Abstract
The study aims to evaluate the efficacy of ondansetron in preventing post-spinal headache, considering the high prevalence of the headache in pregnant women and the common use of the adjuvants for prophylaxis against post-operative nausea and vomiting (PONV). This double-blind clinical trial included the 195 patients who were referred to Taleghani Hospital (in Arak, Iran) for cesarean section (C/S) under spinal anesthesia, and then the subjects were assigned to three equally sized groups using block randomization. Participants in the first, second, and control groups received 8 mg, 4 mg of ondansetron, and normal saline, respectively, 5 minutes before surgery. A final volume of 5 cc was prepared by adding normal saline. Participants were examined for headache one week after surgery, and then data analysis was performed using SPSS 20. The incidence of post-spinal headache was significantly higher in the placebo group than in the ondansetron 8-mg and 4-mg groups at 24 hours after surgery (P < 0.010). But, no significant difference was observed between two ondansetron groups (P ≤ 0.05). The overall incidence of the headache was generally lower in ondansetron 8-mg (26.66% vs. 33.68.05%) and 31.66% in ondansetron 4-mg (P < 0.001). Moreover, the PONV incidence was significantly higher in the placebo group than in the other two groups at 24 hours (P < 0.001). The hemodynamic variables were same in three groups. The ondansetron 8-mg dose can be effective to prevent headache after spinal anesthesia for C/S. Moreover, the ondansetron 8-mg and ondansetron 4-mg have same effect in control of PONV after spinal anesthesia for C/S.
Keywords: cesarean, ondansetron, post spinal headache, post-operative nausea, vomiting
INTRODUCTION
Spinal anesthesia remains the most common anesthetic technique for cesarean section (C/S),1,2 but associated with several complications including, importantly, headache which has differential postoperative effects on the well-being of patients.3,4,5 The prevalence of this complication is estimated at 11% and higher.2,6 Post-spinal headache is typically throbbing and patient will experience photophobia and diplopia. The headache is worse on standing, not responding to common analgesics.4,6 Unfortunately, the prevalence of this headache among pregnant women is higher than other patients due to their gender and age.2,7 Thus, the treatment and prophylaxis of headache after spinal anesthesia is of great significance to anesthetists.2,8,9 The mechanism for prophylaxis of headache after spinal anesthesia must be well understood to choose the best treatment option.10 However, the exact mechanism and cause of the complication are unclear, and an old hypothesis, i.e. cerebrospinal fluid leak (CSFL) is believed to create traction on the pain-sensitive meningeal vessels.4,11 Thus, gravity-dependent traction on pain sensitive vessels causes a headache when the patient is standing up.10,12
Based literatures, ondansetron can lead to severe migraine-like headaches due to 5-hydroxytryptamine-3 (5-HT3) receptors in the brain. Given the effects, the medication seems to effectively reduce the headache after spinal anesthesia by directly preventing dilation of cerebral vessels, or by maintaining mean arterial pressure (MAP) and by directly preventing cerebrovascular vasodilation. Previous study by Fattahi et al.8 has investigated the anti-headache effect of 0.15 mg/kg ondansetron in patients undergoing cesarean section and showed reduce the incidence of post-dural puncture headache, hypotension and PONV. Other studies also showed that ondansetron could significantly reduce dural puncture headache in patients undergoing C/S.13,14 However, given the high prevalence of post-spinal headache in pregnant women and the common use of the adjuvants to prevent nausea and vomiting (N/V), we aimed to evaluate the efficiency of two other doses (8 mg and 4 mg) of ondansetron in preventing post-spinal headache and PONV using different doses.
SUBJECTS AND METHODS
This double-blind clinical trial was conducted in 195 pregnant women with American Society of Anesthesiology Physical status (ASA) classes I and II diagnosis that aged 20-35 years and who were scheduled for elective C/S under spinal anesthesia. The patients were recruited for study from all mothers who referred to gynecology department of Taleghani Hospital, Arak, Iran. Sample size calculation conducted based on alpha error 0.05, study power 80% and the proportion difference of ondansetron efficacy and placebo in other studies. Participants were excluded if had any history of cardiovascular disease, migraine headache, use of selective serotonin reuptake inhibitors (SSRIs), sensitivity to ondansetron and local anesthetics, and finally contraindication for spinal anesthesia.
Inclusion criteria were including ASA I-II,15 age of 20-35 years, patient consent, no history of cardiovascular disease (cardiac arrhythmia, ischemia and heart failure), no SSRI prescriptions, no opioid prescriptions, lack of preeclampsia and eclampsia, and no history of migraine and diabetes.16,17,18 In addition, the exclusion criteria were including lack of patient participation, sensitivity to ondansetron and local anesthetics, contraindication for spinal anesthesia, repeated dural puncture, failed spinal anesthesia which requires adjuvants, and pre-pregnancy body mass index (BMI) less than 30. Verbal and written informed consent was obtained from all patients and the study protocol was approved by Ethical Committee of Arak University of Medical Sciences (approved number IR.ARAKMU.REC.1394.274), registered in the Iranian Registry of Clinical Trials with registration number ID: IRCT2016090629732N1. Subjects were assigned to three equal-sized groups using block random allocation.
Participants in the first (n = 65), second (n = 65), and control groups (n = 65) received 8 mg, 4 mg of intravenous ondansetron and normal saline, respectively, five minutes before the surgery. Chossing the dosage was according to results of other studies.8,19,20,21 Those studies showed that the ondansetron 4 mg has lower effect in prevention of PONV in compared to dexamethasone and the combining dexamethasone with ondansetron is more helpful. Therefore, ondansetron with two doses was used in this study. A final volume of 5 mL was prepared by adding normal saline, and no differences were found among them. Medications were coded and administered to three groups A, B and C by an anesthetist who was not involved in the data collection, while the patients and the resident collecting information were unaware of patient grouping.
Standard monitoring included non-invasive blood pressure, pulse oximetry and electrocardiography in all patients from admission to the operating room, throughout the surgery and to recovery period. All patients were administered 500 mL of Ringer's solution just before spinal anesthesia which was performed with a 25-gauge Quincke needle at the L3–4 or L4–5 interspace, in sitting position. Patients received an intrathecal injection of 12 mg marcaine 0.5%. After the spinal anesthesia, patient is immediately placed in the supine position. With a small pillow placed under the hips, patient rotates 10° to the left to prevent the compression on the aorta and vena cava by the gravid uterus. The blood pressure and heart rate were measured before and immediately after the spinal anesthesia, while being recorded every 5-15 minutes. 12 mg of intravenous ephedrine was injected when blood pressure decreased by more than 20% of the baseline value. In case of bradycardia (below 42 beats/minute), 0.5 mg intravenous atropine was administered. The crystalloid, Ringer's solution, was administered in a volume of 10 mL per kg body weight during the surgery. Patients were excluded from the study if there was hypotension more than 20% of the baseline value which cannot be inhibited by ephedrine and atropine.
The main variable evaluated in this study was headache due to spinal anesthesia, which is a pulsating headache in the frontal or occipital areas, spreading to the neck and right shoulder. This is usually associated with photophobia, diplopia, blurred vision, dizziness, hearing loss, N/V and usually begins 24-48 hours after dural puncture, and worsens by standing up while relieves by lying down, and aggravates by head movements. Though patients with migraine were excluded, they were characterized by relief from headache due to spinal anesthesia by lying down. At 24 hours, 48 hours, 4th day, and 7th day, patients were asked to sit on their beds for 3 minutes and then inquire about what they feel. The patients were asked about headache severity and the complained headache due to spinal anesthesia, was graded by visual analogue scale (VAS) score as mild, 0-3; moderate, 4-6; and severe, 8-10. Moreover, the incidence of PONV was assessed in all groups as secondary outcome.22 The following scores were assigned to scoring the severity of N/V, ranging from 0 to 3: 0 = no N/V, 1 = nausea alone, 2 = N/V, and 3 = vomiting more than twice in 30 minutes).23 Moreover, mean blood pressure (MBP), mean heart rate (MHR) and oxygen saturation (SaO2) was measured at the time of anesthesia induction, 5th, 10th, 15th, 20th, 25th and 30th minutes after induction. The flow chart is depicted as Figure 1.
Figure 1.
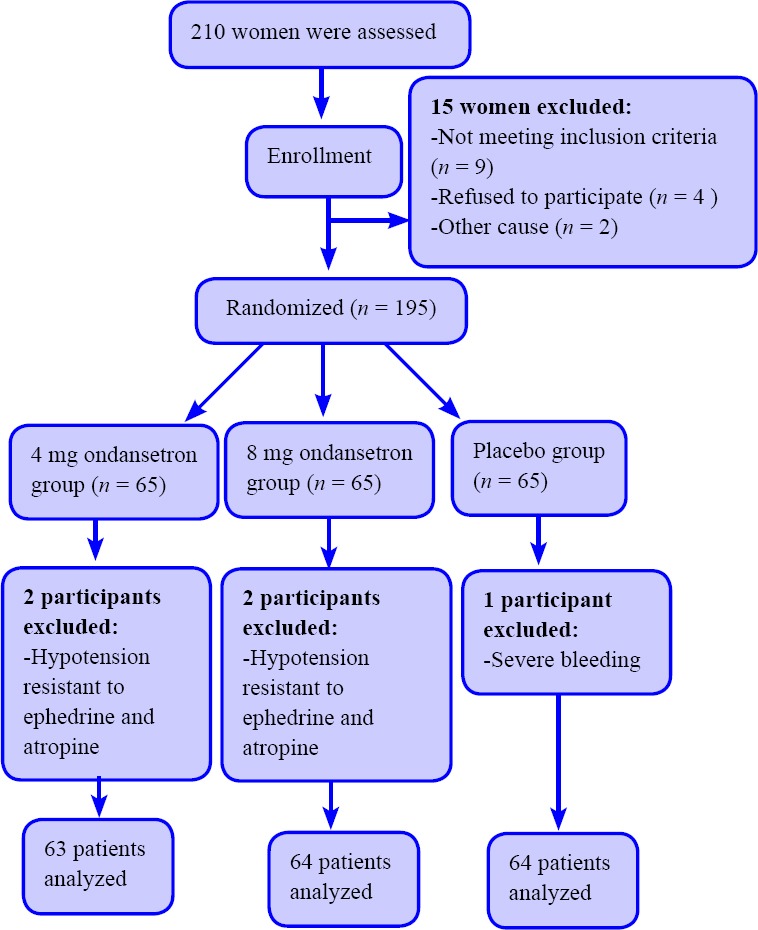
Flow chart of the trial.
Data were analyzed using SPSS 20 (IBM, Armonk, NY, USA) by Chi-square test, analyasis of varience and Tukey post hoc test. Moreover, the trend of hypo-dynamic parameters was assessed by analysis of variance for repeated measures.
RESULTS
The patients’ average age was 29.53 ± 5.10 years and the mean duration of surgery was 64.03 ± 1.79 minutes. The baseline measurements conducted in three groups and the demographic variables such as age and BMI and hemodynamic characteristics was compared among three groups by analysis of variance (Table 1). There was no significant difference in patients of all groups regarding to age, ASA, BMI, first MAP, first heart rate, first SaO2 and surgery duration (P ≥ 0.05). Therefore, the random allocation was good and three groups were comparable. Four patients were excluded form study due to hypotension resistant to ephedrine and atropine in three groups that showed in Figure 1.
Table 1.
Baseline characteristics of pregnant women under spinal anesthesia for cesarean section in both ondansetron and placebo groups
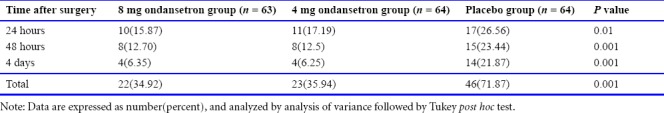
As shown in Table 2, comparing the post-spinal headache in mothers undergoing C/S showed significantly higher severity of post-spinal headache in the placebo group than in the ondansetron 8 mg and 4 mg ondansetron groups at 24 hours after surgery (P < 0.010). However, no significant difference was observed between both 8 mg and 4 mg ondansetron groups based on Tukey post hoc test (P ≤ 0.05). In addition, the post-spinal headache was higher in the placebo group than in the others at 48 hours after surgery (P = 0.001). Moreover, the post-spinal headache though was lower in the 8 mg ondansetron group than in the 4 mg ondansetron group, their difference was not significant (P ≥ 0.05). At 4 days after surgery, the incidence of post-spinal headache was higher in the placebo group than the other groups (P = 0.01). Post-spinal headache in the placebo group was generally more than the other groups at all times, and their distributions were 34.92%, 35.94%, and 71.87% in the 8 mg ondansetron, 4 mg ondansetron and placebo groups, respectively. The overall incidence of the headache was generally lower in 8 mg ondansetron group (P < 0.001).
Table 2.
Comparison of the incidence of headache in pregnant women after spinal anesthesia for cesarean section at 24 hours, 48 hours and 4 days after surgery in the 8 mg, 4 mg ondansetron groups and the placebo group
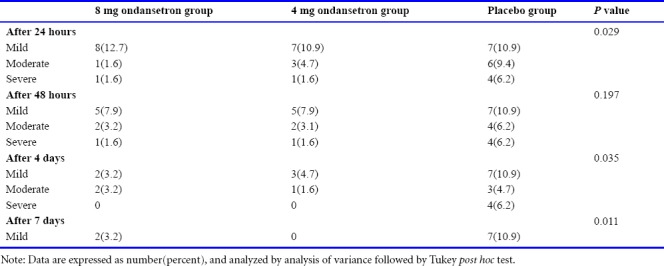
As shown in Table 3, comparing the severity of post-spinal headache at 24, 48 hours and 4 and 7 days after surgery in the three studied groups showed that the headache severity was statistically significant among groups at 1, 4 and 7 days (P < 0.05). The most severity of post-spinal headache was generally mild, and then the severity of the headache was moderate in all groups in at different times. In addition, at all times including 24 hours, 48 hours and 4 days after surgery, lower severity of headache was observed in 8 mg ondansetron group and the highest severity was occurred in placebo group (P < 0.05).
Table 3.
Comparison of severity of headache after spinal anesthesia for cesarean section at 24 hours, 48 hours and 4 days after surgery in 8 mg, 4 mg ondansetron and placebo groups
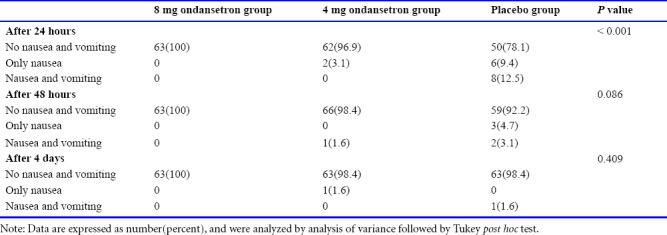
As shown in Table 4, PONV incidence was significantly higher in the placebo group than in the other two groups at 24 hours (P < 0.001). However, the PONV was not significant among studied groups after 48 hours (P = 0.086) and 4 days (P = 0.409) after surgery. The PONV incidence was same between 8 mg and 4 mg ondansetron groups. However, the nausea incidence was higher in placebo than 8 mg and 4 mg ondansetron groups, while the nausea and/or vomiting did not occur in 8 mg ondansetron group at all studied times after C/S surgery. The most incidence of nausea and both nausea and vomiting occurred in placebo group after 24 hours, 2 and 4 days.
Table 4.
Comparison of the frequency of post-operative nausea and vomiting in pregnant women undergoing cesarean section at 24 hours, 48 hours and 4 days after surgery in 8 mg, 4 mg ondansetron and placebo groups
The analysis of variance showed that there was no significant difference in MBP among three study groups (Figure 2) at the time of anesthesia, 5th, 10th, 15th, 20th, 25th and 30th minutes after induction (P > 0.05) and it was almost same in all groups. Nevertheless, based on repeated measurement test, a decreasing trend was observed in MBP in all groups (P < 0.05) and this was not statistically significant between groups (P > 0.05). Based on analysis of variance no differences were found in MHR among the groups at all times (P ≥ 0.05). Moreover, the repeated measurement showed that the trend of deceasing MHR in three groups was not statistically significant (Figure 3).
Figure 2.
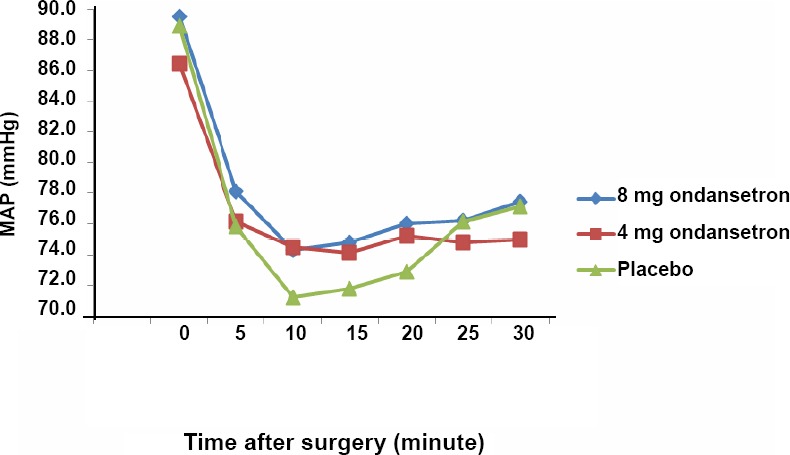
Comparison of mean arterial pressure (MAP) in the 8 mg and 4 mg ondansetron and placebo groups.
Note: Data are expressed as the mean, and were analyzed by analysis of variance followed by Tukey post hoc test.
Figure 3.
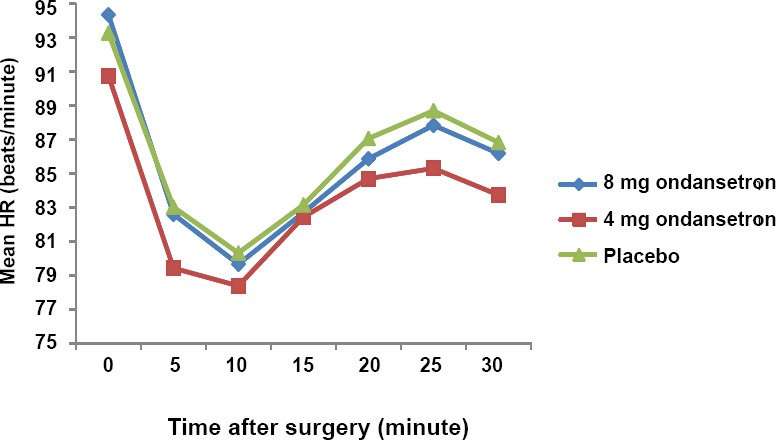
Comparison of mean heart rate (HR) in the 8 mg and 4 mg ondansetron and placebo groups.
Note: Data are expressed as the mean, and were analyzed by analysis of variance followed by Tukey post hoc test.
DISCUSSION
This study evaluated the effect of different doses of ondansetron on headache after spinal anesthesia and showed that ondansetron improved postpartum headache at 24 hours, 48 hours and 4 days after cesarean section, so that the effect of 8 mg ondansetron was more than that of 4 mg ondansetron. In general, post-spinal headache in the placebo group was higher than the other groups at all times, and its frequency distribution in ondansetron 8 mg and 4 mg ondansetron groups and placebo group was 34.92%, 35.94%, and 71.87%, respectively. Moreover, a significant difference was found in nausea, and similarly in PONV among the placebo group and other groups. Vomiting more than twice in 30 minutes was not seen in any group, while PONV was seen less in the 8 mg ondansetron group compared to the 4 mg ondansetron group. A study by Fattahi et al.8 in 2015, aimed to explore the effect of ondansetron on postdural puncture headache in women undergoing C/S, and they stated that ondansetron can reduce the incidence of headache after spinal anesthesia and PONV, while preventing the decrease in blood pressure. This is consistent with our study on headache, nausea and vomiting. While our study uses two ondansetron doses, which both improved headaches and N/V, a single dose level of 15.0 ppm was used in the Fattahi et al. study.8 In the latter study, blood pressure decreased in the ondansetron group, but no difference was found between both intervention and control groups in our study. For investigating the effects of prophylactic ondansetron on spinal anesthesia-induced hypotension, a review study by Gao et al.24 in 2015 showed that ondansetron preventively attenuates the onset of spinal anesthesia-induced hypotension, and decrease the use of vasodilator drugs. Moreover, this reduced complications such as bradycardia and N/V. The results from this study on the effect of reducing N/V are consistent with ours. However, a newly proposed hypothesis suggests that CSFL and volume reduction result in a mechanism, i.e. compensatory intracranial vasodilation, which is responsible for post-spinal headache.6,10 The 5-HT3 receptors play a role in various physiological processes, comprising vasomotor reflexes, control of gastrointestinal function, pain mechanisms, cardiovascular regulation, neuronal function, and limbic-cortical functioning.10 Ondansetron is a highly selective 5-HT3 receptor antagonist commonly prescribed for prophylaxis against and treatment of PONV.12
A study compared the preventing effect of ondansetron and other drugs on PONV after spinal anesthesia for cesarean delivery and showed that same antiemetic efficacy of propofol versus ondansetron.25 In our study, 8 mg ondansetron showed a significant effect on reducing N/V than 4 mg ondansetron. A study by Marashi et al.26 in 2012 aimed to compare two intravenous doses of 6 and 12 mg ondansetron with placebo in suppressing post-spinal anesthesia shivering and hypotension, and found that intravenous administration of different doses of ondansetron significantly reduced spinal anesthesia-induced hypotension, bradycardia and shivering, compared to the control group, whereas no differences were found in blood pressure between our studies. The difference could be due to differences in doses of ondansetron used in both studies. The age range in the Marashi study was 20-50 years, whereas that in ours is 20-35 years. A study by Yazigi et al.9 in 2002, which aimed to determine the effect of prophylactic ondansetron in the treatment of N/V, showed a decrease in N/V when using 8 mg ondansetron. Their results are in line with ours. However, the preventing effect of ondansetron in shivering also is demonstrated in different studies.26,27
We compared the 4 mg and 8 mg ondansetron against post-operative headache and nausea/vomiting after spinal anesthesia in parturient undergoing cesarean section. However, comparing the ondansetron with other drugs such as granisetron on hemodynamic changes is recommended that more extensive studies be done, exploring the complications of ondansetron in patients, the type of drug and the total dose of analgesic drugs used to alleviate headache at home. The current study is also suggested to be used in the procedures in other surgeries that the spinal anesthesia is administered.
Based on our findings, ondansetron can improve postpartum headache at 24 hours, 48 hours and 4 days after cesarean section. However, the 8 mg ondansetron was more effective than the 4-mg dose, but the difference was not significant. Moreover, the PONV was lower in 8 mg ondansetron than 4 mg ondansetron. The decreasing effect of MBP and MHR was same in all groups.
Acknowledgements
We would like to thank the Deputy of Research of Arak University of Medical Sciences, the Clinical Research Center of Valiasr Hospital and all those who participated and helped us in this study
Footnotes
Conflicts of interest
There is no conflict of interest.
Financial support
The work was supported by a grant from Arak University of Medical Sciences.
Institutional review board statement
The study protocol was approved by the Ethical Committee of Arak University of Medical Sciences (approved number IR.ARAKMU. REC.1394.274), and registered in the Iranian Registry of Clinical Trials with registration number ID: IRCT2016090629732N1.
Declaration of patient consent
The authors certify that they have obtained patient consent forms. In the form, patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Biostatistics statement
The statistical methods of this study were reviewed by the biostatistician of Arak University of Medical Sciences, Iran.
Copyright license agreement
The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement
Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
Open peer review reports
Reviewer 1: Lei Huang, Loma Linda University, USA.
Comments to authors: In this double blind and randomized clinical study, the authors investigated the prophylactic effects of ondasetron against post-spinal headache, post-operative nausea and vomiting (PONV) in cesarean section patients. The selected participants were randomized to receive saline control, ondansetron 4 mg or ondansetron 8 mg 5 minutes before surgery. They found that ondansetron significantly reduced the incidence/severity of post-spinal headache and the incidence of PONV compared to saline control pretreated patients. The ondansetron dose of 8 mg showed better efficacy than 4 mg in reduction of incidence of headache.
Reviewer 2: Qin Hu, Shanghai Jiao Tong University, China.
Comments to authors: The authors aim to evaluate the efficacy of ondansetron in post-operative headache and nausea/vomiting after spinal anesthesia. The double-blind clinical trial was conducted on pregnant women. Patients were asked about headache severity at 24 hours, 48 hours, and fourth day. Moreover, the incidence of PONV was assessed in all groups as secondary outcome. The study is well designed and the manuscript is properly organized.
Funding: The work was supported by a grant from Arak University of Medical Sciences.
REFERENCES
- 1.Chooi C, Cox JJ, Lumb RS, et al. Techniques for preventing hypotension during spinal anaesthesia for caesarean section. Cochrane Database Syst Rev. 2017;8:CD002251. doi: 10.1002/14651858.CD002251.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yousefshahi F, Dahmardeh AR, Khajavi M, Najafi A, Khashayar P, Barkhordari K. Effect of dexamethasone on the frequency of postdural puncture headache after spinal anesthesia for cesarean section: a double-blind randomized clinical trial. Acta Neurol Belg. 2012;112:345–350. doi: 10.1007/s13760-012-0065-6. [DOI] [PubMed] [Google Scholar]
- 3.Mehrabi S, Karimzadeh Shirazi K. Results and complications of spinal anesthesia in percutaneous nephrolithotomy. Urol J. 2010;7:22–25. [PubMed] [Google Scholar]
- 4.Jabbari A, Alijanpour E, Mir M, Bani Hashem N, Rabiea SM, Rupani MA. Post spinal puncture headache, an old problem and new concepts: review of articles about predisposing factors. Caspian J Intern Med. 2013;4:595–602. [PMC free article] [PubMed] [Google Scholar]
- 5.Sachs A, Smiley R. Post-dural puncture headache: the worst common complication in obstetric anesthesia. Semin Perinatol. 2014;38:386–394. doi: 10.1053/j.semperi.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Ghaleb A, Khorasani A, Mangar D. Post-dural puncture headache. Int J Gen Med. 2012;5:45–51. doi: 10.2147/IJGM.S17834. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Wadud R, Laiq N, Qureshi FA, Jan AS. The frequency of postdural puncture headache in different age groups. J Coll Physicians Surg Pak. 2006;16:389–392. [PubMed] [Google Scholar]
- 8.Fattahi Z, Hadavi SM, Sahmeddini MA. Effect of ondansetron on post-dural puncture headache (PDPH) in parturients undergoing cesarean section: a double-blind randomized placebo-controlled study. J Anesth. 2015;29:702–707. doi: 10.1007/s00540-015-2000-5. [DOI] [PubMed] [Google Scholar]
- 9.Yazigi A, Chalhoub V, Madi-Jebara S, Haddad F, Hayek G. Prophylactic ondansetron is effective in the treatment of nausea and vomiting but not on pruritus after cesarean delivery with intrathecal sufentanil-morphine. J Clin Anesth. 2002;14:183–186. doi: 10.1016/s0952-8180(01)00381-6. [DOI] [PubMed] [Google Scholar]
- 10.Bezov D, Lipton RB, Ashina S. Post-dural puncture headache: part I diagnosis, epidemiology, etiology, and pathophysiology. Headache. 2010;50:1144–1152. doi: 10.1111/j.1526-4610.2010.01699.x. [DOI] [PubMed] [Google Scholar]
- 11.Arevalo-Rodriguez I, Ciapponi A, Roqué i Figuls M, Muñoz L, Bonfill Cosp X. Posture and fluids for preventing post-dural puncture headache. Cochrane Database Syst Rev. 2016;3:CD009199. doi: 10.1002/14651858.CD009199.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bezov D, Ashina S, Lipton R. Post-dural puncture headache: Part II--prevention, management, and prognosis. Headache. 2010;50:1482–1498. doi: 10.1111/j.1526-4610.2010.01758.x. [DOI] [PubMed] [Google Scholar]
- 13.Heesen M, Klimek M, Hoeks SE, Rossaint R. Prevention of spinal anesthesia-induced hypotension during cesarean delivery by 5-hydroxytryptamine-3 receptor antagonists: a systematic review and meta-analysis and meta-regression. Obstetric Anesthesia Digest. 2017;37:103–104. doi: 10.1213/ANE.0000000000001511. [DOI] [PubMed] [Google Scholar]
- 14.Zheng Y, Yu T, Tang Y, et al. Efficacy and safety of 5-hydroxytryptamine 3 receptor antagonists in irritable bowel syndrome: A systematic review and meta-analysis of randomized controlled trials. PLoS One. 2017;12:e0172846. doi: 10.1371/journal.pone.0172846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doyle DJ, Garmon EH. StatPearls. Treasure Island (FL): Stat-Pearls Publishing LLC; 2018. American Society of Anesthesiologists Classification (ASA Class) [PubMed] [Google Scholar]
- 16.Mohammadbeigi A, Tabatabaee H, Narges MY, Maryam Y. Gestational diabetes and its association with unpleasant outcomes of pregnancy. Pak J Medicalences. 2008;24:566–570. [Google Scholar]
- 17.Memari F, Jadidi R, Noroozi A, Mohammadbeigi A, Falahati J. Protecting effect of gabapentin for nausea and vomiting in the surgery of cesarean after spinal anesthesia. Anesth Essays Res. 2015;9:401–404. doi: 10.4103/0259-1162.157469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohammadbeigi A, Kazemitabaee M, Etemadifar M. Risk factors of early onset of MS in women in reproductive age period: survival analysis approach. Arch Womens Ment Health. 2016;19:681–686. doi: 10.1007/s00737-016-0600-1. [DOI] [PubMed] [Google Scholar]
- 19.D’Souza N, Swami M, Bhagwat S. Comparative study of dexamethasone and ondansetron for prophylaxis of postoperative nausea and vomiting in laparoscopic gynecologic surgery. Int J Gynaecol Obstet. 2011;113:124–127. doi: 10.1016/j.ijgo.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 20.Song JW, Park EY, Lee JG, Park YS, Kang BC, Shim YH. The effect of combining dexamethasone with ondansetron for nausea and vomiting associated with fentanyl-based intravenous patient-controlled analgesia. Anaesthesia. 2011;66:263–267. doi: 10.1111/j.1365-2044.2011.06648.x. [DOI] [PubMed] [Google Scholar]
- 21.Fattahi Z, Hadavi S, Sahmeddi M. Effect of ondansetron on post-dural puncture headache (PDPH) in parturients undergoing cesarean section: a double-blind randomized placebo-controlled study. J Anesth. 2015;29:702–707. doi: 10.1007/s00540-015-2000-5. [DOI] [PubMed] [Google Scholar]
- 22.Boonstra AM, Stewart RE, Koke AJ, et al. Cut-off points for mild, moderate, and severe pain on the numeric rating scale for pain in patients with chronic musculoskeletal pain: variability and influence of sex and catastrophizing. Front Psychol. 2016;7:1466. doi: 10.3389/fpsyg.2016.01466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meek R, Egerton-Warburton D, Mee MJ, Braitberg G. Measurement and monitoring of nausea severity in emergency department patients: a comparison of scales and exploration of treatment efficacy outcome measures. Acad Emerg Med. 2015;22:685–693. doi: 10.1111/acem.12685. [DOI] [PubMed] [Google Scholar]
- 24.Gao L, Zheng G, Han J, Wang Y, Zheng J. Effects of prophylactic ondansetron on spinal anesthesia-induced hypotension: a meta-analysis. Int J Obstet Anesth. 2015;24:335–343. doi: 10.1016/j.ijoa.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 25.Meneses LAR, Torres OC, Navarro PG, Garcia JAG. Comparison of the antiemetic efficacy of propofol versus ondansetron in nasal surgery. Randomised clinical trial. Rev Med Hosp Gen (Mex) 2016;81:72–78. [Google Scholar]
- 26.Marashi SM, Soltani-Omid S, Soltani Mohammadi S, Aghajani Y, Movafegh A. Comparing two different doses of intravenous ondansetron with placebo on attenuation of spinal-induced hypotension and shivering. Anesth Pain Med. 2014;4:e12055. doi: 10.5812/aapm.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Safavi M, Honarmand A, Mohammadsadeqie S. Prophylactic use of intravenous ondansetron versus ketamine - midazolam combination for prevention of shivering during spinal anesthesia: A randomized double-blind placebo-controlled trial. Adv Biomed Res. 2015;4:207. doi: 10.4103/2277-9175.166143. [DOI] [PMC free article] [PubMed] [Google Scholar]


