LL Prochlorococcus clades, respectively. Recently, the CRD1 clade of Synechococcus was found to be prevalent in the iron-depleted areas (16, 18, 50) and to be more abundant in the upper layer than in the lower layer of water columns (16, 50). These areas are often upwelling waters, such as the Costa Rica Dome, equatorial Pacific upwelling, and the Benguela upwelling regions (16). Our results suggest that in the stratified water column clade CRD1 Synechococcus may be present in low abundance in the upper layer but become more abundant near the DCM layer.
In the Sanya Bay samples, only Synechococcus sequences but no Prochlorococcus sequences were retrieved from the clone libraries (Fig. 2A). Synechococcus clade II within the marine subcluster 5.1 was the dominant lineage across the four seasons, making up nearly 80% of sequenced clones in each library. Clade IX of subcluster 5.1 and subcluster 5.2 were also present. These results are consistent with the qPCR data in which Synechococcus clade II was most abundant, outnumbering the other ecotypes by one to two orders of magnitude (Fig. 1K and L). Despite the lack of seasonal variation in diversity, picocyanobacteria in this coastal site still exhibited seasonal variation in cell abundance. Synechococcus clade II was one order of magnitude less abundant in the winter (∼108 cells · liter−1) than in other seasons (∼109 cells · liter−1). HL Prochlorococcus ecotypes were detected in much lower abundance (103 to 106 cells · liter−1) than Synechococcus, and LL Prochlorococcus ecotypes were not detectable. Our results are consistent with previous studies revealing that clade II is the dominant Synechococcus ecotype in the warm tropical area (15–18, 51, 52).
Phylogenetic diversity of cyanopodoviruses.
A total of 1,406 pol environmental sequences were retrieved from clone libraries for the 18 viral concentrate (VC) samples. Similar to ITS sequences, OTU numbers of pol sequences also reached a steady state below the definition cutoff of 95% DNA sequence similarity (Fig. S1A). With the OTU definition of 90%, rarefaction curves for near half of the samples are close to saturated (Fig. S2B). Although it seems that our sequences do not cover a broad diversity, at least 80% of the retrieved sequences in each sample were affiliated with several major phylotypes (see below). Therefore, by using phylotype as the taxon definition, the majority of the cyanopodovirus lineages should have been sampled.
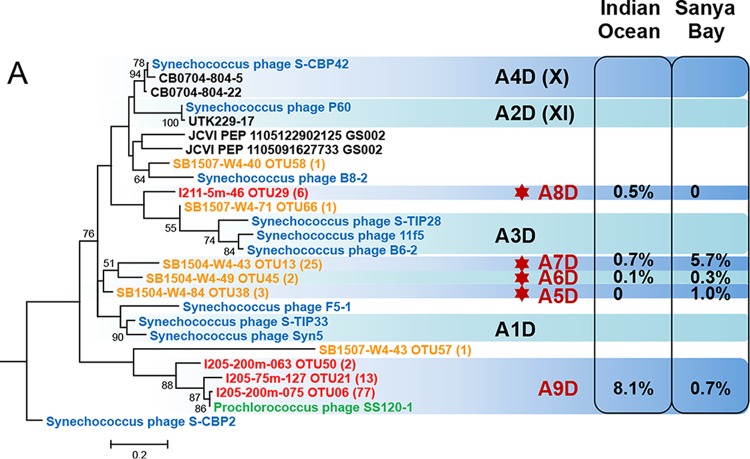
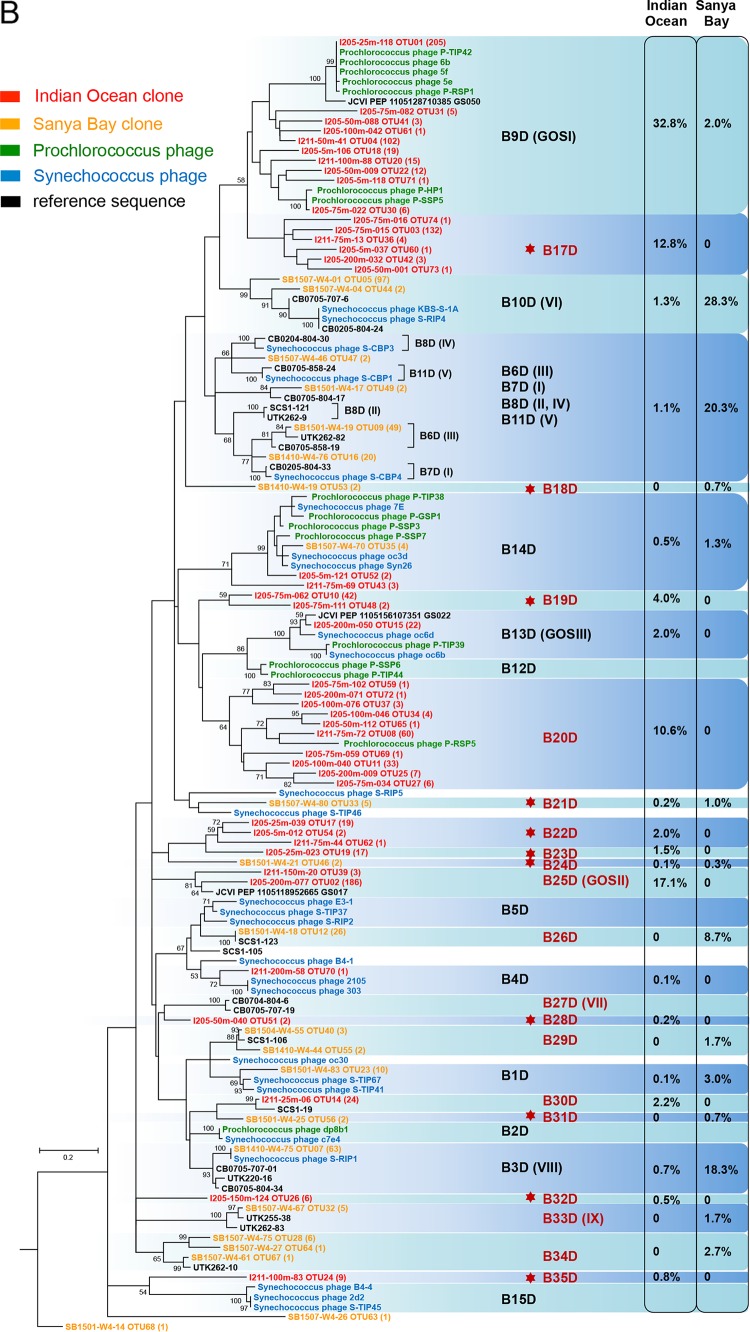
All the pol sequences were grouped into either the MPP-A cluster (131 sequences, ∼10% of the total) (Fig. 3A) or the MPP-B cluster (1,275 sequences, ∼90% of the total) (Fig. 3B). Similar to previous studies (30, 38, 40, 41), the MPP-A sequences are much less present than the MPP-B sequences. All these data support the hypothesis that MPP-B cyanopodoviruses are more successful than the MPP-A cyanopodoviruses in the natural environments (30). Each of the two clusters can be further divided into a few subclusters. We use the subcluster labels established by Dekel-Bird et al. (30), while the earlier labels with Roman numerals (40, 41) are also shown in parentheses if necessary. Compared to previous studies (30, 40, 41, 53), nearly half of the subclusters were newly defined (labeled in red in Fig. 3), among which many were not described previously (indicated by red stars in Fig. 3). Hence, our environmental sequences greatly extend the diversity of marine cyanopodoviruses.
FIG 3.
Maximum likelihood phylogenetic trees based on the viral DNA polymerase protein sequence of cyanopodoviruses. Trees for the MPP-A cluster (A) and the MPP-B cluster (B) were built independently. Environmental sequences obtained in this study are labeled in the format “representative sequence identifier (ID) OTU (no. of sequences assigned to the OTU).” Bootstrap values for 100 sampling replicates are listed only for those higher than 50%. Reference sequence shown in green is from phage isolates infecting Prochlorococcus, those in blue are from phage isolates infecting Synechococcus, and those in black are from environmental clones or from the Global Ocean Sampling metagenomes. Percentages of sequences assigned to each of the genotypes are listed to the right. Genotype labels are according to the numbering by Dekel-Bird and colleagues (30). Genotypes labeled in red indicate those defined in this study, and red star symbols indicate those newly detected here.
Within cluster MPP-A, subclusters A7D and A9D contained a large portion of environmental sequences (Fig. 3A). Most A7D sequences came from the Sanya Bay samples, while most A9D sequences came from the Indian Ocean samples (Fig. 3A). Interestingly, among the cultured MPP-B phages, only one Prochlorococcus phage, P-SSP9, fell into subcluster A9D. This phage infects an LL Prochlorococcus strain, SS-120 (30, 35). Except for P-SSP9, other isolated phages in cluster MPP-A infect Synechococcus (27, 30). It will be interesting to investigate in the future whether MPP-A phages that infect Prochlorococcus are more common than previously thought.
Cluster MPP-B consisted of most of the retrieved environmental sequences for each sample (Fig. 2B). In Sanya Bay, sequences of subclusters B3D (VIII), B10D (VI), and B6D (III) were dispersed in most of the samples and abundant, representing the major genotypes of cyanopodoviruses in this coastal environment (Fig. 3B). Previously, B3D (VIII) was also found to be a major phylogenetic cluster in Chesapeake Bay (41), as well as in some open ocean surface waters (40). Recently, 44 cyanopodoviruses were isolated from Synechococcus strain WH7803 from seawaters of the East Coast (Rhode Island) and the West Coast (Washington) of North America. Most of them were grouped into either B3D (VIII) or B10D (VI) subclusters based on the pol phylogeny (53). Synechococcus podoviruses S-RIP1 and S-RIP4 fell into B3D and B10D, respectively, as representatives of the two major subclusters (53). Isolated phages within subclusters B3D and B10D thus far all infect Synechococcus (30, 53). Therefore, on the basis of our results and previous findings, we speculate that B3D and B10D cyanopodoviruses are prevalent in coastal waters, and they more likely infect Synechococcus.
Four major subclusters, B9D (GOSI), B17D, B20D, and B25D (GOSII), in total constituted 73% of the sequences from the Indian Ocean samples (Fig. 2B and 3B). Depth-related distribution preference was observed among these lineages. B9D was relatively more abundant in the surface mixed layer, and others became more prevalent in deeper layers (see Fig. S6). Among these major groups, B9D and B20D contained sequences from isolated Prochlorococcus phages, while B17D and B25D did not have isolated phage representatives. Interestingly, the dominant clusters found in our study were not the dominant ones in other surface water samples from the Atlantic and Pacific Oceans (40). Instead, clusters B3D (VIII), B8D (II), and A2D (XI) made up the majority in those samples (40). Furthermore, there appears to be another discrepancy that subclusters B12D, B13D, and B14D were barely detected in this study, while numerous phages within these phylotypes have been isolated (30). Previous metagenomic surveys also showed that sequences similar to that of Prochlorococcus podovirus P-SSP7 in B14D are present at a high abundance (5, 38). This variance suggests that cyanopodoviral communities across the broad open oceans are likely quite variable.
Comparing picocyanobacterial and cyanopodoviral communities.
To investigate the relationship between picocyanobacterial and cyanopodoviral communities, we compared diversity indices (alpha diversity) and composition (beta diversity) for the ITS clone libraries and the pol clone libraries. Since we analyzed both picocyanobacterial and cyanopodoviral diversities from the same sample, we referred to them as dual alpha diversity and dual beta diversity.
To gain a broader view, 14 pol clone libraries (40, 41) and 14 ITS clone libraries (52, 54) from the published studies (Fig. 4A; metadata of these samples are listed in Table S2) were compared to those obtained here. These selected libraries are also from the samples that have both pol and ITS sequence data. These additional samples include Chesapeake Bay surface water (41, 54) and the surface water in the Atlantic and Pacific Oceans over a wide latitudinal range (40, 52).
FIG 4.
Alpha diversity and beta diversity of ITS and pol clone libraries. Previously published ITS and pol clone libraries from the Chesapeake Bay surface water samples (CB0205-858, CB0205-804, CB0205-707, CB0705-858, CB0705-804, and CB0705-707) (41, 54) and the Atlantic and Pacific surface ocean samples (UTK202, UTK211, UTK220, UTK229, UTK240, UTK250, UTK255, and UTK262) (40, 52) were compared to those derived here. (A) Locations of the samples are shown. The map was created using Ocean Data View, version 4. Alpha diversity Chao indices (B) and Shannon indices (C). Pearson correlation analysis results that indicate the relationship between diversity levels of picocyanobacteria and cyanopodoviruses are listed under the bar charts. Nonmetric multidimensional scaling (nMDS) plot based on compositions of ITS (D) and pol (E) OTUs. The inset plot in panel D covers all the 32 samples, and the outside plot shows the detailed relationship of a portion of 29 samples (dashed box in the inset plot). ANOSIM was used to test the significance of grouping, and the Mantel test (Spearman correlation) was used to assess the correlation between picocyanobacterial and cyanopodoviral communities. Coefficients (global R and ρ) and P values are shown.
Dual alpha diversity.
At stations I205 and I211, the Chao (indicating OTU richness) and Shannon (indicating both richness and evenness) diversity indices of ITS and pol clone libraries displayed similar vertical patterns, showing that both picocyanobacterial and cyanopodoviral communities in the middle-to-deep euphotic zone are generally more diverse than those in the surface layers (Fig. 4B and C; see Fig. S7A, B, D, and E). We used the Pearson correlation test to assess the relationship between diversity indices of the viral and host communities as well as between the viral or host diversity indices and host abundances. The results (Table 1) show that the species richness (Chao index) of cyanopodoviruses was positively correlated with the species richness of cooccurring picocyanobacteria but was not correlated with the abundance of them. The Shannon index did not show a significant correlation as the Chao index did. Interestingly, picocyanobacterial diversity seems to be negatively correlated with their abundances (Table 1). Note again that the abundance of Prochlorococcus in a low-light zone could be underestimated by our qPCR assays. At station W4, the diversity indices of picocyanobacteria and cyanopodoviruses are both relatively low except for the cyanopodoviral community in the summer of 2015 (Fig. S7C and F).
TABLE 1.
Correlation analysis for the Indian Ocean water column samples (n = 14)
| Variable | Pearson test result (r/P value) |
|||
|---|---|---|---|---|
|
pol |
ITS |
|||
| Chao index | Shannon index | Chao index | Shannon index | |
| ITS | ||||
| Chao index | 0.529/0.052 | |||
| Shannon index | 0.382/0.177 | |||
| Abundance | ||||
| Prochlorococcus | −0.022/0.940 | 0.070/0.812 | −0.377/0.184 | −0.566/0.035 |
| Synechococcus | −0.041/0.888 | 0.015/0.958 | −0.409/0.146 | −0.558/0.038 |
| Prochlorococcus and Synechococcus | −0.033/0.912 | 0.059/0.842 | −0.383/0.177 | −0.571/0.033 |
With more samples involved in the Pearson correlation test, the diversity indices of cyanopodoviruses were still positively correlated with those of picocyanobacteria (r = 0.593, P = 0.001 for the Chao index; r = 0.346, P = 0.052 for the Shannon index) (Fig. 4B and C). Picocyanobacterial communities in the tropical and subtropical surface waters are commonly dominated by only a few genetic lineages, often by one major clade (18). In the middle and lower euphotic zones, more picocyanobacterial lineages cooccurred and, especially, LL Prochlorococcus thrived, with quite genetically divergent members (9, 12, 48). In contrast to diversity, the abundance of picocyanobacteria often reaches the maximum in the upper euphotic zone and decreases toward the bottom euphotic zone (55). Therefore, it is likely that the diversity level of cyanopodoviruses is closely related to the diversity level of picocyanobacteria, suggesting that the host diversity level is a strong constraint on the diversity level of cyanopodoviruses.
Dual beta diversity.
To compare the community composition of picocyanobacteria and cyanopodoviruses, we clustered the samples on the basis of the ITS clone libraries and pol clone libraries, respectively. We also tested the significance of clustering using an analysis of similarity (ANOSIM) and assessed the correlation between the clustering patterns of picocyanobacteria and cyanopodoviruses using the Mantel test. Dendrograms show that the clusterings of samples (using an OTU definition cutoff of 90%) on the basis of the two genetic markers have similar patterns (Spearman correlation coefficient ρ = 0.458, P = 0.001), whereby Synechococcus-dominating waters, HL Prochlorococcus-dominating waters, and LL Prochlorococcus-dominating waters were separated (Fig. 2). An ANOSIM showed that such clustering patterns are statistically significant (global R = 0.89, P = 0.001 for ITS; global R = 0.783, P = 0.001 for pol). Additional Mantel tests (Fig. S1B) showed that most of the ρ coefficients calculated at OTU definition cutoffs of >86% are higher than 0.4 (P < 0.01). Our results support that even at the microdiversity level (using a cutoff of 99%), the viral and host communities were still significantly correlated, reflecting a strong virus-host interaction (56). Moreover, even with small library sizes for each sample (less than 100 sequences), the Mantel tests using different subsets of total sequences resulted in similar correlation levels (Fig. S1C). Nevertheless, the small library size may still have an undetected impact on the robustness of the correlation analysis between the viral and host communities.
However, taking the 14 additional samples into account, the multidimensional scaling (MDS) analysis did not show a high level of correlation between picocyanobacterial and cyanopodoviral communities (Spearman correlation coefficient ρ = 0.233, P = 0.001) (Fig. 4D and E). On the MDS map, picocyanobacteria display a common biogeographic pattern, which separated the samples into groups as summer Chesapeake Bay, winter Chesapeake Bay, tropical and subtropical open ocean, high-latitude North Atlantic, and Sanya Bay (ANOSIM test, global R = 0.8, P = 0.001) (Fig. 4D), while the cyanopodoviral communities appear to be separated by individual studies (ANOSIM test, global R = 0.814, P = 0.001) (Fig. 4E). It seems that the sampling location may play a role in shaping such a pattern. For example, samples from the Indian Ocean, Sanya Bay, and Chesapeake Bay were grouped together (Fig. 4E), reflecting a local pattern. However, it was unexpected that the cyanopodoviral communities of the upper layers (5 m and 25 m) at stations I205 and I211 were not closely related with other surface ocean samples, i.e., UTK samples (Fig. 4E). As mentioned above, the diversity of cyanopodoviruses on the ocean surface is relatively low. Therefore, this discrepancy is likely due to the differences of dominant genetic groups of surface water samples. The complex filtration procedure that concentrates viral particles and the storage of samples in different studies may also impact the comparison between samples from different cruises.
Our findings suggest that environments in similar climate zones or with similar hydrographic conditions may have similar picocyanobacterial populations. In other words, the diversity of picocyanobacteria is predictable from the location (15, 47). In contrast, cyanopodoviral communities are more variable and complex than their host communities.
On one hand, cyanopodoviral communities in estuarine, coastal, and open ocean waters differ from each other, and so do the communities in the upper euphotic zone and in the lower euphotic zone, showing a niche adaptation. Depth was considered to be a driver of viral community structure in the whole water column, where communities in the photic zone differ significantly from those in the aphotic zone (4, 57). However, it was also shown that the total viral communities displayed minimal variation at different euphotic depths (6). Here, we found that the cyanopodoviral communities are structured by depth within the euphotic zone. Similar depth-related community variation was also observed for cyanomyoviruses (58). We speculate that hosts also mediate niche adaptation and play a key role in shaping the regional and vertical community structures of cyanophages. This is also supported by a previous study which suggested that both cyanopodoviral communities and cyanomyoviral communities differ between the Synechococcus-dominating and Prochlorococcus-dominating environments (38). Cyanopodoviruses are commonly host strain specific, while many cyanomyoviruses have broader host ranges. Despite having different host specificities, cyanomyoviral diversity was also shown to be correlated with that of cooccurring Synechococcus in a seasonal time course study (42). Recently, the host range phenotype of cyanomyoviruses was found to be a factor that contributes to their local and regional distribution patterns (53). It is well known that infectious cyanophages are abundant in the field and quantitatively covary with their hosts (44, 59). The virioplankton abundance was found to be highly synchronous with Prochlorococcus distribution, and this suggested that cyanophages contributed to a significant fraction of viruses in the subtropical Atlantic gyre (60). Altogether, these findings imply a tight relationship between wild cyanophages and cyanobacteria, with respect to both abundance and community structure.
On the other hand, one host strain can be infected by diverse podoviruses that fall into distinct phylogenetic groups (30, 41). This could in part explain the randomness of the cyanopodoviral community structures over the broad surface waters of the open ocean. Moreover, the Prochlorococcus community is composed of numerous coexisting subpopulations with a large degree of genomic variation (61). The rapid change of these subpopulations may contribute to a poor correlation between the cyanopodoviral and picocyanobacterial communities across the ocean surface.
Conclusion.
This study demonstrates the highly diverse and variable cyanopodoviral communities in different marine regions and at different depths. Specific phylogenetic lineages of cyanopodoviruses were found to occupy the near-shore waters where Synechococcus organisms are abundant, and other different lineages thrive in the Prochlorococcus-dominating open ocean. Cyanopodoviral communities also change dramatically along the vertical scale in the open ocean, and the changing pattern is correlated to that of the picocyanobacterial community in the water column. Our findings further support that host availability (6, 38, 53, 62) and coevolutionary interactions (63, 64) are the main driving forces of viral diversity and distribution.
MATERIALS AND METHODS
Sampling and parameter measuring.
Seawater samples were collected from Sanya Bay station W4, located in the northern South China Sea, and the Indian Ocean stations I205 and I211. The details of sampling locations, dates, and parameters are shown in Table S1 in the supplemental material. Temperature and salinity data were extracted from the CTD sensor (Sea-Bird Scientific) or the YSI sensor (YSI Inc.) data set. Chlorophyll a concentrations were measured using Turner Designs Trilogy fluorometer (Turner Designs Inc.) according to a protocol described previously (65). N (nitrate), soluble reactive phosphate (SRP), and Si (silicate) were measured with a Seal AA3 autoanalyzer (Bran-Luebbe, GmbH) using long-path spectrophotometry (66, 67) with detection limits of 0.02 μmol · liter−1, 0.02 μmol · liter−1, and 0.03 μmol · liter−1, respectively.
To collect viral concentrates (VCs), 20 liters of seawater was filtered through 0.22-μm-pore-size polycarbonate filter membranes (Millipore). A tangential flow filtration system was used to concentrate the filtrate to a final volume of 200 ml, with a 30-kDa molecular weight cutoff filter (Pall). VCs were stored at 4°C in the dark. The 0.22-μm-pore-size membranes were stored at −80°C until DNA extraction.
DNA extraction, PCR, cloning, and sequencing.
DNA was extracted from filter membranes using the MoBio Powersoil DNA isolation kit according to the manufacturer's protocol. The amplification of picocyanobacterial 16S-23S rRNA internal transcribed spacer (ITS) sequences from the extracted DNA was performed using the primers Picocya16S-F and Picocya23S-R, according to the PCR program described previously (54). A partial sequence of the cyanopodovirus DNA polymerase gene was amplified using 2 μl VC as the template and the primers CP-DNAP-349F, CP-DNAP-533Ra, and CP-DNAP-533Rb, as described previously (40). The PCR products were purified using the TaKaRa agarose gel DNA purification kit (TaKaRa) and cloned using the TaKaRa pMD18-T vector cloning kit (TaKaRa), according to the manufacturer's instructions. Randomly picked clones were sequenced on an ABI 3730 genetic analyzer (Applied Biosystems) at Major Bio-tech Co., Ltd., Shanghai, China.
Phylogenetic diversity analysis.
Raw Sanger sequences were examined and trimmed using BioEdit, and those of low quality were discarded. For the pol sequences, only those that were successfully translated to amino acid sequences were retained. ITS DNA sequences and pol amino acid sequences were aligned using Clustal X2 (68). Chimeras were discarded via a manual examination of the alignment. After quality control, 925 ITS sequences and 1,406 pol sequences were obtained. To reduce the sequence redundancy in the phylogenetic analysis, all the sequences were binned into OTUs using mothur (69) at the cutoff of 90% similarity of DNA sequences for ITS and at 80% for pol. A cutoff of 90% sequence similarity for pol was also tested, while it resulted in a very high degree of sequence redundancy at phylogenetic tree nodes. Thus, the results for the cutoff of 80% are shown. As a result, 149 ITS OTUs and 74 pol OTUs were obtained, and representative sequences for each of the OTUs were imported to build the tree. Note that we used the translated amino acid sequences for constructing the Pol phylogeny. Phylogenetic analysis was performed with MEGA 7 (70). The maximum likelihood (ML) method based on the Jukes-Cantor model and the ML method based on the JTT matrix-based model were used for estimating the ITS phylogeny and the Pol phylogeny, respectively. A discrete gamma distribution was used to model the evolutionary rate differences among sites. The rate variation model enabled some sites to be evolutionarily invariable. The robustness of the phylogeny was tested using a bootstrap method with 100 bootstrap replications. Rarefaction curves were calculated using DOTUR (71) at a 90% DNA sequence similarity cutoff for both ITS and pol.
Previously, we investigated the cyanopodoviral communities (40, 41) and picocyanobacterial communities (52, 54) for the same bulk of seawater samples (see Table S2 for the metadata). However, the viral and host communities were independently assessed in different studies. To compare with those already published data, whole clone libraries for ITS and pol sequences were retrieved from the NCBI GenBank. These retrieved DNA sequences were aligned together with those obtained in this study and the resulting alignment was imported into mothur to calculate the diversity indices of Chao and Shannon at a cutoff of 90% DNA sequence similarity for both ITS and pol sequences. A “shared file” created by the mothur command line “make.shared” was loaded into the software PRIMER 5 (72) to calculate the square-root-transformed Bray-Curtis similarities among clone libraries and to further plot the multidimensional scaling (MDS) map. The clone libraries for pol sequences and for ITS sequences obtained in this study were also analyzed, and a “shared file” was generated and loaded into PRIMER 5 to cluster the samples on the basis of the square-root-transformed Bray-Curtis similarity matrix, using the CLUSTER module and the “complete linkage” mode. ANOSIM implemented in PRIMER 5 was used to test the significance of grouping among samples during the MDS and CLUSTER analyses. The Spearman correlation-based Mantel test (RELATE function in PRIMER 5) was used to assess the correlation between picocyanobacterial and cyanopodoviral communities. The Pearson correlation between diversity indices of picocyanobacteria and cyanopodoviruses was tested using SPSS version 13.
Quantifying ecotypes of Prochlorococcus and Synechococcus by qPCR.
Real-time PCR was used to quantify a total of 14 ecotypes of Prochlorococcus and Synechococcus. We used the published primers and qPCR programs to quantify Prochlorococcus ecotypes eMED4 (HLI) (73), eMIT9312 (HLII) (73), eNATL2A (LLI) (73), eSS120 (LLII) (46), and eMIT9313 (LLIV) (73). Note that the reverse primer for eMED4 is 5′-GAAGCTAGATTCGCTCAGAGC-3′ (Z. Johnson, personal communication). The primers and qPCR programs for quantifying Synechococcus clades I, II, III, IV, X, XV, XVI, CRD1, and CRD2 were according to the study by Ahlgren and Rocap (74). The gene copies of 16S rRNA genes were also quantified using a qPCR assay with the primers EUB338 and EUB518 (75).
To prepare qPCR standards, nested PCR was performed. First, ITS sequences amplified from all the field samples using primers Picocya16S-F and Picocya23S-R were pooled. Second, using the pooled ITS PCR products as the template, ecotype-specific PCR products were generated. Then each of the secondary PCR products was cloned, and randomly picked clones were sequenced to make sure that they indeed represent the corresponding ecotypes. Plasmid DNA was isolated and served as the template to amplify a fragment with the universal M13-47/RV-M primers. Finally, these gel-purified PCR products served as the qPCR standards. Their concentrations were determined with PicoGreen double-stranded DNA quantification reagent (Yeasen Biotech) using 2-fold serially diluted lambda DNA (350 ng/μl; TaKaRa) as the standards. A multimode plate reader (EnSight; PerkinElmer) was used to read the fluorescence intensity. The preparation of standards for 16S rRNA gene quantification was according to the same procedure. The first-round PCR was carried out with the primers EUB338 and EUB518.
Standard curves were generated from triplicate reactions in 25-μl volumes, using the TaKaRa SYBR II agents and the Bio-Rad CFX Connect machine (Bio-Rad Life Science). Theoretical amplification efficiencies for quantifying picocyanobacterial ecotypes ranged from 82% to 96%, consistent with a previous report (73). The amplification efficiency for quantifying 16S rRNA genes was 98%. qPCR assays were performed with 1 μl of template DNA extracted from filter membranes described above.
Accession number(s).
Environmental sequences obtained in this study were deposited in the NCBI GenBank under accession numbers MG427080 to MG427884 and MH034887 to MH035487 for the viral DNA polymerase gene sequences and accession numbers MG427885 to MG428399 and MH034477 to MH034886 for the picocyanobacterial 16S-23S rRNA ITS sequences.
Supplementary Material
ACKNOWLEDGMENTS
We thank the staff of Tropical Marine Biological Research Station in Hainan (CAS) and the crew of the R.V. Shiyan I for assistance in sampling and the Xu Jie Lab and Li Qian Lab at South China Sea Institute of Oceanology for help in measuring chlorophyll and macronutrients.
This study was supported by the NSFC grants 41576126 (S.H.) and 41230962 (S.Z.), the Natural Science Foundation of Guangdong Province grant 2017A030306020 (S.H.), the Youth Innovation Promotion Association CAS (S.H.), and the Strategic Priority Research Program of the Chinese Academy of Sciences, grant no. XDA13020301 (S.H.).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.00591-18.
REFERENCES
- 1.Fuhrman JA. 1999. Marine viruses and their biogeochemical and ecological effects. Nature 399:541–548. doi: 10.1038/21119. [DOI] [PubMed] [Google Scholar]
- 2.Suttle CA. 2005. Viruses in the sea. Nature 437:356–361. doi: 10.1038/nature04160. [DOI] [PubMed] [Google Scholar]
- 3.Suttle CA. 2007. Marine viruses–major players in the global ecosystem. Nat Rev Microbiol 5:801–812. doi: 10.1038/nrmicro1750. [DOI] [PubMed] [Google Scholar]
- 4.Hurwitz BL, Brum JR, Sullivan MB. 2015. Depth-stratified functional and taxonomic niche specialization in the ‘core’ and ‘flexible’ Pacific Ocean virome. ISME J 9:472–484. doi: 10.1038/ismej.2014.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angly FE, Felts B, Breitbart M, Salamon P, Edwards RA, Carlson C, Chan AM, Haynes M, Kelley S, Liu H. 2006. The marine viromes of four oceanic regions. PLoS Biol 4:e368. doi: 10.1371/journal.pbio.0040368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brum JR, Ignacio-Espinoza JC, Roux S, Doulcier G, Acinas SG, Alberti A, Chaffron S, Cruaud C, de Vargas C, Gasol JM, Gorsky G, Gregory AC, Guidi L, Hingamp P, Iudicone D, Not F, Ogata H, Pesant S, Poulos BT, Schwenck SM, Speich S, Dimier C, Kandels-Lewis S, Picheral M, Searson S, Tara Oceans Coordinators, Bork P, Bowler C, Sunagawa S, Wincker P, Karsenti E, Sullivan MB. 2015. Ocean plankton. Patterns and ecological drivers of ocean viral communities. Science 348:1261498. doi: 10.1126/science.1261498. [DOI] [PubMed] [Google Scholar]
- 7.Moore LR, Chisholm SW. 1999. Photophysiology of the marine cyanobacterium Prochlorococcus: ecotypic differences among cultured isolates. Limnol Oceanogr 44:628–638. doi: 10.4319/lo.1999.44.3.0628. [DOI] [Google Scholar]
- 8.Moore LR, Rocap G, Chisholm SW. 1998. Physiology and molecular phylogeny of coexisting Prochlorococcus ecotypes. Nature 393:464–467. doi: 10.1038/30965. [DOI] [PubMed] [Google Scholar]
- 9.Rocap G, Distel DL, Waterbury JB, Chisholm SW. 2002. Resolution of Prochlorococcus and Synechococcus ecotypes by using 16S-23S ribosomal DNA internal transcribed spacer sequences. Appl Environ Microbiol 68:1180–1191. doi: 10.1128/AEM.68.3.1180-1191.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson ZI, Zinser ER, Coe A, McNulty NP, Woodward EM, Chisholm SW. 2006. Niche partitioning among Prochlorococcus ecotypes along ocean-scale environmental gradients. Science 311:1737–1740. doi: 10.1126/science.1118052. [DOI] [PubMed] [Google Scholar]
- 11.Rusch DB, Martiny AC, Dupont CL, Halpern AL, Venter JC. 2010. Characterization of Prochlorococcus clades from iron-depleted oceanic regions. Proc Natl Acad Sci U S A 107:16184–16189. doi: 10.1073/pnas.1009513107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martiny AC, Tai AP, Veneziano D, Primeau F, Chisholm SW. 2009. Taxonomic resolution, ecotypes and the biogeography of Prochlorococcus. Environ Microbiol 11:823–832. doi: 10.1111/j.1462-2920.2008.01803.x. [DOI] [PubMed] [Google Scholar]
- 13.Fuller NJ, Marie D, Partensky F, Vaulot D, Post AF, Scanlan DJ. 2003. Clade-specific 16S ribosomal DNA oligonucleotides reveal the predominance of a single marine Synechococcus clade throughout a stratified water column in the Red Sea. Appl Environ Microbiol 69:2430–2443. doi: 10.1128/AEM.69.5.2430-2443.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Penno S, Lindell D, Post AF. 2006. Diversity of Synechococcus and Prochlorococcus populations determined from DNA sequences of the N-regulatory gene ntcA. Environ Microbiol 8:1200–1211. doi: 10.1111/j.1462-2920.2006.01010.x. [DOI] [PubMed] [Google Scholar]
- 15.Zwirglmaier K, Jardillier L, Ostrowski M, Mazard S, Garczarek L, Vaulot D, Not F, Massana R, Ulloa O, Scanlan DJ. 2008. Global phylogeography of marine Synechococcus and Prochlorococcus reveals a distinct partitioning of lineages among oceanic biomes. Environ Microbiol 10:147–161. [DOI] [PubMed] [Google Scholar]
- 16.Sohm JA, Ahlgren NA, Thomson ZJ, Williams C, Moffett JW, Saito MA, Webb EA, Rocap G. 2016. Co-occurring Synechococcus ecotypes occupy four major oceanic regimes defined by temperature, macronutrients and iron. ISME J 10:333–345. doi: 10.1038/ismej.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zwirglmaier K, Heywood JL, Chamberlain K, Woodward EM, Zubkov MV, Scanlan DJ. 2007. Basin-scale distribution patterns of picocyanobacterial lineages in the Atlantic Ocean. Environ Microbiol 9:1278–1290. doi: 10.1111/j.1462-2920.2007.01246.x. [DOI] [PubMed] [Google Scholar]
- 18.Farrant GK, Dore H, Cornejo-Castillo FM, Partensky F, Ratin M, Ostrowski M, Pitt FD, Wincker P, Scanlan DJ, Iudicone D, Acinas SG, Garczarek L. 2016. Delineating ecologically significant taxonomic units from global patterns of marine picocyanobacteria. Proc Natl Acad Sci U S A 113:E3365–E3374. doi: 10.1073/pnas.1524865113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waterbury JB, Valois FW. 1993. Resistance to co-occurring phages enables marine Synechococcus communities to coexist with cyanophages abundant in seawater. Appl Environ Microbiol 59:3393–3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suttle CA, Chan AM. 1994. Dynamics and distribution of cyanophages and their effect on marine Synechococcus spp. Appl Environ Microbiol 60:3167–3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindell D, Sullivan MB, Johnson ZI, Tolonen AC, Rohwer F, Chisholm SW. 2004. Transfer of photosynthesis genes to and from Prochlorococcus viruses. Proc Natl Acad Sci U S A 101:11013–11018. doi: 10.1073/pnas.0401526101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Avrani S, Lindell D. 2015. Convergent evolution toward an improved growth rate and a reduced resistance range in Prochlorococcus strains resistant to phage. Proc Natl Acad Sci U S A 112:E2191–E2200. doi: 10.1073/pnas.1420347112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Avrani S, Wurtzel O, Sharon I, Sorek R, Lindell D. 2011. Genomic island variability facilitates Prochlorococcus-virus coexistence. Nature 474:604–608. doi: 10.1038/nature10172. [DOI] [PubMed] [Google Scholar]
- 24.Suttle CA, Chan AM. 1993. Marine cyanophages infecting oceanic and coastal strains of Synechococcus: abundance, morphology, cross-infectivity and growth characteristics. Mar Ecol Prog Ser 92:99–109. doi: 10.3354/meps092099. [DOI] [Google Scholar]
- 25.Sullivan MB, Waterbury JB, Chisholm SW. 2003. Cyanophages infecting the oceanic cyanobacterium Prochlorococcus. Nature 424:1047–1051. doi: 10.1038/nature01929. [DOI] [PubMed] [Google Scholar]
- 26.Waterbury JB, Watson SW, Valois FW, Franks DG. 1986. Biological and ecological characterization of the marine unicellular cyanobacterium Synechococcus. Can Bull Fish Aquat Sci 214:71–120. [Google Scholar]
- 27.Wang K, Chen F. 2008. Prevalence of highly host-specific cyanophages in the estuarine environment. Environ Microbiol 10:300–312. doi: 10.1111/j.1462-2920.2007.01452.x. [DOI] [PubMed] [Google Scholar]
- 28.Marston MF, Sallee JL. 2003. Genetic diversity and temporal variation in the cyanophage community infecting marine Synechococcus species in Rhode Island's coastal waters. Appl Environ Microbiol 69:4639–4647. doi: 10.1128/AEM.69.8.4639-4647.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dekel-Bird NP, Sabehi G, Mosevitzky B, Lindell D. 2015. Host-dependent differences in abundance, composition and host range of cyanophages from the Red Sea. Environ Microbiol 17:1286–1299. doi: 10.1111/1462-2920.12569. [DOI] [PubMed] [Google Scholar]
- 30.Dekel-Bird NP, Avrani S, Sabehi G, Pekarsky I, Marston MF, Kirzner S, Lindell D. 2013. Diversity and evolutionary relationships of T7-like podoviruses infecting marine cyanobacteria. Environ Microbiol 15:1476–1491. doi: 10.1111/1462-2920.12103. [DOI] [PubMed] [Google Scholar]
- 31.Wilson WH, Joint IR, Carr NG, Mann NH. 1993. Isolation and molecular characterization of five marine cyanophages propagated on Synechococcus sp. strain WH7803. Appl Environ Microbiol 59:3736–3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sullivan MB, Coleman ML, Weigele P, Rohwer F, Chisholm SW. 2005. Three Prochlorococcus cyanophage genomes: signature features and ecological interpretations. PLoS Biol 3:e144. doi: 10.1371/journal.pbio.0030144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen F, Lu J. 2002. Genomic sequence and evolution of marine cyanophage P60: a new insight on lytic and lysogenic phages. Appl Environ Microbiol 68:2589–2594. doi: 10.1128/AEM.68.5.2589-2594.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pope WH, Weigele PR, Chang J, Pedulla ML, Ford ME, Houtz JM, Jiang W, Chiu W, Hatfull GF, Hendrix RW, King J. 2007. Genome sequence, structural proteins, and capsid organization of the cyanophage Syn5: a “horned” bacteriophage of marine Synechococcus. J Mol Biol 368:966–981. doi: 10.1016/j.jmb.2007.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Labrie SJ, Frois-Moniz K, Osburne MS, Kelly L, Roggensack SE, Sullivan MB, Gearin G, Zeng Q, Fitzgerald M, Henn MR, Chisholm SW. 2013. Genomes of marine cyanopodoviruses reveal multiple origins of diversity. Environ Microbiol 15:1356–1376. doi: 10.1111/1462-2920.12053. [DOI] [PubMed] [Google Scholar]
- 36.Huang SJ, Zhang S, Jiao NZ, Chen F. 2015. Comparative genomic and phylogenomic analyses reveal a conserved core genome shared by estuarine and oceanic cyanopodoviruses. PLoS One 10:e0142962. doi: 10.1371/journal.pone.0142962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bench SR, Hanson TE, Williamson KE, Ghosh D, Radosovich M, Wang K, Wommack KE. 2007. Metagenomic characterization of Chesapeake Bay virioplankton. Appl Environ Microbiol 73:7629–7641. doi: 10.1128/AEM.00938-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang S, Zhang S, Jiao N, Chen F. 2015. Marine cyanophages demonstrate biogeographic patterns throughout the global ocean. Appl Environ Microbiol 81:441–452. doi: 10.1128/AEM.02483-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williamson SJ, Rusch DB, Yooseph S, Halpern AL, Heidelberg KB, Glass JI, Andrews-Pfannkoch C, Fadrosh D, Miller CS, Sutton G, Frazier M, Venter JC. 2008. The Sorcerer II Global Ocean Sampling Expedition: metagenomic characterization of viruses within aquatic microbial samples. PLoS One 3:e1456. doi: 10.1371/journal.pone.0001456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang S, Wilhelm SW, Jiao N, Chen F. 2010. Ubiquitous cyanobacterial podoviruses in the global oceans unveiled through viral DNA polymerase gene sequences. ISME J 4:1243–1251. doi: 10.1038/ismej.2010.56. [DOI] [PubMed] [Google Scholar]
- 41.Chen F, Wang K, Huang S, Cai H, Zhao M, Jiao N, Wommack KE. 2009. Diverse and dynamic populations of cyanobacterial podoviruses in the Chesapeake Bay unveiled through DNA polymerase gene sequences. Environ Microbiol 11:2884–2892. doi: 10.1111/j.1462-2920.2009.02033.x. [DOI] [PubMed] [Google Scholar]
- 42.Mühling M, Fuller NJ, Millard A, Somerfield PJ, Marie D, Wilson WH, Scanlan DJ, Post AF, Joint I, Mann NH. 2005. Genetic diversity of marine Synechococcus and co-occurring cyanophage communities: evidence for viral control of phytoplankton. Environ Microbiol 7:499–508. doi: 10.1111/j.1462-2920.2005.00713.x. [DOI] [PubMed] [Google Scholar]
- 43.Millard AD, Mann NH. 2006. A temporal and spatial investigation of cyanophage abundance in the Gulf of Aqaba, Red Sea. J Mar Biol Assoc U.K. 86:507–515. doi: 10.1017/S0025315406013415. [DOI] [Google Scholar]
- 44.Wang K, Wommack KE, Chen F. 2011. Abundance and distribution of Synechococcus spp. and cyanophages in the Chesapeake Bay. Appl Environ Microbiol 77:7459–7468. doi: 10.1128/AEM.00267-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jameson E, Mann NH, Joint I, Sambles C, Muhling M. 2011. The diversity of cyanomyovirus populations along a North-South Atlantic Ocean transect. ISME J 5:1713–1721. doi: 10.1038/ismej.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malmstrom RR, Coe A, Kettler GC, Martiny AC, Frias-Lopez J, Zinser ER, Chisholm SW. 2010. Temporal dynamics of Prochlorococcus ecotypes in the Atlantic and Pacific oceans. ISME J 4:1252–1264. doi: 10.1038/ismej.2010.60. [DOI] [PubMed] [Google Scholar]
- 47.Zinser ER, Johnson ZI, Coe A, Karaca E, Veneziano D, Chisholm SW. 2007. Influence of light and temperature on Prochlorococcus ecotype distributions in the Atlantic Ocean. Limnol Oceanogr 52:2205–2220. doi: 10.4319/lo.2007.52.5.2205. [DOI] [Google Scholar]
- 48.Zinser ER, Coe A, Johnson ZI, Martiny AC, Fuller NJ, Scanlan DJ, Chisholm SW. 2006. Prochlorococcus ecotype abundances in the North Atlantic Ocean as revealed by an improved quantitative PCR method. Appl Environ Microbiol 72:723–732. doi: 10.1128/AEM.72.1.723-732.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Biller SJ, Berube PM, Lindell D, Chisholm SW. 2015. Prochlorococcus: the structure and function of collective diversity. Nat Rev Microbiol 13:13–27. doi: 10.1038/nrmicro3378. [DOI] [PubMed] [Google Scholar]
- 50.Ahlgren NA, Noble A, Patton AP, Roache-Johnson K, Jackson L, Robinson D, McKay C, Moore LR, Saito MA, Rocap G. 2014. The unique trace metal and mixed layer conditions of the Costa Rica upwelling dome support a distinct and dense community of Synechococcus. Limnol Oceanogr 59:2166–2184. doi: 10.4319/lo.2014.59.6.2166. [DOI] [Google Scholar]
- 51.Mazard S, Ostrowski M, Partensky F, Scanlan DJ. 2012. Multi-locus sequence analysis, taxonomic resolution and biogeography of marine Synechococcus. Environ Microbiol 14:372–386. doi: 10.1111/j.1462-2920.2011.02514.x. [DOI] [PubMed] [Google Scholar]
- 52.Huang S, Wilhelm S, Harvey H, Taylor K, Jiao N, Chen F. 2012. Novel lineages of Prochlorococcus and Synechococcus in the global oceans. ISME J 6:285–297. doi: 10.1038/ismej.2011.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hanson CA, Marston MF, Martiny JBH. 2016. Biogeographic variation in host range phenotypes and taxonomic composition of marine cyanophage isolates. Front Microbiol 7:983. doi: 10.3389/fmicb.2016.00983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cai H, Wang K, Huang S, Jiao N, Chen F. 2010. Distinct patterns of picocyanobacterial communities in winter and summer in the Chesapeake Bay. Appl Environ Microbiol 76:2955–2960. doi: 10.1128/AEM.02868-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Partensky F, Hess WR, Vaulot D. 1999. Prochlorococcus, a marine photosynthetic prokaryote of global significance. Microbiol Mol Biol Rev 63:106–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Needham DM, Sachdeva R, Fuhrman JA. 2017. Ecological dynamics and co-occurrence among marine phytoplankton, bacteria and myoviruses shows microdiversity matters. ISME J 11:1614–1629. doi: 10.1038/ismej.2017.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hurwitz BL, Westveld AH, Brum JR, Sullivan MB. 2014. Modeling ecological drivers in marine viral communities using comparative metagenomics and network analyses. Proc Natl Acad Sci U S A 111:10714–10719. doi: 10.1073/pnas.1319778111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frederickson CM, Short SM, Suttle CA. 2003. The physical environment affects cyanophage communities in British Columbia inlets. Microb Ecol 46:348–357. doi: 10.1007/s00248-003-1010-2. [DOI] [PubMed] [Google Scholar]
- 59.Sandaa RA, Larsen A. 2006. Seasonal variations in virus-host populations in Norwegian coastal waters: focusing on the cyanophage community infecting marine Synechococcus spp. Appl Environ Microbiol 72:4610–4618. doi: 10.1128/AEM.00168-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parsons RJ, Breitbart M, Lomas MW, Carlson CA. 2012. Ocean time-series reveals recurring seasonal patterns of virioplankton dynamics in the northwestern Sargasso Sea. ISME J 6:273–284. doi: 10.1038/ismej.2011.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kashtan N, Roggensack SE, Rodrigue S, Thompson JW, Biller SJ, Coe A, Ding H, Marttinen P, Malmstrom RR, Stocker R, Follows MJ, Stepanauskas R, Chisholm SW. 2014. Single-cell genomics reveals hundreds of coexisting subpopulations in wild Prochlorococcus. Science 344:416–420. doi: 10.1126/science.1248575. [DOI] [PubMed] [Google Scholar]
- 62.Chow CET, Kim DY, Sachdeva R, Caron DA, Fuhrman JA. 2014. Top-down controls on bacterial community structure: microbial network analysis of bacteria, T4-like viruses and protists. ISME J 8:816–829. doi: 10.1038/ismej.2013.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schwartz DA, Lindell D. 2017. Genetic hurdles limit the arms race between Prochlorococcus and the T7-like podoviruses infecting them. ISME J 11:1836–1851. doi: 10.1038/ismej.2017.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marston MF, Pierciey FJ, Shepard A, Gearin G, Qi J, Yandava C, Schuster SC, Henn MR, Martiny JBH. 2012. Rapid diversification of coevolving marine Synechococcus and a virus. Proc Natl Acad Sci U S A 109:4544–4549. doi: 10.1073/pnas.1120310109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Knap AH, Michaels AF, Close AR, Ducklow HW, Dickson AG. 1996. Protocols for the Joint Global Ocean Flux Study (JGOFS) core measurements. JGOFS report no. 19. Reprint of Intergovernmental Oceanic Commission manuals and guides, no. 29 UNESCO, Bergen, Norway. [Google Scholar]
- 66.Li QP, Hansell DA, Zhang JZ. 2008. Underway monitoring of nanomolar nitrate plus nitrite and phosphate in oligotrophic seawater. Limnol Oceanogr Methods 6:319–326. doi: 10.4319/lom.2008.6.319. [DOI] [Google Scholar]
- 67.Li QP, Hansell DA. 2008. Nutrient distributions in baroclinic eddies of the oligotrophic North Atlantic and inferred impacts on biology. Deep Sea Res Part 2 Top Stud Oceanogr 55:1291–1299. doi: 10.1016/j.dsr2.2008.01.009. [DOI] [Google Scholar]
- 68.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 69.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF. 2009. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kumar S, Stecher G, Tamura K. 2016. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schloss PD, Handelsman J. 2005. Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness. Appl Environ Microbiol 71:1501–1506. doi: 10.1128/AEM.71.3.1501-1506.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Clarke KR. 1993. Nonparametric multivariate analyses of changes in community structure. Aust J Ecol 18:117–143. doi: 10.1111/j.1442-9993.1993.tb00438.x. [DOI] [Google Scholar]
- 73.Ahlgren NA, Rocap G, Chisholm SW. 2006. Measurement of Prochlorococcus ecotypes using real-time polymerase chain reaction reveals different abundances of genotypes with similar light physiologies. Environ Microbiol 8:441–454. doi: 10.1111/j.1462-2920.2005.00910.x. [DOI] [PubMed] [Google Scholar]
- 74.Ahlgren NA, Rocap G. 2012. Diversity and distribution of marine Synechococcus: multiple gene phylogenies for consensus classification and development of qPCR assays for sensitive measurement of clades in the ocean. Front Microbiol 3:213. doi: 10.3389/fmicb.2012.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fierer N, Jackson JA, Vilgalys R, Jackson RB. 2005. Assessment of soil microbial community structure by use of taxon-specific quantitative PCR assays. Appl Environ Microbiol 71:4117–4120. doi: 10.1128/AEM.71.7.4117-4120.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.