Abstract
Objective:
To investigate the safety and efficacy of radiofrequency ablation combined with transarterial chemoembolization in patients with specially located small hepatocellular carcinoma.
Materials and Methods:
Between March 2014 and March 2017, a total of 26 patients with 26 lesions (10 perivascular, 6 subdiaphragmatic, 5 subcapsular, 5 perivascular, and subdiaphragmatic location; mean diameter 2.12 (0.62) cm), who received radiofrequency ablation–transarterial chemoembolization treatment, were retrospectively analyzed. Local tumor response was assessed by computed tomography/magnetic resonance imaging 1 month after the procedure. Tumor-free survival was also assessed according to the modified Response Evaluation Criteria in Solid Tumors. Complications were evaluated according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (version 4.0).
Results:
Complete response was achieved in all patients 1 month after the procedure. During a median follow-up duration of 16.76 months (95% confidence interval: 7.78-25.73 months), local tumor recurrence occurred in 2 patients and new intrahepatic lesions developed in 7 patients. The 1-, 2-, and 3-year cumulative local tumor progression rates were 3.84%, 7.69%, and 7.69%, respectively. The median tumor-free survival duration was 21.96 months (95% confidence interval: 17.58-26.34 months). The 1-, 2-, and 3-year tumor-free survival rates were 67.4%, 46.1%, and 39.3%, respectively.
Conclusion:
The radiofrequency ablation–transarterial chemoembolization combination therapy appears to be safe and effective and might be a treatment option for specially located small hepatocellular carcinoma lesions that have a risk of incomplete ablation or major complications.
Keywords: radiofrequency ablation, transarterial chemoembolization, hepatocellular carcinoma, specially located, small
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies and a major cause of mortality worldwide.1 For patients with small HCC lesions (<3 cm), resection or transplantation is usually not employed as radical treatment given its poor liver function and comorbidities.2 Radiofrequency ablation (RFA) or microwave ablation (MWA) has been the established curative therapeutic option that has resulted in similar clinical benefits in such patients.3,4 However, the treatment of specially located lesions, such as perivascular, subdiaphragmatic, and subcapsular lesions, remains challenging, as they may be associated with a higher risk of complications, incomplete ablation, and local tumor recurrence and may be difficult to target during RFA due to their dangerous location.3,4
Transarterial chemoembolization (TACE) has been playing an important role in the management of HCC in unresectable candidates. However, given the nature of TACE as a palliative therapy, complete necrosis of the target lesion is difficult to achieve by TACE alone.5 Recently, the combination of RFA and TACE has been reported to be effective and to provide additional benefit for local control of large as well as small HCC lesions.6–12 However, to the best of our knowledge, few previous studies have assessed the modality of RFA combined with TACE in the treatment of specially located small HCC lesions, and the tumor location was not specified in these randomized controlled studies.13
We speculate that TACE performed immediately after RFA is useful for evaluating the efficacy of ablation and for treating residual lesions or vascular-related complications in patients with specially located lesions. The purpose of the study was to investigate RFA-TACE in treating small HCC lesions which is close to vascular, diaphragmatic, and capsular lesions.
Materials and Methods
Patient Selection
This retrospective study was conducted with Zhongshan Hospital’s institutional review board approval (No: B2014-102). Written informed consent was obtained from each patient before treatment. Between March 2014 and March 2017, a total of 358 consecutive patients with malignant liver tumors (178 with tumors adjoining a large vessel, the liver capsule, or the diaphragm), who were unable or unwilling to receive surgery, underwent RFA-TACE combination therapy at our institution. A perivascular tumor was defined as a tumor located within 5 mm from a vessel such as the portal vein/hepatic vein (≥3 mm) or the inferior vena cava. Lesions <1.0 cm from the hepatic capsule or diaphragm were defined as subcapsular or subdiaphragmatic lesions. Tumor size and location were assessed and determined by 2 radiologists, each with more than 5 years of experience. The exclusion criteria included the following: (1) more than 3 lesions or any lesion >3 cm in diameter, (2) tumor invasion into major intrahepatic blood vessels and extrahepatic metastases, and (3) metastasis or primary liver cancer other than HCC. Hepatocellular carcinoma was diagnosed based on pathology or the following typical clinical features: nodules >2 cm for patients with cirrhosis and coincidental findings by 2 imaging techniques that were considered diagnostic or, alternatively, by one imaging technique along with α-fetoprotein (AFP) levels over 400 ng/mL according to the guidelines of the American Association for the Study of Liver Diseases.
A total of 26 lesions (10 perivascular, 6 subdiaphragmatic, 5 subcapsular, 5 perivascular and subdiaphragmatic) in 26 patients (20 males and 6 females) were included in this study. All patients had a history of hepatitis B virus (HBV) infection and a mean age of 57.73 (10.98) years (range: 35-78 years). The mean tumor diameter was 2.12 (0.62) cm (range: 1.3-2.9 cm). The clinical characteristics of the patients are summarized in Table 1.
Table 1.
Clinical Characteristic of the Patients.a
| Characteristics | Values |
|---|---|
| Age (years) | 57.73 (10.98) |
| Sex | |
| Male | 20 |
| Female | 6 |
| Tumor location | |
| Perivascular | 10 |
| Subdiaphragmatic | 6 |
| Subcapsular | 5 |
| Perivascular and subdiaphragmatic | 5 |
| Maximum tumor diameter (cm) | 2.12 (0.62) |
| AFP | |
| >400 ng/mL | 3 |
| ≤400 ng/mL | 23 |
| ECOG (0/1) | 23/3 |
| Previous treatment history | 12 |
| Surgery | 3 |
| Ablation (RFA or MWA) | 1 |
| TACE | 2 |
| Surgery and TACE | 4 |
| Ablation and TACE | 1 |
| Transplantation, ablation, and TACE | 1 |
Abbreviations: AFP, α-fetoprotein; ECOG, eastern cooperative oncology group; MWA, microwave ablation; RFA, radiofrequency ablation; TACE, transarterial chemoembolization.
a N = 26.
Combination Treatment Procedures
All RFA-TACE procedures were performed by 3 interventional radiologists, each with more than 15 years of experience in performing interventional radiological procedures. The procedures were performed under conscious sedation and local anesthesia. Fentanyl (Yichang Humanwell Pharmaceutical Co, Ltd, Hubei, China) at a dose of 0.1 to 0.2 mg was used for analgesia, and 0.1% lidocaine hydrochloride was used for local anesthesia. To evaluate tumor location and tumor-feeding arteries, celiac and superior mesenteric arteriography was performed through a 5F catheter (RH; Terumo Corp, Tokyo, Japan; Figure 1B). A 2.7F microcatheter (Progreat; Terumo Corp) was then used for superselective catheterization. Ultrasound (ACUSON X300; Siemens, Seoul, Korea) was used to detect the index tumor immediately after arteriography (Figure 2). Radiofrequency ablation was performed under both ultrasound and fluoroscopy guidance and immediately followed by TACE (Figure 1). Blood pressure, respiration, pulse, oxygen saturation, and electrocardiograms were monitored throughout the procedures.
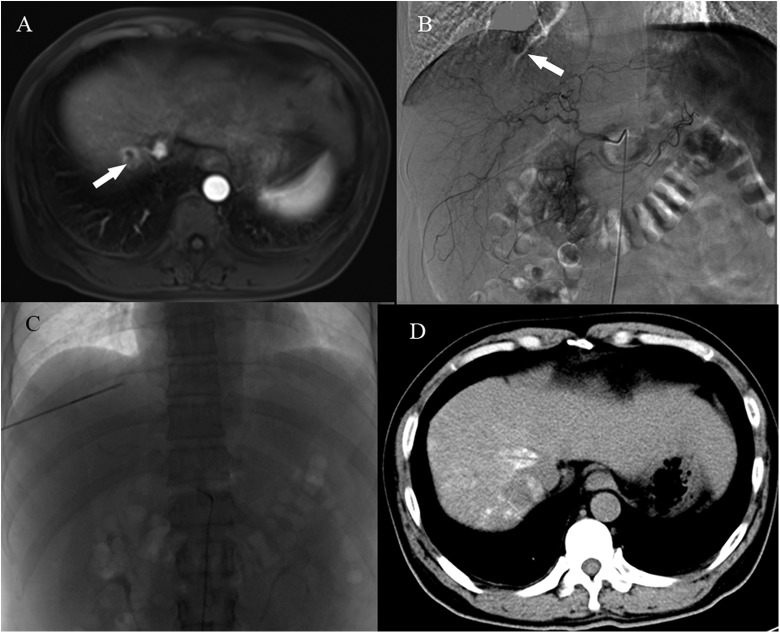
Figure 1.
A, A preprocedure abdominal contrast-enhanced MRI (venous phase) shows a subdiaphragmatic tumor (white arrow). B, Tumor staining is clearly demonstrated by angiography (white arrow). C, An electrode and a microcatheter are in position during the RFA-TACE procedure. D, A noncontrast-enhanced CT scan 3 days after the procedure. CT indicates computed tomography; MRI, magnetic resonance imaging; RFA, radiofrequency ablation; TACE, transarterial chemoembolization.
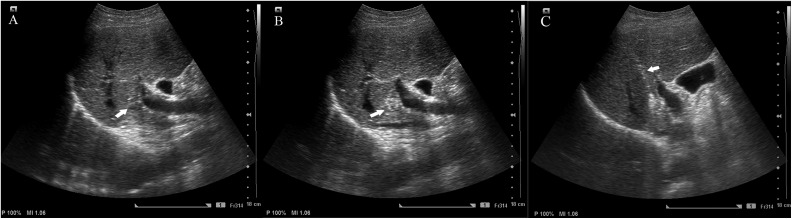
Figure 2.
Ultrasound and fluoroscopy intergraded guidance for tumor targeting. A, A single small HCC lesion is inconspicuous (white arrow) under conventional ultrasonography. B, The index tumor became conspicuous after hepatic angiography with iopromide injection (white arrow). C, The RFA electrode (white arrow) within the index tumor. HCC indicates hepatocellular carcinoma; RFA, radiofrequency ablation.
Percutaneous RFA
An RFA system (S-1500; MedSphere International, Shanghai, China) was used to generate up to 150 W of energy to achieve adequate coagulation necrosis of the target tumor. Expandable electrodes (range: 14-19 gauge; 15 or 20 cm in length; 2, 3, and 4 cm in diameter) with an outer insulated sheath and a core needle with multiple umbrella-shaped electrodes were inserted into the tumors under ultrasound guidance. The electrode diameter was selected according to the size of the target tumor. Power output was set at 60 W and slowly increased by 10 W every 2 minutes until tissue impedance reached 100%. Puncture track ablation at 60 W was conducted during electrode withdrawal in all patients.
Chemoembolization Procedure
Arteriography was performed immediately after RFA to evaluate the coagulated zone, residual tumor vascularity, and postablative complications such as intrahepatic hemorrhage or arteriovenous fistula. An emulsion consisting of 20 mg of epirubicin (Farmorubicin; Pfizer, Wuxi, China) and 2 to 5 mL of iodized oil (Lipiodol; Guerbet, Roissy, France) was slowly injected through the microcatheter. Subsequently, 350 to 510 μm of gelatin sponge particles (Ailikang Medicine, Hangzhou, China) mixed with contrast medium were injected to reduce residual blood flow. If hemorrhage or arteriovenous fistula was observed, the involved artery was embolized using gelatin sponge particles.
Follow-Up
Laboratory tests, including routine blood tests, AFP, liver functions, renal functions, coagulation functions, and noncontrast computed tomography (CT) were performed 3 to 7 days after the RFA-TACE procedure. Patients were examined using triphasic contrast-enhanced CT or magnetic resonance imaging, and routine laboratory tests were conducted at 1 month and then every 3 months after the procedure. Local tumor response was evaluated according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST).14 Tumor-free survival was determined from the time between treatment administration and intrahepatic lesion detection. Complications were evaluated according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events Toxicity Criteria (NCI-CTCAE 4.0).
Statistical Analyses
Continuous variables were expressed as the mean (standard deviation) and category data as frequencies and percentages. A P (2-tailed) value less than .05 was considered statistically significant. Tumor-free survival duration was calculated using the Kaplan-Meier method. Data analysis was performed using SPSS (version 20.0; IBM, Armonk, New York).
Results
Tumor Response
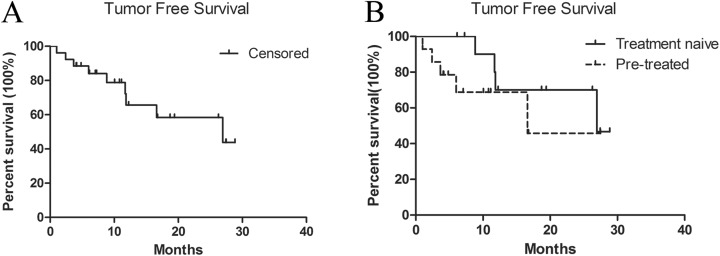
The RFA-TACE procedure was successfully performed in all the patients. Angiography after RFA showed the surrounding hyperemia edema ring and the avascular area of the central ablation zone. According to mRECIST, complete response was achieved in all patients 1 month after treatment. The median follow-up duration was 16.76 months (95% confidence interval [CI]: 7.78-25.73 months). There were 2 cases of local tumor recurrence during the follow-up period. New intrahepatic lesions occurred in 7 patients. The 1-, 2-, and 3-year cumulative local tumor progression rates were 3.84%, 7.69%, and 7.69%, respectively. The median tumor-free survival duration was 21.96 months (95% CI: 17.58-26.34 months; Figure 3A). The mean tumor-free survival duration was 22.99 months (95% CI: 17.97-28.01 months) in 14 (53.85%) patients without previous treatment and 17.49 months (95% CI: 10.91-24.08 months) in 12 (46.15%) patients with previous treatment (P = .272, Figure 3B). The 1-, 2-, and 3-year tumor-free survival rates were 67.4%, 46.1%, and 39.3%, respectively (Table 2). Recurrent or new tumors were treated with repeated RFA-TACE (n = 2), serial TACE (n = 6), or RFA (n = 1). One patient died of liver failure 2 years after the procedure. One month after the procedure, AFP dropped to normal levels (≤20 ng/mL) in all patients, including in 8 of 9 patients who had previously elevated AFP level (n = 9), except in 1 patient whose AFP level decreased from 228.2 to 24 ng/mL.
Figure 3.
Kaplan-Meier curves for tumor-free survival.
Table 2.
The Outcome of Patients.
| Status | Percentage |
|---|---|
| Overall complete ablation | 26/26 (100%) |
| Local recurrence at ablated site | 2/26 (7.69%) |
| New intrahepatic recurrence | 7/26 (26.9%) |
| Death | 1/26 (3.85%) |
| 1-year tumor-free survival | 17/26 (67.4%) |
| 2-year tumor-free survival | 12/26 (46.1) |
| 3-year tumor-free survival | 9/26 (39.3%) |
Complications
No major complications were observed, and the combination therapy was well tolerated in all patients. Postembolization syndromes such as fever, abdominal pain, and vomiting were managed with analgesics, antiemetics, and other symptomatic treatments. Arteriovenous fistulas after RFA were identified by angiography in 3 patients and were promptly treated by embolization. Asymptomatic pleural effusion (CTCAE 4.0 grade 1) was observed in 8 patients and spontaneously resolved within 1 month. A transient impairment of liver function (grade 3) was recorded in 4 of 26 patients (Table 3) and was addressed by supportive treatment within 7 days.
Table 3.
Preprocedure and Postprocedure Laboratory Test Changes.a
| Laboratory Test | Preprocedure, mean (SD) | Postprocedure, mean (SD) | P Valueb | CTCAE Grade, No. of grade: 1/2/3/4 |
|---|---|---|---|---|
| ALT (U/L) | 28.54 (12.15) | 116.81 (73.29) | .000 | 10/5/3/0 |
| AST (U/L) | 30.76 (11.21) | 123.00 (98.52) | .000 | 12/5/4/0 |
| TB (μmol/L) | 13.17 (7.26) | 24.45 (14.54) | .000 | 6/4/1/0 |
| CB (μmol/L) | 5.85 (3.93) | 12.2 (10.01) | .000 | 5/3/1/0 |
| ALB (g/L) | 40.65 (4.74) | 38.42 (5.577) | .022 | 3/0/0/0 |
| WBC (109) | 4.66 (1.71) | 7.20 (3.51) | .000 | 3/1/0/0 |
| Hb (g/L) | 136.15 (16.36) | 129.88 (16.54) | .005 | 10/4/0/0 |
| PLT (109) | 127.27 (63.60) | 119.77 (66.14) | .265 | 9/4/7/0 |
| Cr (μmol/L) | 73.81 (22.86) | 72.54 (24.80) | .650 | 1/0/0/0 |
Abbreviations: ALT, alanine aminotransferase; ALB, albumin; AST, aspartate aminotransferase; CB, conjugated bilirubin; Cr, creatinine; CTCAE, Common Terminology Criteria for Adverse Events; Hb, hemoglobin; PLT, platelet; TB, total bilirubin; WBC, white blood cell.
a N = 26.
b Paired samples t test (2-sided).
Discussion
Radiofrequency ablation is a curative therapeutic modality for patients with early-stage HCC according to Barceona Clinic Liver Cancer strategy.5 Compared to surgical resection, percutaneous RFA may provide similar therapeutic effects with fewer major complications, shorter hospital stays, and lower cost.15–17 Furthermore, RFA is a preferred choice for centrally located tumors that are smaller than 2 cm in diameter.16,18 However, the incidence of local tumor recurrence after RFA for small HCC lesions (<3 cm) ranges from 1.7% to 26%.13,17,19,20 Specially located tumors, such as perivascular, subdiaphragmatic, and subcapsular lesions, pose a challenge for the operators of RFA and confer a high risk of complications, incomplete ablation, and local tumor recurrence.21–23 In an animal study, heat sink effect was identified in 50% of veins greater than 3 mm, and this increased to 100% of veins greater than 5 mm.24 Recently, in a retrospective study of patients with HCC treated using RFA prior to transplantation, histologic examination of explanted human liver tissues revealed that 8 (53%) of 15 perivascular lesions were unsuccessfully treated.25 Furthermore, RFA for subcapsular and subdiaphragmatic lesions can increase complications such as peritoneal seeding, peritoneal bleeding, and pleural effusion.21,23,26 Special attention should be paid to such dangerously located small HCC lesions.
There currently is little evidence in the treatment of these specially located small HCC lesions. It is a great challenge in resection to locate the lesions adjacent to vascular or diaphragm. For RFA of these HCC locations, it is difficult to place an electrode and not being able to obtain enough ablative margin. Therefore, the optimal treatment strategies of these lesions remain controversial. Various combination treatment modalities have been reported for the treatment of high-risk HCC lesions in particular locations. Radiofrequency ablation combined with percutaneous ethanol injection (PEI) was reported for the treatment of high-risk lesions. The local tumor progression rates at 18 months were 21% for RFA-PEI versus 24% for RFA alone for high-risk lesions.27 In another study, the 1- and 3-year disease-free survival rates were 79% and 41% for patients with perivascular lesions treated by RFA alone, respectively.28 A combination therapy modality including TACE was demonstrated to have greater benefits than RFA/MWA alone.6,7 The TACE-RFA combination therapy has been reported to be safe and effective for HCC with tumors located in the caudate lobe or in the subcapsular region.29,30 However, in a recent randomized control study, Toshiya et al reported that combination treatment may not be necessary for small HCC lesions (<3 cm).13 In the study, the actual local tumor progression rate at 15 months was much lower than expected; hence, the results should be interpreted with caution. Furthermore, tumor locations were not clarified in the study. We herein reported a new treatment modality of RFA-TACE for specially located small HCCs. All HCC lesions included in our study were dangerously located. And the main study end point was local tumor response. A good local tumor response was obtained, and no major complication was observed. During the follow-up period, 2 patients experienced local tumor recurrence, and the 1-, 2-, and 3-year cumulative local tumor progression rates were 3.84%, 7.69%, and 7.69%, respectively, which are higher than those reported in previous studies (3-year cumulative local tumor progression rates: 13.2%-18.8%).31 The 1- and 3-year tumor-free survival rates in our study (67.4% and 39.3%, respectively) were similar to those of previous reports that included both centrally and dangerously located small HCC lesions (66%-81% and 31%-54%, respectively).9,13 However, all patients had HBV-related HCC in the present study, and half of them had a history of previous treatment.
Conventionally, RFA is performed after TACE. One of the advantages of this combination modality is that intratumoral lipiodol deposition after TACE can facilitate tumor targeting during subsequent RFA. Meanwhile, embolization reduces the cooling effect of hepatic blood flow and portal veins around the tumor so that RFA can induce a greater extent of necrosis.32–34 In this study, TACE performed immediately after RFA was adopted to treat these specially located small HCC lesions. With regard to tumor location, an increased risk of major complications is often associated with the RFA procedure, and complete ablation of these lesions can be difficult to achieve. Therefore, TACE after RFA could not only evaluate the efficacy of ablation but also treat residual lesions or other microlesions, which are related to long-term recurrence. The anticancer effect of TACE can be enhanced by the administration of a high concentration of chemotherapeutic drugs into a relatively small volume of residual viable neoplastic tissue and a reduction in cellular resistance to the drug due to the exposure to sublethal heating.35 Recently, Hyun D et al reported cone-beam CT-guided percutaneous RFA performed immediately after TACE for caudate lobe HCC. The 1- and 3-year progression-free survival rates were 81.8% and 51.9%.36 This one-step TACE-RFA combination therapy was similar to our treatment modality. The treatment outcomes were better than ours. Notably, the targeted lesions were <2 cm in diameter and thus were smaller than the lesions treated in our study. Masashi et al reported TACE-RFA for caudate HCC lesions <5 cm in diameter. Radiofrequency ablation was performed with a mean interval of 10.5 days after TACE. Local tumor recurrence was observed in 1 (5%) of 20 patients, and new intrahepatic tumor in 8 (40%) of 20 patients. The recurrence-free survival rates were 70.8% at 1 year and 36.9% at 3 years. Hepatocellular carcinoma lesions larger than 3 cm were included in the study, and this may have contributed to the relatively lower tumor control rate than ours.29
Furthermore, angiography after RFA facilitates the prompt management of RFA-related complications. Embolization may reduce bleeding complications associated with RFA.6,37 Dongho et al reported self-limiting capsular bleeding in 1 patient that was noted on an angiogram after RFA.36 Fujimori et al reported embolization of intercostal artery bleeding in one patient and self-limiting subcapsular liver hematoma in another.29 Although abdominal hemorrhage after RFA was not recorded in our study, arteriovenous fistulas were revealed by subsequent angiography in 3 patients. This complication could theoretically lead to intrahepatic or lung metastasis. Technique-related complications can be promptly managed by performing TACE immediately after RFA.
Technically, small HCC lesions are not always visualized on ultrasonography because of their unfavorable locations or isoechogenicity with the surrounding cirrhotic liver parenchyma.38 A laparoscopic ablation technique can provide better visualization of superficial lesions.39–41 However, the difficulty in access caused by adhesion of the postoperative mass may limit the application of laparoscopic ablation in patients with previous treatment history. An integrated guiding method that consists of ultrasonography and fluoroscopy was employed for tumor targeting in our study. Interestingly, tumor visualization under ultrasound guidance was improved, and the tumor boundary was clearly delineated after contrast media injection. Furthermore, some previous invisible lesions were detected after contrast media injection. In these cases, the use of CT guidance can be avoided, as it is both time-consuming and expensive.
This study has some unavoidable limitations. First, it was a retrospective and noncontrolled study at a single institution. Second, the sample size was small, and the follow-up period was relatively short. In addition, complicated clinical factors inevitably influenced the long-term efficacy and overall survival. Therefore, we focused on local tumor control and safety of the combination treatment. Although current evidence seems to favor combination therapy, the potentially curative effect of RFA immediately followed by TACE in the management of patients with specially located small HCC lesions should be further studied. Finally, the study included perivascular, subdiaphragmatic, and subcapsular lesions, and these locations may contribute to different prognosis. In addition, about half of the included patients were not treatment naive. These patients might have poor prognoses. As other alternative methods, MWA creates a larger ablation area within a shorter time, and irreversible electroporation (IRE) is a nonthermal technique that does not produce the heat sink effect. Both MWA and IRE may be better choices for perivascular lesions, but this notion needs further investigation.
In conclusion, US-guided percutaneous RFA immediately followed by TACE appears to be safe and effective and might be a treatment option for specially located small HCC lesions that have a risk of incomplete ablation or major complications. Therefore, a prospective randomized controlled trial with a longer follow-up duration is needed in the future.
Abbreviations
- AFP
α-fetoprotein
- CI
confidence interval
- CT
computed tomography
- CTCAE
Common Terminology Criteria for Adverse Events
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- IRE
irreversible electroporation
- mRECIST
modified Response Evaluation Criteria in Solid Tumors
- MWA
microwave ablation
- RFA
radiofrequency ablation
- TACE
transarterial chemoembolization.
Footnotes
Authors’ Note: W.Y., M-.J.Y., and J.X. contributed equally to this work and should be considered as co-first authors. All authors read and approved the final manuscript. J-.H.W. and S.Q. took equally responsibility for this work and should be identified as co-corresponding authors.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Natural Science Foundation of Shanghai (16ZR1433000 and 15ZR1406700).
References
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. [DOI] [PubMed] [Google Scholar]
- 2. Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cho YK, Kim JK, Kim WT, Chung JW. Hepatic resection versus radiofrequency ablation for very early stage hepatocellular carcinoma: a Markov model analysis. Hepatology. 2010;51(4):1284–1290. [DOI] [PubMed] [Google Scholar]
- 4. Duan C, Liu M, Zhang Z, Ma K, Bie P. Radiofrequency ablation versus hepatic resection for the treatment of early-stage hepatocellular carcinoma meeting Milan criteria: a systematic review and meta-analysis. World J Surg Oncol. 2013;11(1):190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. European Association for the Study of the Liver; European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908–943. [DOI] [PubMed] [Google Scholar]
- 6. Si ZM, Wang GZ, Qian S, et al. Combination therapies in the management of large (≥5 cm) hepatocellular carcinoma: microwave ablation immediately followed by transarterial chemoembolization. J Vasc Interv Radiol. 2016;27(10):1577–1583. [DOI] [PubMed] [Google Scholar]
- 7. Peng ZW, Zhang YJ, Chen MS, et al. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol. 2013;31(4):426–432. [DOI] [PubMed] [Google Scholar]
- 8. Peng ZW, Zhang YJ, Liang HH, Lin XJ, Guo RP, Chen MS. Recurrent hepatocellular carcinoma treated with sequential transcatheter arterial chemoembolization and RF ablation versus RF ablation alone: a prospective randomized trial. Radiology. 2012;262(2):689–700. [DOI] [PubMed] [Google Scholar]
- 9. Kang SG, Yoon CJ, Jeong SH, et al. Single-session combined therapy with chemoembolization and radiofrequency ablation in hepatocellular carcinoma less than or equal to 5 cm: a preliminary study. J Vasc Interv Radiol. 2009;20(12):1570–1577. [DOI] [PubMed] [Google Scholar]
- 10. Ni JY, Liu SS, Xu LF, Sun HL, Chen YT. Meta-analysis of radiofrequency ablation in combination with transarterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol. 2013;19(24):3872–3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang DJ, Luo KL, Liu H, et al. Meta-analysis of transcatheter arterial chemoembolization plus radiofrequency ablation versus transcatheter arterial chemoembolization alone for hepatocellular carcinoma. Oncotarget. 2017;8(2):2960–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lu Z, Wen F, Guo Q, Liang H, Mao X, Sun H. Radiofrequency ablation plus chemoembolization versus radiofrequency ablation alone for hepatocellular carcinoma: a meta-analysis of randomized-controlled trials. Eur J Gastroenterol Hepatol. 2013;25(2):187–194. [DOI] [PubMed] [Google Scholar]
- 13. Shibata T, Isoda H, Hirokawa Y, Arizono S, Shimada K, Togashi K. Small hepatocellular carcinoma: is radiofrequency ablation combined with transcatheter arterial chemoembolization more effective than radiofrequency ablation alone for treatment? Radiology. 2009;252(3):905–913. [DOI] [PubMed] [Google Scholar]
- 14. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. [DOI] [PubMed] [Google Scholar]
- 15. Kang TW, Kim JM, Rhim H, et al. Small hepatocellular carcinoma: radiofrequency ablation versus nonanatomic resection—propensity score analyses of long-term outcomes. Radiology. 2015;275(3):908–919. [DOI] [PubMed] [Google Scholar]
- 16. Cucchetti A, Piscaglia F, Cescon M, et al. Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J Hepatol. 2013;59(2):300–307. [DOI] [PubMed] [Google Scholar]
- 17. Kim GA, Shim JH, Kim MJ, et al. Radiofrequency ablation as an alternative to hepatic resection for single small hepatocellular carcinomas. Br J Surg. 2016;103(1):126–135. [DOI] [PubMed] [Google Scholar]
- 18. Peng ZW, Lin XJ, Zhang YJ, et al. Radiofrequency ablation versus hepatic resection for the treatment of hepatocellular carcinomas 2 cm or smaller: a retrospective comparative study. Radiology. 2012;262(3):1022–1033. [DOI] [PubMed] [Google Scholar]
- 19. Shiina S, Teratani T, Obi S, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005;129(1):122–130. [DOI] [PubMed] [Google Scholar]
- 20. Huang J, Yan L, Cheng Z, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010;252(6):903–912. [DOI] [PubMed] [Google Scholar]
- 21. Tang T, Feng X, Yan J, et al. Predictive value of indocyanine green retention rate with respect to complications of radiofrequency ablation in 878 patients with hepatocellular carcinoma. Int J Hyperthermia. 2014;30(6):402–407. [DOI] [PubMed] [Google Scholar]
- 22. Feng K, Yan J, Li X, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012;57(4):794–802. [DOI] [PubMed] [Google Scholar]
- 23. Chen MH, Yang W, Yan K, et al. Radiofrequency ablation of problematically located hepatocellular carcinoma: tailored approach. Abdom Imaging. 2008;33(4):428–436. [DOI] [PubMed] [Google Scholar]
- 24. Lu DS, Raman SS, Vodopich DJ, Wang M, Sayre J, Lassman C. Effect of vessel size on creation of hepatic radiofrequency lesions in pigs: assessment of the “heat sink” effect. AJR Am J Roentgenol. 2002;178(1):47–51. [DOI] [PubMed] [Google Scholar]
- 25. Lu DS, Yu NC, Raman SS, et al. Radiofrequency ablation of hepatocellular carcinoma: treatment success as defined by histologic examination of the explanted liver. Radiology. 2005;234(3):954–960. [DOI] [PubMed] [Google Scholar]
- 26. Komorizono Y, Oketani M, Sako K, et al. Risk factors for local recurrence of small hepatocellular carcinoma tumors after a single session, single application of percutaneous radiofrequency ablation. Cancer. 2003;97(5):1253–1262. [DOI] [PubMed] [Google Scholar]
- 27. Wong SN, Lin CJ, Lin CC, Chen WT, Cua IH, Lin SM. Combined percutaneous radiofrequency ablation and ethanol injection for hepatocellular carcinoma in high-risk locations. AJR Am J Roentgenol. 2008;190(3): W187–W195. [DOI] [PubMed] [Google Scholar]
- 28. Kang TW, Lim HK, Lee MW, Kim YS, Choi D, Rhim H. Perivascular versus nonperivascular small HCC treated with percutaneous RF ablation: retrospective comparison of long-term therapeutic outcomes. Radiology. 2014;270(3):888–899. [DOI] [PubMed] [Google Scholar]
- 29. Fujimori M, Takaki H, Nakatsuka A, et al. Combination therapy of chemoembolization and radiofrequency ablation for the treatment of hepatocellular carcinoma in the caudate lobe. J Vasc Interv Radiol. 2012;23(12):1622–1628. [DOI] [PubMed] [Google Scholar]
- 30. Morimoto M, Numata K, Kondo M, et al. Radiofrequency ablation combined with transarterial chemoembolization for subcapsular hepatocellular carcinoma: a prospective cohort study. Eur J Radiol. 2013;82(3):497–503. [DOI] [PubMed] [Google Scholar]
- 31. Kang TW, Lim HK, Lee MW, et al. Long-term therapeutic outcomes of radiofrequency ablation for subcapsular versus nonsubcapsular hepatocellular carcinoma: a propensity score matched study. Radiology. 2016;280(1):300–312. [DOI] [PubMed] [Google Scholar]
- 32. Morimoto M, Numata K, Nozawa A, et al. Radiofrequency ablation of the liver: extended effect of transcatheter arterial embolization with iodized oil and gelatin sponge on histopathologic changes during follow-up in a pig model. J Vasc Interv Radiol. 2010;21(11):1716–1724. [DOI] [PubMed] [Google Scholar]
- 33. Rossi S, Garbagnati F, Lencioni R, et al. Percutaneous radio-frequency thermal ablation of nonresectable hepatocellular carcinoma after occlusion of tumor blood supply. Radiology. 2000;217(1):119–126. [DOI] [PubMed] [Google Scholar]
- 34. Sugimori K, Nozawa A, Morimoto M, et al. Extension of radiofrequency ablation of the liver by transcatheter arterial embolization with iodized oil and gelatin sponge: results in a pig model. J Vasc Interv Radiol. 2005;16(6):849–856. [DOI] [PubMed] [Google Scholar]
- 35. Lencioni R, Crocetti L, Petruzzi P, et al. Doxorubicin-eluting bead-enhanced radiofrequency ablation of hepatocellular carcinoma: a pilot clinical study. J Hepatol. 2008;49(2):217–222. [DOI] [PubMed] [Google Scholar]
- 36. Hyun D, Cho SK, Shin SW, Rhim H, Koh KC, Paik SW. Treatment of small hepatocellular carcinoma (≤2 cm) in the caudate lobe with sequential transcatheter arterial chemoembolization and radiofrequency ablation. Cardiovasc Intervent Radiol. 2016;39(7):1015–1022. [DOI] [PubMed] [Google Scholar]
- 37. Kowal CD, Bertino JR. Possible benefits of hyperthermia to chemotherapy. Cancer Res. 1979;39(6 pt 2):2285–2289. [PubMed] [Google Scholar]
- 38. Kim J, Yoon CJ, Seong NJ, Jeong SH, Kim JW. Fluoroscopy-guided radiofrequency ablation for small hepatocellular carcinoma: a retrospective comparison with ultrasound-guided ablation. Clinical Radiol. 2015;70(9):1009–1015. [DOI] [PubMed] [Google Scholar]
- 39. Santambrogio R, Barabino M, Bruno S, et al. Long-term outcome of laparoscopic ablation therapies for unresectable hepatocellular carcinoma: a single European center experience of 426 patients. Surg Endosc. 2016;30(5):2103–2113. [DOI] [PubMed] [Google Scholar]
- 40. Sakoda M, Ueno S, Iino S, et al. Endoscopic versus open radiofrequency ablation for treatment of small hepatocellular carcinoma. World J Surg. 2013;37(3):597–601. [DOI] [PubMed] [Google Scholar]
- 41. Ding H, Su M, Zhu C, Wang L, Zheng Q, Wan Y. CT-guided versus laparoscopic radiofrequency ablation in recurrent small hepatocellular carcinoma against the diaphragmatic dome. Sci Rep. 2017;7:44583. [DOI] [PMC free article] [PubMed] [Google Scholar]