Abstract
Aspergillus flavus produces mycotoxins especially aflatoxin B1 and infects crops worldwide. As a PHD transcription factor, there is no report on the role of Rum1 in the virulence of Aspergillus spp. yet. This study explored the biological function of Rum1 in A. flavus through the construction of rum1 deletion mutants and rum1 complementation strains with the method of homologous recombination. It was found, in the study, that Rum1 negatively regulates conidiation through abaA and brlA, positively regulates sclerotia formation through nsdC, nsdD, and sclR, triggers aflatoxin biological synthesis, and enhances the activity of amylase. Our findings suggested that Rum1 plays a major role in the growth of mycelia, conidia, and sclerotia production along with aflatoxin biosynthesis in A. flavus.
Keywords: Aspergillus flavus, Rum1, conidiation, sclerotia, aflatoxin
1. Introduction
Aspergillus flavus is a famous opportunistic soil-borne pathogen and its harmfulness to immunosuppressed patients is only second to A. fumigatus [1]. A. flavus is the main producer of aflatoxin B1 (AFB1), which is the most toxic, tetratogenic, and carcinogenic (especially to liver) mycotoxins known [2]. IARC (the International Agency for Research on Cancer) has put AFB1 into Group 1 carcinogen [3]. By food contamination (including maize kernels, peanuts, cottonseeds, and tree nuts), A. flavus could cause diseases such as aflatoxicosis and hepatocellular carcinoma in human and animals through daily diets [1,4]. Aflatoxicosis caused by the intake of high dose of AFs could even lead to death [5]. As the most important agricultural mycotoxins producer, A. flavus has given rise to colossal economic losses, grain shortages, and health issues around the world [3,6]. To protect human and animal health and to allay the losses of agricultural economy, effective measures should be resorted to reduce the contamination and virulence of A. flavus.
The morphogenesis and secondary metabolism of pathogenic fungi are regulated by some conserved regulatory factors [7,8] in which VeA (a global regulator) regulates the production of aflatoxin by AflR in A. flavus and mediates sclerotia and cleistothecia formation in A. flavus and A. nidulans, respectively [9,10]. It was further identified that the heterogenous trimeric complex VelB/VeA/LaeA was involved in the connection of light-responding development and secondary metabolism regulation [11]. The master transcription factor MtfA and a VeA-dependent element involved in the regulation of ST biological synthesis in A. nidulans was found to take part in the AFB1 biosynthesis, fungal development, conidiation, and sclerotia formation in A. flavus [12]. Environmental factors including nutritional status and environmental stresses also play a role in the development and secondary metabolism in Aspergillus spp. [13,14].
Fungal cellular development and AFs biosynthesis are complicated processes involving many different types of transcription factors including the PHD family transcription factors, which are less abundant (about one kind) in the A. flavus genome (Figure S1) [15]. The PHD finger domain (plant homeodomain finger) was first discovered in the HAT3.1 protein from Arabidopsis [16]. PHD containing transcriptional factors have been found in various eukaryotic cells especially in plant and animal cells [17]. In recent years, a number of new PHD-finger protein homologs have been identified in different species. However, most putative PHD family members are still unrecognized and unidentified [18]. It was found that the PHD-finger protein DUET of Arabidopsis plays a regulatory role in chromosome organization and progression [19]. Some PHD proteins of mammals (such as UHRF1, ATX1, and ATX2) are essential for DNA methylation and the activity of histone methyl-transferase [20,21]. In addition, it was reported that the PHD-finger protein is closely related to the protein-protein interactions in human beings [22]. Since the PHD-finger protein homologs have potentially important functions in many species, it is important to study PHD family members in pathogenic fungi. As a PHD domain containing protein, Rum1 (regulator U. maydis 1) was found to play a critical role in the sporulation of Ustilago maydis and, according to its domains, it was speculated to function as a repressor in the process of transcription through chromatin structure modulation [23]. However, the biological function of PHD fingers containing Rum1 in the morphogenesis and mycotoxin biosynthesis of Aspergillus spp. has not been explored yet.
In view of the serious impacts of A. flavus on the safety and development of global society and economy and in the light of the putative critical role of the PHD transcriptional factor Rum1 in epigenetic regulation, it is of great importance to explore the biological function of Rum1 in A. flavus. Through sequence alignment with rum1 sequence from U. maydis, we located a Rum1 ortholog (NCBI Gene bank No: AFLA_006240) in A. flavus. The study was carried out to explore the biological functions of Rum1 in the growth of mycelia, sporulation, sclerotial development, and aflatoxin production of A. flavus.
2. Results
2.1. Characterization of the PHD Transcription Factor Rum1 in A. flavus
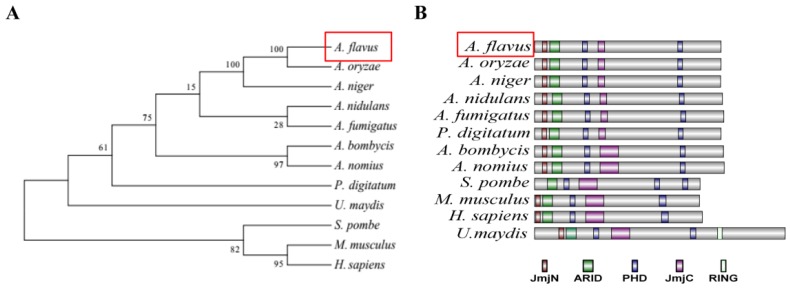
The homologs of 12 Rum1 proteins (from A. flavus, A. niger, A. oryzae, A. nidulans, A. fumigatus, A. bombycis, A. nomius, P. digitatum, S. pombe, M. musculus, H. sapiens, and U. maydis) were obtained from NCBI (http://www.ncbi.nlm.nih.gov), and the evolutionary relationship of these homologs was analyzed using MEGA5.0. The result showed that Rum1 in Aspergillus spp. was classified into a cluster in which the highest identity was found between A. flavus and A. oryzae (Ident 99%, Query cover 100%) and the lowest identity was found between A. flavus and A. nidulans (Ident 79%, Query cover 99%) (Figure 1A). Among non-Aspergillus spp., the highest identity was 76% (between A. flavus and P. digitatum, Query cover 98%) and the lowest identity was 30% (between A. flavus and H. sapiens, Query cover 77%). The protein domains in Rum1 were further analyzed with the use of software SMART, and IBS 1.0. A JmjN, an ARID, a JmjC, and two PHD fingers domains were found in almost all Rum1 homologs. Rum1 homolog from S. pombe has three PHD fingers domains, but lacks a JmjN domain, which is shown in Figure 1B. These results reflected that Rum1 is very conservative in fungi and mammals.
Figure 1.
Characterization of the PHD transcription factor Rum1 in A. flavus. (A) Phylogenetic relationship of 12 Rum1 homologs from different species was analyzed with MEGA5.0. (B) The domain structure of Rum1 homologs was identified by SMART and were visualized using software IBS 1.0.
2.2. Construction of the Deleted Mutant (Δrum1) and Complemented Mutant (Δrum1-C)
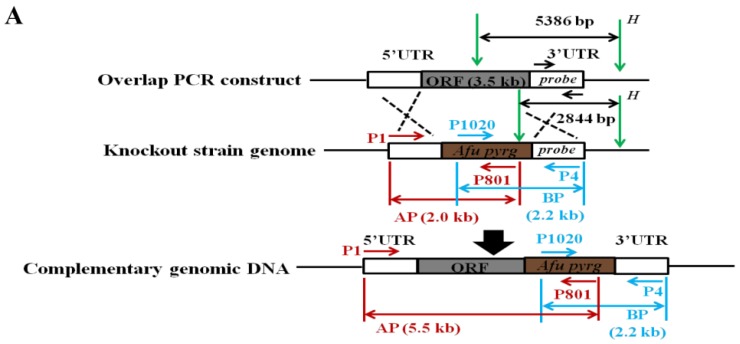
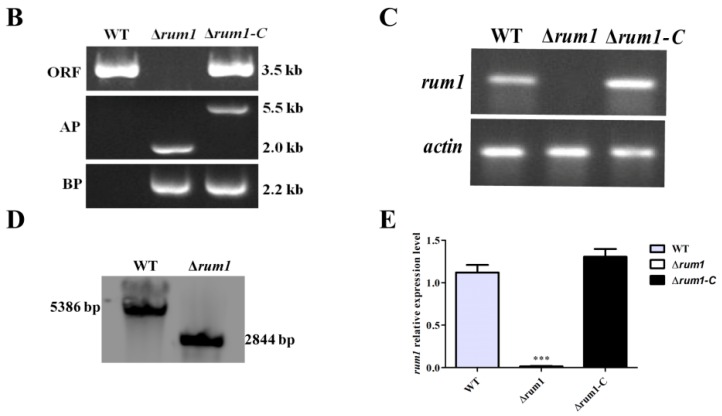
The rum1 deletion mutant (∆rum1) was constructed through homologous recombination (Figure 2A) and was verified with PCR analysis through the amplification of rum1 ORF, AP, and BP fragment using genomic DNA as a template in which a 3.5 kb ORF fragment was only amplified from WT and complementation (∆rum1-C) strains, a BP fragment (2.2 kb) was amplified from both ∆rum1 and ∆rum1-C strains, a 2.0 kb AP fragment was amplified from ∆rum1 strain, and a 5.5 kb AP fragment was amplified from ∆rum1-C strain (Figure 2B). Afterward, with cDNA as a template, a 220 bp DNA fragment from the ORF of rum1 was amplified from WT and ∆rum1-C but not from ∆rum1 mutant (Figure 2C). These results verified that the rum1 gene had been deleted from the ∆rum1 mutant and ∆rum1-C had been successfully constructed. The ∆rum1 mutant was further verified with Southern blot analysis (Figure 2D). The result of q-PCR showed that no transcriptional activity of rum1 could be detected in the ∆rum1 mutant (Figure 2E), which confirmed that rum1 had been deleted from the rum1 mutant and the expression level of rum1 recovered in ∆rum1-C was compared with the WT strain. Therefore, both the rum1 deletion mutant (∆rum1) and complementation strain (∆rum1-C) were correctly constructed.
Figure 2.
Strategy and confirmation of the mutant strains. (A) The scheme of rum1 deletion and complementation strategy (H: HindIII, probe: the probe used in southern blot analysis). (B) Gene knockout and complemented strains were verified by PCR analysis. (The rum1 ORF was confirmed by primers rum1-p9 and rum1-p10, AP fragment was confirmed by primers rum1-p1 and P801, and fragment BP was confirmed by primers P1020 and rum1-p4). (C) q-PCR verification of rum1 gene deletion and complementation strains. The actin gene was used as an inner reference. (D) Southern blot analysis for ∆rum1 mutant. Genomic DNA from above strains was digested with HindIII and hybridized with a 1.4 kb probe of the downstream region fragment of rum1 (3′-UTR) (The probe fragment was amplified with primers rum1-p3 and rum1-p4), (E) q-PCR analysis of the expression level of rum1 gene in ∆rum1 WT and ∆rum1-C strains. *** represents significant difference (p < 0.001).
2.3. Rum1 Is Involved in Mycelium Growth and Conidiation
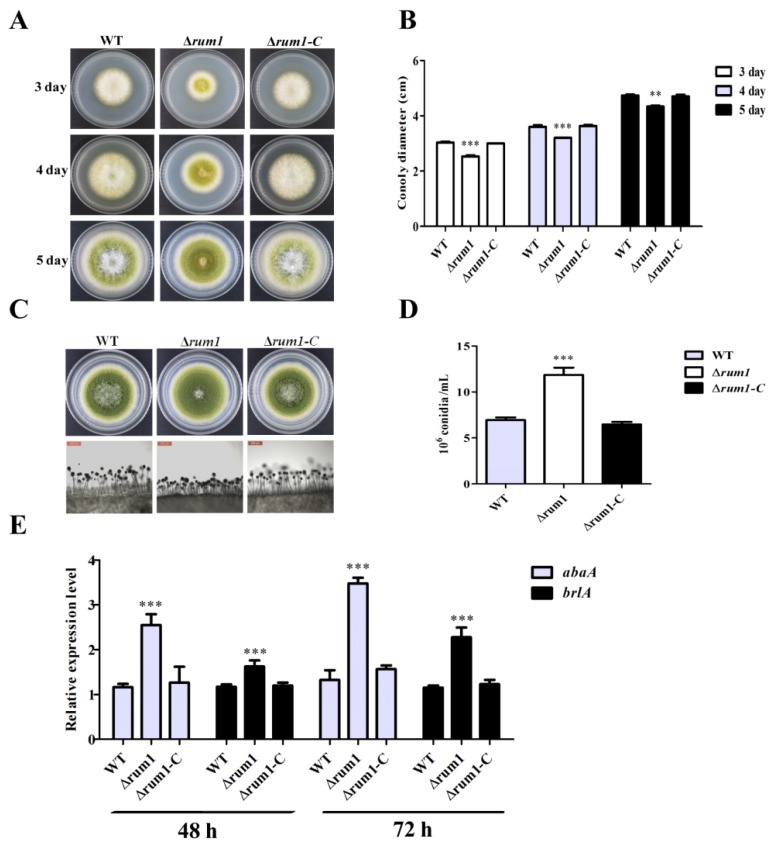
To research the role of Rum1 in the development of A. flavus, the WT, ∆rum1, and ∆rum1-C strains were incubated on PDA medium in the dark at 29 °C for 5 days, 37 °C for 5 days, and 42 °C for 10 days, respectively. The results showed that the ∆rum1 mutant displayed no significant difference in colony growth compared to WT and ∆rum1-C strains under 37 °C and 42 °C (data not shown), but the diameter of the ∆rum1 colony was significantly smaller than that of WT and the ∆rum1-C strains at 29 °C (Figure 3A,B). The results also revealed that the conidiation of the ∆rum1 mutant was remarkably increased and the conidiophores of the ∆rum1 mutant were remarkably shorter and denser than those of WT and the ∆rum-C strains (Figure 3C,D). Results of q-PCR analysis revealed that both brlA and abaA (the transcriptional factor genes for conidiation regulation) were significantly up-regulated in the ∆rum1 mutant (Figure 3E). The above results suggested that Rum1 negatively regulates A. flavus conidiation through transcriptional factor genes brlA and abaA.
Figure 3.
The roles of Rum1 in mycelium growth and conidiation in A. flavus. (A) The colonies of A. flavus strains grew on PDA medium at 29 °C. (B) The histogram of colony diameter calculated according to the result of A. (C) The conidiophores of A. flavus strains were observed under a microscope (20×). (D) The conidia of each plate were suspended with 5 mL of spore eluate. The conidia number of A. flavus strains in 1 ml spore eluate was calculated using hemocytometer. (E) The q-PCR analysis of brlA and abaA and the transcriptional factor genes for conidiation in the related A. flavus strains. ** and *** denote statistical significant levels of p < 0.01 and p < 0.001.
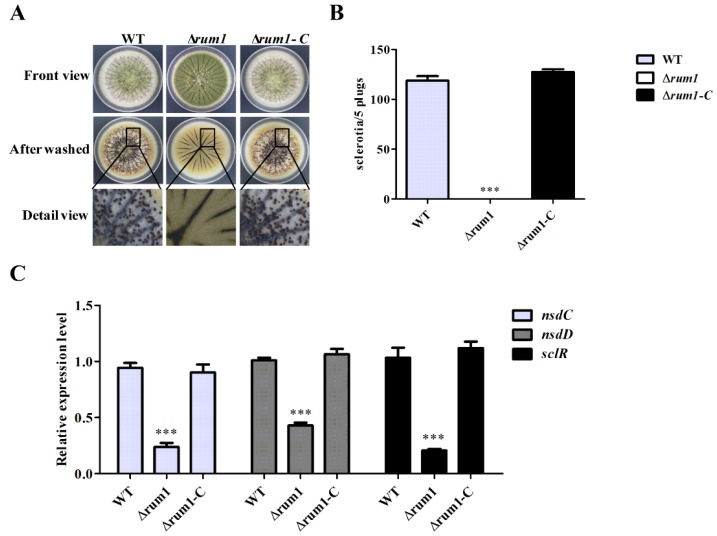
2.4. Rum1 Is Essential for Sclerotial Generation
To explore the biological function of Rum1 in sclerotia formation, the WT, ∆rum1, and ∆rum1-C strains were incubated on the WKM and GMM medium (data not shown) at 37 °C in the dark for a week. We found that there was no sclerotia formed in the ∆rum1 mutant strain compared to WT and ∆rum1-C strains on both media, which indicated that the deletion of rum1 prominently declined the capability of sclerotia formation (Figure 4A,B). The results of the q-PCR analysis showed that the expression levels of sclR, nsdC, and nsdD genes (the positive regulators for sclerotia formation) were all transcriptionally down-regulated in the absence of Rum1 (Figure 4C). These results suggested that Rum1 positively regulates sclerotia formation through sclerotia formation regulators SclR, NsdC, and NsdD.
Figure 4.
The role of Rum1 in sclerotia formation. (A) WT, ∆rum1, and ∆rum1-C strains were inoculated on WKM medium for 7 days and then the plates were sprayed with 75% ethanol to make sclerotia visible. (B) The histogram showing the amount of sclerotia according to (A), (C) Transcriptional expression levels of nsdC, nsdD, and sclR, which are the genes of positive regulators in sclerotia formation. *** represents significant difference (p < 0.001).
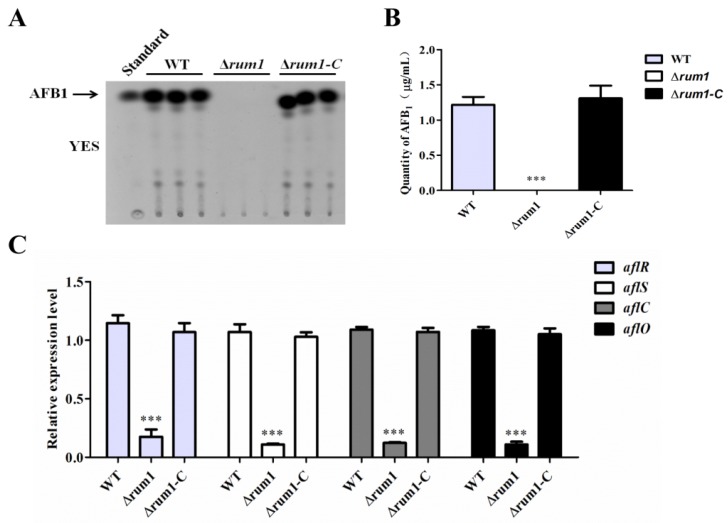
2.5. Rum1 Positively Regulates Aflatoxin Biosynthesis
To examine the impact of Rum1 in the biosynthesis of AFB1, all related A. flavus strains were cultivated in liquid YES medium at 29 °C for 6 days and the samples were collected and prepared on the sixth day. Afterward, these samples were further analyzed by TLC. The results indicated that no AFB1 production could be found from the ∆rum1 mutant compared to WT and ∆rum1-C strains (Figure 5A,B). We performed q-PCR to analyze the expression levels of AFB1 synthesis related genes (aflR, aflS, aflC, and aflO), which revealed that the expression levels of these genes were significantly decreased in the ∆rum1 mutant compared to findings from the WT and ∆rum1-C strains (Figure 5C). These results revealed that Rum1 is crucial for AFB1 biosynthesis in A. flavus.
Figure 5.
The role of Rum1 in aflatoxin biosynthesis. (A) AFB1 production of WT, ∆rum1, and ∆rum1-C strains was detected by Thin-Layer Chromatography (TLC). All strains were cultivated in liquid YES medium for 6 days at 29 °C in the dark (5 μL of each sample was allotted for the TLC analysis. Three lanes of the same strain represent biological repeats, respectively). (B) The quantity of AFB1 produced by related A. flavus strains was quantitated according to (A), (C) Transcriptional levels of aflatoxin biosynthesis related gene aflR, aflS, aflC, and aflO in the strains mentioned above. *** represents a significant difference (p < 0.001).
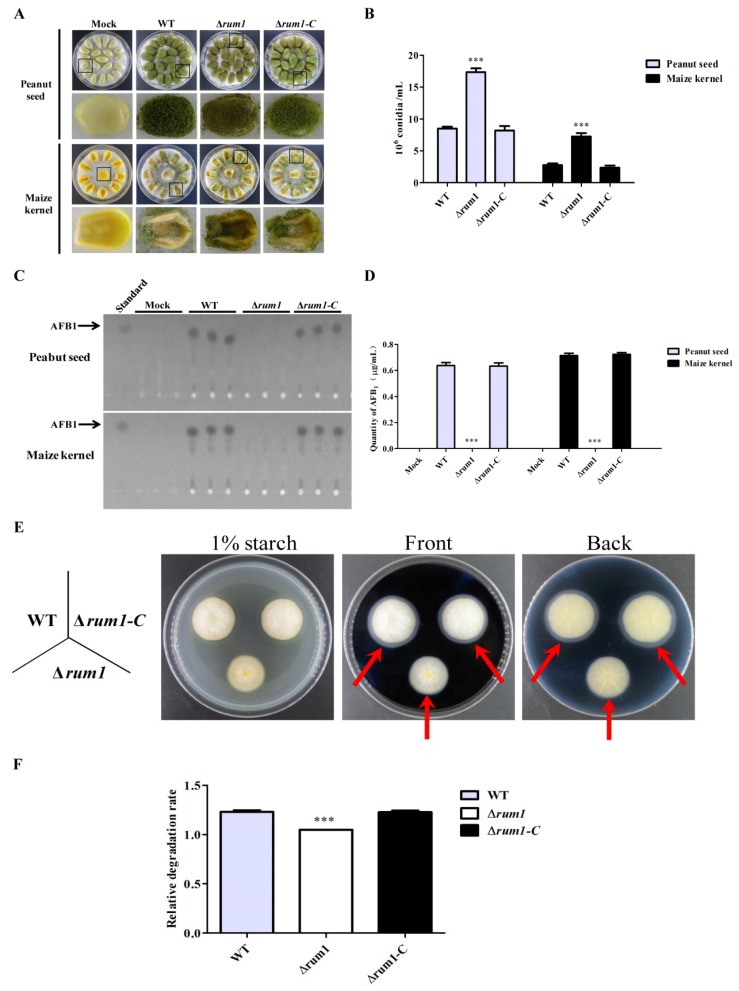
2.6. Rum1 is Involved in the Colonization of A. flavus to Crop Kernels
To detect the biological function of Rum1 in colonization of A. flavus, maize and peanut kernels were incubated with conidia suspension of WT, ∆rum1 mutant, and ∆rum1-C strains. The results (Figure 6A,B) showed that the conidiophore of the ∆rum1 mutant was shorter when colonized on maize and peanut kernels. The ∆rum1 mutant produced more conidia compared to WT and ∆rum1-C strains as the state found on the PDA medium (Figure 3C,D). The production of AFB1 from A. flavus colonized kernels was also explored, which showed that AFB1 production was severely restrained in the ∆rum1 mutant (Figure 6C,D). These results demonstrated that Rum1 is crucial for conidiation and aflatoxin biosynthesis in the colonization process of A. flavus on crop kernels.
Figure 6.
The role of Rum1 in A. flavus colonization. (A) Photographs presented the maize and peanut kernels colonized by WT, ∆rum1, and ∆rum1-C strains. (B) Conidia production of all the strains on seeds (The conidia of each plate were suspended with 5 mL of spore eluate and the conidia number of A. flavus strains in 1 mL spore eluate was calculated with hemocytometer). (C) The production of AFB1 from colonized kernels was detected by TLC (5 μL from each sample was loaded for the TLC analysis. Three lanes of the same strain represent three biological repeats, respectively). (D) Relative AFB1 production in (C) was quantitated. (E) Amylase activity was analyzed with iodine solution and 1% starch (Red arrows point to the degradation zones). (F) The relative degradation rate of starch of WT, ∆rum1, and ∆rum1-C strains, according to the results of (E). *** represents significant difference (p < 0.001).
It was found that the ability of fungal colonization is closely related to changes in hydrolase activity [24,25]. To explore the biological function of Rum1 in A. flavus virulence, the role of Rum1 in the activity of amylase in A. flavus was explored with WT, ∆rum1, and ∆rum1-C strains. The results showed that the relative degradation rate of the ∆rum1 mutant to starch was significantly smaller compared to that of WT and ∆rum1-C strains (p < 0.001) (Figure 6E,F. The relative degradation rate = diameter of degradation zone/diameter of colony). The findings suggested that Rum1 enhances the activity of amylase in fungi.
3. Discussion
The results of phylogenetic analysis in the study indicated that Rum1 is quite conserved from fungi to mammals, which suggested that Rum1 plays important and similar roles in fungal biological activity. The analysis of domain architectures also revealed that Rum1 is composed of a series of functional domains (including a JmjN, an ARID, a JmjC, and two PHD fingers domains). JmjC (Jumonji C) is a demethylase-related domain [26,27] and JmjN (Jumonji N) is always located adjacent to JmjC to form one functional unit [28,29]. ARID (AT-Rich Interacting Domain) is a DNA binding domain participating in the target-specific reaction in a Jumonji domain containing KDMs (histone-lysine demethylase) [30,31,32] and a host of PHD fingers have been characterized as a novel family of histone code readers recently [17,33]. Nevertheless, no reports about the biological function of the PHD transcription factor Rum1 in filamentous fungi were given until now. Our work revealed that Rum1 played important roles in the development, asexual reproduction, sclerotia formation, and aflatoxin biosynthesis in A. flavus.
This study showed that Rum1 improved the growth of mycelia at 29 °C, but negatively regulated conidiation by down-regulating the expression levels of brlA and abaA (Figure 3E). Both abaA and brlA are believed to play a key role in conidia development in which brlA is the first key transcription factor activated in the process of conidiation [34,35,36]. Afterward, abaA is activated by the brlA gene during the middle stage of conidiation [37]. In the process of A. flavus colonization of crops kernels (Figure 6A,B), the asexual reproduction of A. flavus was also obviously negatively regulated by Rum1. The reduction in asexual reproduction in pathogenic Aspergillus spp. in the presence of Rum1 lowers the chances of the plant pathogenic fungus to transfer from one host to the other. It is inferred in the study that Rum1 improves the formation of sclerotia by increasing the expression levels of nsdC, nsdD, and sclR. The genes nsdC, nsdD, and sclR were reported to promote sclerotial formation in A. nidulans and A. oryzae [38,39]. It was found recently that the sexual reproduction structure-ascocarps were embedded in the sclerotia of A. flavus [40], which illuminated that the lack of Rum1 led to the abortion of sclerotia formation in the ∆rum1 mutant in the study. This could mean that Rum1 might play an important role in promoting the genetic variation of filamentous fungi by sexual reproduction to improve environmental adaptability of the plant pathogenic fungus. Sclerotia are commonly considered survival structures against unfavorable conditions. Therefore, the presence of Rum1 is clearly in favor of the survival of the plant pathogenic fungus against an adverse environment.
In YES liquid medium (also in PDB medium, data not shown) and crop colonization models (maize and peanut), the results showed that Rum1 dramatically enhanced the AFB1 biosynthesis by up-regulating the expression levels of aflatoxin biosynthesis structural genes aflC and aflO as well as regulatory genes aflR and aflS (Figure 5C). As a 47 kDa zinc-finger transcriptional factor, AflR is a positive regulator for aflatoxin synthesis and the transcription of most structural genes in the aflatoxin gene cluster require the positive regulation of AflR [10]. AflS, regulated by AflR, is indispensable for aflatoxin bio-synthesis [40]. In the aflS knockout mutants, the expression levels of some structural genes (e.g., aflC, aflD, aflM, and aflP) in aflatoxin pathway were found to have a five-fold to 20-fold reduction. In addition, genes known as aflC and aflO catalyze the chemical reaction of acetate to norsolorinic acid and DMST to ST in the biosynthesis pathway of AFB1, respectively [41]. In this study, it was found that the expression level of aflR dramatically decreased by approximately five-fold in ∆rum1 mutant (p < 0.001) (Figure 5C). Meanwhile, the possible responsible elements (RE) of the aflR promoter were predicted with MEME online (http://meme-suite.org/tools/meme), which revealed that Rum1 might bind to the possible RE on the promoter of aflR to regulate the synthesis of aflatoxin (Figure S2). Above results suggested that Rum1 triggered the biosynthesis of aflatoxin by activating the aflatoxin biosynthesis-relevant gene cluster in A. flavus.
The role of Rum1 in the colonization and virulence of A. flavus on crop kernels is critical and complicated. In the study, Rum1 was found to significantly repress conidiation and to initiate sclerotial development (Figure 4 and Figure 6, p < 0.001), which seemed that Rum1 attempted to initiate sexual reproduction by depressing asexual activity to some degree in the colonization of A. flavus to crops. Similar to the results from YES liquid medium (Figure 5) and PDB medium (data no shown), the absence of Rum1 deprived the plant pathogenic fungus of aflatoxin biosynthesis ability in the colonization of A. flavus on crops (Figure 6), which revealed that Rum1 is an important virulence factor in the fungus. Plant pathogenic fungi might encounter some stresses inside host crops. The role of Rum1 under the stresses was also explored in this study. No statistical significance was found between the inhibition rate of the ∆rum1 mutant and the inhibition rates of WT and the ∆rum1-C strains under stresses tested in the study (Figure S3), which suggested that Rum1 didn’t participate in the stress responses of A. flavus. It was reported that the capacity of fungal colonization is bound up with the changes of the hydrolytic enzymes’ activity [24,25]. The tests on hydrolytic enzymes in the study suggested that Rum1 may affect colonization of plant pathogenic fungi by increasing the activity of amylase in fungi (Figure 6E,F). In summary, the results of our study suggested that Rum1 might be involved in the colonization and virulence of A. flavus on crop kernels through its roles in fungal development, secondary metabolism, and in the activity of some hydrolytic enzymes.
4. Conclusions
In conclusion, the PHD transcription factor Rum1 of A. flavus is deeply involved in the morphogenesis and AFs biosynthesis of the fungus. Our findings provided meaningful information that could improve our understanding of the roles of Rum1 in the regulatory mechanisms of secondary metabolism and fungal morphogenesis in the plant pathogenic fungus, which might lay a foundation for developing new control strategies against AFs producing fungus.
5. Materials and Methods
5.1. Fungal Strains and Primers
All A. flavus strains used in the study were listed in Table 1 and the primers were shown in Table 2 and Table 3. The Wickerham medium (WKM, 2 g/L yeast extract, 3 g/L peptone, 5 g/L cornteep solids, 2 g/L dextrose, 30 g/L sucrose, 2 g/L NaNO3, 1 g/L K2HPO4•3H2O, 0.5 g/L MgSO4•7H2O, 0.2 g/L KCl, 0.1 g/L FeSO4•7H2O), YES medium (20 g/L yeast extract, 150 g/L sucrose, 1 g/L MgSO4•7H2O), potato dextrose agar (PDA, 39 g/L, BDDifco, Franklin, NJ, USA), potato dextrose broth (PDB, 24 g/L, BDDifco, Franklin, NJ, USA), and GMM medium (10 g/L dextrose, 10 mM/L ammonium tartrate, 1.52 g/L KH2PO4, 0.52 g/L MgSO4•7H2O, 0.52 g/L KCl, and 1 mL trace elements) were prepared for fungal cultivation in this study. For solid medium, agar was added at 15 g/L. For the auxotrophic marker (pyrG-), the medium was supplemented with 1 mg/mL uracil and 1 mg/mL uridine [12].
Table 1.
Fungal strains used in the study.
| Strain | Genotype Description | Reference |
|---|---|---|
| A. flavus CA14 | ∆ku70, ∆pyrG | purchased from FGSC |
| wild-type (WT) | ∆ku70, ∆pyrG::AfpyrG | This study |
| ∆rum1 | ∆ku70, ∆rum1::AfpyrG | This study |
| ∆rum1-C | ∆ku70, ∆rum1::AfpyrG, rum1::AfpyrG | This study |
Table 2.
Primers used for the strain construction in this study.
| Primer Name | Sequence (5′-3′) | Fragment |
|---|---|---|
| rum1-p1 | GGCACGAGCTATTAGTGATATTAGTCGAGTCCGA | 5′UTR of rum1 |
| rum1-p2 | CAAGTGAGCCGACCGATTGAGGGAAGTAGT | |
| rum1-p3 | TCCCTATCAACAAATTGGCGCTTCATGGGTTC | 3′UTR of rum1 |
| rum1-p4 | TGGATTCCTTCGGGGGCTAGTTTGCATC | |
| rum1-p5 | ACTACTTCCCTCAATCGGTCGGCTCACTT | A. fumigatus pyrG |
| GGCCTCAAACAATGCTCTTCACCC | ||
| rum1-p6 | GAACCCATGAAGCGCCAATTTGTTGATA | |
| GGGAGTCTGAGAGGAGGCACTGATGC | ||
| rum1-p7 | GACCTGTGAAGATGCTTGGTAGAGCTATTTCAG | Nesting primers |
| rum1-p8 | TATCTCATTGGACTGGACCCTGAGCGGGA | |
| rum1-p9 | CAACTCGACTGGCGGACAGCCT | A fragment from rum1 |
| rum1-p10 | TCATTTGCCGGAGAATATGTTCCAGTCCTTC | |
| P801 | CAGGAGTTCTCGGGTTGTCG | A. fumigatus pyrG |
| P1020 | CAGAGTATGCGGCAAGTCA | |
| rum-p3 + pyrg-F | CTTCATCGCGAGATAACACCCCCGATGG | 5′UTR of Δrum1-C |
| rum-p3 + pyrg-R | GGGTGAAGAGCATTGTTTGAGGCCCCATG | |
| pyrg-F | GCCTCAAACAATGCTCTTCACCC | A. fumigatus pyrG |
| pyrg-R | GTCTGAGAGGAGGCACTGATGC | |
| rum-p4 + pyrg-F | GCATCAGTGCCTCCTCTCAGACAGATTCTT | 3′UTR of Δrum1-C |
| GCCTTGCGCATTCATGACAAC |
Table 3.
Primers for q-PCR analysis in the study.
| Gene | Forward Sequences (5′-3′) | Reverse Sequences (3′-5′) |
|---|---|---|
| rum1 | CTTGATGCATCTCTCTTT | CTTCCAGAGCCTCATTA |
| AGCTCTCCACGGTTC | GCATGTGTGTTCTCC | |
| brlA | GCCTCCAGCGTCAACCTTC | TCTCTTCAAATGCTCTTGCCTC |
| abaA | TCTTCGGTTGATGGATGATTTC | CCGTTGGGAGGCTGGGT |
| nsdC | GCCAGACTTGCCAATCAC | CATCCACCTTGCCCTTTA |
| nsdD | GGACTTGCGGGTCGTGCTA | AGAACGCTGGGTCTGGTGC |
| sclR | CAATGAGCCTATGGGAGTGG | ATCTTCGCCCGAGTGGTT |
| aflR | AAAGCACCCTGTCTTCCCTAAC | GAAGAGGTGGGTCAGTGTTTGTAG |
| aflS | CGAGTCGCTCAGGCGCTCAA | GCTCAGACTGACCGCCGCTC |
| aflC | GTGGTGGTTGCCAATGCG | CTGAAACAGTAGGACGGGAGC |
| aflO | GATTGGGATGTGGTCATGCGATT | GCCTGGGTCCGAAGAATGC |
| actin | ACGGTGTCGTCACAAACTGG | CGGTTGGACTTAGGGTTGATAG |
5.2. Phylogenetic Analysis
Rum1 orthologs from A. flavus, A. niger, A. nidulans, A. oryzae, A. fumigatus, A. bombycis, A. nomius, P. digitatum, S. pombe, M. musculus, H. sapiens, and U. maydis were obtained from the National Center for Biotechnology Information (NCBI, https://www.ncbi.nlm.nih.gov/). The phylogenetic tree was constructed for the above 12 Rum1 orthologs by MEGA 5.0 with an algorithm of 1000 times Neighboring comparison. In the study, SMART (http://smart.embl-heidelberg.de/smart/set_mode.cgi?NORMAL=1) was used to identify the domains in Rum1 and the framework of domains was mapped with software IBS 1.0 (Lab of Cell Dynamics, and Lab of Nanobiology, University of Science & Technology of China, Hefei, Anhui, China) [42].
5.3. Mutant Strains Construction
The ∆rum1 mutants were prepared by a homologous recombination approach [39]. Three fragments (1200 bp 5′-UTR of rum1, 1411 bp 3′-UTR and 1890 bp pyrG) were amplified and overlapped by Overlap PCR and the primers used in the Overlap PCR were shown in Table 2. Afterward, the transformation of protoplasts (CA14 Δku70 ΔpyrG) was carried out, which was mediated by PEG (polyethylene glycol) [12]. The pyrG prototroph strain (∆rum1, Table 1) was further tested with the Southern blot method and PCR analysis (related primers shown in Table 2).
The complementation strains for the ∆rum1 mutants were constructed following the method provided by Yang [43]. First, the rum1 fragment amplified from A. flavus with rum1-p7/rum1-p8 primers were transformed into the protoplasts of ∆rum1 with 2 mg/mL 5-FOA (5-fluoroorotic acid) to replace pyrG [44]. PCR analysis was performed to confirm that the pyrg gene was removed from the first step of complemented strains (Figure S4). Lastly, the Pyrg gene was inserted behind the rum1 to produce the pyrG prototroph complementation strains (∆rum1-C) by using a homologous recombination. The complementation strain was tested by using PCR analysis. Both of the verified ∆rum1 mutant and ∆rum1-C strains were further confirmed by a q-PCR assay with an actin gene as the inner reference. The primers used in the analysis are listed in Table 3.
5.4. Real-Time Quantitative Reverse Transcription PCR
The analysis was implemented according to the protocol used by Nie [45]. RNA was extracted from 0.1 g of a paper towel dried mycelium using an RNA isolation kit (Promega, Madison, WI, USA). Then, the synthesis of first-strand cDNA from 3 μg RNA was performed using the Revert Aid First-strand cDNA Synthesis kit (TransGen Biotech, Beijing, China). The SYBR Green Supermix (Takara, Waltham, MA, USA) was used for the qRT-PCR reaction with the PikoReal 96 Real-time PCR system. All primers used in the assay were listed in Table 3. All experiments were repeated three times.
5.5. Morphological Analysis
The analysis was carried out according to the method used by Lan [46]. The WT, ∆rum1, and ∆rum1-C strains were incubated onto PDA medium in the dark at 37 °C and the diameter of each colony was measured after 5 days. Conidia were homogenized in 5 mL water solution (0.05% Tween-20) and the conidia number was counted haemocytometrically from 1 mL conidia mixture. For sclerotia production analysis, sclerotia inducing WKM medium was prepared and all cultures were grown at 37 °C for a week in the dark. Lastly, 75% ethanol was used to spray the surface of each plate to wash conidia away. Then sclerotia were collected and the number of sclerotia was counted with the Asana microscope. Above experiments were completed with three repetitions.
5.6. Aflatoxin Analysis
The WT, ∆rum1, and ∆rum1-C strains were incubated into 15 mL PDB and YES media at 29 °C in the dark for 6 days, respectively. Then AFB1 was extracted from the culture, which was previously described by Yang [43]. AFB1 production was analyzed with thin layer chromatography (TLC) (5 μL for each sample was used for the TLC analysis) and examined under a UV light. The pictures of the TLC plates were inverted by the PS software to get clearer data. The experiments were repeated three times.
5.7. Stress Assays
The WT, the ∆rum1, and the ∆rum1-C strains were inoculated onto PDA agar with hyperosmotic stress mediators (1 M NaCl, 1 M KCl, or 1.2 M D-Sorbitol), oxidative stress agent (5–10 mM H2O2), DNA damaging agent (0.02% MMS (Methyl methanesulfonate)), and cell wall stress agents (200 μg/mL CFW (Calcofluor white) or 300 μg/mL CR (Congo red)) at 37 °C in the dark for 3 days, respectively. To analyze the role of Rum1 in stress response of A. flavus, the relative inhibition rates were calculated, according to the formula listed in the brackets (diameter of colony without inhibitor—diameter of colony with inhibitor/diameter of colony without inhibitor). The experiments were performed in three repetitions.
5.8. Crop Kernels Colonization Assays
To explore the role of Rum1 in crop colonization, the assay was carried out following the methods used by Lan with minor modification [46]. Kernels were sterilized with 0.05% sodium hypochlorite and inoculated with conidia at 29 °C for 5 days. Afterward, the colonized kernels were collected in 50 mL Falcon tubes, mixed with 10 mL sterile 0.05% Tween 80, and followed by 2 min in a vortex state to release the spores. Additionally, 1 mL aliquot of spore suspension was diluted and counted haemocytometrically. A total of 15 mL of chloroform was added to each Falcon tube for AFB1 extraction and these tubes were placed on the 29 °C shaker and shaken at 180 r/min for 30 min. The lower chloroform layer (10 mL) was collected and dried by airing. After re-dissolving in 1 mL chloroform, 5 µL of each sample was allotted for the TLC assay. The experiments were repeated three times.
5.9. The Analysis on the Activity of Amylase
The activity of amylase was analyzed according to the protocol used by Li [47]. The WT, the ∆rum1, and the ∆rum1-C strains were incubated on the amylase screening medium (10 g/L peptone, 10 g/L yeast extract, 1% soluble starch, 15 g/L Agar) in the dark at 29 °C for 3 days. Iodine solution was added evenly in the plate and the diameter of the degradation zone was measured. The relative degradation rate equals the diameter of degradation zone/colony diameter.
5.10. Statistical Analysis
In this study, all data were presented with the means ± SD (standard deviation). The significant differences (statistical significances) among groups were calculated with ANOVA and LSD (least significant difference) tests. The analysis of statistical and significance was implemented with the software GraphPad Prism5 (La Jolla, CA, USA) and the difference is regarded to be statistically significant when p < 0.05.
Acknowledgments
We especially thank Perng Kuang Chang (Southern Regional Research Center, United States Department of Agriculture, New Orleans, LA, USA), Yang Liu (Institute of Food Science and Technology CAAS), and Kong Qing for their kindness in providing the strains. We also thank Kunlong Yang, Guangshan Yao, Opemipo E. Fasoyin, and Lianghuan Wu (Master degree candidate of School of Life Sciences, Fujian Agriculture and Forestry University, Fuzhou, China) for their kind help in related experiments, and English editing.
Supplementary Materials
The following are available online at http://www.mdpi.com/2072-6651/10/7/301/s1, Figure S1: Classes of transcriptional factors in A. flavus [15], Figure S2: Predicted RE of Rum1 in transcriptional factor genes of A. flavus, Figure S3: Growth of WT, the ∆rum1 strains, and the ∆rum1-C strains under multiple stresses, Figure S4. Strategy and confirmation of the complemented strain.
Author Contributions
Z.Z. and S.W. designed the experiments. Z.Z. and Y.H. wrote the manuscript. Y.H. and G.Y. performed all the experiments. D.Z., Y.L. (Yaju Liu), Y.L. (Yu Li), G.L. and Z.G. performed several experiments and completed the data analysis. All authors read and approved the final manuscript.
Funding
The research was supported by the National Natural Science Foundation of China (31772105), the Fund of Outstanding Youth of Fujian Province (2018J07002), the Nature Science Foundation of Fujian Province (2017J01426), and the fund of Cultivation of Outstanding Youth Science and Technology Talents in Fujian Agriculture and Forestry University (xjq201410).
Conflicts of Interest
No conflicts of interest are reported by the authors. The authors listed in the manuscript alone are responsible for the related work and writing of this paper.
Key Contribution
The PHD transcription factor Rum1 is identified in Aspergillus flavus and found to be indispensable for sclerotia formation and aflatoxin biosynthesis.
References
- 1.Hedayati M.T., Pasqualotto A.C., Warn P.A., Bowyer P., Denning D.W. Aspergillus flavus: Human pathogen, allergen and mycotoxin producer. Microbiology. 2007;153:1677–1692. doi: 10.1099/mic.0.2007/007641-0. [DOI] [PubMed] [Google Scholar]
- 2.Xing F., Ding N., Liu X., Selvaraj J.N., Wang L., Zhou L., Zhao Y., Wang Y., Liu Y. Variation in fungal microbiome (mycobiome) and aflatoxins during simulated storage of in-shell peanuts and peanut kernels. Sci. Rep. 2016;6:25930. doi: 10.1038/srep25930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohd Redzwan S., Rosita J., Mohd Sokhini A.M., Nurul Aqilah A.R., Wang J.S., Kang M.S., Zuraini A. Detection of serum AFB1-lysine adduct in Malaysia and its association with liver and kidney functions. Int. J. Hyg. Environ. Health. 2014;217:443–451. doi: 10.1016/j.ijheh.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Yu J. Current Understanding on Aflatoxin Biosynthesis and Future Perspective in Reducing Aflatoxin Contamination. Toxins. 2012;4:1024–1057. doi: 10.3390/toxins4111024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Misihairabgwi J.M., Ezekiel C.N., Sulyok M., Shephard G.S., Krska R. Mycotoxin contamination of foods in Southern Africa: A 10-year review (2007–2016) Crit. Rev. Food Sci. Nutr. 2017;11:1–16. doi: 10.1080/10408398.2017.1357003. [DOI] [PubMed] [Google Scholar]
- 6.Amaike S., Keller N.P. Aspergillus flavus. Ann. Rev. Phytopathol. 2011;49:107–133. doi: 10.1146/annurev-phyto-072910-095221. [DOI] [PubMed] [Google Scholar]
- 7.Cary J.W., Ehrlich K.C. Aflatoxigenicity in Aspergillus: Molecular genetics, phylogenetic relationships and evolutionary implications. Mycopathologia. 2006;162:167–177. doi: 10.1007/s11046-006-0051-8. [DOI] [PubMed] [Google Scholar]
- 8.Kale S.P., Milde L., Trapp M.K., Frisvad J.C., Keller N.P., Bok J.W. Requirement of LaeA for secondary metabolism and sclerotial production in Aspergillus flavus. Fungal Genet. Biol. 2008;45:1422–1429. doi: 10.1016/j.fgb.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehrlich K.C., Mack B.M., Wei Q., Li P., Roze L.V., Dazzo F., Cary J.W., Bhatnagar D., Linz J.E. Association with AflR in endosomes reveals new functions for AflJ in aflatoxin biosynthesis. Toxins. 2012;4:1582–1600. doi: 10.3390/toxins4121582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masanga J.O., Matheka J.M., Omer R.A., Ommeh S.C., Monda E.O., Alakonya A.E. Downregulation of transcription factor aflR in Aspergillus flavus confers reduction to aflatoxin accumulation in transgenic maize with alteration of host plant architecture. Plant Cell Rep. 2015;34:1379–1387. doi: 10.1007/s00299-015-1794-9. [DOI] [PubMed] [Google Scholar]
- 11.Bayram O., Krappmann S., Ni M., Bok J.W., Helmstaedt K., Valerius O., Braus-Stromeyer S., Kwon N.J., Keller N.P., Yu J.H., et al. VelB/VeA/LaeA complex coordinates light signal with fungal development and secondary metabolism. Science. 2008;320:1504–1506. doi: 10.1126/science.1155888. [DOI] [PubMed] [Google Scholar]
- 12.Zhuang Z., Lohmar J.M., Satterlee T., Cary J.W., Calvo A.M. The Master Transcription Factor mtfA Governs Aflatoxin Production, Morphological Development and Pathogenicity in the Fungus Aspergillus flavus. Toxins. 2016;8:29. doi: 10.3390/toxins8010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fountain J.C., Bajaj P., Pandey M., Nayak S.N., Yang L., Kumar V., Jayale A.S., Chitikineni A., Zhuang W., Scully B.T., et al. Oxidative stress and carbon metabolism influence Aspergillus flavus transcriptome composition and secondary metabolite production. Sci. Rep. 2016;6:38747. doi: 10.1038/srep38747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang B., Han X., Bai Y., Lin Z., Qiu M., Nie X., Wang S., Zhang F., Zhuang Z., Yuan J., et al. Effects of nitrogen metabolism on growth and aflatoxin biosynthesis in Aspergillus flavus. J. Hazard Mater. 2017;324:691–700. doi: 10.1016/j.jhazmat.2016.11.043. [DOI] [PubMed] [Google Scholar]
- 15.Ehrlich K.C., Chang P.K., Yu J., Cary J.W., Bhatnagar D. Aflatoxins—Biochemistry and Molecular Biology. In Tech; New Orleans, LA, USA: 2011. Control of Aflatoxin Biosynthesis in Aspergilli. [Google Scholar]
- 16.Schindler U., Beckmann H., Cashmore A.R. HAT3.1, a novel Arabidopsis homeodomain protein containing a conserved cysteine-rich region. Plant J. 1993;4:137–150. doi: 10.1046/j.1365-313X.1993.04010137.x. [DOI] [PubMed] [Google Scholar]
- 17.Aasland R., Gibson T.J., Stewart A.F. The PHD finger: Implications for chromatin-mediated transcriptional regulation. Trends Biochem. Sci. 1995;20:56–59. doi: 10.1016/S0968-0004(00)88957-4. [DOI] [PubMed] [Google Scholar]
- 18.Wang Q., Liu J., Wang Y., Zhao Y., Jiang H., Cheng B. Systematic Analysis of the Maize PHD-Finger Gene Family Reveals a Subfamily Involved in Abiotic Stress Response. Int. J. Mol. Sci. 2015;16:23517–23544. doi: 10.3390/ijms161023517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reddy T.V., Kaur J., Agashe B., Sundaresan V., Siddiqi I. The duet gene is necessary for chromosome organization and progression during male meiosis in arabidopsis and encodes a PHD finger protein. Development. 2003;130:5975–5987. doi: 10.1242/dev.00827. [DOI] [PubMed] [Google Scholar]
- 20.Liu X., Gao Q., Li P., Zhao Q., Zhang J., Li J., Koseki H., Wong J. UHRF1 targets DNMT1 for DNA methylation through cooperative binding of hemi-methylated DNA and methylated H3K9. Nat. Commun. 2013;4:1563. doi: 10.1038/ncomms2562. [DOI] [PubMed] [Google Scholar]
- 21.Saleh A., Alvarez-Venegas R., Yilmaz M., Le O., Hou G., Sadder M., Al-Abdallat A., Xia Y., Lu G., Ladunga I. The highly similar arabidopsis homologs of trithorax ATX1 and ATX2 encode proteins with divergent biochemical functions. Plant Cell. 2008;20:568–579. doi: 10.1105/tpc.107.056614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merkel D.J., Wells S.B., Hilburn B.C., Elazzouzi F., Pérez-Alvarado G.C., Lee B.M. The C-terminal region of cytoplasmic polyadenylation element binding protein is a ZZ domain with potential for protein–protein interactions. J. Mol. Biol. 2013;425:2015–2026. doi: 10.1016/j.jmb.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Quadbeck-Seeger C., Wanner G., Huber S., Kahmann R., Kamper J. A protein with similarity to the human retinoblastoma binding protein 2 acts specifically as a repressor for genes regulated by the b mating type locus in Ustilago maydis. Mol. Microbiol. 2000;38:154–166. doi: 10.1046/j.1365-2958.2000.02128.x. [DOI] [PubMed] [Google Scholar]
- 24.Hanson S.J., Stelzer C.P., Welch D.B., Logsdon J.M., Jr. Comparative transcriptome analysis of obligately asexual and cyclically sexual rotifers reveals genes with putative functions in sexual reproduction, dormancy, and asexual egg production. BMC Genom. 2013;14:412. doi: 10.1186/1471-2164-14-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yabe K., Nakajima H. Enzyme reactions and genes in aflatoxin biosynthesis. Appl. Microbiol. Biotechnol. 2004;64:745–755. doi: 10.1007/s00253-004-1566-x. [DOI] [PubMed] [Google Scholar]
- 26.Park S.Y., Park J.W., Chun Y.S. Jumonji histone demethylases as emerging therapeutic targets. Pharmacol. Res. 2016;105:146–151. doi: 10.1016/j.phrs.2016.01.026. [DOI] [PubMed] [Google Scholar]
- 27.Shmakova A., Batie M., Druker J., Rocha S. Chromatin and oxygen sensing in the context of JmjC histone demethylases. Biochem. J. 2014;462:385–395. doi: 10.1042/BJ20140754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slama P. Two-domain analysis of JmjN-JmjC and PHD-JmjC lysine demethylases: Detecting an inter-domain evolutionary stress. Proteins. 2018;86:3–12. doi: 10.1002/prot.25394. [DOI] [PubMed] [Google Scholar]
- 29.Quan Z., Oliver S.G., Zhang N. JmjN interacts with JmjC to ensure selective proteolysis of Gis1 by the proteasome. Microbiology. 2011;157:2694–2701. doi: 10.1099/mic.0.048199-0. [DOI] [PubMed] [Google Scholar]
- 30.Hansen F.T., Madsen C.K., Nordland A.M., Grasser M., Merkle T., Grasser K.D. A novel family of plant DNA-binding proteins containing both HMG-box and AT-rich interaction domains. Biochemistry. 2008;47:13207–13214. doi: 10.1021/bi801772k. [DOI] [PubMed] [Google Scholar]
- 31.Wilsker D., Patsialou A., Dallas P.B., Moran E. ARID proteins: A diverse family of DNA binding proteins implicated in the control of cell growth, differentiation, and development. Cell Growth Differ. 2002;13:95–106. [PubMed] [Google Scholar]
- 32.Wang X., Nagl N.G., Wilsker D., Van Scoy M., Pacchione S., Yaciuk P., Dallas P.B., Moran E. Two related ARID family proteins are alternative subunits of human SWI/SNF complexes. Biochem. J. 2004;383:319–325. doi: 10.1042/BJ20040524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanchez R., Zhou M.M. The PHD finger: A versatile epigenome reader. Trends Biochem. Sci. 2011;36:364–372. doi: 10.1016/j.tibs.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park H.S., Yu J.H. Genetic control of asexual sporulation in filamentous fungi. Curr. Opin. Microbiol. 2012;15:669–677. doi: 10.1016/j.mib.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 35.Yamada O., Lee B.R., Gomi K., Iimura Y. Cloning and functional analysis of the Aspergillus oryzae conidiation regulator gene brlA by its disruption and misscheduled expression. J. Biosci. Bioeng. 1999;87:424–429. doi: 10.1016/S1389-1723(99)80089-9. [DOI] [PubMed] [Google Scholar]
- 36.Adams T.H., Boylan M.T., Timberlake W.E. BrlA is necessary and sufficient to direct conidiophore development in Aspergillus nidulans. Cell. 1988;54:353–362. doi: 10.1016/0092-8674(88)90198-5. [DOI] [PubMed] [Google Scholar]
- 37.Han S., Adams T.H. Complex control of the developmental regulatory locus brlA in Aspergillus nidulans. Mol. Genet. Genom. 2001;266:260–270. doi: 10.1007/s004380100552. [DOI] [PubMed] [Google Scholar]
- 38.Jin F.J., Takahashi T., Matsushima K., Hara S., Shinohara Y., Maruyama J., Kitamoto K., Koyama Y. SclR, a basic helix-loop-helix transcription factor, regulates hyphal morphology and promotes sclerotial formation in Aspergillus oryzae. Eukaryot. Cell. 2011;10:945–955. doi: 10.1128/EC.00013-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee M.K., Kwon N.J., Choi J.M., Lee I.S., Jung S., Yu J.H. NsdD is a key repressor of asexual development in Aspergillus nidulans. Genetics. 2014;197:159–173. doi: 10.1534/genetics.114.161430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cary J.W., Han Z., Yin Y., Lohmar J.M., Shantappa S., Harris-Coward P.Y., Mack B., Ehrlich K.C., Wei Q., Arroyo-Manzanares N., et al. Transcriptome Analysis of Aspergillus flavus Reveals veA-Dependent Regulation of Secondary Metabolite Gene Clusters, Including the Novel Aflavarin Cluster. Eukaryot. Cell. 2015;14:983–997. doi: 10.1128/EC.00092-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu J.H., Butchko R.A., Fernandes M., Keller N.P., Leonard T.J., Adams T.H. Conservation of structure and function of the aflatoxin regulatory gene aflR from Aspergillus nidulans and A. flavus. Curr. Genet. 1996;29:549–555. doi: 10.1007/BF02426959. [DOI] [PubMed] [Google Scholar]
- 42.Yang K., Liang L., Ran F., Liu Y., Li Z., Lan H., Gao P., Zhuang Z., Zhang F., Nie X., et al. The DmtA methyltransferase contributes to Aspergillus flavus conidiation, sclerotial production, aflatoxin biosynthesis and virulence. Sci. Rep. 2016;6:23259. doi: 10.1038/srep23259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang K., Liu Y., Liang L., Li Z., Qin Q., Nie X., Wang S. The high-affinity phosphodiesterase PdeH regulates development and aflatoxin biosynthesis in Aspergillus flavus. Fungal Genet. Biol. 2017;101:7–19. doi: 10.1016/j.fgb.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 44.Chang P.K., Scharfenstein L.L., Wei Q., Bhatnagar D. Development and refinement of a high-efficiency gene-targeting system for Aspergillus flavus. J. Microbiol. Methods. 2010;81:240–246. doi: 10.1016/j.mimet.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 45.Nie X., Yu S., Qiu M., Wang X., Wang Y., Bai Y., Zhang F., Wang S. Aspergillus flavus SUMO Contributes to Fungal Virulence and Toxin Attributes. J. Agric. Food Chem. 2016;64:6772–6782. doi: 10.1021/acs.jafc.6b02199. [DOI] [PubMed] [Google Scholar]
- 46.Lan H., Sun R., Fan K., Yang K., Zhang F., Nie X.Y., Wang X., Zhuang Z., Wang S. The Aspergillus flavus Histone Acetyltransferase AflGcnE Regulates Morphogenesis, Aflatoxin Biosynthesis, and Pathogenicity. Front. Microbiol. 2016;7:1324. doi: 10.3389/fmicb.2016.01324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Y., He Y., Li X., Fasoyin O.E., Hu Y., Liu Y., Yuan J., Zhuang Z., Wang S. Histone Methyltransferase aflrmtA gene is involved in the morphogenesis, mycotoxin biosynthesis, and pathogenicity of Aspergillus flavus. Toxicon. 2017;127:112–121. doi: 10.1016/j.toxicon.2017.01.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.