Abstract
As trophic adaptations, rattlesnake venoms can vary in composition depending on several intrinsic and extrinsic factors. Ontogenetic changes in venom composition have been documented for numerous species, but little is known of the potential age-related changes in many rattlesnake species found in México. In the current study, venom samples collected from adult and neonate Crotalus polystictus from Estado de México were subjected to enzymatic and electrophoretic analyses, toxicity assays (LD50), and MALDI-TOF mass spectrometry, and a pooled sample of adult venom was analyzed by shotgun proteomics. Electrophoretic profiles of adult males and females were quite similar, and only minor sex-based variation was noted. However, distinct differences were observed between venoms from adult females and their neonate offspring. Several prominent bands, including P-I and P-III snake venom metalloproteinases (SVMPs) and disintegrins (confirmed by MS/MS) were present in adult venoms and absent/greatly reduced in neonate venoms. Age-dependent differences in SVMP, kallikrein-like, phospholipase A2 (PLA2), and L-amino acid oxidase (LAAO) activity levels were confirmed by enzymatic activity assays, and like many other rattlesnake species, venoms from adult snakes have higher SVMP activity than neonate venoms. Conversely, PLA2 activity was approximately 2.5 × greater in venoms from neonates, likely contributing to the increased toxicity (neonate venom LD50 = 4.5 μg/g) towards non-Swiss albino mice when compared to adult venoms (LD50 = 5.5 μg/g). Thrombin-like (TLE) and phosphodiesterase activities did not vary significantly with age. A significant effect of sex (between adult male and adult female venoms) was also observed for SVMP, TLE, and LAAO activities. Analysis of pooled adult venom by LC-MS/MS identified 14 toxin protein families, dominated by bradykinin-inhibitory peptides, SVMPs (P-I, P-II and P-III), disintegrins, PLA2s, C-type-lectins, CRiSPs, serine proteinases, and LAAOs (96% of total venom proteins). Neonate and adult C. polystictus in this population consume almost exclusively mammals, suggesting that age-based differences in composition are related to physical differences in prey (e.g., surface-to-volume ratio differences) rather than taxonomic differences between prey. Venoms from adult C. polystictus fit a Type I pattern (high SVMP activity, lower toxicity), which is characteristic of many larger-bodied rattlesnakes of North America.
Keywords: bradykinin-inhibitory peptide, enzyme, evolution, phenotypic variation, toxins, venomics
1. Introduction
The rattlesnakes Crotalus and Sistrurus comprise a monophyletic lineage of vipers unique to the Americas, and they are easily recognized by the presence of the caudal rattle. Rattlesnakes appear to be most closely related to the New World pitvipers, with American Agkistrodon being their sister group [1]. It has been hypothesized that rattlesnakes originated in México [2,3], diversifying and dispersing to the north and south, and they have a current range extending over 70 degrees of latitude, from southern Canada to central Argentina and southern Uruguay [4]. However, even with this wide geographical distribution, the highest diversity of rattlesnakes occurs on the Central Mexican Plateau and surrounding highlands [4]. Despite this diversity, venoms of most Mexican rattlesnakes, particularly the montane species, are poorly known. Exceptions include species that represent the greatest risks to humans, such as C. simus [5,6] and C. scutulatus scutulatus [7]. Several recent studies have also examined the venom compositional patterns of the diminutive C. lepidus [8,9,10] and C. willardi [10] subspecies.
Snake venoms are complex secretions of proteins and peptides produced in specialized cephalic glands [11,12]. The venom from a single individual may contain up to 100 proteins, including isoforms, although most venom compounds can be classified into 10–15 protein families [13,14]. For rattlesnakes these families are predominately the enzymatic L-amino acid oxidases (LAAO), phosphodiesterases (PDE), metalloproteinases (SVMP), serine proteases (SVSP), and phospholipases A2 (PLA2), and the non-enzymatic myotoxin a homologs, disintegrins, cysteine-rich secretory proteins (CRiSPs), and C-type lectins [15,16]. Rattlesnake venoms have been shown to vary in composition as a function of species [13,15,17], age [18,19,20,21,22,23,24,25,26,27], and geography [10,28,29,30]. Several other factors, such as season and sex, may also affect individual venom composition [15,16]. However, of all identified factors, phylogeny and ontogeny appear to have the most profound influences on venom phenotypes.
Snakes are gape-limited predators that often show distinct, age-related changes in prey preferences likely associated with prey availability, handling capacity or both [31]. For many species, this change includes a shift from consuming lizards and small/neonate rodents by juvenile snakes, to taking larger rodents as adults [2,21]. This dietary shift often coincides with an age-related shift in venom composition, as has been demonstrated for several northern latitude species and several clades of Central and South American rattlesnakes [20,21,25,26,27,32]. However, to date, little is known of the venom ontogeny patterns among Mexican rattlesnake species. A long-term ecological study of the Mexican Lance-headed Rattlesnake (Crotalus polystictus) in Estado de México [33,34,35] afforded the opportunity to obtain venom samples from neonate, juvenile, and adult rattlesnakes. This medium-sized species averages 600–700 mm in length and occupies native and modified grasslands in central México, and is a mid-elevation species, occurring from approximately 1450–2600 m ([4]; Figure 1). In this population, all age classes appear to feed primarily on rodents, with neonates gape-limited to utilizing only smaller species and individuals (Mociño-Deloya et al., in prep). Following the type I (high SVMP activity and low toxicity, >1.0 mg/g mouse body weight) and type II (low SVMP activity and high toxicity, <1.0 mg/g mouse body weight) classification put forth by Mackessy [15,16], adult C. polystictus possess a type I venom [15]. Transitions from a type-II-like venom in neonates to a type I venom in adults are observed for several rattlesnake species found in the United States [15,21], and therefore it was hypothesized that C. polystictus would also show prominent venom ontogenetic changes in composition. We report here results of a detailed analysis of ontogenetic changes in venom composition, sex-related differences in toxin activities, and LC-MS-MS analysis of the venom proteome in this Mexican rattlesnake.
Figure 1.
(A) Distribution of Crotalus polystictus (red) in México (after Campbell and Lamar [4]). The approximate location of México City is shown by the black dot. (B) Adult C. polystictus; photo by EMD.
2. Results
2.1. Enzyme Analyses
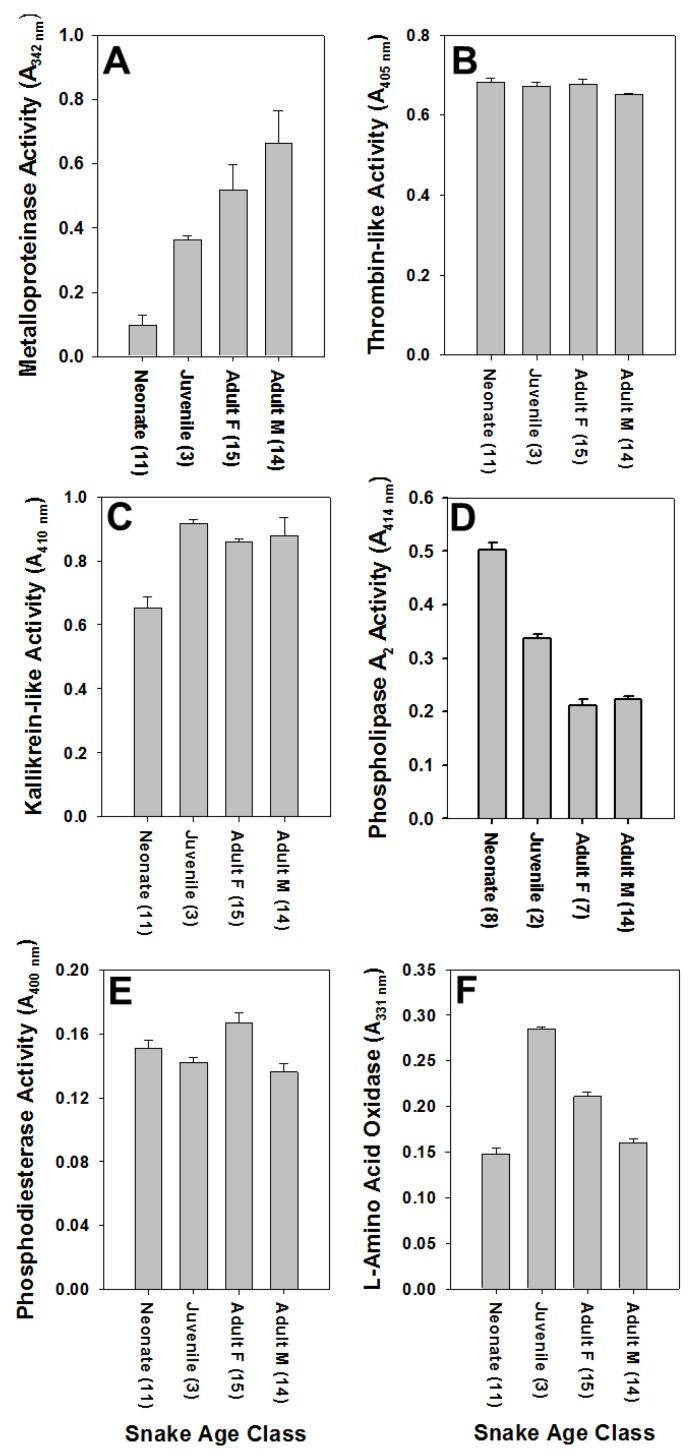
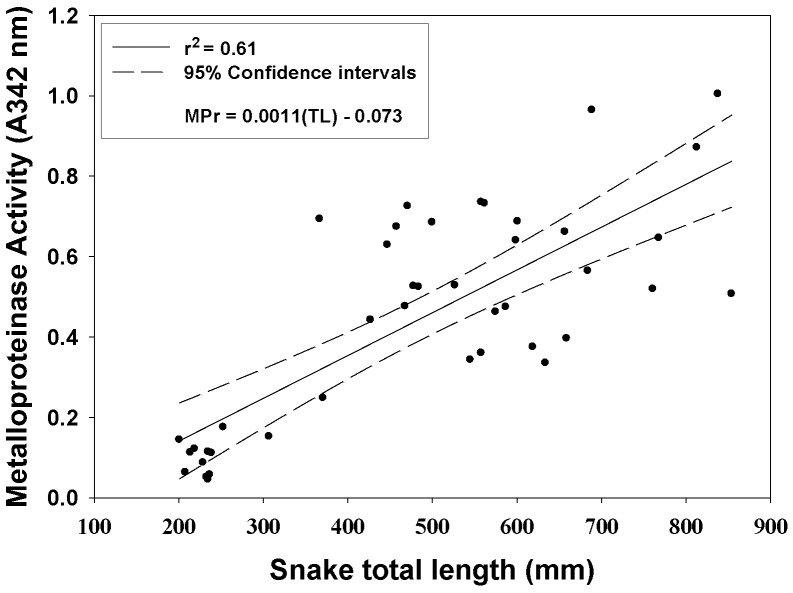
All C. polystictus venoms exhibited activities of six enzymes commonly found in rattlesnake venoms (Figure 2). Metalloproteinases activity showed a statistically significant difference with age (Figure 3; F2,40 = 37.94, p < 0.001), typical of many rattlesnake venoms [21]; average activity of adult venoms was approximately 6.5 × that of neonate venoms (p < 0.001). Juvenile and neonate venoms were also significantly different for SVMP activity (p < 0.05), whereas a comparison of juvenile and adult venoms was not significantly different (p = 0.065). Kallikrein-like activity differences were statistically significant (F2,40 = 14.28, p < 0.001), with neonates also having significantly less activity compared to both juvenile and adult age classes (both p’s < 0.01); juvenile and adult activity differences were not significant (p = 0.795). Phospholipase A2 activity showed an inverse relationship with age that was statistically significant (F2,29 = 41.42, p < 0.001), and activity in neonate venoms was 2.5 × greater than in adult venoms (p < 0.001). A comparison of juvenile and neonate PLA2 activity also showed significant differences (p < 0.01); differences in juvenile and adult activity was again not statistically significant (p = 0.083). A statistically significant difference in relation to age class was observed as well for LAAO activity (F2,39 = 3.894, p < 0.05), with a significant difference being observed between neonate and juvenile age groups (p < 0.05). TLE and PDE activities did not show statistically significant differences for any age classes (all p’s > 0.05).
Figure 2.
Enzyme activities in Crotalus polystictus venoms as a function of age class and sex; sample size for each is given parenthetically. Note that metalloproteinases (SVMP) activity (A) is lowest and PLA2 activity (D) is highest in neonate venoms; adult venoms show the opposite trend. Values are shown as averages ± SD. (A) Metalloproteinase activity; (B) Thrombin-like activity; (C) Kallikrein-like activity; (D) Phospholipase A2 activity; (E) Phosphodiesterase activity; (F) L-amino acid oxidase activity.
Figure 3.
SVMP activity of C. polystictus venoms as a function of total snake length; a significant ontogenetic effect is apparent. n = 43.
Metalloproteinases, TLE, and LAAO activities also showed a significant effect of sex (Student’s t-test). Venoms from adult male snakes exhibited significantly higher SVMP activity than venoms of adult female snakes (t = 2.5057, df = 27, p < 0.05), whereas adult females had significantly higher TLE (t = 3.6904, df = 27, p < 0.01) and LAAO (t = 2.1026, df = 27, p < 0.05) activities than venoms of adult male snakes. There was no apparent effect of sex on kallikrein-like, PDE, or PLA2 activities (all p’s > 0.05).
2.2. 1-D SDS-PAGE
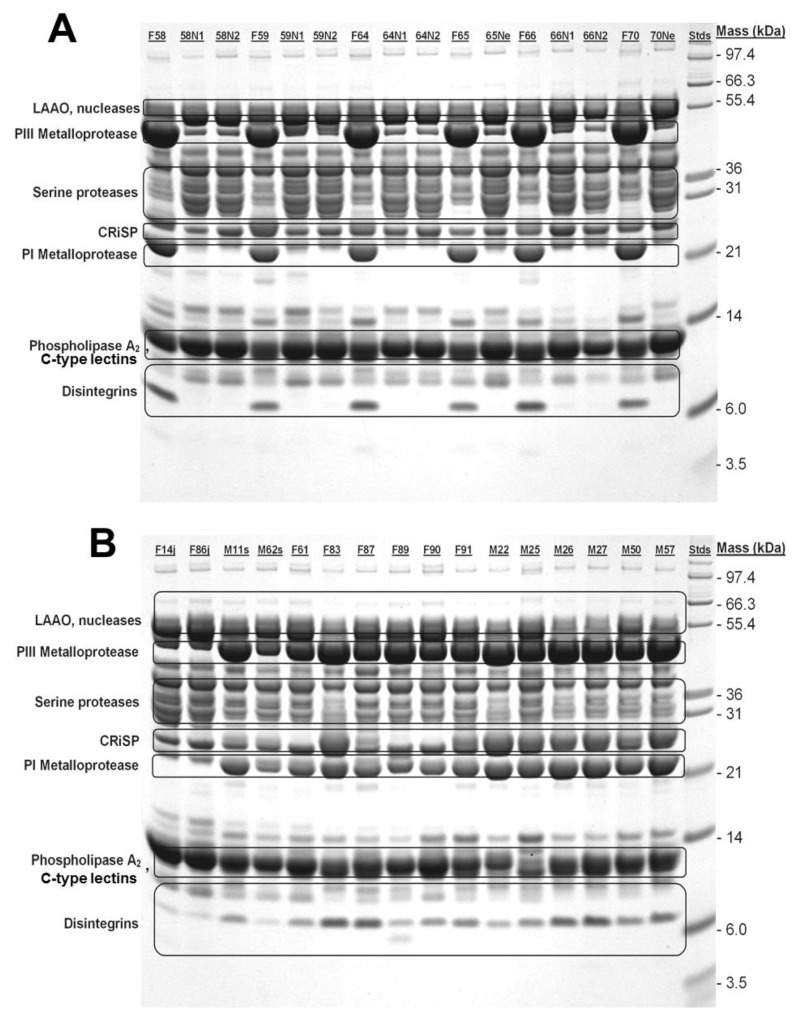
Typical of many venoms from within a species, C. polystictus venoms shared most components as revealed by 1D SDS-PAGE (Figure 4). Enzymatic LAAOs, nucleases (PDEs), SVSPs, and PLA2, in addition to the non-enzymatic CRiSP and C-type lectins appear in all individuals examined. Electrophoretic analysis demonstrated that at least two subtypes of SVMPs, P-I and P-III [36,37], vary ontogenetically, and these components were much more prevalent (high band density) in adult snake venoms. These results are consistent with enzyme data reported above. In addition, a low-mass component (approx. 7 kDa), putatively a disintegrin, appears to be absent from neonate venoms, faint in juvenile venoms, and prominent in venoms of adult snakes.
Figure 4.
SDS-PAGE analysis of neonate, juvenile, and adult C. polystictus venoms. (A) Adult female and neonate venoms. (B) Juvenile, subadult and adult venoms. While most bands are shared between samples, note the absence of P-I SVMP and disintegrin bands from neonate venoms; P-III SVMP are also greatly reduced in intensity in neonate venoms. Identical numbers (i.e., F58 and 58n1) indicate venoms from a female and her neonate offspring. Abbreviations: F, female; M, male; N, neonate; j, juvenile.
2.3. MALDI-TOF-MS Mass Fingerprinting of Venoms
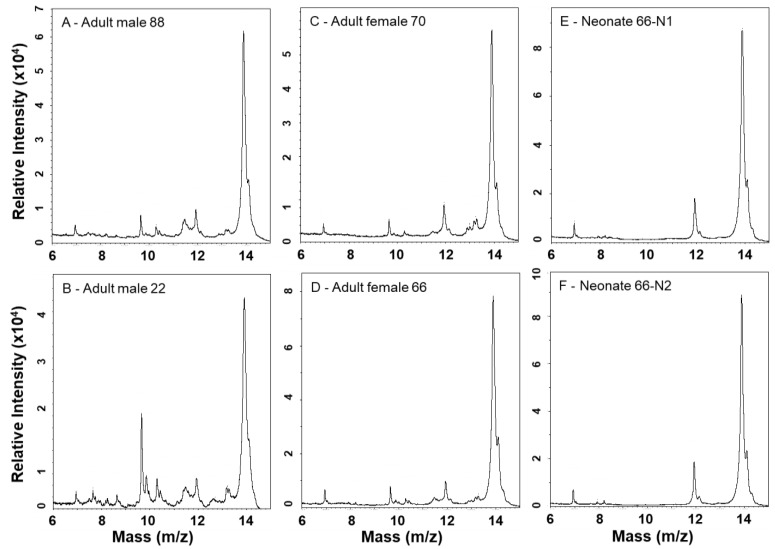
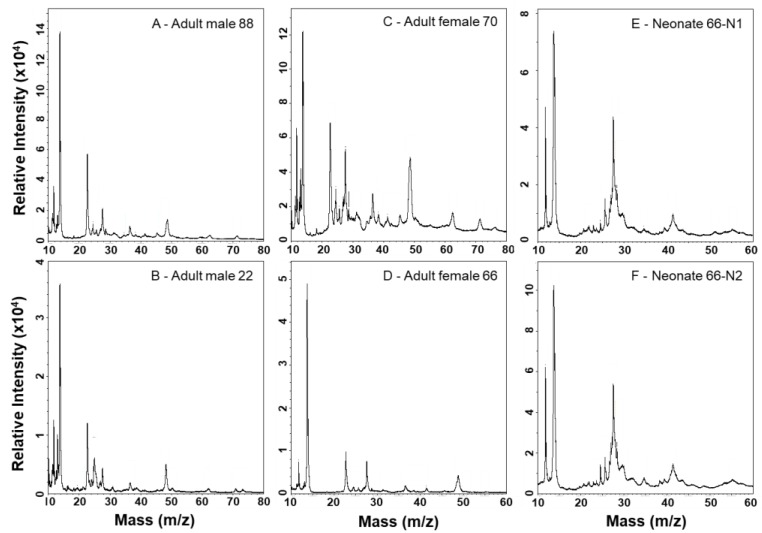
MALDI-TOF mass spectrometry provides more detailed analyses of lower mass components and is complementary to SDS-PAGE (Figure 5 and Figure 6). A PLA2 of 13,905 Da dominates all spectra, and numerous other small peaks are seen between approx. 6.9–11.9 kDa; the prominence of these lower mass components appears muted in neonate venoms, suggesting a lower abundance of these proteins as also observed by SDS-PAGE. Adult venoms showed prominent peaks at approx. 22.6 kDa (likely a P-I SVMP) that were absent from neonate venoms (Figure 6), again consistent with the enzymatic activity assay and SDS-PAGE data. Clusters of several peaks in the 24–30 kDa range are consistent with masses seen for CRiSPs and SVSPs, and these were variable in overall intensity between samples. In general, neonate venoms appeared to contain fewer peaks than adult venoms.
Figure 5.
MALDI-TOF-MS analysis of C. polystictus venoms; 6–15 kDa mass window.
Figure 6.
MALDI-TOF-MS analysis of C. polystictus venoms; 10–80 kDa mass window.
2.4. MALDI-TOF-MS/MS Identification of Specific Proteins
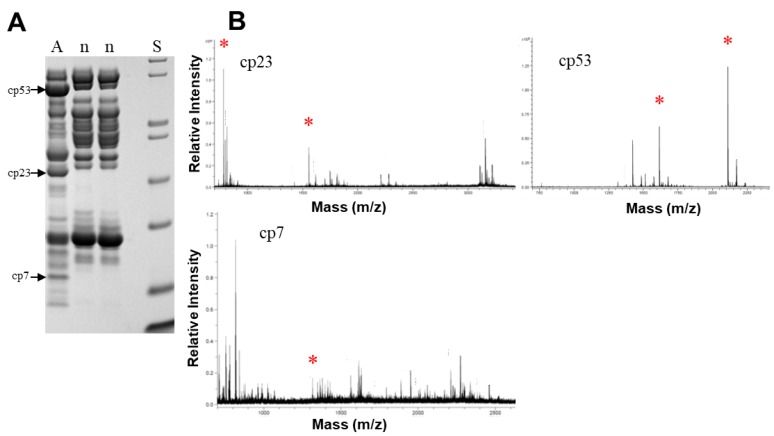
Identity of three venom proteins that varied with age class (SDS-PAGE) was confirmed by MALDI-TOF-MS/MS (Figure 7). These proteins were present in the adult female venom but were absent from venoms of her neonate offspring. Positive hits for the protein band at 53 kDa in adult venom were obtained: peptide 1 (mass = 2110.09; ITVKPEADYTLNAFGEWR) showed identity with a P-III SVMP (T1E6U1) from Crotalus oreganus helleri venom. Shotgun proteomic analysis (see below) also identified this peptide at m/z 1055.53 (2+) and m/z 704.02 (3+) (Table S1). Peptide 2 had a mass of 1615.81, and yielded the sequence MYELANTVNEIYR matching to a P-III SVMP (Q2QA02) from Crotalus d. durissus venom. This peptide was also identified at m/z 808.39 (2+). Similarly, two peptides from the digest of the 23 kDa band showed positive hits: peptide 1 (mass = 1554.79; STGVVQDHSEINLR) showed identity with a P-II SVMP from Crotalus atrox venom (atrolysin E; (P34182)). This peptide was also identified at m/z 777.90 (2+), 518.94 (3+), 570.97 (3+), and 428.48 (4+), with the latter two ions containing an additional N-terminal arginine accounting for the mass difference of 156.1 (Table S1). Peptide 2 (mass = 781.37; SFGEWR) also showed identity with atrolysin E. It should be noted that atrolysin E is a P-II SVMP that is processed to an active proteinase and a disintegrin-like protein [38,39], and therefore, an estimated mass of 23 kDa (by SDS-PAGE) is consistent with this enzyme in its active form.
Figure 7.
MALDI-TOF-MS/MS identification of several venom proteins only expressed in adult venoms of C. polystictus. (A) SDS-PAGE showing three bands excised from gel. (B) Bands indicated were identified as a PIII SVMP (cp53), a P-I SVMP (cp23), and a SVMP degradation fragment (cp7). A, adult female; n, two different neonates; S, mass standards. *, indicates peptides providing positive ID.
The 7 kDa band yielded a peptide (mass = 1313.79; RYIELVVVADHRV) which showed identity with several Crotalus venom SVMPs (Q90392.1, Q90391.1, P15167.3, all from C. atrox). This band, which was also observed via shotgun proteomics (below), appears to be a degradation fragment of a larger SVMP. For all protein hits, MASCOT identity scores of ≥16 were obtained, strongly supporting identity (p < 0.05) with the fragment regions of the respective proteins.
2.5. Mass Spectroscopy: Orbitrap LC-MS/MS Identification of Venom Proteome
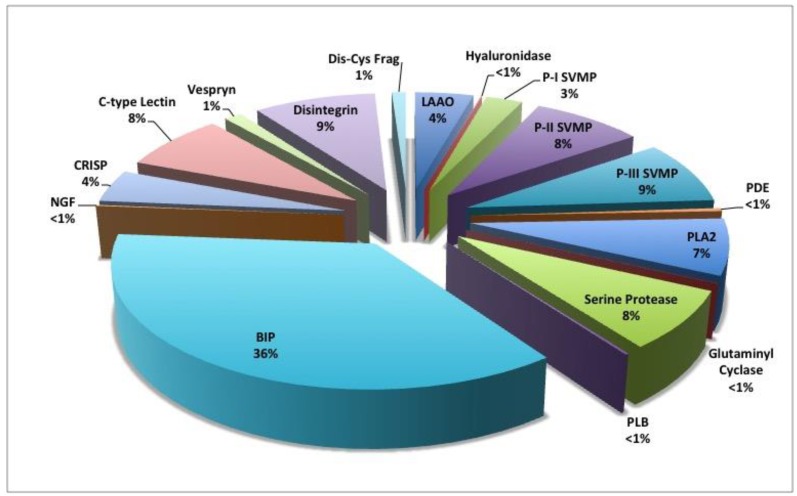
Shotgun proteomic analysis of C. polystictus venom identified 14 protein families (Table 1 and Figure 8; Table S1); relative abundance (% of total proteins) was based on normalized spectral abundance factor (NSAF), which takes into account both spectral counts and parent protein mass. The peptide TPPAGPDVGPR, which is identical to a bradykinin-inhibitory peptide (P0CJ34) accounts for over 36% of the total venom proteome. Consistent with other type I venoms, C. polystictus venom exhibits moderate SVMP levels, (~19%) representing all three SVMP subfamilies: P-I—2.7%, P-II—8.2%, and P-III—8.6%. Serine proteinases comprise 7.8% of the entire venom proteome, with thrombin-like (5.7%), kallikrein-like (1.9%), and a plasminogen activator (T1D6M5) (0.25%) all being identified by proteomic analysis. Basic (6.0%) and acidic (1.5%) PLA2s, and LAAOs (4.4%) were present at moderate levels. The non-enzymatic disintegrins, C-type lectins, and CRiSPs accounted for approximately 9.0%, 7.8%, and 4.4% of the entire venom proteome, respectively. The remaining seven protein families accounted for a total of approximately 4% of venom proteins.
Table 1.
Relative occurrence of the different protein families present in the venom of adult Crotalus polystictus.
| Protein Family | % of Total Venom Proteins |
|---|---|
| L-Amino Acid Oxidase | 4.35 |
| Snake Venom Metalloproteinase (SVMP) | 19.53 |
| • P-I SVMP | 2.72 |
| • P-II SVMP | 8.23 |
| • P-III SVMP | 8.58 |
| Phosphodiesterase (Exonuclease) | <1.0 |
| Phospholipase A2 | 7.49 |
| • Acidic PLA2 | 1.47 |
| • Basic PLA2 | 6.02 |
| Serine Protease | 7.84 |
| • Thrombin-like | 5.69 |
| • Kallikrein-like | 1.90 |
| • Plasminogen Activator | <1.0 |
| Glutaminyl Cyclase | <1.0 |
| Hyaluronidase | <1.0 |
| Phospholipase B | <1.0 |
| Bradykinin-Inhibitory Peptide | 36.32 |
| Cysteine-rich Secretory Protein | 4.41 |
| C-type Lectin | 7.80 |
| Vespryn | 1.31 |
| Disintegrin | 9.07 |
| Dis-Cys Fragments | 1.04 |
| Nerve Growth Factor | <1.0 |
Figure 8.
Venom proteome of adult C. polystictus. Abbreviations: BIP, bradykinin-inhibitory peptide; CRiSP, cysteine-rich secretory protein (helveprin); Dis-Cys Frag, cysteine-containing disintegrin fragment; LAAO, L-amino acid oxidase; NGF, nerve growth factor; PDE, phosphodiesterase (exonuclease); PLA2, phospholipase A2; PLB, phospholipase B; P-I SVMP, class P-I snake venom metalloproteinase; P-II SVMP, class P-II snake venom metalloproteinase; P-III SVMP, class P-III snake venom metalloproteinase.
2.6. Toxicity of Neonate and Adult Venom to Mice
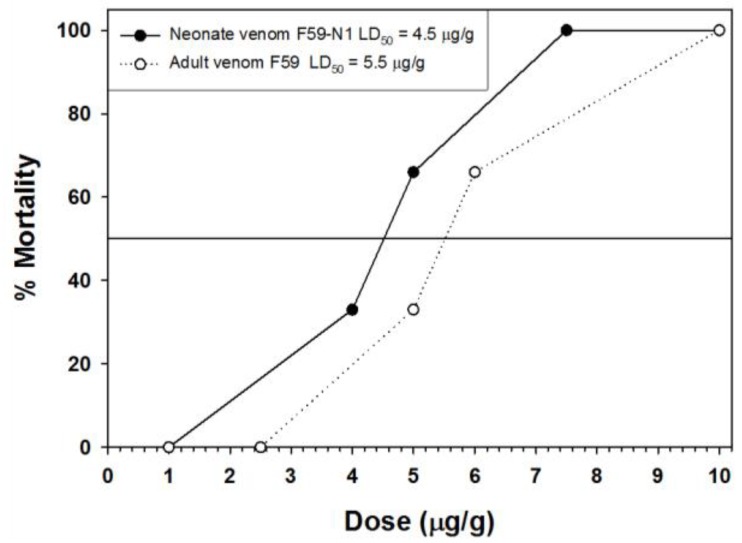
As shown in Figure 9, venom from neonate snakes was somewhat more toxic to NSA mice (LD50 = 4.5 μg/g) than adult venom (LD50 = 5.5 μg/g; Figure 9), a pattern typically observed with type I venoms.
Figure 9.
Lethal toxicity (24 h post-injection) of C. polystictus venoms toward NSA mice.
3. Discussion
As trophic adaptations, snake venoms have allowed for the transition from a mechanical (constriction) to a chemical (venom) means of incapacitating and killing prey. Ontogenetic shifts in venom enzymatic and toxic activities often coincide with shifts in prey preference, and the general shift from low SVMP activity and high toxicity in neonates, to high SVMP activity and low toxicity in adult rattlesnakes is frequently observed. The increased SVMP content in adult rattlesnakes likely facilitates tissue degradation of larger, more metabolically favorable prey items, such as rodents. However, the opposite relationship has also been documented in some populations of C. viridis viridis [26], in which adult venoms have significantly lower SVMP but higher myotoxin a levels than neonates. The increased concentration of myotoxin a is in support of an increase in lethal toxicity towards mammals, as adult C. v. viridis consume almost entirely small mammals and this toxin is specific toward rodents [40]. In addition, paedomorphic trends in venom compositional patterns, where adults retain juvenile venom characteristics, have also been documented in C. o. concolor [24], C. simus, and C. durissus [25].
Like the venoms of several temperate species of rattlesnakes, venoms from C. polystictus undergo prominent shifts in composition during postnatal development. Most striking is the change in SVMP activity, which in adult snake venoms occurs via increased expression of at least two classes of SVMPs (P-I and P-III) as detected by SDS-PAGE and MALDI-TOF MS/MS. Proteomic analysis further confirmed the presence of all three subfamilies of SVMPs (P-I, P-II, and P-III) in pooled adult C. polystictus venom. This compositional pattern appears to be common among rattlesnakes producing type I venoms. In C. o. helleri and C. o. oreganus [21,23], this shift is correlated with a change in diet, from lizards and neonate rodents to larger mammalian prey; venoms from these taxa also show a significant increase in thrombin-like serine protease activity (unpubl. data). However, C. polystictus in this population feeds primarily on small mammals (mammals comprised 87.9% of 545 prey items) throughout their ontogeny, taking juvenile rodents (pygmy mice, Baiomys taylori, and shrews) when neonates and expanding their diet to include larger rodents, especially Microtus mexicanus, as adults (Mociño-Deloya et al., in prep.). This suggests that the main impetus for increases in SVMP activities is utilization of larger, bulkier prey, as has been previously suggested [21], rather than a shift to different taxa of prey. This conclusion is further supported by the fact that adult male C. polystictus consume a greater proportion of larger mammalian prey compared to adult female C. polystictus; venoms from adult males had significantly higher SVMP activity compared to the venoms from adult females, perhaps reflecting the importance of larger rodents and lagomorphs in the diet of adult males compared to females (24.0% vs. 5.1% of prey mass) [35].
As was also observed with C. o. oreganus and C. o. helleri venoms, neonate C. polystictus venoms contained over 2× the levels of PLA2 activity seen in venoms of adult snakes. In Crotalus simus simus, this differential was found manifested as the presence of crotoxin homologs in neonate venoms [41]. However, in C. polystictus, all dominant venom PLA2s showed identical masses of 13,906 Da by MALDI-TOF MS, strongly indicating that a crotoxin homolog is not present in these venoms. Shotgun proteomic analysis also failed to identify crotoxin or Mojave toxin in C. polystictus venom, although several acidic and basic PLA2s are clearly present in the pooled sample. Most notable are peptides that matched a PLA2 (A0A0K8RYR4) from C. horridus, which comprises approximately 5% of the adult C. polystictus venom proteome. The lack of crotoxin is also supported by lethality assays in mice, as rattlesnake venoms containing crotoxin homologs show lethal toxicities below 1.0 µg/g; venoms from neonate C. polystictus are considerably less toxic. The slight increase observed with neonate venom toxicity, as compared to adults, may compensate for the significantly smaller venom bolus that can be injected into prey during a predatory strike, and a more toxic venom enables neonate snakes to dispatch prey items more rapidly. However, the similarity in toxicity seen in C. polystictus venoms may also be a reflection of their mammals-only diet; in C. oreganus helleri and C. o. oreganus, juvenile venoms were approximately 2× as toxic as adult venoms, and this species utilizes lizards as a dominant prey of neonate and young snakes [21].
Shotgun proteomic analysis indicates that the venom proteome of adult C. polystictus comprises just over 9% disintegrins, and SDS-PAGE analysis shows a clear distinction in disintegrin band intensity between adult and neonate venoms. Disintegrins are small (4–16 kDa) non-enzymatic proteins that often result from a post-translational processing of the P-II class of SVMPs [42,43,44]. Therefore, the lack of a disintegrin band in neonate venoms may be correlated with the lower SVMP content and activity observed in these venoms. By blocking integrin αIIbβ3, disintegrins inhibit platelet aggregation and facilitate the circulation of toxins throughout prey [45]. In C. atrox, and likely other species, disintegrins also appear to act as a molecular “tag” by altering the chemical scent of prey and allowing for successful prey recovery by strike-induced chemosensory searching [46]. For adult C. polystictus, the increased disintegrin concentration may assist with successful prey relocation during predatory episodes, as adults frequently release rodent prey following the envenomating strike. Neonates, on the other hand, often strike-and-hold prey, and therefore an abundance of disintegrin(s) in their venom is not necessary. The lack of disintegrins in neonate C. polystictus venom is also likely compensated by the increased toxicity, as venom would more rapidly debilitate prey before it retaliated or could wander far from the attack site. The concentration of disintegrin required for successful prey recovery remains unknown. However, in C. o. concolor, which exhibits a type II venom phenotype [24] and has significantly less disintegrin compared to type I species such as C. atrox, C. o. oreganus/helleri and C. polystictus, successful discrimination between envenomated and non-envenomated prey cues has been documented [47].
In addition to the marked ontogenetic shift discussed above, significant age-related differences were also observed for kallikrein-like and LAAO activities, with neonate venoms having significantly lower activity levels for both enzymes. On the other hand, there does not appear to be a significant age-dependent trend for TLE and PDE activities, although TLE and LAAO activities did demonstrate a sex-based difference in adult snakes; adult females had statistically higher activity levels when compared to adult males, but overall levels were similar between age classes. This lack of age-related variation is consistent with a mammals-only diet, as TLE serine proteinases are likely more effective toward mammals, which generally have much narrower tolerances than reptiles for variation in blood pressure and clotting. TLE and kallikrein-like serine proteases target the blood-coagulation cascade, and TLEs have received significant attention for their ability to cleave fibrinogen and deplete the major clotting factors that would normally assist with clot formation [22,23,48,49,50]. Phosphodiesterases, which comprises <1% of the C. polystictus venom proteome, generates purine nucleosides and may assist with prey immobilization via hypotension [51,52]. L-amino acid oxidases, which make up approximately 4% of the venom proteome, appear to have multiple roles in subduing prey. L-amino acid oxidase activity likely contributes to overall toxicity via production of hydrogen peroxide during the enzymatic deamidation of amino acids [53], induction and inhibition of platelet aggregation [54,55], hemorrhage [56] and cell apoptosis [55,57,58]. Examination of SDS-PAGE electrophoretic patterns showed that neonate, juvenile, and adult venoms shared most other major venom protein components.
The major toxin present in adult C. polystictus venom was the bradykinin-inhibitory peptide (BIP) TPPAGPDVGPR [P0CJ34], which accounts for 36% of the venom proteome using NSAF. Bradykinin-inhibitory peptides result from the proteolytic processing of a larger (approx. 180 residue) bradykinin-potentiating peptide (BPP)/C-type natriuretic peptide (CNP) precursor [59], and they are broadly distributed in the venoms of New World pit vipers [25,26,59,60,61,62], albeit at significantly lower concentrations than in C. polystictus venom. Bradykinin-inhibitory peptides appear to antagonize the vasodilatory actions of bradykinin at the B2 receptor, suggesting that they may disrupt normal cardiovascular function and enhance the overall toxicity of venom [59]. The high abundance of this peptide in C. polystictus venom suggests that they likely play a pivotal role in the action of snake venoms. Interestingly, Pla et al. [63] identified 12 BPP-like peptides, accounting for 28% of the total venom proteome of Lachesis muta rhombeata; however, an incredibly high dose of pooled BPP-like peptides had no effect on mice following intravenous and intraperitoneal administration, so their role in envenomation remains obscure.
In addition to the venom components discussed above, shotgun proteomic analysis of C. polystictus venom identified various proteins of lesser abundance (≤1% each), including glutaminyl cyclase (M9NCD3), phospholipase B (PLB) (A0A077L7E7), vespryn (F8S122), a disintegrin-cysteine fragment (E9JG43), hyaluronidase (U3TBU1), and a nerve-growth factor (T1DEA2). It should be noted, however, that our analysis identified only a single peptide matching to hyaluronidase (U3TBU1) and nerve-growth factor (T1DEA2) (Table S1). Although present in low concentrations in snake venoms, glutaminyl cyclases may play a significant role in post-translational modifications by enzymatically converting the N-terminal formation of pyroglutamate of several snake venom protein families [64,65]. This may stabilize these compounds and therefore contribute to overall venom toxicity. Glutaminyl cyclases may also be involved in the biosynthesis of pyroglutamyl peptides such as BPPs [66,67] and tripeptide inhibitors of SVMPs [68]. Phospholipase B has been correlated with high direct hemolytic activity of venoms from several Australian elapids [69,70,71]; however, it remains unknown if they have similar roles in viperid venoms. Hyaluronidase increases venom potency and degrades local tissue by breaking down hyaluronic acid in the extracellular matrix and facilitating the diffusion of additional venom compounds throughout prey [72]. Vespryn and nerve-growth factors, although becoming more frequently identified in venom, have elusive biological roles. Additionally, many trace components are detected in venoms by mass spectrometry, but whether they have any role during envenomation seems doubtful, as most are of low toxicity and their effects, if any, would be greatly overshadowed by more prominent toxins.
4. Conclusions
Venom ontogeny has been widely documented in viperid [21,24,25,26,73,74,75,76,77], elapid [78,79,80], and several rear-fanged “colubrid” [81,82] snakes, and it appears to be largely driven by natural selection for different diets [21,83]. Crotalus polystictus shows clear distinction between neonate and adult venoms, with SVMP, kallikrein-like, LAAO activities, and disintegrin content being significantly higher in adult venoms, whereas neonates exhibit higher PLA2 activity. Surprisingly, with adult venoms, there is also a significant sex-based difference in SVMP, LAAO, and TLE activities. Although both neonate and adult C. polystictus exhibit a type I venom phenotype, the differences in venom composition are likely driven by prey size rather than shifts in prey type, as both neonate and adult C. polystictus consume rodent prey. Results of this and other studies indicate that there are several variations in the basic patterns of rattlesnake venom ontogeny, suggesting that venom ontogeny, like venom compositional variation generally, is shaped by multifactorial determinants.
5. Materials and Methods
5.1. Reagents
Invitrogen NuPage 12% bis-tris electrophoretic gels, buffers, and Mark 12 molecular weight standards were obtained from ThermoFisher Scientific (Waltham, MA, USA). Protein concentration reagents were purchased from BioRad, Inc. (San Diego, CA, USA). Soluble phospholipase A2 assay kit (765001) was purchased from Cayman Chemical Co. (Ann Arbor, MI, USA). All other reagents used (analytical grade or better) were obtained from Sigma Chemical Corp. (St. Louis, MO, USA).
5.2. Venoms
Venoms from 43 snakes (adults (>450 mm SVL):15 females, 14 males; juveniles (250–325 mm):1 female, 2 males; neonates (<240 mm):6 females, 5 males) were collected in the field and stabilized via desiccation over silica gel. Venoms were then stored with desiccant at −20 °C until used. All snake handling and venom collection was conducted under permit NUM/SGPA/DGVS/06320 from México (issued to E. Mociño-Deloya).
5.3. Enzyme Assays
All venom samples were solubilized in Millipore-filtered ddH2O at 4 mg/mL. Before experimental use, venom samples were brought to room temperature, vortexed, and centrifuged. Protein concentrations were determined [84] using bovine γ globulin as standard, and all enzyme assays (run in triplicate) were based on these values.
Metalloproteinase (SVMP) activity was determined using azocasein as substrate [85]. Serine proteinase (SVSP) activities (thrombin-like and kallikrein-like) were assayed using p-nitroaniline tripeptide substrates [22]. Phosphodiesterase (PDE) activity was determined using Ca-bis-nitrophenyl phosphate [86,87]. L-amino acid oxidase (LAAO) assays followed methods described previously using L-kynurenine as a substrate [21]. All results are reported as product formed/min/mg of venom protein.
Phospholipase A2 (PLA2) activity was determined by a modification of the assay procedure recommended by the supplier (Cayman Chemical Co.); all samples were run in duplicate, but due to limited samples, only 31 venoms were assayed (all age classes represented). Substrate (diheptanoyl thio-phosphatidyl choline) was solubilized at 1.66 mM in assay buffer (25 mM tris-HCl, pH 7.5 with 10 mM CaCl2, 100 mM NaCl, and 0.3 mM Triton X-100) and diluted 1:3 with assay buffer (=working substrate solution) just before use. Ten µL assay buffer (blank), bee venom PLA2 (positive control) or 0.5 µg venom was added to wells of a 96-well plate and then 10 µL DTNB (10 mM) was added. Two hundred µL working substrate solution was then added to a column of assays on the plate to initiate reactions and the plate was placed in a Spectramax 190 plate reader at 37 °C; absorbance readings (414 nm) were taken every minute for 10 min. Enzyme activity was calculated from the linear portion of reaction rate curves (between 2.0 and 3.0 min), and specific activity was expressed as µmol product formed/min/mg protein.
5.4. One-dimensional SDS Gel Electrophoresis
Dithiothreitol (DTT) reducing sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was conducted using Nu Page 12% acrylamide gels and Mark 12 standards (Life Technologies, Inc. (Grand Island, NY, USA) as described previously [88]. Twenty-four μg of venom was loaded into each lane. Gels were stained in 0.1% Coomassie brilliant blue R-250, destained with 30% methanol/7% acetic acid, and photographed using a Bio-Rad gel imaging system. Protein class was identified based on mass and published accounts [15,16].
5.5. Mass Spectrometry: MALDI-TOF
Two representatives each of neonate, adult female, and adult male venoms were subjected to mass fingerprinting. Approximately 0.5 μg of venom in 1.0 μL 50% acetonitrile (ACN) in ddH2O was mixed with 1 μL sinapinic acid matrix (10 mg/mL 50% ACN in ddH2O), spotted onto MALDI target plates, allowed to air dry and analyzed using an Ultraflex-TOF/TOF mass spectrometer (Bruker Daltonics, Billerica, MA, USA) in operating in linear mode using a 25 kV accelerating voltage.
5.6. Mass Spectrometry: Orbitrap LC-MS/MS
One pooled venom sample (3 male, 3 female, adults) was subjected to shotgun proteomic analysis (Florida State University College of Medicine Translational Science Laboratory) in order to obtain an overview of the total venom proteome of C. polystictus. Samples were digested using the Calbiochem ProteoExtract All-in-one Trypsin Digestion kit (Merck, Darmstadt, Germany) with LC/MS grade solvents according to the manufacturer’s instructions. The LC-MS/MS analyses were performed using an LTQ Orbitrap Velos equipped with a Nanospray Flex ion source and interfaced to an Easy nanoLC II HPLC (Thermo Scientific). Peptide fragments were separated using a vented column configuration consisting of a 0.1 × 20 mm, 3 mm C18 trap column, and a 0.075 × 100 mm, 3 mm C18 analytical column (SC001 and SC200 Easy Column respectively, Thermo Scientific). The elution gradient consisted of 5% buffer B (0.1% formic acid in HPLC grade acetonitrile), and 95% buffer A (0.1% formic acid) at the run start, to 35% B at 60 min, to 98% B from 63 to 78 min with a flow rate of 600 nL/min from 64 to 78 min, and 5% B at 300 nL/min at 79 min. The mass spectrometer was operated in positive mode nanoelectrospray with a spray voltage of + 2300 V. A “Top 9” method was used with precursor ion scans in the Orbitrap at 60 K resolving power and fragment ion scans in the linear ion trap. Precursor ion selection using MIPS was enabled for charge states of 2+, 3+ and 4+. Dynamic exclusion was applied for 60 s at 10 ppm. ITMS scans were performed using collision-induced dissociation (CID) at 35% normalized collision energy. MS/MS peptide spectra produced were interpreted using Mascot (Matrix Science, London, UK; version 1.4.0.288), SEQUEST (Thermo Fisher Scientific, San Jose, CA, USA; version 1.4.0.288), and X! Tandem (thegpm.org; version CYCLONE 2010.12.01.1), assuming a trypsin digestion. The Mascot5_Trembl_bony vertebrate database and the SEQUEST and X! Tandem Uniprot Serpentes (A8570) databases were used for homology searches. SEQUEST and X! Tandem were searched with a fragment ion mass tolerance set to 0.6 Da, and a parent ion tolerance of 10 ppm. Mascot was searched with a fragment ion mass tolerance of 0.8 Da, and a parent ion tolerance of 10 ppm. Glu/pyro-Glu of the N-terminus, ammonia loss of the N-terminus, carbamidomethylation of cysteines and carboxymethylation of cysteines were specified as variable posttranslational modifications within X! Tandem. Oxidations of methionine, carbamidomethyl cysteine, and carboxymethyl cysteine were specified as variable post-translational modifications within Mascot and SEQUEST. Results were viewed and validated within Scaffold (Proteome Software Inc., Portland, OR, U.S.A; version 4.4.6), and protein identities were accepted if they could be established at >99.9% probability and contained at least one identified peptide.
Protein family identification was based on these criteria, and label free quantification of unique members of each family was performed according to normalized spectral abundance factor (NSAF) [89,90]. Percent of total proteome was then calculated for each protein family (and subgroups for SVMPs, PLA2s, and serine proteases).
5.7. Mass Spectrometry: MALDI-TOF-MS/MS
Adult and neonate venoms were electrophoresed as above, and select bands prevalent only in adult venoms (approximate masses: 7, 23, and 53 kDa) were excised and subjected to in-gel trypsin digestion. Briefly, excised bands were destained with 100% ACN in an eppendorf tube, washed with a second change of ACN, reduced and alkylated with DTT and iodoacetamide respectively, and then digested overnight with 0.6 μg Pierce Gold trypsin at 37 °C (in a total volume of 100 μL 50 mM ammonium bicarbonate buffer). Peptides generated were concentrated to 10 μl and contaminating reactants were removed via C18 ZipTip cleaning. One μL of cleaned sample was mixed with 1 μL of a-cyano-4-hydroxycinnamic acid (10 mg/mL in 50% ACN, 0.1% TFA). The mixture was then spotted onto the MALDI target plate, allowed to air dry and analyzed using an Ultraflex-TOF/TOF mass spectrometer (Bruker Daltonics, Billerica, MA) in positive ion, reflector mode using a 25 kV accelerating voltage (Proteomics and Metabolomics Facility, Colorado State University). Mass spectrograms and MS/MS data generated were searched using the MASCOT ver. 2.2 search engine [91,92] and the NCBI database [93]. The peptide mass tolerance was set at 0.15 Da and the peptide fragment ion mass tolerance was set at 0.8 Da. Peptides characteristic of trypsin autolysis and keratin hydrolysis were removed before analysis.
5.8. Venom Lethality
Toxicity of venom from one adult female (F59) and one of her offspring (F59-N1) was assayed in 25–30 g female non-Swiss albino (NSA) mice [81]. Venom doses were delivered intraperitoneally (IP) in sterile saline (100 μL bolus), with doses adjusted to individual animal body masses. Three animals per dose were utilized, and all animals were monitored for 24 h. Lethality was expressed as micrograms of venom per gram body mass (μg/g) producing 50% mortality after 24 h and was calculated from the raw mortality-dose data using the Trimmed Spearman–Karber (TSK) Program version 1.5 [94]. Animal numbers were minimized, and all procedures were approved by the University of Northern Colorado IACUC (protocol #9401). Date of approval: 11 February 2015.
5.9. Statistical Analyses
Venom enzymatic activities between the different age classes were analyzed with analysis of variance (ANOVA) followed by a Tukey HSD post-hoc test. Comparison of the enzymatic activity levels between adult male and adult female snakes was completed using a Student’s t-test. Both statistical tests were performed with R version 3.4.2. p-values < 0.05 were considered statistically significant.
Acknowledgments
We thank the late F. Mendoza-Quijano for his early contributions to this project.
Supplementary Materials
The following are available online at http://www.mdpi.com/2072-6651/10/7/271/s1, Table S1: Full list of peptides identified following shotgun proteomic analysis of a pooled (3 adult and 3 female) Crotalus polystictus venom sample.
Author Contributions
S.P.M. conceived and designed experiments. E.M.-D., K.S., and R.W.B. provided all venom samples. J.L. performed all enzymes assays and gel electrophoresis. S.P.M. and A.J.S. conducted mass spectrometry experiments. S.P.M. and A.J.S. analyzed data and wrote the manuscript. All authors have read and contributed to the revision of this article.
Funding
Funding for this study was provided in part by the Colorado Office for Economic Development and International Trade (to SPM). Additional funds were provided by the UNC Office of Research.
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
We provide a detailed analysis of the venom of a rattlesnake that occupies mid-elevation habitat in México. Venoms from neonate and adult Crotalus polystictus show distinct differences in SVMP, kallikrein-like, LAAO, and PLA2 activities and disintegrin content. Venomics of pooled adult C. polystictus venom identified many major venom components that are common in rattlesnake venoms, with bradykinin-inhibitory peptide constituting 36% of the venom proteome. Crotalus polystictus shows a pattern of venom ontogeny similar to other rattlesnakes that produce a type I venom.
References
- 1.Castoe T.A., Parkinson C.L. Bayesian mixed models and the phylogeny of pitvipers (Viperidae: Serpentes) Mol. Phylogenet. Evol. 2006;39:91–110. doi: 10.1016/j.ympev.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 2.Klauber L.M. Rattlesnakes. Volume 2. University of California Press; Berkeley, CA, USA: 1956. Their habits, life histories and influences on Mankind; p. 153. [Google Scholar]
- 3.Blair C., Sanchez-Ramirez S. Diversity-dependent cladogenesis throughout western Mexico: Evolutionary biogeography of rattlesnakes (Viperidae: Crotalinae: Crotalus and Sistrurus) Mol. Phylogenet. Evol. 2016;97:145–154. doi: 10.1016/j.ympev.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 4.Campbell J.A., Lamar W.W. The Venomous Reptiles of the Western Hemisphere. Cornell University Press; Ithaca, NY, USA: 2004. [Google Scholar]
- 5.Castro E.N., Lomonte B., del Carmen Gutiérrez M., Alagón A., Gutiérrez J.M. Intraspecies variation in the venom of the rattlesnake Crotalus simus from Mexico: Different expression of crotoxin results in highly variable toxicity in the venoms of three subspecies. J. Proteom. 2013;87:103–121. doi: 10.1016/j.jprot.2013.05.024. [DOI] [PubMed] [Google Scholar]
- 6.Durban J., Sanz L., Trevisan-Silva D., Neri-Castro E., Alagón A., Calvete J.J. Integrated venomics and venom gland transcriptome analysis of juvenile and adult Mexican rattlesnakes Crotalus simus, C. tzabcan, and C. culminatus revealed miRNA-modulated ontogenetic shifts. J. Proteome Res. 2017;16:3370–3390. doi: 10.1021/acs.jproteome.7b00414. [DOI] [PubMed] [Google Scholar]
- 7.Borja M., Neri-Castro E., Castañeda-Gaytán G., Strickland J.L., Parkinson C.L., Castañeda-Gaytán J., Ponce-López R., Lomonte B., Olvera-Rodríguez A., Alagón A., et al. Biological and Proteolytic variation in the venom of Crotalus scutulatus scutulatus from México. Toxins. 2018;10:35. doi: 10.3390/toxins10010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martínez-Romero G., Rucavado A., Lazcano D., Gutiérrez J.M., Borja M., Lomonte B., Garza-García Y., Zugasti-Cruz A. Comparison of venom composition and biological activities of the subspecies Crotalus lepidus lepidus, Crotalus lepidus klauberi and Crotalus lepidus morulus from Mexico. Toxicon. 2013;71:84–95. doi: 10.1016/j.toxicon.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Rivas E., Neri-Castro E., Bénard-Valle M., Hernánez-Dávila A.I., Zamudio F., Alagón A. General characterization of the venoms from two species of rattlesnakes and an intergrade population (C. lepidus x aquilus) from Aguascalientes and Zacatecas, Mexico. Toxicon. 2017;138:191–195. doi: 10.1016/j.toxicon.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Saviola A.J., Gandara A.J., Bryson R.W., Jr., Mackessy S.P. Venom phenotypes of the Rock Rattlesnake (Crotalus lepidus) and the Ridge-nosed Rattlesnake (Crotalus willardi) from México and the United States. Toxicon. 2017;138:119–129. doi: 10.1016/j.toxicon.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 11.Mackessy S.P. Morphology and ultrastructure of the venom glands of the northern Pacific rattlesnake Crotalus viridis oreganus. J. Morphol. 1991;208:109–128. doi: 10.1002/jmor.1052080106. [DOI] [PubMed] [Google Scholar]
- 12.Mackessy S.P., Baxter L.M. Bioweapons synthesis and storage: The venom gland of front-fanged snakes. Zool. Anz. 2006;245:147–159. doi: 10.1016/j.jcz.2006.01.003. [DOI] [Google Scholar]
- 13.Sanz L., Gibbs H.L., Mackessy S.P., Calvete J.J. Venom proteomes of closely related Sistrurus rattlesnakes with divergent diets. J. Proteome Res. 2006;5:2098–2112. doi: 10.1021/pr0602500. [DOI] [PubMed] [Google Scholar]
- 14.Calvete J.J., Juárez P., Sanz L. Snake venomics. Strategy and applications. J. Mass Spectrom. 2007;42:1405–1414. doi: 10.1002/jms.1242. [DOI] [PubMed] [Google Scholar]
- 15.Mackessy S.P. Venom composition in rattlesnakes: Trends and biological significance. In: Hayes W.K., Beaman K.R., Cardwell M.D., Bush S.P., editors. The Biology of Rattlesnakes. Loma Linda University Press; Loma Linda, CA, USA: 2008. pp. 495–510. [Google Scholar]
- 16.Mackessy S.P. The field of reptile toxinology: Snakes, lizards and their venoms. In: Mackessy S.P., editor. Handbook of Venoms and Toxins of Reptiles. CRC Press/Taylor & Francis Group; Boca Raton, FL, USA: 2010. pp. 3–23. [Google Scholar]
- 17.Tu A.T. Rattlesnake venoms: Their actions and treatments. Can. Vet. J. 1983;24:203–204. [Google Scholar]
- 18.Minton S.A. Observations on toxicity and antigenetic makeup of venoms from juvenile snakes. In: Russell F.E., Saunders P.R., editors. Animal Toxins. Pergamon Press; Oxford, UK: 1967. pp. 211–222. [Google Scholar]
- 19.Reid H.A., Theakston R.D.G. Changes in coagulation effects by venoms of Crotalus atrox as snakes age. Am. J. Trop. Med. Hyg. 1978;27:1053–1057. doi: 10.4269/ajtmh.1978.27.1053. [DOI] [PubMed] [Google Scholar]
- 20.Lomonte B., Gené J.A., Gutiérrez J.M., Cerdas L. Estudio comparativo de los venenos de serpiente Cascabel (Crotalus durissus durissus) de ejemplares adultos y recien nacidos. Toxicon. 1983;21:379–384. doi: 10.1016/0041-0101(83)90094-6. [DOI] [PubMed] [Google Scholar]
- 21.Mackessy S.P. Venom ontogeny in the Pacific rattlesnakes Crotalus viridis helleri and C. v. oreganus. Copeia. 1988;1988:92–101. doi: 10.2307/1445927. [DOI] [Google Scholar]
- 22.Mackessy S.P. Kallikrein-like and thrombin-like proteases from the venoms of juvenile and adult northern Pacific rattlesnakes (Crotalus viridis oreganus) J. Nat. Toxins. 1993;2:223–239. doi: 10.1016/0305-0491(93)90025-z. [DOI] [PubMed] [Google Scholar]
- 23.Mackessy S.P. Fibrinogenolytic proteases from the venoms of juvenile and adult northern Pacific rattlesnakes (Crotalus viridis oreganus) Comp. Biochem. Physiol. 1993;106B:181–189. doi: 10.1016/0305-0491(93)90025-Z. [DOI] [PubMed] [Google Scholar]
- 24.Mackessy S.P., Williams K., Ashton K.G. Ontogenetic variation in venom composition and diet of Crotalus oreganus concolor: A case of venom paedomorphosis? Copeia. 2003;2003:769–782. doi: 10.1643/HA03-037.1. [DOI] [Google Scholar]
- 25.Calvete J.J., Sanz L., Cid P., de la Torre P., Flores-Díaz M., Dos Santos M.C., Borges A., Bremo A., Angulo Y., Lomonte B., et al. Snake venomics of the Central American rattlesnake Crotalus simus and the South American Crotalus durissus complex points to neurotoxicity as an adaptive paedomorphic trend along Crotalus dispersal in South America. J. Proteome Res. 2010;9:528–544. doi: 10.1021/pr9008749. [DOI] [PubMed] [Google Scholar]
- 26.Saviola A.J., Pla D., Sanz L., Castoe T.A., Calvete J.J., Mackessy S.P. Comparative venomics of the Prairie Rattlesnake (Crotalus viridis viridis) from Colorado: Identification of a novel pattern of ontogenetic changes in venom composition and assessment of the immunoreactivity of the commercial antivenom CroFab®. J. Proteom. 2015;121:28–43. doi: 10.1016/j.jprot.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 27.Wray K.P., Margres M.J., Seavy M., Rokyta D.R. Early significant ontogenetic changes in snake venoms. Toxicon. 2015;96:74–81. doi: 10.1016/j.toxicon.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 28.Glenn J.L., Straight R. Mojave rattlesnake Crotalus scutulatus venom: Variation in toxicity with geographical origin. Toxicon. 1978;16:81–84. doi: 10.1016/0041-0101(78)90065-X. [DOI] [PubMed] [Google Scholar]
- 29.Núñez V., Cid P., Sanz L., De La Torre P., Angulo Y., Lomonte B., Gutiérrez J.M., Calvete J.J. Snake venomics and antivenomics of Bothrops atrox venoms from Colombia and the Amazon regions of Brazil, Perú and Ecuador suggest the occurrence of geographic variation of venom phenotype by a trend towards paedomorphism. J. Proteom. 2009;73:57–78. doi: 10.1016/j.jprot.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 30.Boldrini-França J., Corrêa-Netto C., Silva M.M., Rodrigues R.S., De La Torre P., Pérez A., Soares A.M., Zingali R.B., Nogueira R.A., Rodrigues V.M., et al. Snake venomics and antivenomics of Crotalus durissus subspecies from Brazil: Assessment of geographic variation and its implication on snakebite management. J. Proteom. 2010;73:1758–1776. doi: 10.1016/j.jprot.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Glaudas X., Jezkova T., Rodríguez-Robles J.A. Feeding ecology of the Great Basin Rattlesnake (Crotalus lutosus, Viperidae) Can. J. Zool. 2008;86:723–734. doi: 10.1139/Z08-049. [DOI] [Google Scholar]
- 32.Durban J., Pérez A., Sanz L., Gómez A., Bonilla F., Rodríguez S., Chacón D., Sasa M., Angulo Y., Gutiérrez J.M., et al. Integrated “omics” profiling indicates that miRNAs are modulators of the ontogenetic venom composition shift in the Central American rattlesnake, Crotalus simus simus. BMC Genet. 2013;14:234. doi: 10.1186/1471-2164-14-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mociño-Deloya E., Setser K., Pleguezuelos J.M., Kardon A., Lazcano D. Cannibalism of nonviable offspring by postparturient Mexican lance-headed rattlesnakes, Crotalus polystictus. Anim. Behav. 2009;77:145–150. doi: 10.1016/j.anbehav.2008.09.020. [DOI] [Google Scholar]
- 34.Setser K., Mociño-Deloya E., Pleguezuelos J.M., Lazcano D., Kardon A. Reproductive ecology of female Mexican lance-headed rattlesnakes. J. Zool. 2010;281:175–182. doi: 10.1111/j.1469-7998.2010.00692.x. [DOI] [Google Scholar]
- 35.Meik J.M., Setser K., Mociño-Deloya E., Lawing A.M. Sexual differences in head form and diet in a population of Mexican lance-headed rattlesnakes, Crotalus polystictus. Biol. J. Linn. Soc. 2012;106:633–640. doi: 10.1111/j.1095-8312.2012.01881.x. [DOI] [Google Scholar]
- 36.Fox J.W., Serrano S.M. Insights into and speculations about snake venom metalloproteinase (SVMP) synthesis, folding and disulfide bond formation and their contribution to venom complexity. FEBS J. 2008;275:3016–3030. doi: 10.1111/j.1742-4658.2008.06466.x. [DOI] [PubMed] [Google Scholar]
- 37.Fox J.W., Serrano S.M. Snake venom metalloproteinases. In: Mackessy S.P., editor. Handbook of Venoms and Toxins of Reptiles. CRC Press/Taylor & Francis Group; Boca Raton, FL, USA: 2010. pp. 95–113. [Google Scholar]
- 38.Jia L.G., Shimokawa K.I., Bjarnason J.B., Fox J.W. Snake venom metalloproteinases: Structure, function and relationship to the ADAMs family of proteins. Toxicon. 1996;34:1269–1276. doi: 10.1016/S0041-0101(96)00108-0. [DOI] [PubMed] [Google Scholar]
- 39.Fox J.W., Serrano S.M.T. Structural considerations of the snake venom metalloproteinases, key members of the M12 reprolysin family of metalloproteinases. Toxicon. 2005;45:969–985. doi: 10.1016/j.toxicon.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 40.Mackessy S.P., Saviola A.J. Understanding biological roles of venoms among the Caenophidia: The importance of rear-fanged snakes. Integr. Comp. Biol. 2016;56:1004–1021. doi: 10.1093/icb/icw110. [DOI] [PubMed] [Google Scholar]
- 41.Saravia P., Rojas E., Arce V., Guevara C., López J.C., Chaves E., Velásquez R., Rojas G., Gutiérrez J.M. Geographic and ontogenic variability in the venom of the neotropical rattlesnake Crotalus durissus: Pathophysiological and therapeutic implications. Rev. Biol. Trop. 2002;50:337–346. [PubMed] [Google Scholar]
- 42.Kini R.M., Evans H.J. Structural domains in venom proteins: Evidence that metalloproteinases and nonenzymatic platelet aggregation inhibitors (disintegrins) from snake venoms are derived by proteolysis from a common precursor. Toxicon. 1992;30:265–293. doi: 10.1016/0041-0101(92)90869-7. [DOI] [PubMed] [Google Scholar]
- 43.Calvete J.J., Marcinkiewicz C., Monleón D., Esteve V., Celda B., Juárez P., Sanz L. Snake venom disintegrins: Evolution of structure and function. Toxicon. 2005;45:1063–1074. doi: 10.1016/j.toxicon.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 44.Casewell N.R., Wagstaff S.C., Harrison R.A., Renjifo C., Wüster W. Domain loss facilitates accelerated evolution and neofunctionalization of duplicate snake venom metalloproteinase toxin genes. Mol. Biol. Evol. 2011;28:2637–2649. doi: 10.1093/molbev/msr091. [DOI] [PubMed] [Google Scholar]
- 45.Scarborough R.M., Rose J.W., Naughton M.A., Phillips D.R., Nannizzi L., Arfsten A., Campbell A.M., Charo I.F. Characterization of the integrin specificities of disintegrins isolated from American pit viper venoms. J. Biol. Chem. 1993;268:1058–1065. [PubMed] [Google Scholar]
- 46.Saviola A.J., Chiszar D., Busch C., Mackessy S.P. Molecular basis for prey relocation in viperid snakes. BMC Biol. 2013;11:20. doi: 10.1186/1741-7007-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saviola A.J., Mackessy S.P. Observations on the chemosensory responses of the midget faded rattlesnake (Crotalus oreganus concolor): Discrimination of envenomated prey in a type II venom species. J. Ethol. 2017;35:245–250. doi: 10.1007/s10164-017-0511-2. [DOI] [Google Scholar]
- 48.Mackessy S.P. Thrombin-like enzymes in snake venoms. In: Kini R., Clemetson K., Markland F., McLane M., Morita T., editors. Toxins and Hemostasis. Springer; Dordrecht, The Netherlands: 2010. pp. 519–557. [Google Scholar]
- 49.Swenson S., Markland F.S. Snake venom fibrin(ogen)olytic enzymes. Toxicon. 2005;45:1021–1039. doi: 10.1016/j.toxicon.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 50.Markland F.S., Swenson S. Snake venom metalloproteinases. Toxicon. 2013;62:3–18. doi: 10.1016/j.toxicon.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 51.Russell F.E., Beuss F.W., Woo M.Y. Zootoxicological properties of venom phosphodiesterase. Toxicon. 1963;1:99–108. doi: 10.1016/0041-0101(63)90070-9. [DOI] [Google Scholar]
- 52.Aird S.D. Ophidian envenomation strategies and the role of purines. Toxicon. 2002;40:335–393. doi: 10.1016/S0041-0101(01)00232-X. [DOI] [PubMed] [Google Scholar]
- 53.Izidoro L.F.M., Sobrinho J.C., Mendes M.M., Costa T.R., Grabner A.N., Rodrigues V.M., da Silva S.L., Zanchi F.B., Zuliani J.P., Fernandes C.F., et al. Snake venom L-amino acid oxidases: Trends in pharmacology and biochemistry. BioMed Res. Int. 2014;2014 doi: 10.1155/2014/196754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rodrigues R.S., da Silva J.F., França J.B., Fonseca F.P., Otaviano A.R., Silva F.H., Hamaguchi A., Magro A.J., Braz A.S.K., dos Santos J.I., et al. Structural and functional properties of Bp-LAAO, a new L-amino acid oxidase isolated from Bothrops pauloensis snake venom. Biochimie. 2009;91:490–501. doi: 10.1016/j.biochi.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 55.Samel M., Vija H., Rönnholm G., Siigur J., Kalkkinen N., Siigur E. Isolation and characterization of an apoptotic and platelet aggregation inhibiting L-amino acid oxidase from Vipera berus berus (common viper) venom. BBA Proteins Proteom. 2006;1764:707–714. doi: 10.1016/j.bbapap.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 56.Souza D.H., Eugenio L.M., Fletcher J.E., Jiang M.S., Garratt R.C., Oliva G., Selistre-de-Araujo H.S. Isolation and structural characterization of a cytotoxic L-amino acid oxidase from Agkistrodon contortrix laticinctus snake venom: Preliminary crystallographic data. Arch. Biochem. Biophys. 1999;368:285–290. doi: 10.1006/abbi.1999.1287. [DOI] [PubMed] [Google Scholar]
- 57.Lee M.L., Fung S.Y., Chung I., Pailoor J., Cheah S.H., Tan N.H. King cobra (Ophiophagus hannah) venom L-amino acid oxidase induces apoptosis in PC-3 cells and suppresses PC-3 solid tumor growth in a tumor xenograft mouse model. Int. J. Med. Sci. 2014;11:593–601. doi: 10.7150/ijms.8096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mukherjee A.K., Saviola A.J., Burns P.D., Mackessy S.P. Apoptosis induction in human breast cancer (MCF-7) cells by a novel venom L-amino acid oxidase (Rusvinoxidase) is independent of its enzymatic activity and is accompanied by caspase-7 activation and reactive oxygen species production. Apoptosis. 2015;20:1358–1372. doi: 10.1007/s10495-015-1157-6. [DOI] [PubMed] [Google Scholar]
- 59.Graham R.L.J., Graham C., McClean S., Chen T., O’Rourke M., Hirst D., Theakston D., Shaw C. Identification and functional analysis of a novel bradykinin inhibitory peptide in the venoms of New World Crotalinae pit vipers. Biochem. Biophys. Res. Commun. 2005;338:1587–1592. doi: 10.1016/j.bbrc.2005.10.130. [DOI] [PubMed] [Google Scholar]
- 60.Calvete J.J., Fasoli E., Sanz L., Boschetti E., Righetti P.G. Exploring the venom proteome of the western diamondback rattlesnake, Crotalus atrox, via snake venomics and combinatorial peptide ligand library approaches. J. Proteome Res. 2009;8:3055–3067. doi: 10.1021/pr900249q. [DOI] [PubMed] [Google Scholar]
- 61.Fernández J., Lomonte B., Sanz L., Angulo Y., Gutiérrez J.M., Calvete J.J. Snake venomics of Bothriechis nigroviridis reveals extreme variability among palm pitviper venoms: Different evolutionary solutions for the same trophic purpose. J. Proteome Res. 2010;9:4234–4241. doi: 10.1021/pr100545d. [DOI] [PubMed] [Google Scholar]
- 62.Lomonte B., Tsai W.C., Ureña-Diaz J.M., Sanz L., Mora-Obando D., Sánchez E.E., Fry B.G., Gutiérrez J.M., Gibbs H.L., Sovic M.G., et al. Venomics of New World pit vipers: Genus-wide comparisons of venom proteomes across Agkistrodon. J. Proteom. 2014;96:103–116. doi: 10.1016/j.jprot.2013.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pla D., Sanz L., Molina-Sánchez P., Zorita V., Madrigal M., Flores-Díaz M., Alape-Girón A., Núñez V., Andrés V., Gutiérrez J.M., Calvete J.J. Snake venomics of Lachesis muta rhombeata and genus-wide antivenomics assessment of the paraspecific immunoreactivity of two antivenoms evidence the high compositional and immunological conservation across Lachesis. J. Proteom. 2013;89:112–123. doi: 10.1016/j.jprot.2013.05.028. [DOI] [PubMed] [Google Scholar]
- 64.Pawlak J., Kini R.M. Snake venom glutaminyl cyclase. Toxicon. 2006;48:278–286. doi: 10.1016/j.toxicon.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 65.Wang Y.M., Huang K.F., Tsai I.H. Snake venom glutaminyl cyclases: Purification, cloning, kinetic study, recombinant expression, and comparison with the human enzyme. Toxicon. 2014;86:40–50. doi: 10.1016/j.toxicon.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 66.Wermelinger L.S., Dutra D.L.S., Oliveira-Carvalho A.L., Soares M.R., Bloch C., Jr., Zingali R.B. Fast analysis of low molecular mass compounds present in snake venom: Identification of ten new pyroglutamate-containing peptides. Rapid Commun. Mass Spectrom. 2005;19:1703–1708. doi: 10.1002/rcm.1973. [DOI] [PubMed] [Google Scholar]
- 67.Xu X., Li B., Zhu S., Rong R. Hypotensive peptides from snake venoms: Structure, function and mechanism. Curr. Top. Med. Chem. 2015;15:658–669. doi: 10.2174/1568026615666150217113835. [DOI] [PubMed] [Google Scholar]
- 68.Munekiyo S.M., Mackessy S.P. Presence of peptide inhibitors in rattlesnake venoms and their effects on endogenous metalloproteases. Toxicon. 2005;45:255–263. doi: 10.1016/j.toxicon.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 69.Bernheimer A.W., Weinstein S.A., Linder R. Isoelectric analysis of some Australian elapid snake venoms with special reference to phospholipase B and hemolysis. Toxicon. 1986;24:841–849. doi: 10.1016/0041-0101(86)90109-1. [DOI] [PubMed] [Google Scholar]
- 70.Bernheimer A.W., Linder R., Weinstein S.A., Kim K.S. Isolation and characterization of a phospholipase B from venom of Collett’s snake, Pseudechis colletti. Toxicon. 1987;25:547–554. doi: 10.1016/0041-0101(87)90290-X. [DOI] [PubMed] [Google Scholar]
- 71.Takasaki C., Tamiya N. Isolation and properties of lysophospholipases from the venom of an Australian elapid snake, Pseudechis australis. Biochem. J. 1982;203:269–276. doi: 10.1042/bj2030269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kemparaju K., Girish K.S. Snake venom hyaluronidase: A therapeutic target. Cell Biochem. Funct. 2006;24:7–12. doi: 10.1002/cbf.1261. [DOI] [PubMed] [Google Scholar]
- 73.Guércio R.A., Shevchenko A., Shevchenko A., López-Lozano J.L., Paba J., Sousa M.V., Ricart C.A. Ontogenetic variations in the venom proteome of the Amazonian snake Bothrops atrox. Proteome Sci. 2006;4:11. doi: 10.1186/1477-5956-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alape-Girón A., Sanz L., Escolano J., Flores-Díaz M., Madrigal M., Sasa M., Calvete J.J. Snake venomics of the lancehead pitviper Bothrops asper: Geographic, individual, and ontogenetic variations. J. Proteome Res. 2008;7:3556–3571. doi: 10.1021/pr800332p. [DOI] [PubMed] [Google Scholar]
- 75.Madrigal M., Sanz L., Flores-Díaz M., Sasa M., Núñez V., Alape-Girón A., Calvete J.J. Snake venomics across genus Lachesis. Ontogenetic changes in the venom composition of Lachesis stenophrys and comparative proteomics of the venoms of adult Lachesis melanocephala and Lachesis acrochorda. J. Proteom. 2012;77:280–297. doi: 10.1016/j.jprot.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 76.Rokyta D.R., Margres M.J., Ward M.J., Sanchez E.E. The genetics of venom ontogeny in the eastern diamondback rattlesnake (Crotalus adamanteus) Peer J. 2017;5:3249. doi: 10.7717/peerj.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pla D., Sanz L., Sasa M., Acevedo M.E., Dwyer Q., Durban J., Pérez A., Rodriguez Y., Lomonte B., Calvete J.J. Proteomic analysis of venom variability and ontogeny across the arboreal palm-pitvipers (genus Bothriechis) J. Proteom. 2017;152:1–12. doi: 10.1016/j.jprot.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 78.Modahl C.M., Mukherjee A.K., Mackessy S.P. An analysis of venom ontogeny and prey-specific toxicity in the Monocled Cobra (Naja kaouthia) Toxicon. 2016;119:8–20. doi: 10.1016/j.toxicon.2016.04.049. [DOI] [PubMed] [Google Scholar]
- 79.Jackson T.N., Koludarov I., Ali S.A., Dobson J., Zdenek C.N., Dashevsky D., Masci P.P., Nouwens A., Josh P., Goldenberg J., et al. Rapid radiations and the race to redundancy: An investigation of the evolution of Australian elapid snake venoms. Toxins. 2016;8:309. doi: 10.3390/toxins8110309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cipriani V., Debono J., Goldenberg J., Jackson T.N., Arbuckle K., Dobson J., Koludarov I., Li B., Hay C., Dunstan N., et al. Correlation between ontogenetic dietary shifts and venom variation in Australian brown snakes (Pseudonaja) Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2017;197:53–60. doi: 10.1016/j.cbpc.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 81.Mackessy S.P., Sixberry N.M., Heyborne W.H., Fritts T. Venom of the Brown Treesnake, Boiga irregularis: Ontogenetic shifts and taxa-specific toxicity. Toxicon. 2006;47:537–548. doi: 10.1016/j.toxicon.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 82.Pla D., Petras D., Saviola A.J., Modahl C.M., Sanz L., Pérez A., Juárez E., Frietze S., Dorrestein P.C., Mackessy S.P., et al. Transcriptomics-guided bottom-up and top-down venomics of neonate and adult specimens of the arboreal rear-fanged Brown Treesnake, Boiga irregularis, from Guam. J. Proteom. 2018;174:71–84. doi: 10.1016/j.jprot.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 83.Barlow A., Pook C.E., Harrison R.A., Wüster W. Coevolution of diet and prey-specific venom activity supports the role of selection in snake venom evolution. Proc. R. Soc. Lond. B. Biol. Sci. 2009;276:2443–2449. doi: 10.1098/rspb.2009.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–251. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 85.Aird S.D., da Silva N.J., Jr. Comparative enzymatic composition of Brazilian coral snake (Micrurus) venoms. Comp. Biochem. Physiol. 1991;99B:287–294. doi: 10.1016/0305-0491(91)90043-D. [DOI] [PubMed] [Google Scholar]
- 86.Björk W. Purification of phosphodiesterase from Bothrops atrox venom, with special consideration of the elimination of monophosphatases. J. Biol. Chem. 1963;238:2487–2490. [PubMed] [Google Scholar]
- 87.Laskowski M. Purification and properties of venom phosphodiesterase. Meths. Enzymol. 1980;65:276–284. doi: 10.1016/s0076-6879(80)65037-x. [DOI] [PubMed] [Google Scholar]
- 88.Smith C.F., Mackessy S.P. The effects of hybridization on divergent venom phenotypes: Characterization of venom from Crotalus scutulatus scutulatus × Crotalus oreganus helleri hybrids. Toxicon. 2016;120:110–123. doi: 10.1016/j.toxicon.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 89.Zhu W., Smith J.W., Huang C.M. Mass spectrometry-based label-free quantitative proteomics. J. Biomed. Biotechnol. 2010;6 doi: 10.1155/2010/840518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McIlwain S., Mathews M., Bereman M.S., Rubel E.W., MacCoss M.J., Noble W.S. Estimating relative abundances of proteins from shotgun proteomics data. BMC Bioinform. 2012;13:308. doi: 10.1186/1471-2105-13-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.MASCOT Ver. 2.2 Search Engine. [(accessed on 10 September 2017)]; Available online: http://www.matrixscience.com.
- 92.Perkins D.N., Pappin D.J.C., Creasy D.M., Cottrell J.S. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis. 1999;20:3551–3567. doi: 10.1002/(SICI)1522-2683(19991201)20:18<3551::AID-ELPS3551>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 93.NCBI BLAST program. [(accessed on 10 September 2017)]; Available online: http://www.ncbi.nlm.nih.gov/BLAST/
- 94.U.S. Environmental Protection Agency Trimmed Spearman–Karber (TSK) Program Version 1.5. Ecological Monitoring Research Division, Environmental Monitoring Systems Laboratory, Cincinnati, OH. [(accessed on 15 September 2017)];1990 Available online: http://www.scirp.org/%28S%28i43dyn45teexjx455qlt3d2q%29%29/reference/ReferencesPapers.aspx?ReferenceID=1130679.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.