Abstract
Dental caries is a common disease caused by oral bacteria. Streptococcus mutans and Streptococcus sobrinus are the primary cariogenic microbes that often survive as biofilms on teeth. In this study, we evaluated the activity of ClyR, a well-known chimeric lysin with extended streptococcal host range, against common Gram-positive oral microbes and its anticaries efficacy in rat models. ClyR demonstrated high lytic activity against S. mutans MT8148 and S. sobrinus ATCC6715, with minor activity against Streptococcus sanguinis, Streptococcus oralis, and Streptococcus salivarius, which are considered as harmless commensal oral bacteria. Confocal laser scanning microscopy showed that the number of viable cells in 72-h aged S. mutans and S. sobrinus biofilms are significantly (p < 0.05) decreased after treatment with 50 µg/mL ClyR for 5 min. Furthermore, continuous administration of ClyR for 40 days (5 µg/day) significantly (p < 0.05) reduced the severity of caries in rat models infected with a single or a mixed bacteria of S. mutans and S. sobrinus. Therefore, ClyR could be a promising agent or additive for the prevention and treatment of dental caries.
Keywords: dental caries, bacteriophage lysin, biofilm, antibacterial, anticaries
1. Introduction
Dental caries remains a significant problem all over the world despite improved oral hygiene awareness [1]. For more than a century, it has been known that caries is initiated by demineralization of the enamel as a result of fermentation and acidogenesis by oral bacteria in biofilm [2]. Among cariogenic microbes, Streptococcus mutans is identified as the primary cariogenic pathogen because of its unique acid-producing and aciduric ability [3,4]. Glucan produced by S. mutans plays a fundamental role in biofilm formation, providing binding sites for bacteria colonization on enamel surface [5]. Streptococcus sobrinus, another important cariogenic microorganism, is less frequently detected than S. mutans in the oral cavity but is considered more virulent than S. mutans due to its high acidogenicity and acid tolerance [6]. Previous studies have observed that co-existence of both species is associated with higher incidence of dental caries and higher scores in the DMFT (decayed missing and filled teeth) index in children with early childhood caries (ECC) [7,8]. The amounts of oral S. mutans and S. sobrinus are used as risk indicators for dental caries [9].
There are presently a few ways to reduce dental caries, where fluoride therapy is the most common method to reduce the risk of the disease [10]. However, fluoride toothpaste (with concentrations of 1000 ppm and above) or fluoride supplements may exposure children under six, especially under three to higher risks of dental fluorosis [11], fluoride toxicity [12], and even fatality caused by accidental ingestion (http://fluoridealert.org/articles/kennerly/). Cariogenic biofilms can be removed by traditional nonspecific mechanical brushing or flossing or by rinsing with a broad-spectrum antibiotic such as chlorhexidine. Derivatives and extractives from natural products, such as fractions of barley coffee [13], cranberry constituents [14], and α-mangostin with/or lawsone methyl ether [15], have been shown to prevent S. mutans biofilm formation. In addition, numerous small molecules, including chitosan, 2-amino-imidazole/triazole conjugate [16], and apigenin [17], have shown good antibiofilm activity. However, few of these treatments confer selectivity against S. mutans biofilms. In recent years, antimicrobials capable of selectively eliminating S. mutans have been designed to achieve targeted killing with minimal effect on other oral microbes [18,19]. This has opened up alternative methods to prevent caries without disturbing the ecological balance to commensal bacteria in the oral cavity [20].
Endolysins are bacteriophage-encoded enzymes that degrade bacterial peptidoglycan at the end of the lytic cycle. Exogenous application of recombinant endolysins to Gram-positive bacteria rapidly induces osmotic lysis and, consequently, cell death. In recent years, endolysins and their derivatives have emerged as a novel class of antibacterial [21,22], and some of them have now entered clinical trial phases [23,24]. Many lysins are reported to be superior to antibiotics in biofilm eradication [25,26]. Their rapid mode of action and specificity differentiate them from antibiotics. Another advantage of endolysins is that they have a low risk of resistance because they target the essential conserved elements on bacterial cell walls that maintain cell viability [27,28].
Recently, our cooperator engineered a chimeolysin ClyR (with its catalytic domain from the CHAP domain of PlyC lysin and the cell wall binding domain from PlySs2 lysin), which was found by an induced lysis-based rapid screening method with broad activity against various Gram-positive pathogens, including multiple strains of streptococci, part of staphylococci, and enterococci [29]. The bactericidal activity of ClyR against S. mutans has been further demonstrated in vitro and in a dental colonization mouse model previously [30], however, its bactericidal activity and anticaries efficacy against mixed oral microbes still need to be established. In this study, we determined the efficacy of ClyR against multiple oral Gram-positive bacteria, including S. sobrinus and S. mutans isolated from children with severe early childhood caries (SECC) in China. The anticaries efficacy of ClyR was further investigated in rat models infected with a single or mixed bacteria of S. mutans and S. sobrinus.
2. Materials and Methods
2.1. Bacterial Strains
S. mutans MT8148, S. sobrinus ATCC6715, Streptococcus oralis ATCC10557, Streptococcus salivarius ATCC7073, and Streptococcus sanguinis ATCC10556 were supplied by the Key Laboratory of Oral Biomedicine Ministry of Education, School of Stomatology, Wuhan University. The clinical strains of S. mutans and S. sobrinus were isolated from dental plaques of different children with SECC, which were confirmed by biochemistry identification (Tianhe Microbial, Inc., Hangzhou, China) and 16S rDNA sequencing analysis (the primers used were 27F: 5′-AGAGTTTGATCATGGCTCAG-3′; 1492R: 5′-TAGGGTTACCTTGTTACGACTT-3′). The isolates were also genotyped by arbitrarily primed polymerase chain reaction (AP-PCR) [31]. The animal experiment was approved by the Medical Ethics Committee of School of Stomatology of Wuhan University (approval no. 2016-30, 26 February 2016). All streptococci were routinely cultivated in brain heart infusion (BHI) broth (Alpha Bioscience) and grown at 37 °C. For production of biofilms, BHI medium was supplemented with 1% sucrose (BHIS). Luria broth (LB) was used to cultivate Escherichia coli.
2.2. Cloning, Expression, and Purification of ClyR
The Escherichia coli BL21(DE3) strain containing plasmid pET28a-clyR, originated from Wuhan Scithera Microbial Technologies Co Ltd. (Wuhan, China), expresses a chimeolysin consisting of the CHAP catalytic domain of PlyC (PlyCAC, amino acids 314–465) and the cell wall binding domain of PlySs2 (PlySb, C-terminal 99 amino acids). As detailed by Yang et al. [29], E. coli strain was cultured to an optical density (OD600) of ~0.6, induced by adding IPTG to 0.2 mM, and allowed to grow overnight at 16 °C. Purification was performed following the instructions of HisTrap FF columns (GE Healthcare, Chicago, IL, USA). Briefly, columns loaded with sample were washed and eluted with 20 and 250 mM imidazole, respectively. After being dialyzed against phosphate-buffered saline (PBS, containing 137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.4 mM KH2PO4, pH 7.4), the purified protein was passed through a Detoxi-Gel™ Endotoxin Removing Gel (Thermo Scientific, Waltham, MA, USA) and quantified by a ToxinSensor Chromogenic LAL Endotoxin Assay Kit (GenScript, Nanjing, China) to confirm a low level of endotoxin (<0.2 EU/mL). After quantitation by Bradford assay, the protein solution was stored at −20 °C until use.
2.3. Bactericidal Assay
In order to know the lytic activity of ClyR against oral common streptococci, a screening experiment was performed. S. mutans MT8148, S. sobrinus ATCC6715, S. oralis ATCC10557, S. salivarius ATCC7073, and S. sanguinis ATCC10556 were grown to log-phase at 37 °C and adjusted to an OD600 of ~0.8 with PBS buffer as measured in 96-well plates (NEST, Nanjing, China) using a Synergy H1 microplate reader (BioTek, Winooski, VT, USA). After mixing 190 μL of bacterial suspension with 10 μL of the lysin (1 mg/mL) or PBS in a 96-well plate, the drop of OD600 in each well was monitored immediately by the microplate reader for 60 min at 37 °C. Meanwhile, viable cell numbers after each treatment were determined by serial ten-fold dilution and plating on BHI agar.
To determine the dose-dependent and time-dependent lytic activity of ClyR, S. mutans MT8148 and S. sobrinus ATCC6715 resuspended in PBS were adjusted to an OD600 of ~0.8. Aliquots of 190 µL of the cell suspension was distributed in 96-well microplates and treated with 10µL of ClyR at different concentrations (0, 25, 50, and 100 µg/mL) for 10 min or 50 µg/mL ClyR for 0, 1, 2, 3, and 5 min at 37 °C. At the end of the reaction, 10-fold serial dilutions of each well were plated on BHI agar and incubated at 37 °C. Resultant colonies were enumerated after 18 h. The bactericidal effect was expressed as the difference in colony-forming units (CFU) from PBS-treated and ClyR-treated groups. To rule out degradation or aggregation of ClyR by salivary proteins, we also tested the activity of ClyR in human saliva. S. mutans MT8148 resuspended in PBS buffer or human saliva were treated with 25 µg/mL of ClyR for 1 h at 37 °C, then serially diluted and plated onto BHI agar for CFU enumeration. All experiments were performed independently three times in triplicate.
2.4. ClyR Activity against Clinical S. mutans and S. sobrinus Isolates
Clinical S. mutans and S. sobrinus isolates were grown overnight and brought to an OD600 of ~0.8 with PBS. From these bacterial stocks, 190 μL were added to each well of a 96-well microplate. For each strain, each of the well received 10 μL of ClyR at 1 mg/mL (10 μg, resulting in a final concentration of 50 μg/mL). Corresponding triplicate wells received 10 μL of PBS as controls. The Synergy H1 microplate reader was used to measure the drop of OD600 of the mixture at 37 °C for 10 min.
2.5. Transmission Electron Microscopy (TEM)
TEM was used to visualize the effects of ClyR on the bacterial cell wall. Log phase S. mutans MT8148 and S. sobrinus ATCC6715 were washed with PBS and adjusted to an OD600 of 0.8, then treated with 50 µg/mL ClyR or PBS at 37 °C for 5 min and resuspended in PBS. After fixation by 2.5% glutaraldehyde, samples were analyzed by a transmission electron microscope (Tecnai G2 20 Twin; Fei, Hillsboro, OR, USA).
2.6. Scanning Electron Microscopy (SEM)
S. mutans MT8148 and S. sobrinus ATCC6715 were inoculated into BHIS in a 6-well plate with a glass coverslip in each well. After incubation for 72 h, biofilms formed on the glass coverslips were rinsed three times with sterile PBS to remove planktonic and loosely adherent cells. The biofilms were treated with either 1 mL of 50 μg/mL ClyR or PBS for 5 min simultaneously, followed by prefixing with 2.5% glutaraldehyde for 10 min before fixation in 1% osmium tetroxide for 1 h. After that, the coverslips were subjected to a series of ethanol (30%, 50%, 60%, 70%, 80%, 90% and 100%) for dehydration. Finally, samples were coated with gold (Hummer VI; Technic Inc., Anaheim, CA, USA) before observation under SEM (SEM, SU8010, Hitachi, Japan) at an accelerating voltage of 5 kv using 5000× and 10,000× magnifications.
2.7. Confocal Laser Scanning Microscopy (CLSM)
The 72-h biofilms exposed to 50 μg/mL of ClyR or PBS for 5 min were also stained by LIVE/DEAD® Bac-LightTM Bacterial Viability kit L-7012 (Invitrogen, Carlsbad, CA, USA) for 15 min and washed twice with PBS. The stained biofilms were viewed using a confocal laser scanning microscope (UltraVIEW VoX; PerkinElmer, Waltham, MA, USA) at a 1024 × 1024 pixel scan area using a 60× lens. Four random areas of each biofilm on each coverslip were scanned. A stack of 10–20 slices in 0.5 μm step sizes was captured from the top to the bottom of the biofilm. All acquired images were analyzed by the Volocity (version 6.3.0, PerkinElmer, Waltham, MA, USA) software supplied with the instrument.
2.8. Quantify Recovered Biofilm Bacteria in Vitro
To determine the viable cell number within biofilm after ClyR treatment, 10 µL overnight culture of S. mutans MT8148 or S. sobrinus ATCC6715 was mixed with 250 µL BHIS in a 48-well polystyrene plate (Tissue culture treated, Nest, China), and incubated at 37 °C for 72 h to allow biofilm formation. After removing the supernatant and washing twice with sterile PBS, biofilms were treated with 200 µL of 50 μg/mL ClyR or PBS for 5 min. After washing twice with PBS, adherent cells from the biofilm were resuspended by vigorous pipetting and vortexing and, finally, serially diluted and plated on BHI agar for CFU enumeration.
2.9. Animal Study
Rat models were used to evaluate the anticaries activity of ClyR in vivo as previously described with some modifications [20]. Thirty six female Sprague Dawley (SD) rats (21 days old) were purchased from Hubei Medical Laboratory Animal Center (Wuhan, China). Animals were housed in the State Key Laboratory Breeding Base of Basic Science of Stomatology (Hubei-MOST) and Key Laboratory of Oral Biomedicine of Ministry of Education (KLOBM) under specific pathogen-free conditions. All animal experiments were conducted with the approval of the Animal Experiments Committee of Wuhan University (approval no. 2016-72). The rats were fed with Keyes 2000 cariogenic diet supplemented with antibiotics (1.0 g/kg each of ampicillin, chloramphenicol, and carbenicillin) and water containing 4000 U/mL penicillin G for the first 3 days to temporarily suppress the proliferation of oral flora to facilitate bacterial infection. At day 25, 36 SD female rats were randomly divided into three groups and infected with 200 µL overnight cultures of S. mutans MT8148, S. sobrinus ATCC6715, or a mixture of S. mutans MT8148 and S. sobrinus, respectively, for the next 6 days. At day 31, half of the rats in each group were treated by pipetting 100 µL ClyR (50 μg/mL) solution per day into the mouth of each rat for 40 days continuously; the rest of the rats were treated with PBS as control. All the rats were euthanized at day 120. After removal of flesh from the jaws, the teeth were observed by stereomicroscope (Olympus, Tokyo, Japan) and stained for caries scoring by Keyes’ method [32].
2.10. Statistical Analysis
Experimental data are presented as means ± standard errors of means. One-way ANOVA test is performed for the statistical analysis. p value of <0.05 is considered to be statistically significant.
3. Results
3.1. High Bactericidal Activity of ClyR against Planktonic S. mutans and S. sobrinus
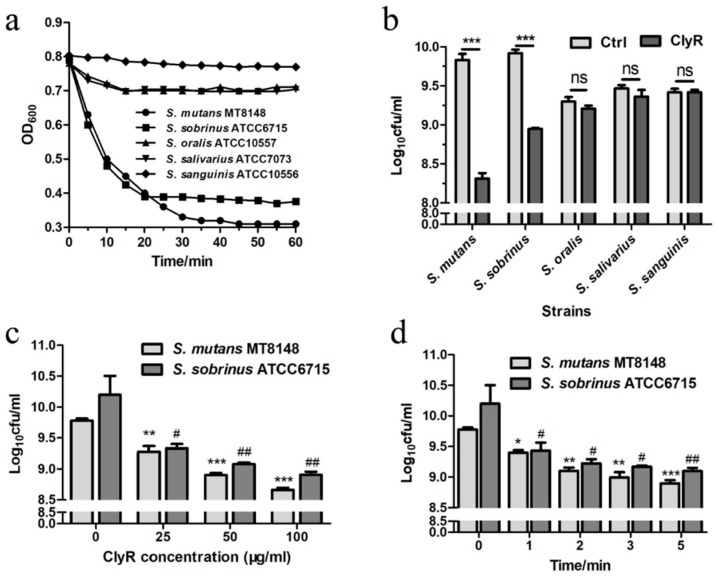
The screening experiment showed that the major cariogenic bacteria S. mutans MT8148 and S. sobrinus ATCC6715 are susceptible to ClyR, with rapid OD600 decrease from 0.8 to 0.3 or 0.4, after treatment with 50 µg/mL ClyR for 1 h (Figure 1a). A corresponding ~1.5 logs or ~1 logs decrease in cell viability was achieved (Figure 1b). However, minor lytic activity against S. oralis ATCC10557, S. salivarius ATCC7073, and S. sanguinis ATCC10556 was observed (Figure 1a,b). These bacteria are believed to be harmless, normal oral bacteria. Then, the dose-dependent (Figure 1c) and time-dependent (Figure 1d) killing efficacy of ClyR were performed. As shown in Figure 1c, ClyR was highly active against S. mutans and S. sobrinus, with reduction of 92.37 ± 1.19% and 93.33 ± 3.41% bacteria, respectively, after treatment with 100 µg/mL of ClyR for 10 min. Notably, a rapid mode of action was observed in the first 5 min, with only 13.18 ± 1.1% S. mutans and 7.94 ± 2.9% S. sobrinus remaining after treatment with 50 µg/mL of ClyR (Figure 1d). Moreover, the activity of ClyR was rare affected by human saliva (Figure S1). Because lysin is intended to be applied directly in mouth, it is preferred that ClyR could kill utmost bacteria in a short time under a low concentration. Therefore, a relatively low ClyR concentration (50 µg/mL) and short treatment time (5 min) were chosen for the biofilm experiments shown below.
Figure 1.
Activity of ClyR against oral microbes. (a) Activity of ClyR against common oral bacteria. S. mutans MT8148, S. sobrinus ATCC6715, S. oralis ATCC10557, S. salivarius ATCC7073, and S. sanguinis ATCC10556 were treated with 50 µg/mL ClyR for 1 h; the Y-axis represents the bacteria turbidity at optical density (OD600); (b) Viable cell number after each treatment was determined by serially ten-fold dilution and plating on brain heart infusion (BHI) agar; (c) Dose-dependent bactericidal activity of ClyR. S. mutans and S. sobrinus were treated with different concentrations of ClyR (0, 25, 50, and 100 µg/mL) for 10 min, viable cell numbers were determined by plating to BHI agar; (d) Time-dependent bactericidal activity of ClyR. Bacteria were treated with 50 µg/mL of ClyR for 0, 1, 2, 3, and 5 min, viable cell numbers were determined by plating on BHI agar. ns: not significant; # and *: p < 0.05; ## and **: p < 0.01; ***: p < 0.001 compared to the control group.
3.2. Broad Lytic Activity
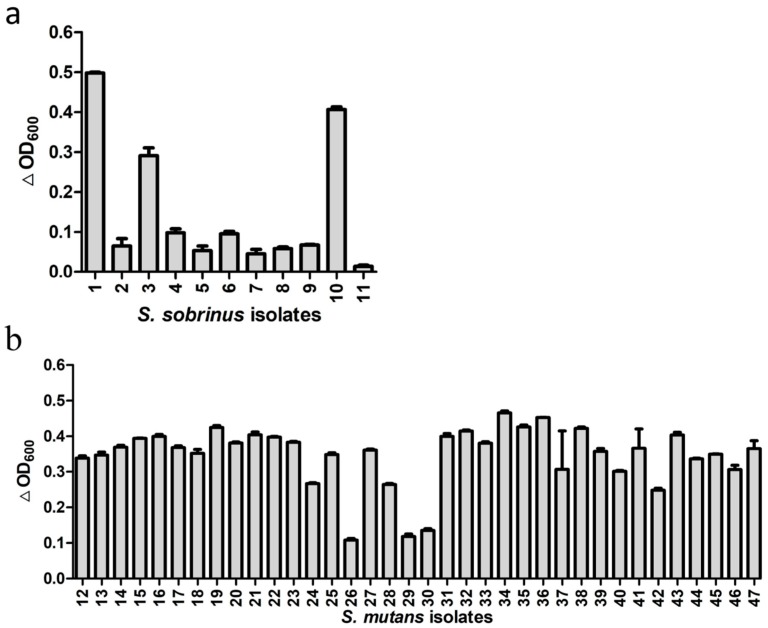
Besides the standard strains, we also tested activity of ClyR against 11 S. sobrinus isolates from dental plaque samples of eight children with SECC and 36 S. mutans isolates from 22 children with SECC. As shown in Table S1 and Figure S2, 8 genotypes of S. sobrinus and 27 genotypes of S. mutans were identified in these isolates, suggesting the diversity of these isolates. The lytic assay (Figure 2) showed that ClyR has varied activities against these isolates, with 3 of the 11 S. sobrinus (Figure 2a) and 33 of the 36 S. mutans (Figure 2b) isolates found to be highly susceptible, causing significant drop in OD600. The minimum bactericidal concentration (MBC) values (Table S2) of S. mutans and S. sobrinus isolates ranged from 125 to >1000 μg/mL. Among them, 42 isolates were completely killed with 500 μg/mL or even lower ClyR concentration, while the MBCs of S. mutans MT8148 and S. sobrinus ATCC6715 were 1000 μg/mL.
Figure 2.
Activities of ClyR against multiple clinical isolates. S. sobrinus (a) and S. mutans (b) isolates were grown to logarithmic phase, suspended in PBS buffer to an OD600 of ~0.8, and then treated with 50 µg/mL of ClyR at 37 °C for 10 min. The relative change in OD600 (∆OD600) of each well was determined by subtracting the decrease of OD600 in the PBS-treated control well.
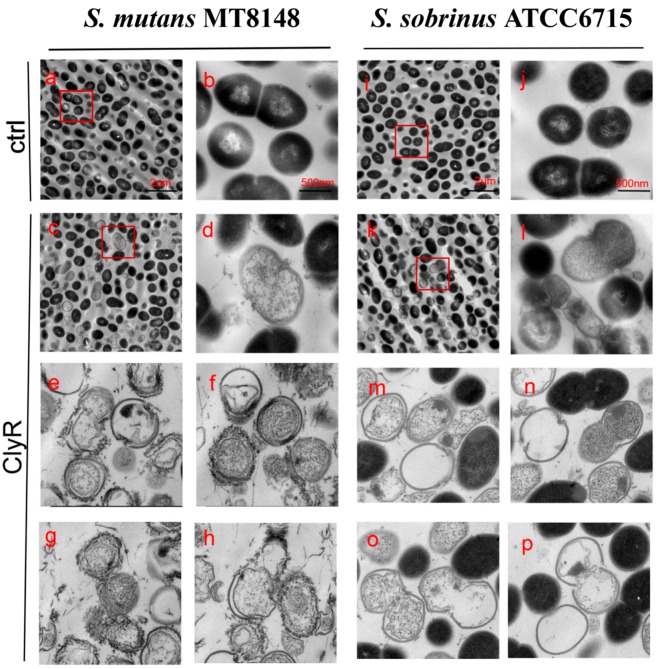
3.3. TEM Analysis of S. mutans and S. sobrinus Exposed to ClyR
TEM Images showed that intact S. mutans MT8148 and S. sobrinus ATCC6715 cells have normal cellular morphology with integral peptidoglycan layers (Figure 3a,b,i,j). However, when cells were exposed to ClyR, distinct localized degradation of the cell wall was observed, resulting in the loss of their cytoplasmic contents (Figure 3c–h,k–p).
Figure 3.
TEM images of S. mutans and S. sobrinus exposed to ClyR. Bacterial cells were treated with PBS (Ctrl, a,b,i,j) or 50 µg/mL of ClyR (c–h,k–p) at 37 °C for 5 min and then fixed by 2.5% glutaraldehyde.
3.4. Activity of ClyR against S. mutans and S. sobrinus Biofilms
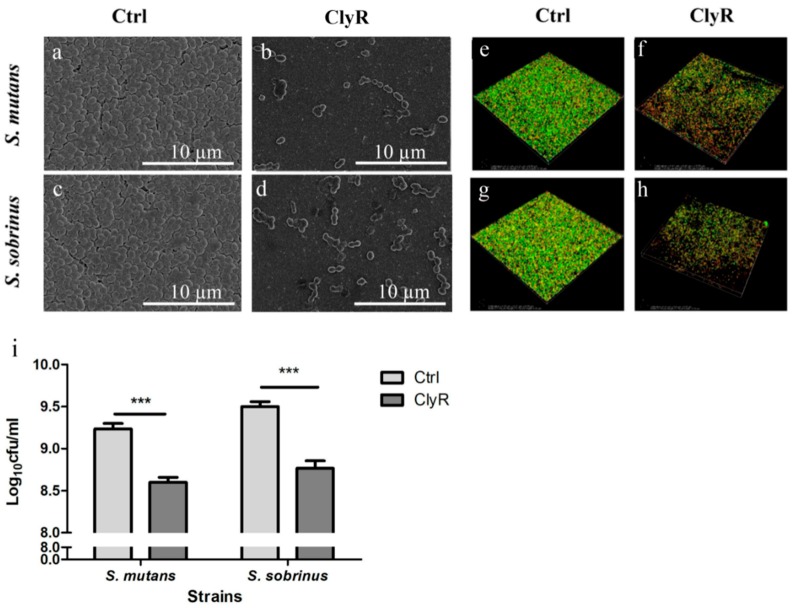
The impacts of ClyR on S. mutans and S. sobrinus biofilms were investigated by SEM, CLSM, and direct CFUs counting assay. The SEM images in Figure 4a–d showed that viable bacteria in 72-h biofilms decreased significantly after treatment with 50 µg/mL of ClyR for 5 min compared with those of the PBS-treated control biofilms. Morphologically, cells after ClyR treatment exhibited an irregular shape and cell wall damage, indicating that ClyR may remove biofilms through its bactericidal activity.
Figure 4.
Effect of ClyR on S. mutans and S. sobrinus biofilms. (a–d) SEM analysis of bacterial biofilms after treatment with ClyR. 72-h biofilms of S. mutans MT8148 and S. sobrinus ATCC6715 were treated with PBS (Ctrl) or 50 µg/mL of ClyR for 5 min and then analyzed by SEM; (e–h) CLSM analysis of bacterial biofilms after treatment with ClyR. 72-h biofilms were analyzed by CLSM to calculate the viable cell percentage after treatment with PBS (Ctrl) or 50 µg/mL of ClyR for 5 min; (i) Effect of ClyR on viable cell number within biofilms. 72 h S. mutans MT8148 and S. sobrinus ATCC6715 biofilms were treated with 50 µg/mL ClyR for 5 min, viable cell number after each treatment was determined and compared with that of the PBS-treated controls (Ctrl). Data is shown as mean ± standard deviation, and *** p < 0.001.
To test whether ClyR affects the viability of S. mutans and S. sobrinus cells within the biofilm, we performed the CLSM analysis by staining live bacteria with green fluorescence and dead bacteria with red fluorescence. As shown in Figure 4e–h, after treatment with PBS (Figure 4e,g), the percentages of viable bacterial cells within 72 h S. mutans and S. sobrinus biofilms were 88.10 ± 1.23% and 87.66 ± 1.27%, respectively, and the average thicknesses of the corresponding biofilms were 11.5 ± 2.50 µm and 9.75 ± 0.88 µm, respectively. Meanwhile, after treatment with 50 µg/mL ClyR for 5 min (Figure 4f,h), the percentages of viable bacterial cells were decreased to 47.49 ± 10.34% and 47.01 ± 10.77% for S. mutans and S. sobrinus biofilms, respectively, with the average thicknesses of the biofilms of 8.40 ± 1.52 µm and 7.75 ± 1.75 µm, respectively. All these reductions were significant (p < 0.05) compared with the PBS-treated control groups (Figure 4e,g).
In accordance with the CLSM results, direct CFUs counting assay (Figure 4i) showed that ClyR decrease the number of viable cells in the S. mutans and S. sobrinus biofilms to 32.51 ± 1.33% and 25.41 ± 1.52%, respectively, compared with the PBS controls (p < 0.001).
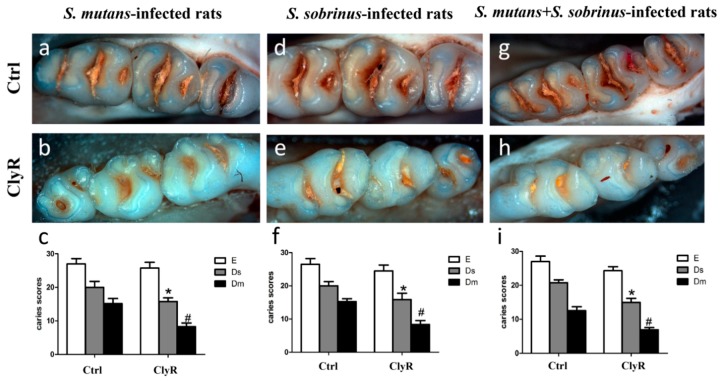
3.5. Anticaries Efficacy of ClyR in Rat Models
Our earlier study [30] showed that repeated use of ClyR for 21 days (5 µg/day) significantly reduce the number of colonized S. mutans cells in the dental plaques and has no harmful effects on the mice. In the present study, we applied 5 µg/day of ClyR in a longer time (40 days) to further demonstrate its anticaries efficacy in rat models. As shown in Figure 5, severe carious lesions were observed in the PBS-treated control groups (Figure 5a,d,g), while, in ClyR-treated groups, mild occlusal caries were occurred in both single-bacterial and mixed-bacterial infected rat models (Figure 5b,e,h). As for the caries scores, ClyR treated rats infected with S. mutans (Figure 5c), S. sobrinus (Figure 5f), or a mixture of S. mutans and S. sobrinus (Figure 5i) demonstrated 25%, 25%, or 33.33% of the dentinal slight (Ds) and 55.56%, 46.67%, or 50% dentinal moderate (Dm) lesions reduction, compared with the PBS-treated groups (p < 0.05). However, no significant difference was observed in enamel (E) lesions in all three groups.
Figure 5.
Effect of ClyR on the development of dental caries in rats (representative teeth images and Keyes’ score). Rats were infected with S. mutans (a–c), S. sobrinus (d–f) or a mixture of S. mutans and S. sobrinus (g–i) for six days, then treated with 50 μg/mL of ClyR or PBS (Ctrl) for 40 days. The teeth images were recorded and Keyes’ score were calculated on day 120 (a,b,d,e,g,h); Magnification: ×50; E, enamel caries; Ds: dentin exposed; Dm: 3/4 of the dentin affected. * and #: p < 0.05 compared to control group.
4. Discussion
In the last two decades, the bactericidal efficacy of endolysins and their derivatives have been proven in different animal models [33,34] and foods [35]. These insights have paved the road for the clinical development of endolysins, with a few leads already in different phases of preclinical and clinical trials. In a recent comparative analysis, Czaplewski and colleagues ranked endolysins as the alternative class of antibacterial with the greatest potential based on their clinical impact and technical feasibility (reviewed in Reference [36]). Therefore, the development of endolysins as antibacterials accommodates an important societal need as antibiotic-resistant bacteria have been emerging and spreading, while the antibiotic development pipeline is seeing a significant decline. A unique feature of endolysins is their specificity. The results of our study showed that ClyR has high antibacterial activity against the two major cariogenic pathogens S. mutans and S. sobrinus but low activity against the harmless commensal S. sanguinis, S. oralis, and S. salivarius (Figure 1a,b). In our subsequent study, we have assessed ClyR’s lytic activity against several other important oral non-streptococci, including Enterococcus faecalis ATCC51299. Its activity against E. faecalis (the main pathogen of refractory periapical endodontic lesions) was robust [37]. The normal flora usually play an important role in maintaining oral health. For example, even in a relatively low pH environment, S. sanguinis and S. salivarius can produce ammonia and other alkaline substances using their own urease or arginine deiminase. This reduces the rapid decline in the pH of the plaque, thereby lowering the incidence of caries [38,39]. Meanwhile, S. sanguinis can antagonize certain periodontopathogens by delaying its colonization, which is beneficial to prevent periodontal disease [40]. Moreover, noncariogenic flora have been shown to inhibit, and even prevent, exogenous S. mutans colonization in vitro and in vivo [41]. In contrast to current broad-spectrum antibiotics, this specificity minimizes the damage to the ecological balance between commensal residents and pathogens in the oral cavity. In addition to standard strains, we also tested the susceptibility of multiple clinical isolates of S. mutans and S. sobrinus to ClyR (Figure 2). ClyR showed good activities against most S. mutans isolates and 3 out of 11 S. sobrinus isolates (1, 3, 10), but minor to no activity against the other 8 S. sobrinus isolates. The detailed mechanism behind the tolerance is currently unknown, one possible reason may be that the differences in structure or composition of the bacterial cell wall either prevents lysin from getting access to the peptidoglycan or attenuates the affinity of lysin for its binding or catalytic substrates [42]. The varied susceptibility of S. mutans and S. sobrinus isolates to ClyR and the relevant mechanism still needs further study.
Cariogenic biofilm is mainly composed of oral bacteria including cariogenic bacterium S. mutans and S. sobrinus, exopolysaccharide, and bacterial debris [43]. Generally, biofilms can tolerate to antimicrobial agents in two ways: the physical barrier and the bacterial communication. The former is formed by the extracellular matrix, which hinders the penetration of antimicrobial agents into the biofilm [44]; the latter involves stimulating bacteria to produce enzymes and proteins to maintain homeostasis in the microbial community [45]. The bactericidal concentration of antibiotics for biofilm may be 100–1000 folds higher than that for planktonic bacteria. However, our study showed that there is only a minor difference in bactericidal concentrations for ClyR to remove biofilm and planktonic S. mutans and S. sobrinus cells, which indicates that ClyR might be able to penetrate the biofilms. Since oral biofilms are much more complicated than the models studied here, further study is needed to assess the efficacy of ClyR in real oral environments.
Considering the fact that co-existence of S. mutans and S. sobrinus is an important risk factor in the development of dental caries [46] and that mixed bacteria colonization is more likely to mimic the physiological condition of oral cavity, we evaluated the protective efficiency of ClyR in rats infected with S. mutans and S. sobrinus separately or together. To our surprise, the protective efficacies in these three groups were almost the same. ClyR-treated rats demonstrated fewer Ds and Dm lesions, indicating that ClyR possesses the ability to lower the severity of dental caries (Figure 5). We speculated that S. mutans and S. sobrinus are partly killed by ClyR and the formations of oral biofilms containing these two bacteria are also reduced after the treatment, which in turn lowered the formation of carious lesions. Compared with other treatments, a reduction in total caries (total score = score of E + Ds + Dm) of 24% was observed after treatment with ClyR in S. mutans infected rats model (Figure 5c), which is among the best efficacies achieved so far. For example, recently reported glucosyltransferase (Gtf) inhibitor [47] and proanthocyanidins (PAC) [14] showed about 26% and 17% reduction in total caries, respectively. In addition, we did not observe any adverse effects in our animals. Based on its robust lytic activity (Figure 1d) and good safety profile, ClyR might be a promising additive in mouth rinse, toothpaste, or oral spray to prevent caries. However, how to maintain the high activity of ClyR in different formulations still needs to be considered in the future.
In conclusion, we report here that the chimeric lysin ClyR is capable of killing S. mutans and S. sobrinus under both planktonic and biofilm conditions, with rare effect on other harmless commensal oral bacteria. Good anticaries efficacy of ClyR is further demonstrated in S. mutans- and/or S. sobrinus-infected rat models. Taken together, ClyR could be a promising agent or ingredient for dental caries prevention and long-term dental treatment. Its potential remains to be further explored.
Supplementary Materials
Supplementary materials can be found at http://www.mdpi.com/1999-4915/10/7/380/s1.
Author Contributions
H.W. and Y.L. conceived the study. J.X., H.Y., Y.B., and W.L. performed the experiments. J.X., H.Y., Y.B., and Y.L. analyzed the data. J.X., H.W., and Y.L. wrote the paper.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81670981) to Y.L. and the Youth Innovation Promotion Association CAS (to H.Y.).
Conflicts of Interest
The chimeolysin reported here originated from Wuhan Scithera Microbial Technologies Co., Ltd. in which H.Y. and H.W. have financial interests.
References
- 1.Petersen P.E., Kwan S. World Health Organization global oral health strategies for oral health promotion and disease prevention in the twenty-first century. Prvention Und Gesundheitsfrderung. 2009;4:100–104. doi: 10.1007/s11553-009-0169-x. [DOI] [Google Scholar]
- 2.Miller W.D. The Microorganisms of Human Mouth. Am. J. Med. Sci. 1970;98:275–276. doi: 10.1097/00000441-188909000-00010. [DOI] [Google Scholar]
- 3.Tanzer J.M., Livingston J., Thompson A.M. The microbiology of primary dental caries in humans. J. Dent. Educ. 2001;65:1028–1037. [PubMed] [Google Scholar]
- 4.Hamilton I.R., Buckley N.D. Adaptation by Streptococcus mutans to acid tolerance. Oral Microbiol. Immunol. 1991;6:65–71. doi: 10.1111/j.1399-302X.1991.tb00453.x. [DOI] [PubMed] [Google Scholar]
- 5.Monchois V., Willemot R., Monsan P. Glucansucrases: Mechanism of action and structure–function relationships. FEMS Microbiol. Rev. 1999;23:131–151. doi: 10.1111/j.1574-6976.1999.tb00394.x. [DOI] [PubMed] [Google Scholar]
- 6.De Soet J.J., Van L.C., Lammens A.J., Pavicić M.J., Homburg C.H., ten Cate J.M., de G.J. Differences in cariogenicity between fresh isolates of Streptococcus sobrinus and Streptococcus mutans. Caries Res. 1991;25:116–122. doi: 10.1159/000261353. [DOI] [PubMed] [Google Scholar]
- 7.Sánchezacedo M., Montielcompany J.M., Dasífernández F., Almerichsilla J.M. Streptococcus mutans and Streptococcus sobrinus detection by Polymerase Chain Reaction and their relation to dental caries in 12 and 15 year-old schoolchildren in Valencia (Spain) Med. Oral Patol. Oral Cir. Bucal. 2013;18:E839–E845. doi: 10.4317/medoral.18941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okada M., Kawamura M., Oda Y., Yasuda R., Kojima T., Kurihara H. Caries prevalence associated with Streptococcus mutans and Streptococcus sobrinus in Japanese schoolchildren. Int. J. Paediatr. Dent. 2012;22:342–348. doi: 10.1111/j.1365-263X.2011.01203.x. [DOI] [PubMed] [Google Scholar]
- 9.Kishi M., Abe A., Kishi K., Ohara-Nemoto Y., Kimura S., Yonemitsu M. Relationship of quantitative salivary levels of Streptococcus mutans and S. sobrinus in mothers to caries status and colonization of mutans streptococci in plaque in their 2.5-year-old children. Commun. Dent. Oral Epidemiol. 2009;37:241–249. doi: 10.1111/j.1600-0528.2009.00472.x. [DOI] [PubMed] [Google Scholar]
- 10.Featherstone J.D. Prevention and reversal of dental caries: Role of low level fluoride. Commun. Dent. Oral Epidemiol. 1999;27:31–40. doi: 10.1111/j.1600-0528.1999.tb01989.x. [DOI] [PubMed] [Google Scholar]
- 11.Osuji O.O., Leake J.L., Chipman M.L., Nikiforuk G., Locker D., Levine N. Risk factors for dental fluorosis in a fluoridated community. J. Dent. Res. 1988;67:1488–1492. doi: 10.1177/00220345880670120901. [DOI] [PubMed] [Google Scholar]
- 12.Gessner B.D., Beller M., Middaugh J.P., Whitford G.M. Acute fluoride poisoning from a public water system. New Engl. J. Med. 1994;330:95–99. doi: 10.1056/NEJM199401133300203. [DOI] [PubMed] [Google Scholar]
- 13.Stauder M., Papetti A., Daglia M., Vezzulli L., Gazzani G., Varaldo P.E., Pruzzo C. Inhibitory activity by barley coffee components towards Streptococcus mutans biofilm. Curr. Microbiol. 2010;61:417–421. doi: 10.1007/s00284-010-9630-5. [DOI] [PubMed] [Google Scholar]
- 14.Koo H., Duarte S., Murata R.M., Scottanne K., Gregoire S., Watson G.E., Singh A.P., Vorsa N. Influence of Cranberry Proanthocyanidins on Formation of Biofilms by Streptococcus mutans on Saliva-Coated Apatitic Surface and on Dental Caries Development in vivo. Caries Res. 2010;44:116–126. doi: 10.1159/000296306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nittayananta W., Limsuwan S., Srichana T., Sae-Wong C., Amnuaikit T. Oral spray containing plant-derived compounds is effective against common oral pathogens. Arch. Oral Biol. 2018;90:80–85. doi: 10.1016/j.archoralbio.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Pan W., Fan M., Wu H., Melander C., Liu C. A new small molecule inhibits Streptococcus mutans biofilms in vitro and in vivo. J. Appl. Microbiol. 2015;119:1403–1411. doi: 10.1111/jam.12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koo H., Hayacibara M.F., Schobel B.D., Cury J.A., Rosalen P.L., Park Y.K., Vacca-Smith A.M., Bowen W.H. Inhibition of Streptococcus mutans biofilm accumulation and polysaccharide production by apigenin and tt-farnesol. J. Antimicrob. Chemother. 2003;52:782–789. doi: 10.1093/jac/dkg449. [DOI] [PubMed] [Google Scholar]
- 18.Li L., Guo L., Lux R., Eckert R., Yarbrough D., He J., Anderson M., Shi W. Targeted Antimicrobial Therapy Against Streptococcus mutans Establishes Protective Non-cariogenic Oral Biofilms and Reduces Subsequent Infection. Int. J. Oral Sci. 2010;2:66–73. doi: 10.4248/IJOS10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaplan C.W., Sim J.H., Shah K.R., Kolesnikovakaplan A., Shi W., Eckert R. Selective Membrane Disruption: Mode of Action of C16G2, a Specifically Targeted Antimicrobial Peptide. Antimicrob. Agents Chemother. 2011;55:3446–3452. doi: 10.1128/AAC.00342-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu C., Worthington R.J., Melander C., Wu H. A new small molecule specifically inhibits the cariogenic bacterium Streptococcus mutans in multispecies biofilms. Antimicrob. Agents Chemother. 2011;55:2679–2687. doi: 10.1128/AAC.01496-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roach D.R., Donovan D.M. Antimicrobial bacteriophage-derived proteins and therapeutic applications. Bacteriophage. 2015;5:e1062590. doi: 10.1080/21597081.2015.1062590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmelcher M., Shen Y., Nelson D.C., Eugster M.R., Eichenseher F., Hanke D.C., Loessner M.J., Dong S., Pritchard D.G., Lee J.C. Evolutionarily distinct bacteriophage endolysins featuring conserved peptidoglycan cleavage sites protect mice from MRSA infection. J. Antimicrob. Chemother. 2015;70:1453–1465. doi: 10.1093/jac/dku552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilmer D.B., Schmitz J.E., Euler C.W., Fischetti V.A. Novel bacteriophage lysin with broad lytic activity protects against mixed infection by Streptococcus pyogenes and methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2013;57:2743–2750. doi: 10.1128/AAC.02526-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poonacha N., Nair S., Desai S., Tuppad D., Hiremath D., Mohan T., Vipra A., Sharma U. Efficient Killing of Planktonic and Biofilm-Embedded Coagulase-Negative Staphylococci by Bactericidal Protein P128. Antimicrob. Agents Chemother. 2017;61:e00457-17. doi: 10.1128/AAC.00457-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nair S., Poonacha N., Desai S., Hiremath D., Tuppad D., Mohan T., Chikkamadaiah R., Durgaiah M., Kumar S., Channabasappa S. Restoration of sensitivity of a diverse set of drug-resistant Staphylococcus clinical strains by bactericidal protein P128. J. Med. Microbiol. 2018;67:296–307. doi: 10.1099/jmm.0.000697. [DOI] [PubMed] [Google Scholar]
- 26.Vázquez R., Domenech M., Iglesiasbexiga M., Menéndez M., García P. Csl2, a novel chimeric bacteriophage lysin to fight infections caused by Streptococcus suis, an emerging zoonotic pathogen. Sci. Rep. 2017;7:16506. doi: 10.1038/s41598-017-16736-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Becker S.C., Roach D.R., Chauhan V.S., Yang S., Juli F.F., Powell A.M., Gary B., Lease R.A., Homan M., Harty W.J. Triple-acting Lytic Enzyme Treatment of Drug-Resistant and Intracellular Staphylococcus aureus. Sci. Rep. 2016;6:25063. doi: 10.1038/srep25063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernández L., González S., Campelo A.B., Martínez B., Rodríguez A., García P. Downregulation of Autolysin-Encoding Genes by Phage-Derived Lytic Proteins Inhibits Biofilm Formation in Staphylococcus aureus. Antimicrob. Agents Chemother. 2017;61:e02724-16. doi: 10.1128/AAC.02724-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hang Y., Linden S.B., Jing W., Yu J., Nelson D.C., Wei H. A chimeolysin with extended-spectrum streptococcal host range found by an induced lysis-based rapid screening method. Sci. Rep. 2015;5:17257. doi: 10.1038/srep17257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang H., Bi Y., Shang X., Wang M., Linden S.B., Li Y., Li Y., Nelson D.C., Wei H. Antibiofilm Activities of a Novel Chimeolysin against Streptococcus mutans under Physiological and Cariogenic Conditions. Antimicrob. Agents Chemother. 2016;60:7436–7443. doi: 10.1128/AAC.01872-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y., Caufield P.W. Arbitrarily primed polymerase chain reaction fingerprinting for the genotypic identification of mutans streptococci from humans. Mol. Oral Microbiol. 2010;13:17–22. doi: 10.1111/j.1399-302X.1998.tb00745.x. [DOI] [PubMed] [Google Scholar]
- 32.Keyes P.H. Dental caries in the molar teeth of rats. 2. A method for diagnosing and scoring several types of lesions simultaneously. J. Dent. Res. 1958;37:1088–1099. doi: 10.1177/00220345580370060901. [DOI] [PubMed] [Google Scholar]
- 33.Yang H., Zhang H., Wang J., Yu J., Wei H. A novel chimeric lysin with robust antibacterial activity against planktonic and biofilm methicillin-resistant Staphylococcus aureus. Sci. Rep. 2017;7:40182. doi: 10.1038/srep40182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gilmer D.B., Schmitz J.E., Thandar M., Euler C.W., Fischetti V.A. The Phage Lysin PlySs2 Decolonizes Streptococcus suis from Murine Intranasal Mucosa. PLoS ONE. 2017;12:e0169180. doi: 10.1371/journal.pone.0169180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmelcher M., Loessner M.J. Bacteriophage endolysins: Applications for food safety. Curr. Opin. Biotechnol. 2015;37:76–87. doi: 10.1016/j.copbio.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 36.Czaplewski L., Bax R., Clokie M., Dawson M., Fairhead H., Fischetti V.A., Foster S., Gilmore B.F., Hancock R.E.W., Harper D. Alternatives to antibiotics—A pipeline portfolio review. Lancet Infect. Dis. 2016;16:239–251. doi: 10.1016/S1473-3099(15)00466-1. [DOI] [PubMed] [Google Scholar]
- 37.Li W., Yang H., Gong Y., Wang S., Li Y., Wei H. Effects of a Chimeric Lysin against Planktonic and Sessile Enterococcus faecalis Hint at Potential Application in Endodontic Therapy. Viruses. 2018;10:290. doi: 10.3390/v10060290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burne R.A., Parsons D.T., Marquis R.E. Cloning and expression in Escherichia coli of the genes of the arginine deiminase system of Streptococcus sanguis NCTC 10904. Infect. Immun. 1989;57:3540–3548. doi: 10.1128/iai.57.11.3540-3548.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y.H., Chen Y.Y., Burne R.A. Regulation of urease gene expression by Streptococcus salivarius growing in biofilms. Environ. Microbiol. 2010;2:169–177. doi: 10.1046/j.1462-2920.2000.00088.x. [DOI] [PubMed] [Google Scholar]
- 40.Lee S.H. Antagonistic effect of peptidoglycan of Streptococcus sanguinis on lipopolysaccharide of major periodontal pathogens. J. Microbiol. 2015;53:553–560. doi: 10.1007/s12275-015-5319-6. [DOI] [PubMed] [Google Scholar]
- 41.Kreth J., Zhang Y., Herzberg M.C. Streptococcal antagonism in oral biofilms: Streptococcus sanguinis and Streptococcus gordonii interference with Streptococcus mutans. J. Bacteriol. 2008;190:4632–4640. doi: 10.1128/JB.00276-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hardie J.M., Bowden G.H. Cell Wall and Serological Studies on Streptococcus mutans. Caries Res. 2009;8:301–316. doi: 10.1159/000260120. [DOI] [PubMed] [Google Scholar]
- 43.Chałas R., Wójcikchęcińska I., Woźniak M.J., Grzonka J., Święszkowski W., Kurzydłowski K.J. Dental plaque as a biofilm—A risk in oral cavity and methods to prevent. Postȩpy Higieny I Medycyny Doświadczalnej. 2015;69:1140–1148. doi: 10.5604/17322693.1173925. [DOI] [PubMed] [Google Scholar]
- 44.Costerton W., Veeh R., Shirtliff M., Pasmore M., Post C., Ehrlich G. The application of biofilm science to the study and control of chronic bacterial infections. J. Clin. Investig. 2003;112:1466–1477. doi: 10.1172/JCI200320365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Corbin A., Pitts B., Parker A., Stewart P.S. Antimicrobial penetration and efficacy in an in vitro oral biofilm model. Antimicrob. Agents Chemother. 2011;55:3338–3344. doi: 10.1128/AAC.00206-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fragkou S., Balasouli C., Tsuzukibashi O., Argyropoulou A., Menexes G., Kotsanos N., Kalfas S. Streptococcus mutans, Streptococcus sobrinus and Candida albicans in oral samples from caries-free and caries-active children. Eur. Arch. Paediatr. Dent. 2016;17:1–9. doi: 10.1007/s40368-016-0239-7. [DOI] [PubMed] [Google Scholar]
- 47.Ren Z., Cui T., Zeng J., Chen L., Zhang W., Xu X., Cheng L., Li M., Li J., Zhou X., et al. Molecule Targeting Glucosyltransferase Inhibits Streptococcus mutans Biofilm Formation and Virulence. Antimicrob. Agents Chemother. 2015;60:126–135. doi: 10.1128/AAC.00919-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.