Abstract
Sequence databases and transcriptome-wide mapping have revealed different reversible and dynamic chemical modifications of the nitrogen bases of RNA molecules. Modifications occur in coding RNAs and noncoding-RNAs post-transcriptionally and they can influence the RNA structure, metabolism, and function. The result is the expansion of the variety of the transcriptome. In fact, depending on the type of modification, RNA molecules enter into a specific program exerting the role of the player or/and the target in biological and pathological processes. Many research groups are exploring the role of RNA modifications (alias epitranscriptome) in cell proliferation, survival, and in more specialized activities. More recently, the role of RNA modifications has been also explored in stem cell biology. Our understanding in this context is still in its infancy. Available evidence addresses the role of RNA modifications in self-renewal, commitment, and differentiation processes of stem cells. In this review, we will focus on five epitranscriptomic marks: N6-methyladenosine, N1-methyladenosine, 5-methylcytosine, Pseudouridine (Ψ) and Adenosine-to-Inosine editing. We will provide insights into the function and the distribution of these chemical modifications in coding RNAs and noncoding-RNAs. Mainly, we will emphasize the role of epitranscriptomic mechanisms in the biology of naïve, primed, embryonic, adult, and cancer stem cells.
Keywords: N6-methyladenosine, N1-methyladenosine, 5-methylcytosine, writers, readers, erasers proteins, epigenetics, mitochondrial transfer RNA, mitochondrial ribosomal RNA, stem cells self-renewal and differentiation, cancer stem cells, naïve and primed stem cells, bioinformatics predictive tools
1. Epigenetics and Epitranscriptomics
Current knowledge highlights epigenetics as the extra level of the genetic code [1,2,3,4,5]. Epigenetic mechanisms orchestrate molecular events that govern changes in gene expression without changes in the DNA sequence. Epigenetic mechanisms include remodeling of chromatin conformation, methylation and demethylation of DNA, post-translational modification of histones, and the activity of many regulatory proteins [1,2,3,4,5]. The overall events set a gene expression signature that is maintained stable through many cell divisions and is reprogrammed under the effects of specific signals, such as development [6,7,8,9,10,11] and tissue specification [7,8,9,12,13]. Thenceforth, alterations of one of above epigenetic mechanisms are often associated with several diseases, such as cancer and the immunodeficiency–centromeric instability–facial anomalies syndrome (ICF) [14,15].
Recent new knowledge of RNA allows associating the functions of the different RNA classes with the post-transcriptional chemical modifications in the RNAs structure [16,17,18,19] and with the networks of competition between RNA molecules [19,20]. Overall, the interplays are called epitranscriptomics and, definitely, RNA molecules are one of the epigenetic players [16,17,18].
Epitranscriptomic modifications target all types of RNAs and they enclose chemical changes in more than 140 nucleotides [21,22,23,24]. Studies are ongoing to map RNA modifications, to elucidate the biological functions of such modifications and to identify the molecules involved in each modification [1,16,17,25,26,27,28,29,30,31,32].
In this review, we describe the most frequents five RNA modifications, namely N6-methyladenosine, N1-methyladenosine, 5-methylcytosine, Pseudouridine (Ψ) and Adenosine-to-Inosine (A-to-I) editing, together with selected enzymes. We highlight the relevance of bioinformatics tools in the identification of the above-mentioned modifications. Finally, we give insight into the molecular effects of such RNA modifications in stem cell biology.
2. RNA Modifications
2.1. State of the Art
Biotechnological progress has boosted the understanding of the intricate scenario of modifications in RNA molecules [33,34,35]. Next-generation sequencing and mass spectrometry technologies have revealed widespread messenger RNA (mRNA) modifications together with their effects on mammalian transcriptome [33,34,35]. Many proteins introducing, deleting, or recognizing specific modifications have also been identified and, according to epigenetic language, they have been called as writers, erasers, and readers, respectively [34,35].
The first RNA modification has been identified almost 60 years ago in yeasts [36]. After that, more than 100 modifications in ribonucleotides have been described [36]. These included either common chemical modifications in mRNAs and in noncoding-RNAs or distinct modifications in each type of RNA [36]. The bioinformatics approach is helpful to provide comprehensive databases (e.g., MODOMICS, [36]) with information about the chemical modifications in RNAs and with the biological effects of such modifications.
In this chapter, are reported the latest findings describing the chemical modifications influencing the structure and the function of the different RNA classes: coding RNAs and noncoding-RNAs. Modifications are designed based on the type of chemical changes: N6-methyladenosine (m6A) [37,38,39,40]; Pseudouridine (Ψ), 5-methylcytosine (m5C) [41]; 5-hydroxymethylcytidine (hm5C) [42,43,44], N1-methyladenosine (m1A) [45,46], and A-to-I editing [47,48,49]. The mechanism of each modification is described in the mRNA section, as this is the first in this review.
2.2. Coding RNA Modifications: The Messenger RNA
2.2.1. Canonical Modifications in mRNA
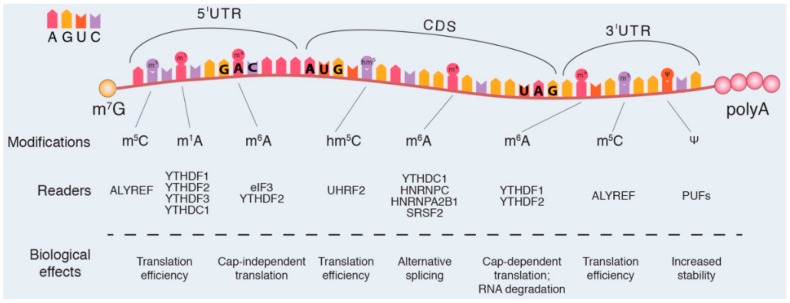
The early canonical modifications in mRNA described in eukaryotes and in some viruses refer to specific changes at the 5′-end and at the 3′-end of this molecule [50,51,52] (Figure 1).
Figure 1.
The figure illustrates the possible chemical modifications at the different messenger RNA (mRNA) nitrogen bases. The preferential location of each mark within the mRNA sequence and the reader proteins are also shown (see the text for details). N6-methyladenosine: m6A; Pseudouridine: Ψ; 5-methylcytosine: m5C; 5-hydroxymethylcytidine: hm5C; N1-methyladenosine: m1A.
The first modification, called as cap-0, comprises the addition of an N7-methylguanosine (m7G) via a 5′–5′ triphosphate bond to the 5′-terminal nucleotide of the pre-mRNA [53,54]. Alternatively, in small nuclear RNAs, the guanosine is modified by the addition of 2 methyl groups at the N2 position. The three-methylguanosine cap allows the interaction with U1, U2, U4, and U5 ribonucleoproteins (snRNPs) and it is required for pre-mRNA splicing and pre-ribosomal RNA (rRNA) processing if the modification interacts with U3 and U8 snRNPs [55].
Further modifications at 5′-end mRNA (called as cap-1 and cap-2) consist of a ribose 2′-O-methylation on the first and second transcribed nucleoside [56]. Cap-1 and Cap-2 are critical for RNA processing, translation, and stability [50,51].
The second main mRNA modification arises at the 3′-end and it is called as poly(A)-tail. It comprises at least 150–200 adenylate residues and a conserved AAUAAA sequence bordered by less conserved sequences (e.g., downstream elements composed of U-rich sequences). The poly(A) tail guarantees mRNA stability through the export out of the nucleus and during the translation process [51,52,57].
In higher eukaryotes, a third well-documented modification occurs. It relates to the intron excision from pre-mRNAs through the splicing mechanism achieved by the splicing machinery comprising of U1, U2, U4, U5, and U6 snRNPs, in addition to other non-snRNP proteins [52,58,59]. Thanks to the splicing process, pre-mRNA precursors enter into the post-transcriptional regulation of gene expression and give rise the landscape of proteome in the cells [60].
2.2.2. Non-Canonical mRNA Modifications
In addition to the above mentioned modifications, other nucleosides carrying chemical modifications at the nitrogen bases, have been discovered in mature mRNAs, in cytoplasmatic mRNA (Figure 1 and Figure 2) [33,47,50,61,62] and in mitochondrial mRNA (mt-mRNA) [63,64].
Figure 2.
The figure illustrates the chemical modifications at the Adenosine, Cytosine, and Uridine nitrogen bases. In green are the enzymes catalyzing the reaction of addition of a moiety group (writers). In red are the enzymes catalyzing the reaction of deletion of the modification (erasers). The modification sites are colored in blue. The abbreviations of the modified nucleosides are shown in parentheses.
The molecular characteristics of each modification, together with the writers, readers, and erasers, are detailed below.
N6-methyladenosine consists of the addition of a methyl group at the nitrogen-6 position of adenosine base in the sequence GAC [18] (Figure 1). The m6A modification in 5′-untranslated regions (5′-UTRs) was the first internal mRNA modification uncovered, several decades ago [18,33,43,44,45,65]. At the same time, the methyltransferases involved in the methylation process (writers) [66,67] and the proteins specifically recognizing the m6A decoy (readers) [18,68] were identified (Figure 1 and Figure 2).
METTL3 methyltransferase (alias MT-A70), was the first enzyme identified as part of a large multimeric protein complex involved in the mRNA methylation, in 1997 [66]. Now, it is known that this enzyme (belongs to the DNA m6A methyltransferases subfamily) contains the sequence D/N/S/H]PP[Y/F/W, highly conserved among yeasts, plants, Drosophila, and mammals [69]. Recent studies found the METTL14 as another component of the writer complex. METTL3 serves as a catalytic subunit while METTL14 functions as a stabilizer for the reaction [70]. However, both proteins expose a binding site for RNA in the consensus sequence RRACH (R is a purine, A corresponds to the m6A modification and H is a non-guanine base [69]).
The reader proteins specifically recognize the m6A decoy, due to the presence of YTH sequence, (the specific m6A -reader domain [18,68]) and control the mRNA homeostasis. For instance, the reader YTHDF2 regulates the mRNA stability, the YTHDC1 controls the splicing of mRNA, whereas the YTHDF1 and YTHDF3 supervise the translation process [71,72,73] (Figure 2).
The fat mass obesity-associated protein (FTO) [22,23] and AlkB Homolog 5 RNA Demethylase (ALKBH5) [74,75], two members of the Fe(II)-dependent and 2-oxoglutarate-dependent oxygenase superfamily, are involved in the removal of m6A modification in RNAs in vitro and in vivo [22,23] (Figure 2). FTO binds to the m6A moiety in mRNAs and converts m6A to N6-hydroxymethyladenosine (hm6A) and N6-formyladenosine (f6A) in a step-wise manner. Studies are in progress to uncover additional proteins that might be involved in promoting the FTO-mediated demethylation of m6A [22,23].
Noteworthy, m6A has also been observed in the 3′-UTR region of mRNA. This modification, together with the readers YTHDF1 and YTHDF2, controls the mRNA stability during the stem cell differentiation or the circadian clock control [18,76]. Additionally, YTHDF2 binds to m6A in the 5′-UTR of stress-responsive mRNAs, prevents the FTO-mediated demethylation through a direct bond via eIF3 complex (eukaryotic translation initiation factor 3) and permits a rapid cap-independent translation of these mRNAs [77,78,79,80].
m6A demethylation on NANOG mRNA was observed in breast cancer stem cells in response to hypoxia. In these cells, the hypoxia-inducible factor activates the demethylase ALKBH5 and thereby the removal of m6A [81]. Indeed, the m6A methylation of mRNA seems a mechanism that cells employ in response to various stresses, such as deprivation of nutrients, deficit of oxygen, exposure to extreme temperatures or toxins [80,82]. Thus, budding yeasts respond to nitrogen starvation entering meiosis by the m6A methylation of key transcripts [80].
N1-methyladenosine. The N1-methyladenosine modification, uncovered in all classes of RNAs several decades ago [83], has been revealed in mRNA signature in eukaryotic cells from yeast and mammals (Figure 1 and Figure 2). The m1A modification consists of the addition of a methyl group at the nitrogen-1 position of adenosine [83]. m1A is copious in GC-rich regions of the 5′-UTRs regions but it’s about ten times less abundant than m6A. The m1A modification is specifically recognized by YTHDF1, YTHDF2, YTHDF3, and YTHDC1 reader proteins [84]. The role of m1A modification is under elucidation, however, first evidence suggests that the addition of m1A to mRNA is critical for the initiation of translation process through the binding with unknown readers [45] (Figure 2).
5-Methylcytosine. Currently, the potential roles of m5C in mRNA remain elusive. Methylation at position 5′ of cytosine increases the hydrophobicity of mRNA and probably increases base stacking but has a minimal effect on base pairing [26] (Figure 1 and Figure 2). The m5C modification is specifically recognized by the reader enzyme ALYREF [85]. m5C sites seem to be widespread randomly in coding regions, although an enrichment has been also observed in the 5′-UTR and the 3′-UTR regions (Figure 2). For instance, the methylation of p16 mRNA by NSUN2 (an RNA m5C methyltransferase) increases the stability of the p16 transcript and prevents the degradation [42,86].
5-Hydroxymethylcytosine modification is highest in exon sequences and increases the efficiency of translation (Figure 1 and Figure 2). The exact mechanism is still unknown but it is likely that the m5C might be subjected to oxidative processing forming hm5C [77,87]. In Drosophila melanogaster, levels of hm5C are highest in the brain, suggesting a potential role in brain development [77,87].
Pseudouridine modification in the mRNA is a recent finding, contrasting with evidence showing that pseudouridylation is the most abundant modification in the noncoding-RNAs [88,89]. The Ψ modification consists of the isomerization of uridine in which the uracil is attached via a carbon–carbon bond instead of a nitrogen–carbon glycosidic bond [88,89]. Ψ reader proteins have not yet been identified, although the PUFs family (Pumilio family proteins) seems a good candidate [90]. The Ψ in mRNA 3′-UTRs has been shown to increase the stability of modified transcripts during heat shock processes [88,89] (Figure 1 and Figure 2). It was also suggested that mRNA pseudouridylation could alter the efficiency of the translation initiation and it could influence ribosome pausing and RNA localization [88,89].
Adenosine-to-inosine editing. Inosine modification, also referred to as Adenosine-to-Inosine (A-to-I) editing, consisting of different base-pairing properties, has been described in mRNA transcripts [77,91]. Inosine pairs were also observed in the combination Inosine-to-Cytosine (I-to-C), that is more stable [77,91]. This modification is critical for the editing of mRNA and therefore for the fate of mRNA [77,91,92].
2.2.3. Mitochondrial mRNA
Modifications in the mRNAs of mammalian mitochondrial have revealed recently by transcriptome-wide analyses [93]. It is suggested that mitochondrial mRNA (mt-mRNA) modifications are necessary for facilitating the mitochondrial gene expression machinery, due to the minimal set of transfer RNA (tRNAs) and to the non-conventional genetic code [63,64,93,94]. For instance, the methyltransferase TRMT10C adds m1A in a single site in the NADH-ubiquinone oxidoreductase chain 5 mt-mRNA, leading its post-translation modification [95]. In yeasts the presence of the m6A in key transcripts, by mitochondrial m6A methyltransferase IME4 activity, is necessary for meiosis and sporulation in yeasts [96]. The C-to-U editing in mt-mRNAs seems a characteristic of transcripts in the plant model moss Physcomitrella [97].
2.3. Noncoding-RNA Modifications
To offer a comprehensive landscape of the epitranscriptome, here, we summarized the post-translation modifications in noncoding-RNAs:tRNAs, rRNAs, and regulatory RNAs (such as long noncoding RNAs [lncRNAs], microRNA [miRNA], and circleRNA [circRNA]) [40,75,98,99]. The five main modifications, m6A, m1A, m5C, and Ψ and A-to-I editing, have been documented in noncoding-RNAs.
Apart from tRNA and rRNA, the knowledge on the modifications occurring in other types of noncoding-RNAs and their effects on cellular functions is still restricted to a few studies.
2.3.1. Transfer RNA
The presence of chemical modifications in numerous nucleotides in mature tRNA is largely studied. The role of modifications in the function of tRNA has been demonstrated (see Table 1 for more details and for review [100,101,102]). Different modifications have been identified in the tRNA anticodon loop (e.g., inosine, queuosine, 5-methylcytosine, 5-methoxycarbonylmethyl-2-thiouridine, threonyl-carbamoyl-adenosine and wybutosine), as required for the interaction of tRNA and mRNA within the ribosome [74,92,103,104,105,106,107,108,109]. Other modifications have been found associated with stability, the localization, ribosome binding, and translational dynamic processes [100,110]. For instance, A-to-I editing at wobble positions was found in at least eight human tRNA samples and it was correlated with the expansion of the base pairing capability from A34-U to I34-U/I34-C enhancing the codon–anticodon interactions in the ribosome [110]. The uracil methylation at position 5 (m5U) by the enzyme hTRMT9 has been also described in tRNA and it was associated with an increase in decoding activity [111,112]. More recently, the presence of the m1A modification at nucleotides 9, 14, 22, 57, and 58 was associated with increased tRNA stability and with the correct tRNA folding [113]. Other modifications in tRNA nucleotides (such as N1-methylguanosine, N6-threonylcarbamoyladenosine, N6-isopentenyladenosine) have been involved in preventing frameshifting or in helping the tRNA on the ribosome accommodation during the translational elongation process [100,114].
Table 1.
Summary of the main characteristics and the more abundant epitranscriptomic modifications in coding RNA (mRNA), and noncoding-RNAs (tRNA, rRNA, lncRNA, miRNA, circRNA).
| RNA Species | Post-Transcriptional Modifications | RNA Characteristics | Ref. |
|---|---|---|---|
| mRNA | Cap 5′-end Poly-A tail 3′-end Splicing N6-methyladenosine N1-methyladenosine 5-methylcytidine Pseudouridine Adenosine to Inosine editing |
Structure: The mature messenger RNA (mRNA) is a single linear polymer of ribonucleotides containing the 3′-UTR and 5′-UTR regions that border the coding sequence Function: mRNA carries the genetic information from the DNA to the ribosome where it is translated into a polymer of amino acids (protein) |
[19,20,21,24,25,27,28,33] |
| tRNA | Anticodon loop: inosine, queuosine, 5-methylcytosine, 5-methoxycarbonylmethyl-2-thiouridine, threonyl-carbamoyl-adenosine and wybutosine D-loop: dihydrouridine T-loop: pseudouridine |
Structure: The mature transfer RNA (tRNA) is a polymer of ribonucleotides with a characteristic three-dimensional structure. Here, the anticodon loop has the three pairing nucleotides with the codon in the mRNA; the 3′-end has the CCA sequence that allows the binding of the amino acid Function: The tRNA is the translator of the nucleotidic language to aminoacidic language |
[19,20,28,29,91,92,99,106] |
| rRNA | 2′-O-methylation Uridine-to-Pseudouridine isomerization Pseudouridine |
Structure: The mature ribosomal RNA (rRNA) is a polymer of ribonucleotides consisting of several hairpin clusters Function: rRNA together with riboproteins compose the ribosome. Here, rRNAs have a direct role in the formation of the peptic bond between two amino acids |
[19,20,28,127,128] |
| lncRNA | N6-methyladenosine N1-methyladenosine 5-methylcytidine Pseudouridine |
Structure: The mature long noncoding-RNA (lncRNA) is a polymer of ribonucleotides with secondary structure Function: lncRNA has a significant role in different biological functions such as chromatin modifications, post-transcriptional regulation |
[19,20,28,72,98,99,104] |
| miRNA | Pseudouridine 5-methylcytidine Adenosine to Inosine editing |
Structure: The microRNA (miRNA) is a small oligonucleotide containing about 22 nucleotides with a single hairpin structure Function: The miRNA silencing at the post-transcriptional level of regulation of gene expression |
[19,20,28,98,105,137] |
| circRNA | Pseudouridine N6-methyladenosine |
Structure: The circleRNA (circRNA) is a polymer of ribonucleotides with circular conformation, as the 3′-end and 5′-ends have been joined together Function: The circRNA function as a sponge of miRNA, therefore have a key role in the regulation of gene expression |
[19,20,28,98,138] |
The tRNA methyltransferase family is involved in these reactions. Thus, TrmL is the writer that catalyzes the transfer of a methyl group to the 2′-hydroxyl group of the pyrimidines at the wobble position 34 in two tRNA isoacceptors [115,116,117]. However, MS analysis has revealed that METTL2 is the enzyme catalyzing 3-methylcytosine (m3C) methylation in total tRNA and that METTL6 is the enzyme catalyzing m3C in seryl-tRNA [118].
Recent studies have suggested a link between defects in tRNA modifications and neurological disorders, such as Amyotrophic Lateral Sclerosis (ALS) [119]. It was shown that ELP3, a subunit of the elongator complex modifying wobble uridines in tRNA, attenuated the axonopathy of a mutant SOD1 of ALS zebrafish model [120].
Mitochondrial tRNA (mt-tRNA). The role of epitranscriptomic modifications in mt-tRNA have been extensively described [101,121,122]. For example, the eraser ALKBH1 catalyzes 5-hydroxymethyl-2′-O-methylcytidine and 5-formyl-2′-O-methylcytidine modifications at the first position of the anticodon of mitochondrial tRNAMet. These modifications are essential for the translation of the non-universal codon AUA in mammalian mitochondria [114]. Further, the modification at the wobble position of selected mt-tRNAs of the uridine base in taurinomethyluridine is necessary to avoid ribosome stalling at certain AAG (lysine) and UUG (leucine) codons [123]. The reduction of mt-tRNA m5C methylation and formylation impairs the mitochondrial translation and respiration processes [124].
2.3.2. Ribosomal RNA
rRNA modification has been revealed in several nucleotide residues [117,125,126]. During the biogenesis of ribosomes, a large fraction of rRNA nucleotides (approximately 2% on human ribosomes) is specifically modified, either by writer enzymes or by antisense small nucleolar RNAs [117,125,126]. The most abundant modification is represented by 2′-O-methylation (2′-O-Me) or Uridine-to-Pseudouridine isomerization. Methylations of mature rRNA may be achieved through the action of specific methyltransferases, which are responsible for the modification of one or sometimes two specific nucleotides generally during ribosome assembly [127]. Epitranscriptomic rRNA modifications play an important role in the rRNA catalytic activity, folding, and stability and it is likely to modulate the antibiotics action. Interestingly, the presence of unexpected rRNA modification sites and the absence of the predicted 2′-O-Me sites have revealed that rRNA modifications differ among human cell types, as well as between healthy and cancer cells, such as hepatocellular carcinoma and breast cancer [128]. An example of the isomerization of Uridine to Ψ is the modification described in 23S-rRNA, by the pseudouridine sintase RluE, and required for RNA–protein interaction and Ψ formation [129]. More recently, the m1A modification has been documented in yeast 25S-rRNA and mammalian 28S-rRNA, respectively [130]. In particular, the m1A in 28S-rRNA was associated with the control of the translation process during embryogenesis and erythropoiesis. It was shown that KO of the nucleomethylin caused the defective methylation of rRNA and the consequent failure of ribosome formation [131].
Mitochondrial rRNA (mt-rRNA). Epitranscriptomic modifications in mt-rRNA are documented in all type of rRNAs [132]. The 2′-O-methyl modification, at position U1369 in the human 16S-mt-rRNA and at position U279 in the yeast 21S-mt-rRNA, is necessary for the correct respiration machinery [126]. The pseudouridylation of a single residue in the 16S-mt-rRNA is required for the oxidative phosphorylation complex assembly as demonstrated by the depletion of the Pseudouridine synthases, TRUB2, RPUSD3, and RPUSD4 through the CRISPR/Cas9 system [133]. The m5C modification at position 911 of cytosine in 12S rRNA by the methyltransferase NSUN4 activity, is critical to control the final step in ribosome biogenesis and to guarantee that only the mature major and minor ribosomal subunits are assembled [134].
2.3.3. Long Noncoding-RNA
m6A, m5C, A-to-I editing, and Ψ modifications have been documented in mature lncRNAs (Table 1) [104,135]. For instance, a segment with 78 m6A residues was shown critical for regulating the lncRNA X-inactive specific transcript (XIST) activity in silencing genes in the X chromosome [93]. The m6A formation in XIST is mediated by the RNA-binding motif protein 15 (RBM15), which binds the m6A methylation complex recruiting it to specific sites in the lncRNA [93]. m6A modification is also implicated in the destabilization of the lncRNA hairpin stem, allowing the access of heterogeneous nuclear ribonucleoproteins C1/C2 (HNRNPC) to a U-rich tract situated on the hairpin arm opposite of the m6A in Metastasis-Associated Lung Adenocarcinoma Transcript 1 (MALAT1) lncRNA [72].
2.3.4. microRNAs
Chemical modifications have been uncovered in mature miRNAs (Table 1). Methylation of 2′-O-methyl group at 3′-end of miRNA by the HEN1 methyltransferase is necessary for protecting these small RNAs from poly-uridylation and degradation [136]. A-to-I editing has been demonstrated in miRNAs. In this regard, Shoshan and co-workers correlated A-to-I editing of miR-455-5p with the metastatic activity of melanoma cancer [137].
2.3.5. circRNAs
m6A modifications have been revealed in circRNAs (Table 1), combining the computational pipeline AutoCirc and immunoprecipitation assays. A further study demonstrated that the readers YTHDF1 and YTHDF2 also interact with circRNAs [138].
3. Bioinformatics for Epitranscriptomics
To provide an integrative analysis of epitranscriptome-wide landscapes of RNA modifications from high-throughput sequencing, different types of databases have been developed. The availability of large epitranscriptome-wide datasets for m1A, m6A, m5C, and pseudouridine have stimulated the creation of several databases, periodically updated.
3.1. Databases for RNA Modifications
Here are reported the characteristics of the most renowned RNA modifications databases: The RNA Modification Database (RNAMDB) [139], MODOMICS—a database of RNA modifications pathways [78], MeT-DB—a database of transcriptome methylation in mammalian cells [140], and a database dedicated to RNA modifications in normal and disease such as RNA Modification Base (RMBase) [141].
RNAMDB is online from 1997 [142]. Acting as a reference for updating findings on RNA modifications. RNAMDB reports 13 eukarya; mRNA known post-transcriptional modifications that naturally occurring in mRNAs and 11 eukarya; small nuclear RNA (snRNA) in noncoding-RNA species as miRNAs, snRNAs, and small nucleolar (snoRNAs). Presently, the database for each entry provides the information on chemical structures, common name, symbols, molecular weight, Chemical Abstracts index name, and registry numbers [139]. The RNAMDB portal also hosts a number of tools of great benefit in mass spectrometry identification and characterization of natural or modified RNAs. Starting from an RNA sequence, it is possible to calculate the molecular mass, electrospray series, CID fragments, internal fragments, base losses, and fragment digestions [139].
MODOMICS. The MODOMICS database represents the reference database of RNA modifications since it provides the most complete information regarding the chemical structures of modified ribonucleosides, the reaction summary, the functionally characterized enzymes involved in modifications, and the biosynthetic pathways of RNA modifications. The current database version contains 163 different modifications that have been identified in RNA molecules and about 340 functionally characterized proteins involved in RNA modifications [78]. MODOMICS also provides information of (i) Liquid chromatography-Mass Spectrometry (LC-MS) data for modified nucleosides; (ii) Simplified molecular-input line-entry system (SMILES) for each modified base with the tridimensional structure (e.g., MDL Molfile, .mol) and its occurrence in Protein Data Base (PDB) structures. All files can be downloaded from their site and are useful in the field of the molecular dynamics and simulation [143,144,145,146].
MeT-DB. The knowledge about m6A position sites plays an essential role in investigating the mechanisms and functions of this modification.
MeT-DB is a comprehensive database focusing on m6A mammalian methyltranscriptome [140]. MeT-DB includes approximately 300.000 m6A methylation sites detected in samples from different experimental conditions, in human and mouse cells [140]. Data were obtained from methylated RNA immunoprecipitation sequencing (MeRIP-Seq) analyses [147] and were detected by exomePeak and MACS2 algorithm [148,149,150]. To explore the whole information, MeT-DB provides a genome browser to query and visualize specific m6A methylation. MeT-DB also includes in the browser window the data on binding site of microRNA, on splicing factor and on RNA binding proteins for comparison with m6A sites and for exploring the potential functions of m6A.
RMBase. The RMBase is a comprehensive database that integrates epitranscriptome sequencing data for exploring the post-transcriptional modifications of RNAs and their relationships with miRNA, disease-related single-nucleotide polymorphisms (SNPs), and RNA-binding proteins (RBPs). Since its inauguration, RMBase provides a variety of interfaces and graphic visualizations to facilitate analyses of the massive modification sites in normal tissues and cancer cells [151]. Currently, the RMBase v2.0, contains ~5400 m1A, ~1,373,000 m6A, ~5100 2′-O-methylations, ~9600 pseudouridine modifications, ~1000 m5C modifications, and ~2800 modifications of other types, identified from high-throughput sequencing data (MeRIP-seq, m6A-seq, miCLIP, m6A-CLIP, Pseudo-seq, Ψ-seq, CeU-seq, Aza-IP, RiboMeth-seq) [141]. RMBase also includes a motif module providing the visualized logos and position weight matrices (PWMs) of the modification motifs; a modRBP module to study the relationships between RNA modifications and RBPs. RMBase identified thousands of nucleotide modifications within mRNAs, regulatory noncoding-RNAs (e.g., lncRNAs, miRNAs, pseudogenes, circRNAs, snoRNAs, tRNAs), miRNA target sites, and disease-related SNPs.
3.2. Bioinformatic Tools for Predicting RNA Modifications
The development of an in silico approach based on support vector machines (SVM) [152,153,154] to accurately predict post-transcriptional modification sites from sequence information, is very helpful for the scientific community [154]. As good complements of experiments, many computational methods have been proposed to predict RNA modification sites in recent years [153,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169]. In Table 2 are summarized the online computational tools (user-friendly web server) currently existing directed at predicting the RNA modification sites.
Table 2.
Prediction tools of RNA modifications.
| Tools | Source | Prediction of Modifications | Description | Ref. |
|---|---|---|---|---|
| HAMR | http://www.lisanwanglab.org/hamr/ | m1A, m6A, A-to-I, Pseudouridine (Ψ), Dihydrouridine (D) | HAMR (High-throughput Annotation of Modified Ribonucleotides) is a web application that allows to detect and classify modified nucleotides in RNA-seq data | [153] |
| PAI | http://lin.uestc.edu.cn/server/PAI/ | A-to-I | Prediction of Adenosine to Inosine sitesby using pseudo nucleotide compositions | [155] |
| iRNA-AI | http://lin.uestc.edu.cn/server/iRNA-AI/ | A-to-I | Identification of Adenosine to Inosine editing sites | [156] |
| RAMPred | http://lin-group.cn/server/RAMPred/ | m1A | Identification of the N1-methyladenosine sites in eukaryotic transcriptomes | [157] |
| iRNA-3typeA | http://lin-group.cn/server/iRNA-3typeA/ | m1A, m6A, A-to-I | Identification of 3-types of modification at RNA’s Adenosine sites | [158] |
| iRNA-PseColl | http://lin.uestc.edu.cn/server/iRNA-PseColl/ | m1A, m6A, m5C | A seamless platform for identifying the occurrence sites of different RNA modifications by incorporating collective effects of nucleotides into PseKNC | [159] |
| iRNAm5C-PseDNC | http://www.jci-bioinfo.cn/iRNAm5C-PseDNC/ | m5C | Identification of RNA 5-methylcytosine sites by incorporation physical-chemical properties into pseudo dinucleotide composition | [160] |
| iRNA-Methyl | http://lin.uestc.edu.cn/server/iRNA-Methyl/ | m6A | Identification of N6-methyladenosine sites using pseudo nucleotide composition | [161] |
| m6Apred | http://lin.uestc.edu.cn/server/m6Apred/ | m6A | Identification and analysis of the N6-methyladenosine in Saccharomyces cerevisiae transcriptome | [162] |
| MethyRNA | http://lin.uestc.edu.cn/server/methyrna/ | m6A | A sequence-based tool for the identification of N6-methyladenosine sites | [163] |
| SRAMP | http://www.cuilab.cn/sramp/ | m6A | SRAMP (sequence-based RNA Adenosine methylation site predictor), a useful tool to predict m6A modification sites on the RNA sequences of interests | [164] |
| RAM-ESVM | http://server.malab.cn/RAM-ESVM/ | m6A | Identification of M6A Sites in theS. Cerevisiae Transcriptome | [165] |
| PPUS | http://lyh.pkmu.cn/ppus/ | Pseudouridine (Ψ) | PPUS is an online tool to predict Pseudouridine sites recognized by Pseudouridine synthase in RNA | [166] |
| iRNA-PseU | http://lin.uestc.edu.cn/server/iRNA-PseU/ | Pseudouridine (Ψ) | Identification of RNA Pseudouridine sites | [167] |
| tRNAMOD | http://crdd.osdd.net/raghava/trnamod/ | Pseudouridine (Ψ), Dihydrouridine (D) | Web-server for the prediction of transfer RNA (tRNA) modifications | [168] |
4. Epitranscriptomics and Stem Cells
4.1. Stem Cells
Stem cells are the unique cells with the capability to keep the stemness feature (self-renewal property) and to generate differentiate progenies in response to selective signals (pluri/multipotency property). Both activities are the consequence of the asymmetric division of stem cells producing two different daughter cells: one identical to the mother stem cell, and one committed toward a selected cell lineage. In some cases, stem cells divide in a symmetric division and produce two stem cells, both identical to the mother cell [170,171,172].
Naïve and primed stem cells represent the first distinction between the stem cell types [173,174]. Both stem cells originate during the zygotic stage of the mammalian embryo, immediately after the maternal predetermination. This stage, considered the ground zero status for embryogenesis, is a transient window in the preimplantation epiblast where unrestricted stem cells (naïve) are generated and, are then, set to enter into a lineage-commitment process (primed stem cells) [173,175]. This initial difference is extended to stem cells derived from embryos or through reprogramming techniques ex vivo [175]. Thus, embryonic stem cells (ESCs) are the stem cells derived by the immortalization of the naïve epiblast [176], while the primed stem cells, are the stem cells derived from post-implantation epiblasts and therefore are termed epiblast stem cells (EpiSCs) [173,175]. The latter cells express the Oct4, Sox2, and Nanog pluripotency genes and differ from ESCs for the differentiation efficiency [173,175].
The natural stem cell types (ESCs, EpiSCs, and adult stem cells, ASCs, [170,177,178]), and those generated in vitro (induced pluripotent stem cells, iPSCs [179,180,181]) have self-renewal properties but differ for the capability to generate differentiated cells (see Table 3 for details). Moreover, ASCs have the capability to replace damaged cells with new healthy substitutes within the adult tissues/organs where they reside or after therapeutic implantation, as this property is maintained in degenerated tissues/organs [17,181,182,183,184,185,186]. Furthermore, the regenerative potential may be enhanced by the combination of the stem cells with gene therapy technology [187,188,189,190,191,192,193,194,195] or by their association with selected biomaterials [174,196,197,198,199,200].
Table 3.
Source of the origin the diverse types of stem cells and recapitulates the main characteristics of naïve, primed, ESCs, ASCs, iPSCs, and CSCs.
| Stem Cell Types | Origin | Properties | Ref. |
|---|---|---|---|
| Naïve stem cells | Zygotic stage of the mammalian embryo, immediately after the maternal redetermination. Specifically, they originate from the preimplantation epiblast | Self-renewal Pluripotency Unrestricted stem cells |
[173,174,175] |
| Primed stem cells | Zygotic stage of the mammalian embryo, immediately after the maternal redetermination. Specifically, they originate from naïve stem cells that enter into a lineage commitment process | Self-renewal Pluripotency More lineage restricted stem cells compared to naive stem cells |
[173,174,175] |
| Embryonic Stem cells (ESCs) |
ESCs originate from the inner mass of the blastocyst | Self-renewal Pluripotent: multi-lineage differentiation through either asymmetric or symmetric division Generation of all 254 cell types originating adult tissue Generation of mouse chimeras Replacement of damaged cells with newly differentiated progenies if transplanted in degenerated tissues/organs |
[170,177,196,206,207] |
| Adult stem cells (ASCs) | ASCs are created during ontogeny and persist in the adult tissues/organs within the niche | Self-renewal Multipotent: multi-lineage differentiation through either asymmetric or symmetric division Maintenance of the tissue homeostasis in the physiological condition Replacement of damaged cells with newly differentiated progenies if transplanted in degenerated tissues/organs |
[170,178,196,206,207] |
| Induced pluripotentStem cells (iPSCs) | iPSCs originate in vitro from somatic differentiated cells after transduction with cMyc, Klf-4, Oct-3/4 , Sox-2 genes |
Self-renewal Pluripotent Generation of patient-specific stem cells Generation of mouse chimeras Replacement of damaged cells with newly differentiated progenies if transplanted in degenerated tissues/organs |
[170,179,180,196,206,207] |
| Cancer stem cells (CSCs) | CSCs originate from: (i) Malignant transformation of normal stem cells (ii) de-differentiation of cancer cell |
Self-renewal through either asymmetric or symmetric division Multipotent: multi-lineage differentiation. Key role in predicting the biological aggressiveness of the cancer |
[170,201,202,206,207] |
Noteworthy, since the last decade, cancer stem cells (CSCs) have been recognized as the stem cell type causing tumors progression. For these features, CSCs are considered a challenge for cancer therapies [201,202,203]. CSCs have self-renewal and multipotential properties as well as several other characteristics critical for metastatic development (e.g., as motility, invasiveness, and resistance to therapeutics) (Table 3; [201,202,203]).
4.2. RNA Modifications and Stem Cells
Stem cells’ functions are orchestrated by networks of molecular cues including both epigenetic (e.g., reorganization of chromatin architecture, DNA methylation, and demethylation, post-translational modifications of histones), and epitranscriptomic signals (e.g., RNA modifications and related proteins). The whole processes determine genes expression signature and, in turn, stem cells’ maintenance and specification lineage processes [17,186,204,205,206,207,208,209,210,211].
In the last chapter, we describe the role of epitranscriptomic modifications on the stem cells’ processes, highlighting the effects of different RNA modifications on stem cell fate.
4.2.1. N6-methyl-adenosine mRNA Modification and Stem Cells
Among RNA modifications, the presence of m6A in mRNAs has been explored in several types of stem cells. Key roles in modulating stem cell maintenance, differentiation, reprogramming, and controlling of the development stages in mammals have been revealed [38,212,213,214].
Naïve vs. primed stem cells. Recent studies suggest that m6A modification of mRNA could be useful for facilitating the distinction between naïve and primed cells (Table 4). This conclusion primarily comes from the study of Geula and collaborators [215]. This group demonstrated that m6A mRNA methylation is a molecular switch, acting as a regulator during the resolution of murine naïve pluripotency. The methylation seems critical for the safeguarding and timely downregulation of pluripotency factors, which is required for proper lineage priming and differentiation. This mechanism was confirmed by METTL3–/– knockout that leads to the depletion of m6A in mRNAs in the blastocysts. Here, METTL3 ablation retained the normal morphology and expression of pluripotency genes that produced ESCs at the expected ratio. Additionally, METTL3–/– ESCs preserved their naïve pluripotent identity, whereas METTL3 KO ESCs did not support the embryo chimera formation after blastocyst microinjection [215]. More importantly, METTL3 depletion reduced the expression of pluripotency genes, greatly increased the lineage commitment markers and, in turn, lead the tipping off of the balance to differentiation compromising the stability of the primed state [215].
Table 4.
Correlation between RNA modifications and stem cells.
| Stem Cell Types | RNA Modification | Presence/Absence Increase/Decrease |
Effect | Ref. |
|---|---|---|---|---|
| Naïve vs. primed | m6A in mRNAs | Presence | Molecular switches for differentiation and generation of EpiSCs | [215,216] |
| Naïve vs. primed | m6A in mRNAs | Absence | Molecular switches for reating the naïve status | [215,216] |
| Naïve/ESCs | pseudouridylation of tRNA | Presence | Stem cell commitment during the first stage of embryogenesis | [242,243] |
| ESCs | m6A in mRNAs | Presence | Critical steps for keeping ESCs in a stemness status | [213,217,218] |
| ESCs | m6A in mRNAs | Absence | Critical steps for keeping ESCs in a stemness status | [220,221] |
| ESCs | m5C in mRNAs | Increase | Critical steps for keeping ESCs in a stemness status | [241] |
| ESCs | m5C in mt-tRNAs | Presence | Regulator of ESCs fate | [124] |
| ASCs | m6A in mRNAs | Presence | Activation of differentiation process | [224] |
| ASCs | m6A in mRNAs | Decrease | Hamper the HSCs development | [213,227,228,229] |
| ASCs | m6A in mRNAs | Decrease | Myeloid differentiation of HSCs | [230] |
| ASCs | m5C in mRNAs | Presence | Balance of epidermis stem cell self-renewal and differentiation processes | [237,238] |
| CSCs | m6A in mRNAs | Increase | Acute myeloid leukemia | [230] |
| CSCs | m6A in mRNAs | Absence/Decrease | Growth and self-renewal of human glioblastoma stem cells | [232,234] |
| CSCs | m6A in mRNAs | Decrease | Progression of human lung cancer | [235] |
| CSCs | m6A in mRNAs | Decrease | Demethylation on NANOG mRNA in breast cancer stem cells in response to hypoxia | [81] |
Table 4 summarizes the effect of the different RNA modifications in the naïve, primed, ESCs, ASCs, and CSCs.
These findings were also confirmed by the study of Aguilo and co-workers [216]. It was shown that early inactivation of METTL3 during mouse development blocked the reprogramming of differentiated EpiSCs to ESCs. Zinc finger protein 217 is partially responsible for stabilizing key pluripotency and for reprogramming transcripts by inhibiting their METTL3-mediated methylation, thus promoting the self-renewal of ESCs and the reprogramming of somatic cells [216].
Embryonic stem cells. The role of m6A methylation of mRNAs in the pluripotency and in the differentiation processes of ESCs is being currently explored [213,214,217,218]. So far, results are yet contrasting since they support the role of m6A in opposite processes (Table 4).
Some authors endorsed m6A modifications as critical steps for keeping ESCs in a stemness status [213,217,218]. Thus, the knock-down of both METTL3 and METTL14 in mouse ESCs resulted in reduced levels of m6A modification and, in turn, in decreased stemness activity and increased differentiation program of stem cells. In particular, the knock-down of METTL3 and METTL14 caused a decrease in the expression of pluripotency genes (e.g., NANOG and SOX2) and an increase in the expression of developmental regulators, although in part mediated by the human antigen R and several microRNAs (e.g., let-7a-3) [67,217,218]. Supporting this study, Wang and collaborators suggested that m6A modification enhances the self-renewal activity in embryonic neural stem cells. In METTL14 knockout mice, embryonic neural stem cells displayed high decreased proliferation and premature differentiation and, in turn, less number of late-born neurons during cortical neurogenesis [217]. Furthermore, germ cell-specific METTL3 knockout mice impaired the initiation of meiosis and, in turn, male fertility, and spermatogenesis [219].
TGFβ signaling supports these findings. In fact, SMAD2 and SMAD3, through the interaction with the METTL3-METTL14-WTAP complex, promote binding of the m6A methyltransferase complex to transcripts involved in early cell fate decisions. This mechanism destabilizes specific SMAD2/3 transcriptional targets, including the pluripotency factor gene NANOG [220].
Challenging the above reported conclusions, other authors suggested the need of m6A modifications for the transition of ESCs to differentiation stage [215,221]. Depletion of m6A by METTL14 knockout in embryonic mice brains has been shown to prolong the cell cycle of radial glial cells and to extend the cortical neurogenesis into postnatal stages. m6A sequencing of embryonic mice cortex revealed enrichment of mRNAs related to transcription factors involved in neurogenesis, cell cycle, and neuronal differentiation [222]. Convergent in vitro and in vivo studies revealed that gene silencing or depleting of murine and human METTL3 caused the removal of m6A in target genes, sustained the expression of NANOG, and blocked the activation of ESC commitment program toward the diverse differentiation lineages. Therefore, m6A has been proposed as a mark of a transcriptome required for stem cells to enter in the differentiation status [221,223].
Adult stem cells. Many research groups have investigated the effect of m6A mRNA modification in adult stem cells.
An indirect correlation of the presence of m6A modifications in mRNAs and in differentiation processes was observed by Vu et al. [224]. It was shown that silencing of METTL3 by interference RNA transfection induced the differentiation of human hematopoietic stem/progenitor cells (hHSPCs), whereas the overexpression of wild-type METTL3 caused inhibition of stem cell differentiation [224].
Additional evidence comes from the need of m6A methylation in the 5′-UTR of MyoD mRNA for correct expression of MyoD protein in proliferative myoblasts. This transcription factor is necessary for myogenic stem/progenitor cells to retain their skeletal muscle differentiation potential [225]. Using MeRIP-seq [226] to profile mRNA modifications in zebrafish embryos at 28 h post fertilization, Zhang and coworkers studied the role of m6A mRNA methylation in the stem cell fate [227]. Generating METTL3-deficient zebrafish embryos, they observed that the decreased levels of m6A hampered the development of hematopoietic stem cell. In particular, they showed that the reader YTHDF2 mediates the degradation of NOTCH1a RNA [228]. These findings were also confirmed in knockdown METTL3 mice [213,227,229].
Cancer stem cells. The relevance of m6A modification in mRNA functions in stem cell biology is highlighted by the correlation of abnormal m6A profile in malignant cells (Table 4). These findings include also the expression of reader, writer, and eraser proteins.
For instance, Weng and coauthors observed the increase of the expression of m6A methyltransferase METTL14 in healthy HSPCs and acute myeloid leukemia (AML) cells carrying the t(11q23), t(15;17), or t(8;21) mutations, and its downregulation during myeloid differentiation [230]. The parallel increase of m6A modifications and METTL3 expression was also demonstrated in c-MYC, BCL2, and PTEN mRNAs in the human AML MOLM-13 cells line [224]. Furthermore, silencing of METTL14 has been found to promote terminal myeloid differentiation of normal HSPCs and AML cells and to inhibit AML cells survival and proliferation. Like METTL3, METTL14 has been demonstrated to exert its oncogenic activity by regulating the m6A levels in MYB and MYC mRNAs while the methyltransferase itself is inhibited by SPI1. Thus, the SPI1-METTL14-MYB/MYC signaling is suggested as a basic mechanism for myelopoiesis and leukemogenesis, whereas the levels of METTL14 and m6A modification are critical in normal and malignant hematopoiesis [230]. In this context, the protein CEBPZ (CCAAT/Enhancer Binding Protein Zeta) has been identified as the mark required to recruit of METTL3 to chromatin and to maintain the leukemic state [231].
The knockdown of METTL3 or METTL14 was also found to promote the growth and self-renewal of human glioblastoma stem cells (GSCs) and the progression of tumorigenesis. Additionally, the overexpression of METTL3 and the inhibition of the RNA demethylase FTO was shown to arrest GSC growth and the self-renewal processes [232].
Validating these data, Zheng and collaborators showed high levels of the m6A demethylase ALKBH5 in human GSCs [233]. Moreover, the silencing of ALKBH5 suppresses the proliferation of GSCs in vitro through the alteration of m6A methylation of some mRNA target of ALKBH5. In particular, ALKBH5 demethylates FOXM1 mRNA and controls FOXM1 protein expression by the long noncoding-RNA FOXM1-AS that led to blockage of tumorigenesis of GSC [234].
Alteration of m6A methylation, due to the dysfunction of METTL3, has been also associated with the progression of human lung cancer and foremost with growth, survival and invasion of human lung cancer cells. It has been demonstrated that in human cancer cells, METTL3 promotes the translation of certain mRNAs, including the EGFR and the TAZ protein [235].
Of note, deregulation of epitranscriptomic events in cancer biogenesis and progression comprises also adenosine-to-inosine double-stranded RNA editing (e.g., pri-microRNA let-7), by the activity of the enzyme adenosine deaminase [236].
In MDA-MB-231 breast cancer cells, the knockdown of the eraser ALKBH5 caused the demethylation of m6A in NANOG mRNA and significantly decreased metastasis from breast to lungs in immunodeficient mice. A similar mechanism could be induced by the exposure of breast cancer cells to hypoxia that specifically induces ZNF217-dependent inhibition of m6A methylation [81,237].
4.2.2. 5-methylcytosine and 5-hydroxymethylcytosine Modifications and Stem Cells
Knowledge about the prevalence and transcriptome-wide distribution of m5C in stem cells is still limited. Nevertheless, studies in different cell types, tissues, and organisms correlated the m5C and the hm5C modifications with key biological pathways [238] (Table 4). For example, the activity of MISU/NSUN2 (the writer of m5C methylation) was shown to be essential in controlling of the expression of critical transcripts critical in the balance of epidermis stem cell self-renewal and differentiation processes [238,239]. Advances could come from epitranscriptome profile studies. A global analysis of m5C in whole and nuclear poly(A) RNA of mice embryonic stem cells and brain was conducted by Amort and collaborators [240]. In particular, a marked accumulation of m5C sites close to of the translational start codon, a reduction in coding sequences, and an enrichment in the 3′-UTR [240] were observed.
In ESCs, the m5C of NSUN3 mt-tRNA is associated with the stem cell fate regulation. Moreover, NSUN3 inactivation attenuated induction of mitochondrial reactive oxygen species (ROS) upon stress, which may affect gene expression programs upon differentiation. These findings indicated NSUN3 as a central regulator of stem cell fate and also provide a model system to study the correlation of mitochondrial function with stem cell pluripotency and differentiation [124].
4.2.3. Pseudouridine Modification and Stem Cells
As aforementioned, pseudouridine modification has been demonstrated in whole RNA types (Table 4). Nevertheless, evidence correlating this modification and stem cell biology are restricted to few studies. Most of them highlight the correlation of levels of expression of pseudouridylation writers with the control of stem cell differentiation, as well as, with pathways essential for normal development [211,241,242]. A recent study showed that during the first stage of embryogenesis, the pseudouridylation of tRNA is directly involved in the stem cell commitment [242,243]. Guzzi and co-authors identified the PUS7 as the writer of above modifications and found their localization into the tRNA-derived fragment, the region involved in the translation initiation complex [243].
5. Concluding Remarks
The discovery of RNA modifications opened a new frontier in the understanding of the biological processes. Identification of over 100 modifications in coding and noncoding-RNA nucleotides has revealed a variety of interplays involving RNA molecules directly or RNA molecules that get over the competition between mRNAs and noncoding-RNAs. Our knowledge in this field is in the early days. However, it can be predicted that elucidation of the epitranscriptome regulatory mechanisms could help the understanding of how gene expression is finely tuned and regulated across development and in tissues.
Future studies aimed at deciphering the epitranscriptome in stem cells could shed light on the mechanisms of stem cell self-renewal and stem cell differentiation. In particular, the combination of bioinformatic predictive tools together with single-cell epitranscriptomic sequencing could provide insights into the molecular differences underlying naïve and primed stem cells. The same approach might be advantageous for understanding the mechanisms controlling the commitment and the lineage specification of stem cells.
Furthermore, better knowledge of epitranscriptomic mechanisms could help the development of inhibitors to target writers, readers, and erasers, and thereby to explore new routes for controlling gene expression in stem cells in physiology and pathology. Such discoveries might aid the design of innovative therapeutic strategies for complex diseases, including cancer.
Acknowledgments
We thank all researchers cited in this review and we apologize to colleagues in the field for not being able to cite many important papers due to the limitation of the article.
Abbreviations
| 3′-UTR | Three prime untranslated region |
| ALKB | Alpha-ketoglutarate-dependent dioxygenase AlkB |
| ALKBH1 | Nucleic acid dioxygenase |
| ALKBH5 | RNA demethylase ALKBH5 |
| ALYREF | THO complex subunit 4 |
| BCL2 | B-cell lymphoma 2 |
| CDS | CoDing Sequence |
| DKC1 | H/ACA ribonucleoprotein complex subunit 4 |
| DNA | Deoxyribonucleic acid |
| DNMT2 | tRNA (cytosine(38)-C(5))-methyltransferase |
| EGFR | Epidermal growth factor receptor |
| ELP3 | Elongator complex protein 3 |
| FOXM1 | Forkhead box protein M1 |
| HEN1 | Small RNA 2′-O-methyltransferase |
| HNRNPA2B1 | Heterogeneous nuclear ribonucleoproteins A2/B1 |
| hTRMT9 | Human Probable tRNA methyltransferase 9B |
| IME4 | N6-adenosine-methyltransferase |
| MED2 | Mediator of RNA polymerase II transcription subunit 2 |
| MED4 | Mediator of RNA polymerase II transcription subunit 4 |
| METTL2 | Methyltransferase-like protein 2 |
| METTL3 | Methyltransferase-like protein 3 |
| METTL6 | Methyltransferase-like protein 6 |
| METTL14 | Methyltransferase-like protein 14 |
| MYB | Myb proto-oncogene protein |
| MYC | Myc proto-oncogene protein |
| MyoD | Myogenic differentiation 1 |
| NOTCH1 | Neurogenic locus notch homolog protein 1 |
| NSUN2 | NOP2/Sun domain family, member 2 |
| NSUN4 | NOL1/NOP2/Sun domain family member 4 |
| p16 | Cyclin-dependent kinase inhibitor 2A, multiple tumor suppressor 1 |
| PTEN | Phosphatidylinositol 3,4,5-trisphosphate 3-phosphatase and dual-specificity protein phosphatase |
| PUS | Pseudouridine synthase A |
| RBM15 | Putative RNA-binding protein 15 |
| RluE | Ribosomal large subunit Pseudouridine synthase E |
| ROS | Reactive oxygen specie |
| RPUSD3 | Mitochondrial mRNA Pseudouridine synthase RPUSD3 |
| RPUSD4 | Mitochondrial RNA Pseudouridine synthase RPUSD4 |
| SMAD2 | Mothers against decapentaplegic homolog 2 |
| SMAD3 | Mothers against decapentaplegic homolog 2 |
| SOD1 | Superoxide dismutase [Cu-Zn] |
| SPI1 | Transcription factor PU.1 |
| SRSF2 | Serine/arginine-rich splicing factor 2 |
| TAZ | Tafazzin |
| TET1 | methylcytosine dioxygenase TET1 |
| TET3 | methylcytosine dioxygenase TET3 |
| TGFβ | Transforming growth factor beta |
| TRM4 | Multisite-specific tRNA:(cytosine-C(5))-methyltransferase |
| TrmL | tRNA(cytidine(34)-2′-O)-methyltransferase |
| TRMT10C | tRNA methyltransferase 10 homolog C |
| TRMT61A | tRNA (adenine(58)-N(1))-methyltransferase catalytic subunit |
| TRMT61B | tRNA (adenine(58)-N(1))-methyltransferase, mitochondrial |
| TRUB2 | Mitochondrial mRNA Pseudouridine synthase TRUB2 |
| U1 | U1 spliceosomal RNA |
| U2 | U2 spliceosomal RNA |
| U3 | U3 spliceosomal RNA |
| U4 | U4 spliceosomal RNA |
| U5 | U5 spliceosomal RNA |
| U6 | U6 spliceosomal RNA |
| U8 | U8 spliceosomal RNA |
| UHRF2 | E3 ubiquitin-protein ligase UHRF2 |
| WTAP | Wilms tumor 1 associated protein |
| YTDCH1 | YTH domain-containing protein 1 |
| YTHDF1 | YTH domain-containing family protein 1 |
| YTHDF2 | YTH domain-containing family protein 2 |
| YTHDF3 | YTH domain-containing family protein 2 |
| ZNF217 | Zinc finger protein 217 |
Funding
This research was funded by FFABRMARTINO-2018 from MIUR, Italy, to S.M.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Martino S., Morena F., Barola C., Bicchi I., Emiliani C. Proteomics and epigenetic mechanisms in stem cells. Curr. Proteom. 2014;11:193–209. doi: 10.2174/157016461103140922164050. [DOI] [Google Scholar]
- 2.Shakya K., O’Connell M.J., Ruskin H.J. The landscape for epigenetic/epigenomic biomedical resources. Epigenetics. 2012;7:982–986. doi: 10.4161/epi.21493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sen P., Shah P.P., Nativio R., Berger S.L. Epigenetic mechanisms of longevity and aging. Cell. 2016;166:822–839. doi: 10.1016/j.cell.2016.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaenisch R., Bird A. Epigenetic regulation of gene expression: How the genome integrates intrinsic and environmental signals. Nat. Genet. 2003;33:245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 5.Fu Y., He C. Nucleic acid modifications with epigenetic significance. Curr. Opin. Chem. Biol. 2012;16:516–524. doi: 10.1016/j.cbpa.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Q., Zhang Z. Linking DNA replication to heterochromatin silencing and epigenetic inheritance. Acta Biochim. Biophys. Sin. 2012;44:3–13. doi: 10.1093/abbs/gmr107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Francis N.J. Mechanisms of epigenetic inheritance: Copying of polycomb repressed chromatin. Cell Cycle. 2009;8:3521–3526. doi: 10.4161/cc.8.21.9876. [DOI] [PubMed] [Google Scholar]
- 8.Roloff T.C., Nuber U.A. Chromatin, epigenetics and stem cells. Eur. J. Cell Biol. 2005;84:123–135. doi: 10.1016/j.ejcb.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 9.Krishnakumar R., Blelloch R.H. Epigenetics of cellular reprogramming. Curr. Opin. Genet. Dev. 2013;23:548–555. doi: 10.1016/j.gde.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ng R.K., Gurdon J.B. Epigenetic inheritance of cell differentiation status. Cell Cycle. 2008;7:1173–1177. doi: 10.4161/cc.7.9.5791. [DOI] [PubMed] [Google Scholar]
- 11.Probst A.V., Dunleavy E., Almouzni G. Epigenetic inheritance during the cell cycle. Nat. Rev. Mol. Cell Biol. 2009;10:192–206. doi: 10.1038/nrm2640. [DOI] [PubMed] [Google Scholar]
- 12.Li E., Beard C., Jaenisch R. Role for DNA methylation in genomic imprinting. Nature. 1993;366:362–365. doi: 10.1038/366362a0. [DOI] [PubMed] [Google Scholar]
- 13.Groth A., Rocha W., Verreault A., Almouzni G. Chromatin challenges during DNA replication and repair. Cell. 2007;128:721–733. doi: 10.1016/j.cell.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 14.Gopalakrishnan S., Van Emburgh B.O., Robertson K.D. DNA methylation in development and human disease. Mutat. Res. 2008;647:30–38. doi: 10.1016/j.mrfmmm.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamm C.A., Costa F.F. Epigenomes as therapeutic targets. Pharmacol. Ther. 2015;151:72–86. doi: 10.1016/j.pharmthera.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Saletore Y., Chen-Kiang S., Mason C.E. Novel RNA regulatory mechanisms revealed in the epitranscriptome. RNA Biol. 2013;10:342–346. doi: 10.4161/rna.23812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bicchi I., Morena F., Montesano S., Polidoro M., Martino S. MicroRNAs and molecular mechanisms of neurodegeneration. Genes. 2013;4:244–263. doi: 10.3390/genes4020244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyer K.D., Jaffrey S.R. The dynamic epitranscriptome: N6-methyladenosine and gene expression control. Nat. Rev. Mol. Cell Biol. 2014;15:313–326. doi: 10.1038/nrm3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwartz S. Cracking the epitranscriptome. RNA. 2016;22:169–174. doi: 10.1261/rna.054502.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tay Y., Rinn J., Pandolfi P.P. The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014;505:344–352. doi: 10.1038/nature12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng G., Dahl J.A., Niu Y., Fu Y., Klungland A., Yang Y.-G., He C. Sprouts of RNA epigenetics: The discovery of mammalian RNA demethylases. RNA Biol. 2013;10:915–918. doi: 10.4161/rna.24711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jia G., Fu Y., Zhao X., Dai Q., Zheng G., Yang Y., Yi C., Lindahl T., Pan T., Yang Y.-G., et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol. 2011;7:885–887. doi: 10.1038/nchembio.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu Y., Jia G., Pang X., Wang R.N., Wang X., Li C.J., Smemo S., Dai Q., Bailey K.A., Nobrega M.A., et al. FTO-mediated formation of N6-hydroxymethyladenosine and N6-formyladenosine in mammalian RNA. Nat. Commun. 2013;4:1798. doi: 10.1038/ncomms2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore M.J. From birth to death: The complex lives of eukaryotic mRNAs. Science. 2005;309:1514–1518. doi: 10.1126/science.1111443. [DOI] [PubMed] [Google Scholar]
- 25.Jaffrey S.R. An expanding universe of mRNA modifications. Nat. Struct. Mol. Biol. 2014;21:945–946. doi: 10.1038/nsmb.2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Squires J.E., Patel H.R., Nousch M., Sibbritt T., Humphreys D.T., Parker B.J., Suter C.M., Preiss T. Widespread occurrence of 5-methylcytosine in human coding and non-coding RNA. Nucleic Acids Res. 2012;40:5023–5033. doi: 10.1093/nar/gks144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khoddami V., Cairns B.R. Identification of direct targets and modified bases of RNA cytosine methyltransferases. Nat. Biotechnol. 2013;31:458–464. doi: 10.1038/nbt.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ge J., Yu Y.-T. RNA pseudouridylation: New insights into an old modification. Trends Biochem. Sci. 2013;38:210–218. doi: 10.1016/j.tibs.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoang C., Ferré-D’Amaré A.R. Cocrystal structure of a tRNA Psi55 pseudouridine synthase: Nucleotide flipping by an RNA-modifying enzyme. Cell. 2001;107:929–939. doi: 10.1016/S0092-8674(01)00618-3. [DOI] [PubMed] [Google Scholar]
- 30.Xiang Y., Laurent B., Hsu C.-H., Nachtergaele S., Lu Z., Sheng W., Xu C., Chen H., Ouyang J., Wang S., et al. RNA m6A methylation regulates the ultraviolet-induced DNA damage response. Nature. 2017;543:573–576. doi: 10.1038/nature21671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Incarnato D., Oliviero S. The RNA epistructurome: uncovering rna function by studying structure and post-transcriptional modifications. Trends Biotechnol. 2017;35:318–333. doi: 10.1016/j.tibtech.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 32.Gatsiou A., Vlachogiannis N., Lunella F.F., Sachse M., Stellos K. Adenosine-to-Inosine RNA editing in health and disease. Antioxid. Redox Signal. 2017 doi: 10.1089/ars.2017.7295. [DOI] [PubMed] [Google Scholar]
- 33.Gilbert W.V., Bell T.A., Schaening C. Messenger RNA modifications: Form, distribution, and function. Science. 2016;352:1408–1412. doi: 10.1126/science.aad8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ben-Haim M.S., Moshitch-Moshkovitz S., Rechavi G. FTO: Linking m6A demethylation to adipogenesis. Cell Res. 2015;25:3–4. doi: 10.1038/cr.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Licht K., Jantsch M.F. Rapid and dynamic transcriptome regulation by RNA editing and RNA modifications. J. Cell Biol. 2016;213:15–22. doi: 10.1083/jcb.201511041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Machnicka M.A., Milanowska K., Osman Oglou O., Purta E., Kurkowska M., Olchowik A., Januszewski W., Kalinowski S., Dunin-Horkawicz S., Rother K.M., et al. MODOMICS: A database of RNA modification pathways—2013 update. Nucleic Acids Res. 2013;41:D262–D267. doi: 10.1093/nar/gks1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perry R.P., Kelley D.E., Friderici K., Rottman F. The methylated constituents of L cell messenger RNA: Evidence for an unusual cluster at the 5′ terminus. Cell. 1975;4:387–394. doi: 10.1016/0092-8674(75)90159-2. [DOI] [PubMed] [Google Scholar]
- 38.Batista P.J. The RNA Modification N6-methyladenosine and its implications in human disease. Genom. Proteom. Bioinform. 2017;15:154–163. doi: 10.1016/j.gpb.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Helm M., Motorin Y. Detecting RNA modifications in the epitranscriptome: Predict and validate. Nat. Rev. Genet. 2017;18:275–291. doi: 10.1038/nrg.2016.169. [DOI] [PubMed] [Google Scholar]
- 40.Pan T. N6-methyl-adenosine modification in messenger and long non-coding RNA. Trends Biochem. Sci. 2013;38:204–209. doi: 10.1016/j.tibs.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dubin D.T., Taylor R.H. The methylation state of poly A-containing messenger RNA from cultured hamster cells. Nucleic Acids Res. 1975;2:1653–1668. doi: 10.1093/nar/2.10.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Delatte B., Wang F., Ngoc L.V., Collignon E., Bonvin E., Deplus R., Calonne E., Hassabi B., Putmans P., Awe S., et al. RNA biochemistry. Transcriptome-wide distribution and function of RNA hydroxymethylcytosine. Science. 2016;351:282–285. doi: 10.1126/science.aac5253. [DOI] [PubMed] [Google Scholar]
- 43.Rosa-Mercado N.A., Withers J.B., Steitz J.A. Settling the m6A debate: Methylation of mature mRNA is not dynamic but accelerates turnover. Genes Dev. 2017;31:957–958. doi: 10.1101/gad.302695.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roignant J.-Y., Soller M. m6A in mRNA: An ancient mechanism for fine-tuning gene expression. Trends Genet. 2017;33:380–390. doi: 10.1016/j.tig.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 45.Dominissini D., Nachtergaele S., Moshitch-Moshkovitz S., Peer E., Kol N., Ben-Haim M.S., Dai Q., Di Segni A., Salmon-Divon M., Clark W.C., et al. The dynamic N1-methyladenosine methylome in eukaryotic messenger RNA. Nature. 2016;530:441–446. doi: 10.1038/nature16998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li X., Xiong X., Wang K., Wang L., Shu X., Ma S., Yi C. Transcriptome-wide mapping reveals reversible and dynamic N1-methyladenosine methylome. Nat. Chem. Biol. 2016;12:311–316. doi: 10.1038/nchembio.2040. [DOI] [PubMed] [Google Scholar]
- 47.Harcourt E.M., Kietrys A.M., Kool E.T. Chemical and structural effects of base modifications in messenger RNA. Nature. 2017;541:339–346. doi: 10.1038/nature21351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morse D.P., Bass B.L. Detection of inosine in messenger RNA by inosine-specific cleavage. Biochemistry. 1997;36:8429–8434. doi: 10.1021/bi9709607. [DOI] [PubMed] [Google Scholar]
- 49.Copp W., Denisov A.Y., Xie J., Noronha A.M., Liczner C., Safaee N., Wilds C.J., Gehring K. Influence of nucleotide modifications at the C2′ position on the Hoogsteen base-paired parallel-stranded duplex of poly(A) RNA. Nucleic Acids Res. 2017;45:10321–10331. doi: 10.1093/nar/gkx713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Byszewska M., Śmietański M., Purta E., Bujnicki J.M. RNA methyltransferases involved in 5′ cap biosynthesis. RNA Biol. 2014;11:1597–1607. doi: 10.1080/15476286.2015.1004955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bawankar P., Shaw P.J., Sardana R., Babar P.H., Patankar S. 5′ and 3′ end modifications of spliceosomal RNAs in Plasmodium falciparum. Mol. Biol. Rep. 2010;37:2125–2133. doi: 10.1007/s11033-009-9682-4. [DOI] [PubMed] [Google Scholar]
- 52.Crick F. Split genes and RNA splicing. Science. 1979;204:264–271. doi: 10.1126/science.373120. [DOI] [PubMed] [Google Scholar]
- 53.Muthukrishnan S., Filipowicz W., Sierra J.M., Both G.W., Shatkin A.J., Ochoa S. mRNA methylation and protein synthesis in extracts from embryos of brine shrimp, Artemia salina. J. Biol. Chem. 1975;250:9336–9341. [PubMed] [Google Scholar]
- 54.Mao X., Schwer B., Shuman S. Yeast mRNA cap methyltransferase is a 50-kilodalton protein encoded by an essential gene. Mol. Cell. Biol. 1995;15:4167–4174. doi: 10.1128/MCB.15.8.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wurth L., Gribling-Burrer A.-S., Verheggen C., Leichter M., Takeuchi A., Baudrey S., Martin F., Krol A., Bertrand E., Allmang C. Hypermethylated-capped selenoprotein mRNAs in mammals. Nucleic Acids Res. 2014;42:8663–8677. doi: 10.1093/nar/gku580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Furuichi Y., Shatkin A.J. Viral and cellular mRNA capping: Past and prospects. Adv. Virus Res. 2000;55:135–184. doi: 10.1016/S0065-3527(00)55003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao J., Hyman L., Moore C. Formation of mRNA 3′ ends in eukaryotes: Mechanism, regulation, and interrelationships with other steps in mRNA synthesis. Microbiol. Mol. Biol. Rev. 1999;63:405–445. doi: 10.1128/mmbr.63.2.405-445.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Darnell J.E. Implications of RNA-RNA splicing in evolution of eukaryotic cells. Science. 1978;202:1257–1260. doi: 10.1126/science.364651. [DOI] [PubMed] [Google Scholar]
- 59.Schellenberg M.J., Ritchie D.B., MacMillan A.M. Pre-mRNA splicing: A complex picture in higher definition. Trends Biochem. Sci. 2008;33:243–246. doi: 10.1016/j.tibs.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 60.Lee Y., Rio D.C. Mechanisms and regulation of alternative pre-mRNA splicing. Annu. Rev. Biochem. 2015;84:291–323. doi: 10.1146/annurev-biochem-060614-034316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dezi V., Ivanov C., Haussmann I.U., Soller M. Nucleotide modifications in messenger RNA and their role in development and disease. Biochem. Soc. Trans. 2016;44:1385–1393. doi: 10.1042/BST20160110. [DOI] [PubMed] [Google Scholar]
- 62.Zhao B.S., Roundtree I.A., He C. Post-transcriptional gene regulation by mRNA modifications. Nat. Rev. Mol. Cell Biol. 2017;18:31–42. doi: 10.1038/nrm.2016.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuzmenko A., Atkinson G.C., Levitskii S., Zenkin N., Tenson T., Hauryliuk V., Kamenski P. Mitochondrial translation initiation machinery: Conservation and diversification. Biochimie. 2014;100:132–140. doi: 10.1016/j.biochi.2013.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salemi M., Giambirtone M., Barone C., Salluzzo M.G., Russo R., Giudice M.L., Cutuli S., Ridolfo F., Romano C. Mitochondrial mRNA expression in fibroblasts of Down syndrome subjects. Hum. Cell. 2018;31:179–181. doi: 10.1007/s13577-018-0205-2. [DOI] [PubMed] [Google Scholar]
- 65.Perry R., Kelley D. Existence of methylated messenger RNA in mouse L cells. Cell. 1974;1:37–42. doi: 10.1016/0092-8674(74)90153-6. [DOI] [Google Scholar]
- 66.Bujnicki J.M., Feder M., Radlinska M., Blumenthal R.M. Structure prediction and phylogenetic analysis of a functionally diverse family of proteins homologous to the MT-A70 subunit of the human mRNA: m6A methyltransferase. J. Mol. Evol. 2002;55:431–444. doi: 10.1007/s00239-002-2339-8. [DOI] [PubMed] [Google Scholar]
- 67.Wang Y., Li Y., Toth J.I., Petroski M.D., Zhang Z., Zhao J.C. N6-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nat. Cell Biol. 2014;16:191–198. doi: 10.1038/ncb2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dominissini D., Moshitch-Moshkovitz S., Schwartz S., Salmon-Divon M., Ungar L., Osenberg S., Cesarkas K., Jacob-Hirsch J., Amariglio N., Kupiec M., et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485:201–206. doi: 10.1038/nature11112. [DOI] [PubMed] [Google Scholar]
- 69.Wang X., Huang J., Zou T., Yin P. Human m6A writers: Two subunits, 2 roles. RNA Biol. 2017;14:300–304. doi: 10.1080/15476286.2017.1282025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu J., Yue Y., Han D., Wang X., Fu Y., Zhang L., Jia G., Yu M., Lu Z., Deng X., et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat. Chem. Biol. 2014;10:93–95. doi: 10.1038/nchembio.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alarcón C.R., Goodarzi H., Lee H., Liu X., Tavazoie S., Tavazoie S.F. HNRNPA2B1 Is a mediator of m6A-dependent nuclear RNA processing events. Cell. 2015;162:1299–1308. doi: 10.1016/j.cell.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu N., Dai Q., Zheng G., He C., Parisien M., Pan T. N6-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature. 2015;518:560–564. doi: 10.1038/nature14234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Edupuganti R.R., Geiger S., Lindeboom R.G.H., Shi H., Hsu P.J., Lu Z., Wang S.-Y., Baltissen M.P.A., Jansen P.W.T.C., Rossa M., et al. N6-methyladenosine (m6A) recruits and repels proteins to regulate mRNA homeostasis. Nat. Struct. Mol. Biol. 2017;24:870–878. doi: 10.1038/nsmb.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Roundtree I.A., He C. RNA epigenetics—Chemical messages for posttranscriptional gene regulation. Curr. Opin. Chem. Biol. 2016;30:46–51. doi: 10.1016/j.cbpa.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Roundtree I.A., Evans M.E., Pan T., He C. Dynamic RNA modifications in gene expression regulation. Cell. 2017;169:1187–1200. doi: 10.1016/j.cell.2017.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Loos R.J.F., Yeo G.S.H. The bigger picture of FTO: The first GWAS-identified obesity gene. Nat. Rev. Endocrinol. 2014;10:51–61. doi: 10.1038/nrendo.2013.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lewis C.J.T., Pan T., Kalsotra A. RNA modifications and structures cooperate to guide RNA-protein interactions. Nat. Rev. Mol. Cell Biol. 2017;18:202–210. doi: 10.1038/nrm.2016.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Boccaletto P., Machnicka M.A., Purta E., Piatkowski P., Baginski B., Wirecki T.K., de Crécy-Lagard V., Ross R., Limbach P.A., Kotter A., et al. MODOMICS: A database of RNA modification pathways. 2017 update. Nucleic Acids Res. 2018;46:D303–D307. doi: 10.1093/nar/gkx1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Klungland A., Dahl J.A. Dynamic RNA modifications in disease. Curr. Opin. Genet. Dev. 2014;26:47–52. doi: 10.1016/j.gde.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 80.Zhou J., Wan J., Gao X., Zhang X., Jaffrey S.R., Qian S.-B. Dynamic m6A mRNA methylation directs translational control of heat shock response. Nature. 2015;526:591–594. doi: 10.1038/nature15377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang C., Samanta D., Lu H., Bullen J.W., Zhang H., Chen I., He X., Semenza G.L. Hypoxia induces the breast cancer stem cell phenotype by HIF-dependent and ALKBH5-mediated m6A-demethylation of NANOG mRNA. Proc. Natl. Acad. Sci. USA. 2016;113:E2047–E2056. doi: 10.1073/pnas.1602883113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schwartz S., Agarwala S.D., Mumbach M.R., Jovanovic M., Mertins P., Shishkin A., Tabach Y., Mikkelsen T.S., Satija R., Ruvkun G., et al. High-resolution mapping reveals a conserved, widespread, dynamic mRNA methylation program in yeast meiosis. Cell. 2013;155:1409–1421. doi: 10.1016/j.cell.2013.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dunn D.B. The occurrence of 1-methyladenine in ribonucleic acid. Biochim. Biophys. Acta. 1961;46:198–200. doi: 10.1016/0006-3002(61)90668-0. [DOI] [PubMed] [Google Scholar]
- 84.Dai X., Wang T., Gonzalez G., Wang Y. Identification of YTH Domain-containing proteins as the readers for N1-Methyladenosine in RNA. Anal. Chem. 2018;90:6380–6384. doi: 10.1021/acs.analchem.8b01703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang X., Yang Y., Sun B.-F., Chen Y.-S., Xu J.-W., Lai W.-Y., Li A., Wang X., Bhattarai D.P., Xiao W., et al. 5-methylcytosine promotes mRNA export—NSUN2 as the methyltransferase and ALYREF as an m5C reader. Cell Res. 2017;27:606–625. doi: 10.1038/cr.2017.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang S., Kool E.T. Origins of the large differences in stability of DNA and RNA helices: C-5 methyl and 2′-hydroxyl effects. Biochemistry. 1995;34:4125–4132. doi: 10.1021/bi00012a031. [DOI] [PubMed] [Google Scholar]
- 87.Tang H., Fan X., Xing J., Liu Z., Jiang B., Dou Y., Gorospe M., Wang W. NSun2 delays replicative senescence by repressing p27 (KIP1) translation and elevating CDK1 translation. Aging. 2015;7:1143–1158. doi: 10.18632/aging.100860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schwartz S., Bernstein D.A., Mumbach M.R., Jovanovic M., Herbst R.H., León-Ricardo B.X., Engreitz J.M., Guttman M., Satija R., Lander E.S., et al. Transcriptome-wide mapping reveals widespread dynamic-regulated pseudouridylation of ncRNA and mRNA. Cell. 2014;159:148–162. doi: 10.1016/j.cell.2014.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carlile T.M., Rojas-Duran M.F., Zinshteyn B., Shin H., Bartoli K.M., Gilbert W.V. Pseudouridine profiling reveals regulated mRNA pseudouridylation in yeast and human cells. Nature. 2014;515:143–146. doi: 10.1038/nature13802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vaidyanathan P.P., AlSadhan I., Merriman D.K., Al-Hashimi H.M., Herschlag D. Pseudouridine and N6-methyladenosine modifications weaken PUF protein/RNA interactions. RNA. 2017;23:611–618. doi: 10.1261/rna.060053.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lim V.I., Curran J.F. Analysis of codon: Anticodon interactions within the ribosome provides new insights into codon reading and the genetic code structure. RNA. 2001;7:942–957. doi: 10.1017/S135583820100214X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Keegan L., Khan A., Vukic D., O’Connell M. ADAR RNA editing below the backbone. RNA. 2017;23:1317–1328. doi: 10.1261/rna.060921.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bohnsack M.T., Sloan K.E. The mitochondrial epitranscriptome: The roles of RNA modifications in mitochondrial translation and human disease. Cell. Mol. Life Sci. 2018;75:241–260. doi: 10.1007/s00018-017-2598-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liao X., Huang C., Zhang D., Wang J., Li J., Jin H., Huang C. Mitochondrial catalase induces cells transformation through nucleolin-dependent Cox-2 mRNA stabilization. Free Radic. Biol. Med. 2017;113:478–486. doi: 10.1016/j.freeradbiomed.2017.10.387. [DOI] [PubMed] [Google Scholar]
- 95.Safra M., Sas-Chen A., Nir R., Winkler R., Nachshon A., Bar-Yaacov D., Erlacher M., Rossmanith W., Stern-Ginossar N., Schwartz S. The m1A landscape on cytosolic and mitochondrial mRNA at single-base resolution. Nature. 2017;551:251–255. doi: 10.1038/nature24456. [DOI] [PubMed] [Google Scholar]
- 96.Yadav P.K., Rajasekharan R. The m6A methyltransferase Ime4 and mitochondrial functions in yeast. Curr. Genet. 2018;64:353–357. doi: 10.1007/s00294-017-0758-8. [DOI] [PubMed] [Google Scholar]
- 97.Rüdinger M., Funk H.T., Rensing S.A., Maier U.G., Knoop V. RNA editing: Only eleven sites are present in the Physcomitrella patens mitochondrial transcriptome and a universal nomenclature proposal. Mol. Genet. Genom. 2009;281:473–481. doi: 10.1007/s00438-009-0424-z. [DOI] [PubMed] [Google Scholar]
- 98.Adams B.D., Parsons C., Walker L., Zhang W.C., Slack F.J. Targeting noncoding RNAs in disease. J. Clin. Investig. 2017;127:761–771. doi: 10.1172/JCI84424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang D., Xiong M., Xu C., Xiang P., Zhong X. Long noncoding RNAs: An overview. Methods Mol. Biol. 2016;1402:287–295. doi: 10.1007/978-1-4939-3378-5_22. [DOI] [PubMed] [Google Scholar]
- 100.Pan T. Modifications and functional genomics of human transfer RNA. Cell Res. 2018;28:395–404. doi: 10.1038/s41422-018-0013-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chatterjee K., Nostramo R.T., Wan Y., Hopper A.K. tRNA dynamics between the nucleus, cytoplasm and mitochondrial surface: Location, location, location. Biochim. Biophys. Acta. 2018;1861:373–386. doi: 10.1016/j.bbagrm.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sissler M., González-Serrano L.E., Westhof E. Recent advances in mitochondrial aminoacyl-tRNA synthetases and disease. Trends Mol. Med. 2017;23:693–708. doi: 10.1016/j.molmed.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 103.Greco C.M., Condorelli G. Epigenetic modifications and noncoding RNAs in cardiac hypertrophy and failure. Nat. Rev. Cardiol. 2015;12:488–497. doi: 10.1038/nrcardio.2015.71. [DOI] [PubMed] [Google Scholar]
- 104.Shafik A., Schumann U., Evers M., Sibbritt T., Preiss T. The emerging epitranscriptomics of long noncoding RNAs. Biochim. Biophys. Acta. 2016;1859:59–70. doi: 10.1016/j.bbagrm.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 105.Pepin G., Gantier M.P. microRNA Decay: Refining microRNA regulatory activity. Microrna. 2016;5:167–174. doi: 10.2174/2211536605666161027165915. [DOI] [PubMed] [Google Scholar]
- 106.Tuorto F., Lyko F. Genome recoding by tRNA modifications. Open Biol. 2016;6 doi: 10.1098/rsob.160287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Niu Y., Zhao X., Wu Y.-S., Li M.-M., Wang X.-J., Yang Y.-G. N6-methyl-adenosine (m6A) in RNA: An old modification with a novel epigenetic function. Genom. Proteom. Bioinform. 2013;11:8–17. doi: 10.1016/j.gpb.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Choi J., Indrisiunaite G., DeMirci H., Ieong K.-W., Wang J., Petrov A., Prabhakar A., Rechavi G., Dominissini D., He C., et al. 2′-O-methylation in mRNA disrupts tRNA decoding during translation elongation. Nat. Struct. Mol. Biol. 2018;25:208–216. doi: 10.1038/s41594-018-0030-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Asano K., Suzuki T., Saito A., Wei F.-Y., Ikeuchi Y., Numata T., Tanaka R., Yamane Y., Yamamoto T., Goto T., et al. Metabolic and chemical regulation of tRNA modification associated with taurine deficiency and human disease. Nucleic Acids Res. 2018;46:1565–1583. doi: 10.1093/nar/gky068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu R.-J., Zhou M., Fang Z.-P., Wang M., Zhou X.-L., Wang E.-D. The tRNA recognition mechanism of the minimalist SPOUT methyltransferase, TrmL. Nucleic Acids Res. 2013;41:7828–7842. doi: 10.1093/nar/gkt568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Begley U., Sosa M.S., Avivar-Valderas A., Patil A., Endres L., Estrada Y., Chan C.T.Y., Su D., Dedon P.C., Aguirre-Ghiso J.A., et al. A human tRNA methyltransferase 9-like protein prevents tumour growth by regulating LIN9 and HIF1-α. EMBO Mol. Med. 2013;5:366–383. doi: 10.1002/emmm.201201161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lentini J.M., Ramos J., Fu D. Monitoring the 5-methoxycarbonylmethyl-2-thiouridine (mcm5s2U) modification in eukaryotic tRNAs via the γ-toxin endonuclease. RNA. 2018;24:749–758. doi: 10.1261/rna.065581.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Oerum S., Dégut C., Barraud P., Tisné C. m1A Post-transcriptional modification in tRNAs. Biomolecules. 2017;7 doi: 10.3390/biom7010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kawarada L., Suzuki T., Ohira T., Hirata S., Miyauchi K., Suzuki T. ALKBH1 is an RNA dioxygenase responsible for cytoplasmic and mitochondrial tRNA modifications. Nucleic Acids Res. 2017;45:7401–7415. doi: 10.1093/nar/gkx354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pang P., Deng X., Wang Z., Xie W. Structural and biochemical insights into the 2′-O-methylation of pyrimidines 34 in tRNA. FEBS J. 2017;284:2251–2263. doi: 10.1111/febs.14120. [DOI] [PubMed] [Google Scholar]
- 116.Chen H.M., Wang J., Zhang Y.F., Gao Y.H. Ovarian cancer proliferation and apoptosis are regulated by human transfer RNA methyltransferase 9-likevia LIN9. Oncol. Lett. 2017;14:4461–4466. doi: 10.3892/ol.2017.6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bourgeois G., Létoquart J., van Tran N., Graille M. Trm112, a protein activator of methyltransferases modifying actors of the eukaryotic translational apparatus. Biomolecules. 2017;7 doi: 10.3390/biom7010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Xu L., Liu X., Sheng N., Oo K.S., Liang J., Chionh Y.H., Xu J., Ye F., Gao Y.-G., Dedon P.C., et al. Three distinct 3-methylcytidine (m3C) methyltransferases modify tRNA and mRNA in mice and humans. J. Biol. Chem. 2017;292:14695–14703. doi: 10.1074/jbc.M117.798298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bednářová A., Hanna M., Durham I., VanCleave T., England A., Chaudhuri A., Krishnan N. Lost in translation: defects in transfer RNA modifications and neurological disorders. Front. Mol. Neurosci. 2017;10:135. doi: 10.3389/fnmol.2017.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bento-Abreu A., Jager G., Swinnen B., Rué L., Hendrickx S., Jones A., Staats K.A., Taes I., Eykens C., Nonneman A., et al. Elongator subunit 3 (ELP3) modifies ALS through tRNA modification. Hum. Mol. Genet. 2018;27:1276–1289. doi: 10.1093/hmg/ddy043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Suzuki T., Nagao A., Suzuki T. Human mitochondrial tRNAs: Biogenesis, function, structural aspects, and diseases. Annu. Rev. Genet. 2011;45:299–329. doi: 10.1146/annurev-genet-110410-132531. [DOI] [PubMed] [Google Scholar]
- 122.Villanueva M.T. Genetics: The Importance of Mitochondrial tRNA. [(accessed on 12 June 2018)]; Available online: https://www.nature.com/articles/nrc3997.
- 123.Morscher R.J., Ducker G.S., Li S.H.-J., Mayer J.A., Gitai Z., Sperl W., Rabinowitz J.D. Mitochondrial translation requires folate-dependent tRNA methylation. Nature. 2018;554:128–132. doi: 10.1038/nature25460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Trixl L., Amort T., Wille A., Zinni M., Ebner S., Hechenberger C., Eichin F., Gabriel H., Schoberleitner I., Huang A., et al. RNA cytosine methyltransferase Nsun3 regulates embryonic stem cell differentiation by promoting mitochondrial activity. Cell. Mol. Life Sci. 2018;75:1483–1497. doi: 10.1007/s00018-017-2700-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Erales J., Marchand V., Panthu B., Gillot S., Belin S., Ghayad S.E., Garcia M., Laforêts F., Marcel V., Baudin-Baillieu A., et al. Evidence for rRNA 2′-O-methylation plasticity: Control of intrinsic translational capabilities of human ribosomes. Proc. Natl. Acad. Sci. USA. 2017;114:12934–12939. doi: 10.1073/pnas.1707674114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Garone C., D’Souza A.R., Dallabona C., Lodi T., Rebelo-Guiomar P., Rorbach J., Donati M.A., Procopio E., Montomoli M., Guerrini R., et al. Defective mitochondrial rRNA methyltransferase MRM2 causes MELAS-like clinical syndrome. Hum. Mol. Genet. 2017;26:4257–4266. doi: 10.1093/hmg/ddx314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sergiev P.V., Aleksashin N.A., Chugunova A.A., Polikanov Y.S., Dontsova O.A. Structural and evolutionary insights into ribosomal RNA methylation. Nat. Chem. Biol. 2018;14:226–235. doi: 10.1038/nchembio.2569. [DOI] [PubMed] [Google Scholar]
- 128.Natchiar S.K., Myasnikov A.G., Kratzat H., Hazemann I., Klaholz B.P. Visualization of chemical modifications in the human 80S ribosome structure. Nature. 2017;551:472–477. doi: 10.1038/nature24482. [DOI] [PubMed] [Google Scholar]
- 129.Tillault A.-S., Schultz S.K., Wieden H.-J., Kothe U. Molecular Determinants for 23S rRNA Recognition and modification by the E. coli Pseudouridine Synthase RluE. J. Mol. Biol. 2018;430:1284–1294. doi: 10.1016/j.jmb.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 130.Yokoyama W., Hirota K., Wan H., Sumi N., Miyata M., Araoi S., Nomura N., Kako K., Fukamizu A. rRNA adenine methylation requires T07A9.8 gene as rram-1 in Caenorhabditis elegans. J. Biochem. 2018;163:465–474. doi: 10.1093/jb/mvy018. [DOI] [PubMed] [Google Scholar]
- 131.Murakami S., Suzuki T., Yokoyama W., Yagi S., Matsumura K., Nakajima Y., Harigae H., Fukamizu A., Motohashi H. Nucleomethylin deficiency impairs embryonic erythropoiesis. J. Biochem. 2018;163:413–423. doi: 10.1093/jb/mvx086. [DOI] [PubMed] [Google Scholar]
- 132.Bar-Yaacov D., Frumkin I., Yashiro Y., Chujo T., Ishigami Y., Chemla Y., Blumberg A., Schlesinger O., Bieri P., Greber B., et al. Correction: Mitochondrial 16S rRNA is methylated by tRNA methyltransferase TRMT61B in all vertebrates. PLoS Biol. 2017;15:e1002594. doi: 10.1371/journal.pbio.1002594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Antonicka H., Choquet K., Lin Z.-Y., Gingras A.-C., Kleinman C.L., Shoubridge E.A. A pseudouridine synthase module is essential for mitochondrial protein synthesis and cell viability. EMBO Rep. 2017;18:28–38. doi: 10.15252/embr.201643391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chigusa S., Moroi T., Shoji Y. State-of-the-art calculation of the decay rate of electroweak vacuum in the standard model. Phys. Rev. Lett. 2017;119:211801. doi: 10.1103/PhysRevLett.119.211801. [DOI] [PubMed] [Google Scholar]
- 135.Hombach S., Kretz M. Non-coding RNAs: Classification, biology and functioning. Adv. Exp. Med. Biol. 2016;937:3–17. doi: 10.1007/978-3-319-42059-2_1. [DOI] [PubMed] [Google Scholar]
- 136.Zhai J., Meyers B.C. Deep sequencing from hen1 mutants to identify small RNA 3′ modifications. Cold Spring Harb. Symp. Quant. Biol. 2012;77:213–219. doi: 10.1101/sqb.2013.77.014779. [DOI] [PubMed] [Google Scholar]
- 137.Shoshan E., Mobley A.K., Braeuer R.R., Kamiya T., Huang L., Vasquez M.E., Salameh A., Lee H.J., Kim S.J., Ivan C., et al. Reduced adenosine-to-inosine miR-455-5p editing promotes melanoma growth and metastasis. Nat. Cell Biol. 2015;17:311–321. doi: 10.1038/ncb3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhou C., Molinie B., Daneshvar K., Pondick J.V., Wang J., Van Wittenberghe N., Xing Y., Giallourakis C.C., Mullen A.C. Genome-wide maps of m6A circRNAs Identify widespread and Cell-Type-specific methylation patterns that are distinct from mRNAs. Cell Rep. 2017;20:2262–2276. doi: 10.1016/j.celrep.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cantara W.A., Crain P.F., Rozenski J., McCloskey J.A., Harris K.A., Zhang X., Vendeix F.A.P., Fabris D., Agris P.F. The RNA Modification Database, RNAMDB: 2011 update. Nucleic Acids Res. 2011;39:D195–D201. doi: 10.1093/nar/gkq1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liu H., Flores M.A., Meng J., Zhang L., Zhao X., Rao M.K., Chen Y., Huang Y. MeT-DB: A database of transcriptome methylation in mammalian cells. Nucleic Acids Res. 2015;43:D197–D203. doi: 10.1093/nar/gku1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xuan J.-J., Sun W.-J., Lin P.-H., Zhou K.-R., Liu S., Zheng L.-L., Qu L.-H., Yang J.-H. RMBase v2.0: Deciphering the map of RNA modifications from epitranscriptome sequencing data. Nucleic Acids Res. 2018;46:D327–D334. doi: 10.1093/nar/gkx934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Crain P.F., McCloskey J.A. The RNA modification database. Nucleic Acids Res. 1997;25:126–127. doi: 10.1093/nar/25.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Aduri R., Psciuk B.T., Saro P., Taniga H., Schlegel H.B., SantaLucia J. AMBER force field parameters for the naturally occurring modified nucleosides in RNA. J. Chem. Theory Comput. 2007;3:1464–1475. doi: 10.1021/ct600329w. [DOI] [PubMed] [Google Scholar]
- 144.McDowell S.E., Špačková N., Šponer J., Walter N.G. Molecular Dynamics simulations of RNA: An in silico single molecule approach. Biopolymers. 2007;85:169–184. doi: 10.1002/bip.20620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dudek M., Trylska J. Molecular Dynamics Simulations of l-RNA involving homo- and heterochiral complexes. J. Chem. Theory Comput. 2017;13:1244–1253. doi: 10.1021/acs.jctc.6b01075. [DOI] [PubMed] [Google Scholar]
- 146.Verona M.D., Verdolino V., Palazzesi F., Corradini R. Focus on PNA Flexibility and RNA binding using molecular dynamics and metadynamics. Sci. Rep. 2017;7:42799. doi: 10.1038/srep42799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dominissini D., Moshitch-Moshkovitz S., Salmon-Divon M., Amariglio N., Rechavi G. Transcriptome-wide mapping of N6-methyladenosine by m6A-seq based on immunocapturing and massively parallel sequencing. Nat. Protoc. 2013;8:176–189. doi: 10.1038/nprot.2012.148. [DOI] [PubMed] [Google Scholar]
- 148.Meng J., Cui X., Rao M.K., Chen Y., Huang Y. Exome-based analysis for RNA epigenome sequencing data. Bioinformatics. 2013;29:1565–1567. doi: 10.1093/bioinformatics/btt171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang Y., Liu T., Meyer C.A., Eeckhoute J., Johnson D.S., Bernstein B.E., Nusbaum C., Myers R.M., Brown M., Li W., et al. Model-based analysis of ChIP-Seq (MACS) Genome Biol. 2008;9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Feng J., Liu T., Qin B., Zhang Y., Liu X.S. Identifying ChIP-seq enrichment using MACS. Nat. Protoc. 2012;7:1728–1740. doi: 10.1038/nprot.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sun W.-J., Li J.-H., Liu S., Wu J., Zhou H., Qu L.-H., Yang J.-H. RMBase: A resource for decoding the landscape of RNA modifications from high-throughput sequencing data. Nucleic Acids Res. 2016;44:D259–D265. doi: 10.1093/nar/gkv1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Vapnik V.N. An overview of statistical learning theory. IEEE Trans. Neural Netw. 1999;10:988–999. doi: 10.1109/72.788640. [DOI] [PubMed] [Google Scholar]
- 153.Kuksa P.P., Leung Y.Y., Vandivier L.E., Anderson Z., Gregory B.D., Wang L.-S. In silico identification of rna modifications from high-throughput sequencing data using HAMR. Methods Mol. Biol. 2017;1562:211–229. doi: 10.1007/978-1-4939-6807-7_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chen W., Lin H. Recent Advances in Identification of RNA Modifications. Noncoding RNA. 2016;3 doi: 10.3390/ncrna3010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chen W., Feng P., Ding H., Lin H. PAI: Predicting adenosine to inosine editing sites by using pseudo nucleotide compositions. Sci. Rep. 2016;6:35123. doi: 10.1038/srep35123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chen W., Feng P., Yang H., Ding H., Lin H., Chou K.-C. iRNA-AI: Identifying the adenosine to inosine editing sites in RNA sequences. Oncotarget. 2017;8:4208–4217. doi: 10.18632/oncotarget.13758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chen W., Feng P., Tang H., Ding H., Lin H. RAMPred: Identifying the N1-methyladenosine sites in eukaryotic transcriptomes. Sci. Rep. 2016;6:31080. doi: 10.1038/srep31080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chen W., Feng P., Yang H., Ding H., Lin H., Chou K.-C. iRNA-3typeA: Identifying three types of modification at RNA’s adenosine sites. Mol. Ther. Nucleic Acids. 2018;11:468–474. doi: 10.1016/j.omtn.2018.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Feng P., Ding H., Yang H., Chen W., Lin H., Chou K.-C. iRNA-PseColl: Identifying the occurrence sites of different RNA modifications by incorporating collective effects of nucleotides into PseKNC. Mol. Ther. Nucleic Acids. 2017;7:155–163. doi: 10.1016/j.omtn.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Qiu W.-R., Jiang S.-Y., Xu Z.-C., Xiao X., Chou K.-C. iRNAm5C-PseDNC: Identifying RNA 5-methylcytosine sites by incorporating physical-chemical properties into pseudo dinucleotide composition. Oncotarget. 2017;8:41178–41188. doi: 10.18632/oncotarget.17104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chen W., Feng P., Ding H., Lin H., Chou K.-C. iRNA-Methyl: Identifying N6-methyladenosine sites using pseudo nucleotide composition. Anal. Biochem. 2015;490:26–33. doi: 10.1016/j.ab.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 162.Chen W., Tran H., Liang Z., Lin H., Zhang L. Identification and analysis of the N6-methyladenosine in the Saccharomyces cerevisiae transcriptome. Sci. Rep. 2015;5:13859. doi: 10.1038/srep13859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chen W., Tang H., Lin H. MethyRNA: A web server for identification of N6-methyladenosine sites. J. Biomol. Struct. Dyn. 2017;35:683–687. doi: 10.1080/07391102.2016.1157761. [DOI] [PubMed] [Google Scholar]
- 164.Zhou Y., Zeng P., Li Y.-H., Zhang Z., Cui Q. SRAMP: Prediction of mammalian N6-methyladenosine (m6A) sites based on sequence-derived features. Nucleic Acids Res. 2016;44:e91. doi: 10.1093/nar/gkw104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chen W., Xing P., Zou Q. Detecting N6-methyladenosine sites from RNA transcriptomes using ensemble Support Vector Machines. Sci. Rep. 2017;7:40242. doi: 10.1038/srep40242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Li Y.-H., Zhang G., Cui Q. PPUS: A web server to predict PUS-specific pseudouridine sites. Bioinformatics. 2015;31:3362–3364. doi: 10.1093/bioinformatics/btv366. [DOI] [PubMed] [Google Scholar]
- 167.Chen W., Tang H., Ye J., Lin H., Chou K.-C. iRNA-PseU: Identifying RNA pseudouridine sites. Mol. Ther. Nucleic Acids. 2016;5:e332. doi: 10.1038/mtna.2016.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Panwar B., Raghava G.P.S. Prediction of uridine modifications in tRNA sequences. BMC Bioinform. 2014;15:326. doi: 10.1186/1471-2105-15-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Machnicka M.A., Dunin-Horkawicz S., de Crécy-Lagard V., Bujnicki J.M. tRNAmodpred: A computational method for predicting posttranscriptional modifications in tRNAs. Methods. 2016;107:34–41. doi: 10.1016/j.ymeth.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jopling C., Boue S., Izpisua Belmonte J.C. Dedifferentiation, transdifferentiation and reprogramming: Three routes to regeneration. Nat. Rev. Mol. Cell Biol. 2011;12:79–89. doi: 10.1038/nrm3043. [DOI] [PubMed] [Google Scholar]
- 171.Orlacchio A., Bernardi G., Orlacchio A., Martino S. Stem cells and neurological diseases. Discov. Med. 2010;9:546–553. [PubMed] [Google Scholar]
- 172.Orlacchio A., Bernardi G., Orlacchio A., Martino S. Stem cells: An overview of the current status of therapies for central and peripheral nervous system diseases. Curr. Med. Chem. 2010;17:595–608. doi: 10.2174/092986710790416272. [DOI] [PubMed] [Google Scholar]
- 173.Weinberger L., Ayyash M., Novershtern N., Hanna J.H. Dynamic stem cell states: Naive to primed pluripotency in rodents and humans. Nat. Rev. Mol. Cell Biol. 2016;17:155–169. doi: 10.1038/nrm.2015.28. [DOI] [PubMed] [Google Scholar]
- 174.Morena F., Armentano I., Montanucci P., Argentati C., Fortunati E., Montesano S., Bicchi I., Pescara T., Pennoni I., Mattioli S., et al. Design of a nanocomposite substrate inducing adult stem cell assembly and progression toward an Epiblast-like or Primitive Endoderm-like phenotype via mechanotransduction. Biomaterials. 2017;144:211–229. doi: 10.1016/j.biomaterials.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 175.Nichols J., Smith A. Naive and primed pluripotent states. Cell Stem Cell. 2009;4:487–492. doi: 10.1016/j.stem.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 176.Evans M.J., Kaufman M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 177.Doğan A. Embryonic stem cells in development and regenerative medicine. Adv. Exp. Med. Biol. 2018 doi: 10.1007/5584_2018_175. [DOI] [PubMed] [Google Scholar]
- 178.Clevers H., Watt F.M. Defining adult stem cells by function, not by phenotype. Annu. Rev. Biochem. 2018 doi: 10.1146/annurev-biochem-062917-012341. [DOI] [PubMed] [Google Scholar]
- 179.Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 180.Meissner A., Wernig M., Jaenisch R. Direct reprogramming of genetically unmodified fibroblasts into pluripotent stem cells. Nat. Biotechnol. 2007;25:1177–1181. doi: 10.1038/nbt1335. [DOI] [PubMed] [Google Scholar]
- 181.Martin U. Therapeutic application of pluripotent stem cells: challenges and risks. Front. Med. 2017;4:229. doi: 10.3389/fmed.2017.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.De Lázaro I., Kostarelos K. Engineering cell fate for tissue regeneration by in vivo transdifferentiation. Stem Cell Rev. Rep. 2016;12:129–139. doi: 10.1007/s12015-015-9624-6. [DOI] [PubMed] [Google Scholar]
- 183.Wu R., Hu X., Wang J. Concise review: optimized strategies for stem cell-based therapy in myocardial repair: Clinical translatability and potential limitation. Stem Cells. 2018 doi: 10.1002/stem.2778. [DOI] [PubMed] [Google Scholar]
- 184.Satthenapalli V.R., Lamberts R.R., Katare R.G. Concise review: challenges in regenerating the diabetic Heart: A comprehensive review. Stem Cells. 2017;35:2009–2026. doi: 10.1002/stem.2661. [DOI] [PubMed] [Google Scholar]
- 185.Ciccocioppo R., Cangemi G.C., Kruzliak P., Corazza G.R. Concise Review: Cellular Therapies: The potential to regenerate and restore tolerance in immune-mediated intestinal diseases. Stem Cells. 2016;34:1474–1486. doi: 10.1002/stem.2367. [DOI] [PubMed] [Google Scholar]
- 186.Martino S., Montesano S., di Girolamo I., Tiribuzi R., Di Gregorio M., Orlacchio A., Datti A., Calabresi P., Sarchielli P., Orlacchio A. Expression of cathepsins S and D signals a distinctive biochemical trait in CD34+ hematopoietic stem cells of relapsing-remitting multiple sclerosis patients. Mult. Scler. 2013;19:1443–1453. doi: 10.1177/1352458513477230. [DOI] [PubMed] [Google Scholar]
- 187.Sessa M., Lorioli L., Fumagalli F., Acquati S., Redaelli D., Baldoli C., Canale S., Lopez I.D., Morena F., Calabria A., et al. Lentiviral haemopoietic stem-cell gene therapy in early-onset metachromatic leukodystrophy: An ad-hoc analysis of a non-randomised, open-label, phase 1/2 trial. Lancet. 2016;388:476–487. doi: 10.1016/S0140-6736(16)30374-9. [DOI] [PubMed] [Google Scholar]
- 188.Ricca A., Rufo N., Ungari S., Morena F., Martino S., Kulik W., Alberizzi V., Bolino A., Bianchi F., Del Carro U., et al. Combined gene/cell therapies provide long-term and pervasive rescue of multiple pathological symptoms in a murine model of globoid cell leukodystrophy. Hum. Mol. Genet. 2015;24:3372–3389. doi: 10.1093/hmg/ddv086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ungari S., Montepeloso A., Morena F., Cocchiarella F., Recchia A., Martino S., Gentner B., Naldini L., Biffi A. Design of a regulated lentiviral vector for hematopoietic stem cell gene therapy of globoid cell leukodystrophy. Mol. Ther. Methods Clin. Dev. 2015;2:15038. doi: 10.1038/mtm.2015.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Biffi A., Montini E., Lorioli L., Cesani M., Fumagalli F., Plati T., Baldoli C., Martino S., Calabria A., Canale S., et al. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science. 2013;341:1233158. doi: 10.1126/science.1233158. [DOI] [PubMed] [Google Scholar]
- 191.Visigalli I., Ungari S., Martino S., Park H., Cesani M., Gentner B., Sergi Sergi L., Orlacchio A., Naldini L., Biffi A. The galactocerebrosidase enzyme contributes to the maintenance of a functional hematopoietic stem cell niche. Blood. 2010;116:1857–1866. doi: 10.1182/blood-2009-12-256461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Morena F., di Girolamo I., Emiliani C., Gritti A., Biffi A., Martino S. A new analytical bench assay for the determination of arylsulfatase a activity toward galactosyl-3-sulfate ceramide: Implication for metachromatic leukodystrophy diagnosis. Anal. Chem. 2014;86:473–481. doi: 10.1021/ac4023555. [DOI] [PubMed] [Google Scholar]
- 193.Meneghini V., Frati G., Sala D., De Cicco S., Luciani M., Cavazzin C., Paulis M., Mentzen W., Morena F., Giannelli S., et al. Generation of human induced pluripotent stem cell-derived bona fide neural stem cells for ex vivo gene therapy of metachromatic leukodystrophy. Stem Cells Transl. Med. 2017;6:352–368. doi: 10.5966/sctm.2015-0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Martino S., di Girolamo I., Cavazzin C., Tiribuzi R., Galli R., Rivaroli A., Valsecchi M., Sandhoff K., Sonnino S., Vescovi A., et al. Neural precursor cell cultures from GM2 gangliosidosis animal models recapitulate the biochemical and molecular hallmarks of the brain pathology. J. Neurochem. 2009;109:135–147. doi: 10.1111/j.1471-4159.2009.05919.x. [DOI] [PubMed] [Google Scholar]
- 195.Calbi V., Fumagalli F., Consiglieri G., Penati R., Acquati S., Redaelli D., Attanasio V., Marcella F., Cicalese M.P., Migliavacca M., et al. Use of Defibrotide to help prevent post-transplant endothelial injury in a genetically predisposed infant with metachromatic leukodystrophy undergoing hematopoietic stem cell gene therapy. Bone Marrow Transplant. 2018 doi: 10.1038/s41409-017-0085-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Martino S., D’Angelo F., Armentano I., Kenny J.M., Orlacchio A. Stem cell-biomaterial interactions for regenerative medicine. Biotechnol. Adv. 2012;30:338–351. doi: 10.1016/j.biotechadv.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 197.Morena F., Argentati C., Calzoni E., Cordellini M., Emiliani C., D’Angelo F., Martino S. Ex-Vivo tissues engineering modeling for reconstructive surgery using human adult adipose stem cells and polymeric Nanostructured Matrix. Nanomaterials. 2016;6 doi: 10.3390/nano6040057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Martino S., D’Angelo F., Armentano I., Tiribuzi R., Pennacchi M., Dottori M., Mattioli S., Caraffa A., Cerulli G.G., Kenny J.M., et al. Hydrogenated amorphous carbon nanopatterned film designs drive human bone marrow mesenchymal stem cell cytoskeleton architecture. Tissue Eng. Part A. 2009;15:3139–3149. doi: 10.1089/ten.tea.2008.0552. [DOI] [PubMed] [Google Scholar]
- 199.Tarpani L., Morena F., Gambucci M., Zampini G., Massaro G., Argentati C., Emiliani C., Martino S., Latterini L. The influence of modified silica nanomaterials on adult stem cell culture. Nanomaterials. 2016;6 doi: 10.3390/nano6060104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Argentati C., Morena F., Montanucci P., Rallini M., Basta G., Calabrese N., Calafiore R., Cordellini M., Emiliani C., Armentano I., et al. Surface hydrophilicity of poly (l-Lactide) acid polymer film changes the human adult adipose stem cell architecture. Polymers. 2018;10:140. doi: 10.3390/polym10020140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Islam F., Qiao B., Smith R.A., Gopalan V., Lam A.K.-Y. Cancer stem cell: Fundamental experimental pathological concepts and updates. Exp. Mol. Pathol. 2015;98:184–191. doi: 10.1016/j.yexmp.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 202.Sampieri K., Fodde R. Cancer stem cells and metastasis. Semin. Cancer Biol. 2012;22:187–193. doi: 10.1016/j.semcancer.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 203.Badve S., Nakshatri H. Breast-cancer stem cells-beyond semantics. Lancet Oncol. 2012;13:e43–e48. doi: 10.1016/S1470-2045(11)70191-7. [DOI] [PubMed] [Google Scholar]
- 204.Barrero M.J., Boué S., Izpisúa Belmonte J.C. Epigenetic mechanisms that regulate cell identity. Cell Stem Cell. 2010;7:565–570. doi: 10.1016/j.stem.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 205.Tee W.-W., Reinberg D. Chromatin features and the epigenetic regulation of pluripotency states in ESCs. Development. 2014;141:2376–2390. doi: 10.1242/dev.096982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Takata N., Eiraku M. Stem cells and genome editing: Approaches to tissue regeneration and regenerative medicine. J. Hum. Genet. 2018;63:165–178. doi: 10.1038/s10038-017-0348-0. [DOI] [PubMed] [Google Scholar]
- 207.Paksa A., Rajagopal J. The epigenetic basis of cellular plasticity. Curr. Opin. Cell Biol. 2018;49:116–122. doi: 10.1016/j.ceb.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hong C.P., Park J., Roh T.-Y. Epigenetic regulation in cell reprogramming revealed by genome-wide analysis. Epigenomics. 2011;3:73–81. doi: 10.2217/epi.10.72. [DOI] [PubMed] [Google Scholar]
- 209.Basanta-Sanchez M., Temple S., Ansari S.A., D’Amico A., Agris P.F. Attomole quantification and global profile of RNA modifications: Epitranscriptome of human neural stem cells. Nucleic Acids Res. 2016;44:e26. doi: 10.1093/nar/gkv971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hattangadi S.M., Wong P., Zhang L., Flygare J., Lodish H.F. From stem cell to red cell: Regulation of erythropoiesis at multiple levels by multiple proteins, RNAs, and chromatin modifications. Blood. 2011;118:6258–6268. doi: 10.1182/blood-2011-07-356006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Frye M., Blanco S. Post-transcriptional modifications in development and stem cells. Development. 2016;143:3871–3881. doi: 10.1242/dev.136556. [DOI] [PubMed] [Google Scholar]
- 212.Guo M., Liu X., Zheng X., Huang Y., Chen X. m6A RNA modification determines cell fate by regulating mRNA Degradation. Cell Reprogr. 2017;19:225–231. doi: 10.1089/cell.2016.0041. [DOI] [PubMed] [Google Scholar]
- 213.Aguilo F., Walsh M.J. The N6-Methyladenosine RNA modification in pluripotency and reprogramming. Curr. Opin. Genet. Dev. 2017;46:77–82. doi: 10.1016/j.gde.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Weng Y.-L., Wang X., An R., Cassin J., Vissers C., Liu Y., Liu Y., Xu T., Wang X., Wong S.Z.H., et al. Epitranscriptomic m6A Regulation of axon regeneration in the adult mammalian nervous system. Neuron. 2018;97:313–325. doi: 10.1016/j.neuron.2017.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Geula S., Moshitch-Moshkovitz S., Dominissini D., Mansour A.A., Kol N., Salmon-Divon M., Hershkovitz V., Peer E., Mor N., Manor Y.S., et al. m6A mRNA methylation facilitates resolution of naïve pluripotency toward differentiation. Science. 2015;347:1002–1006. doi: 10.1126/science.1261417. [DOI] [PubMed] [Google Scholar]
- 216.Aguilo F., Zhang F., Sancho A., Fidalgo M., Di Cecilia S., Vashisht A., Lee D.-F., Chen C.-H., Rengasamy M., Andino B., et al. Coordination of m6A mRNA methylation and gene transcription by ZFP217 regulates pluripotency and reprogramming. Cell Stem Cell. 2015;17:689–704. doi: 10.1016/j.stem.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wang Y., Li Y., Yue M., Wang J., Kumar S., Wechsler-Reya R.J., Zhang Z., Ogawa Y., Kellis M., Duester G., et al. N6-methyladenosine RNA modification regulates embryonic neural stem cell self-renewal through histone modifications. Nat. Neurosci. 2018;21:195–206. doi: 10.1038/s41593-017-0057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Lu L., Katsaros D., de la Longrais I.A.R., Sochirca O., Yu H. Hypermethylation of let-7a-3 in epithelial ovarian cancer is associated with low insulin-like growth factor-II expression and favorable prognosis. Cancer Res. 2007;67:10117–10122. doi: 10.1158/0008-5472.CAN-07-2544. [DOI] [PubMed] [Google Scholar]
- 219.Xu K., Yang Y., Feng G.-H., Sun B.-F., Chen J.-Q., Li Y.-F., Chen Y.-S., Zhang X.-X., Wang C.-X., Jiang L.-Y., et al. Mettl3-mediated m6A regulates spermatogonial differentiation and meiosis initiation. Cell Res. 2017;27:1100–1114. doi: 10.1038/cr.2017.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Bertero A., Brown S., Madrigal P., Osnato A., Ortmann D., Yiangou L., Kadiwala J., Hubner N.C., de Los Mozos I.R., Sadée C., et al. The SMAD2/3 interactome reveals that TGFβ controls m6A mRNA methylation in pluripotency. Nature. 2018;555:256–259. doi: 10.1038/nature25784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Batista P.J., Molinie B., Wang J., Qu K., Zhang J., Li L., Bouley D.M., Lujan E., Haddad B., Daneshvar K., et al. m6A RNA modification controls cell fate transition in mammalian embryonic stem cells. Cell Stem Cell. 2014;15:707–719. doi: 10.1016/j.stem.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Yoon K.-J., Ringeling F.R., Vissers C., Jacob F., Pokrass M., Jimenez-Cyrus D., Su Y., Kim N.-S., Zhu Y., Zheng L., et al. Temporal control of mammalian cortical neurogenesis by m6A Methylation. Cell. 2017;171:877–889. doi: 10.1016/j.cell.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Yi C., Pan T. Cellular dynamics of RNA modification. Acc. Chem. Res. 2011;44:1380–1388. doi: 10.1021/ar200057m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Vu L.P., Pickering B.F., Cheng Y., Zaccara S., Nguyen D., Minuesa G., Chou T., Chow A., Saletore Y., MacKay M., et al. The N6-methyladenosine (m6A)-forming enzyme METTL3 controls myeloid differentiation of normal hematopoietic and leukemia cells. Nat. Med. 2017;23:1369–1376. doi: 10.1038/nm.4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kudou K., Komatsu T., Nogami J., Maehara K., Harada A., Saeki H., Oki E., Maehara Y., Ohkawa Y. The requirement of Mettl3-promoted MyoD mRNA maintenance in proliferative myoblasts for skeletal muscle differentiation. Open Biol. 2017;7 doi: 10.1098/rsob.170119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Meyer K.D., Saletore Y., Zumbo P., Elemento O., Mason C.E., Jaffrey S.R. Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell. 2012;149:1635–1646. doi: 10.1016/j.cell.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Zhang C., Chen Y., Sun B., Wang L., Yang Y., Ma D., Lv J., Heng J., Ding Y., Xue Y., et al. m6A modulates haematopoietic stem and progenitor cell specification. Nature. 2017;549:273–276. doi: 10.1038/nature23883. [DOI] [PubMed] [Google Scholar]
- 228.Wang X., Lu Z., Gomez A., Hon G.C., Yue Y., Han D., Fu Y., Parisien M., Dai Q., Jia G., et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014;505:117–120. doi: 10.1038/nature12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Wu Y., Zhang S., Yuan Q. N6-Methyladenosine Methyltransferases and Demethylases: New regulators of stem cell pluripotency and differentiation. Stem Cells Dev. 2016;25:1050–1059. doi: 10.1089/scd.2016.0062. [DOI] [PubMed] [Google Scholar]
- 230.Weng H., Huang H., Wu H., Qin X., Zhao B.S., Dong L., Shi H., Skibbe J., Shen C., Hu C., et al. METTL14 inhibits hematopoietic stem/progenitor differentiation and promotes leukemogenesis via mRNA m6A modification. Cell Stem Cell. 2018;22:191.e9–205.e9. doi: 10.1016/j.stem.2017.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Barbieri I., Tzelepis K., Pandolfini L., Shi J., Millán-Zambrano G., Robson S.C., Aspris D., Migliori V., Bannister A.J., Han N., et al. Promoter-bound METTL3 maintains myeloid leukaemia by m6A-dependent translation control. Nature. 2017;552:126–131. doi: 10.1038/nature24678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Cui Q., Shi H., Ye P., Li L., Qu Q., Sun G., Sun G., Lu Z., Huang Y., Yang C.-G., et al. m6A RNA methylation regulates the self-renewal and tumorigenesis of glioblastoma stem cells. Cell Rep. 2017;18:2622–2634. doi: 10.1016/j.celrep.2017.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Zheng G., Dahl J.A., Niu Y., Fedorcsak P., Huang C.-M., Li C.J., Vågbø C.B., Shi Y., Wang W.-L., Song S.-H., et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol. Cell. 2013;49:18–29. doi: 10.1016/j.molcel.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Zhang S., Zhao B.S., Zhou A., Lin K., Zheng S., Lu Z., Chen Y., Sulman E.P., Xie K., Bögler O., et al. m6A Demethylase ALKBH5 maintains tumorigenicity of glioblastoma stem-like cells by sustaining FOXM1 expression and cell proliferation program. Cancer Cell. 2017;31:591.e6–606.e6. doi: 10.1016/j.ccell.2017.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Lin S., Choe J., Du P., Triboulet R., Gregory R.I. The m6A methyltransferase METTL3 promotes translation in human cancer cells. Mol. Cell. 2016;62:335–345. doi: 10.1016/j.molcel.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Jiang Q., Crews L.A., Holm F., Jamieson C.H.M. RNA editing-dependent epitranscriptome diversity in cancer stem cells. Nat. Rev. Cancer. 2017;17:381–392. doi: 10.1038/nrc.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Zhang C., Zhi W.I., Lu H., Samanta D., Chen I., Gabrielson E., Semenza G.L. Hypoxia-inducible factors regulate pluripotency factor expression by ZNF217- and ALKBH5-mediated modulation of RNA methylation in breast cancer cells. Oncotarget. 2016;7:64527–64542. doi: 10.18632/oncotarget.11743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Blanco S., Kurowski A., Nichols J., Watt F.M., Benitah S.A., Frye M. The RNA-methyltransferase Misu (NSun2) poises epidermal stem cells to differentiate. PLoS Genet. 2011;7:e1002403. doi: 10.1371/journal.pgen.1002403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Blanco S., Bandiera R., Popis M., Hussain S., Lombard P., Aleksic J., Sajini A., Tanna H., Cortés-Garrido R., Gkatza N., et al. Stem cell function and stress response are controlled by protein synthesis. Nature. 2016;534:335–340. doi: 10.1038/nature18282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Amort T., Rieder D., Wille A., Khokhlova-Cubberley D., Riml C., Trixl L., Jia X.-Y., Micura R., Lusser A. Distinct 5-methylcytosine profiles in poly(A) RNA from mouse embryonic stem cells and brain. Genome Biol. 2017;18:1. doi: 10.1186/s13059-016-1139-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Fong Y.W., Ho J.J., Inouye C., Tjian R. The dyskerin ribonucleoprotein complex as an OCT4/SOX2 coactivator in embryonic stem cells. eLife. 2014;3 doi: 10.7554/eLife.03573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Bellodi C., McMahon M., Contreras A., Juliano D., Kopmar N., Nakamura T., Maltby D., Burlingame A., Savage S.A., Shimamura A., et al. H/ACA small RNA dysfunctions in disease reveal key roles for noncoding RNA modifications in hematopoietic stem cell differentiation. Cell Rep. 2013;3:1493–1502. doi: 10.1016/j.celrep.2013.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Guzzi N., Cieśla M., Ngoc P.C.T., Lang S., Arora S., Dimitriou M., Pimková K., Sommarin M.N.E., Munita R., Lubas M., et al. Pseudouridylation of tRNA-derived fragments steers translational control in stem cells. Cell. 2018;173:1204.e26–1216.e26. doi: 10.1016/j.cell.2018.03.008. [DOI] [PubMed] [Google Scholar]