Abstract
Objective:
To analyze the clinical, pathological, and sociodemographic aspects between triple-negative breast cancer (TNBC) and non-TNBC in a Brazilian cohort and identify potential prognostic factors.
Methods:
This hospital-based retrospective cohort study included 447 women with breast cancer treated at referral centers in Southeastern Brazil. Overall and disease-free survival were compared; prognostic factors were evaluated.
Results:
Triple-negative breast cancer corresponded to 19.5% of breast cancer diagnosis and was more prevalent among nonwhite and less educated women. The patients with TNBC tended to present with stage III cancer, high p53 expression, lymphocytic infiltration, and multifocality and treated with radical surgery and chemotherapy. The 5-year overall and disease-free survival were 62.1% and 57.5% for TNBC and 80.8% and 75.3% for non-TNBC, respectively (P < .001). The TNBC recurrence was associated with multicentricity, whereas lymph node involvement increased the risk of both recurrence and death. Non-TNBC worse clinical course was associated with nonwhite ethnicity, lower education level, lymph node involvement, and advanced stage.
Conclusions:
Triple-negative breast cancer exhibited a more aggressive behavior, earlier and more frequent recurrence, and worse survival compared with non-TNBC. While biological and social variables were associated with poorer prognosis in non-TNBC, only lymph node involvement and multicentricity were correlated with worse clinical outcomes in TNBC.
Keywords: Breast neoplasms, triple-negative breast cancer, survival analysis
Introduction
The high heterogeneity of breast cancer explains in part the differences in the morbidity and mortality of this disease. In that sense, molecular and genetic profiling of breast tumors, as well as their classification, has led to a greater diagnostic accuracy, better prediction of patients’ clinical evolution, and more appropriate treatment strategies.1,2
Triple-negative breast cancer (TNBC) is a specific subset of tumors characterized by the absence of the 3 most commonly targeted biomarkers considered for breast cancer treatment: estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor (HER2).3 The TNBC accounts for 15% to 20% of all breast cancer diagnoses and usually has a more aggressive clinical course, with worse evolution within the first 3 to 5 years after diagnosis; early and higher rates of distant recurrences, typically visceral; and poor survival.3–5 There are differences in the natural history and prognosis within TNBC. Recent studies, using next-generation sequencing, for instance, have shown that TNBC is actually a group of distinct tumors displaying different clinical and biological features.6–8
It has been proposed to classify TNBC into subgroups: basal-like 1 and 2, mesenchymal, and luminal androgen receptor.6 Novel targets, such as the epithelial growth factor receptor, have emerged as a new drug target. BRCA1 mutation studies have raised expectations for the use of DNA-damaging agents and androgen receptors in the management of TNBC. However, the response to therapy varies considerably, making it difficult to establish a treatment that covers all cases, as a single target has not yet been identified.6–8
Anthracycline and taxane-based chemotherapy has traditionally been the mainstay of TNBC therapy in clinical practice.9 Therefore, part of the patients, notably those with initial tumors, receives aggressive treatment without significant improvement in overall survival but rather a worsening quality of life. This scenario highlights the need to research drugs capable of overcoming chemoresistances and reduce the damage of cytotoxic therapy.9,10
Identifying TNBC with a high probability of responding to traditional chemotherapy poses a challenge in current clinical practice. The role of cell proliferation markers, especially the nuclear antigen Ki67, has been emphasized as a prognostic marker and predictor of response to chemotherapeutic regimens, although an optimal cutoff point has not yet been defined.11–13 Lymphocytic infiltration has also been found to be a prognostic and predictive marker of response to both neoadjuvant and adjuvant therapies.14–16
Therefore, studies are needed to enhance the understanding of the TNBC spectrum, to improve current management and patient outcome. It is possible that tumor grade, histological type, lymphocytic infiltration, and cell division markers would have some interface with the complex information of genetic and molecular assays, thus providing estimates of clinically relevant TNBC subgroups.16–18 How these parameters occur in TNBC and their relation to survival and disease relapse have been inadequately investigated in Brazil. This study thus aimed to identify clinical and pathological features in a cohort of Brazilian women diagnosed with breast cancer, comparing TNBC with non-TNBC tumors, and to assess possible associations of the parameters with recurrence and survival.
Patients and Methods
Study population
This hospital-based retrospective cohort study comprised women diagnosed with breast cancer between 2003 and 2005. They were assisted at a public and a private referral center for cancer care in the city of Juiz de Fora, state of Minas Gerais, Brazil. The patients were identified through the Hospital Registry of Cancer, and data were obtained through a standardized form from the medical records of both cancer and breast specialists.
A total of 563 patients were initially identified. Women diagnosed with carcinoma in situ were excluded (n = 45), as well as those with distant metastasis at diagnosis (n = 39). In addition, 32 patients were excluded because of missing information on the immunochemistry profile. The final cohort consisted of 447 patients, who were classified into 2 groups: TNBC, when tumors were negative for ER, PR, and HER2 (n = 87), and non-TNBC, for all other cases (n = 360).
The immunohistochemical analysis was performed in the hospitals’ accredited laboratory following standard criteria. It started with tissue deparaffinization, followed by antigen recovery using wet heat and buffers. Blockage of peroxidases was performed, and specific antibodies were added: SP1 and SP2 for estrogen and progesterone and SP3 or 4B5 for HER2 (dilution: 1/250). The bound antibodies were displayed by the streptavidin-biotin system. A tumor was classified as positive for ER and PR when >10% of tumor cell nuclei expressed these antigens. For HER2, a positive score of 3+ for membrane staining was required, whereas those with scores of + and 2+ were sent to fluorescence in situ hybridization. This approach meets the American Society of Clinical Oncology 2007 and 2013 recommendations. All laboratory reports were revised.
Study variables
The following sociodemographic variables were analyzed: age at diagnosis (in years); ethnicity (white or nonwhite); education level (high [secondary school or higher], moderate [primary school], or low [incomplete primary school or illiterate]). The nature of the oncology service (public or private) was also analyzed. Variables related to tumor characteristics included anatomopathological tumor size (⩽2.0 and >2.0 cm), histological type, lymph node involvement, staging according to TNM classification,19 lymphocytic infiltration (presence or absence), Ki67 (low ⩽25% and high/moderate ⩾26%), p53, multifocality, and multicentricity. Treatment variables were surgery type (radical or conservative) and additional therapy (chemotherapy).
Follow-up
A 5-year follow-up of each patient was conducted based on the consultation of medical records and was complemented by search in the Brazilian Mortality Information System (SIM) of Minas Gerais, telephone contact, consultation of the National Health Registry, and Brazilian individual registration number (CPF).
The diagnosis date (corresponding to the date of the histopathological disease report) was considered the starting point for the analysis of overall survival, and all-cause mortality was used as failure in this case. Disease-free survival was defined as the interval between the first treatment and recurrence (locoregional recurrence, metastasis, or death from any cause).20 Patients who remained alive until December 31, 2010 (the end of follow-up), were censored. The date of the last contact was used to define the end point for loss to follow-up.
Data analysis
Differences in the distribution of study variables between TNBC and non-TNBC were evaluated by χ2 test. Survival functions were calculated using the Kaplan-Meier method, and the log-rank test was used to compare the survival curves. Prognostic factors of overall survival and disease-free survival were analyzed by the Cox proportional hazards model, and the hazard ratios (HRs) were calculated with a 95% confidence interval (CI). Variables that presented P values of <.25 in the univariate Cox model were included in the multivariate model. The risk proportionality assumption was validated by the Schoenfeld residue analysis. For variables with missing data (tumor grade, Ki67, and p53), a comparative study was performed, considering tumor profile, recurrence, metastasis, and death as dependent variables. No significant differences were found between patients with missing data and other patients, showing that data losses were random, which made the analysis of those variables possible. A 5% level of significance was used for all tests. The IBM Statistical Package for the Social Sciences (SPSS) 21 (IBM Corp., Armonk, NY, USA) was used for all statistical analyses.
The study design was approved by the Ethics Committee of Universidade Federal de Juiz de Fora (protocol no. 151.219 of November 22, 2012). The need for informed consent was waived owing to the noninterventional design of the survey and full data protection.
Results
The TNBC accounted for 19.5% of the 447 patients. The mean age at diagnosis did not differ between the groups, and significant differences were identified for ethnicity and educational level, with TNBC being more prevalent in nonwhite women and those with moderate and low education level. The TNBC tended to present at diagnosis with lymphocytic infiltration (P = .02), multifocality (P = .04), tumor size of >2 cm (P = .06), and stage III tumors (P = .09), and to express high levels of p53 (P = .04) and Ki67 (P = .09). The patients with TNBC showed significantly higher percentages of radical surgery and chemotherapy. When outcomes were compared, the TNBC group presented a higher frequency of early recurrences, defined as up to 24 months (P = .02), and death in 5 years (P < .001) (Table 1).
Table 1.
Distribution of variables according to breast cancer subtype (Juiz de Fora, Brazil, 2003-2005).
| Variable | Breast cancer subtype |
|||
|---|---|---|---|---|
| Total | TNBC, No. (%) | Non-TNBC, No. (%) | P value | |
| No. of patients | 447 | 87 (19.5) | 360 (80.5) | |
| Age at diagnosis | 447 | 57 (±13) | 58 (±14) | .49 |
| Ethnicity | 422 | .001 | ||
| White | 340 | 57 (67.9) | 283 (83.7) | |
| Nonwhite | 82 | 27 (32.1) | 55 (16.3) | |
| Education level | 404 | .007 | ||
| High | 166 | 22 (27.8) | 144 (44.3) | |
| Moderate | 68 | 21 (26.6) | 47 (14.5) | |
| Low | 170 | 36 (45.6) | 134 (41.2) | |
| Family history of cancer | 416 | .3 | ||
| Positive | 120 | 19 (24.1) | 101 (30) | |
| Negative | 296 | 60 (75.9) | 236 (70) | |
| Health service | 447 | .14 | ||
| Public | 220 | 49 (56.3) | 171 (47.5) | |
| Private | 227 | 38 (43.7) | 189 (52.5) | |
| Tumor size, cm | 438 | .06 | ||
| >2 | 257 | 58 (67.4) | 199 (56.5) | |
| ⩽2 | 181 | 28 (32.6) | 153 (43.5) | |
| Lymph node involvement | 437 | .64 | ||
| Positive | 198 | 40 (47.6) | 158 (44.8) | |
| Negative | 239 | 44 (52.4) | 195 (55.2) | |
| Staging | 446 | .09 | ||
| I | 124 | 18 (20.9) | 106 (29.4) | |
| II | 190 | 35 (40.7) | 155 (43.1) | |
| III | 132 | 33 (38.4) | 99 (27.5) | |
| Histological type | 446 | .21 | ||
| Invasive ductal | 352 | 73 (83.9) | 279 (77.7) | |
| Invasive lobular | 56 | 6 (6.9) | 50 (13.9) | |
| Other | 38 | 8 (9.2) | 30 (8.4) | |
| Tumor grade | 337 | <.001 | ||
| I | 111 | 10 (14.9) | 101 (37.4) | |
| II | 158 | 33 (49.3) | 125 (46.3) | |
| III | 68 | 24 (35.8) | 44 (16.3) | |
| Lymphocytic infiltration | 427 | .02 | ||
| Positive | 186 | 44 (55.7) | 142 (40,8) | |
| Negative | 241 | 35 (44.3) | 206 (59.2) | |
| Ki67 | 349 | .09 | ||
| ⩽25% | 94 | 15 (19.5) | 79 (29) | |
| ⩾26% | 255 | 62 (80.5) | 193 (71) | |
| p53 | 336 | .04 | ||
| ⩽25% | 230 | 37 (57.8) | 193 (71) | |
| ⩾26% | 106 | 27 (42.2) | 79 (29) | |
| Multifocality | 423 | .04 | ||
| Positive | 35 | 11 (14.1) | 24 (7) | |
| Negative | 388 | 67 (85.9) | 321 (93) | |
| Multicentricity | 422 | .25 | ||
| Positive | 21 | 6 (7.7) | 15 (4.4) | |
| Negative | 401 | 72 (92.3) | 329 (95.6) | |
| Surgery | 442 | .01 | ||
| Radical | 217 | 53 (61.6) | 164 (46.1) | |
| Conservative | 225 | 33 (38.4) | 192 (53.9) | |
| Chemotherapy | 447 | .001 | ||
| Yes | 308 | 73 (83.9) | 235 (65.3) | |
| No | 139 | 14 (16.1) | 125 (34.7) | |
| Recurrence | 447 | .001 | ||
| Yes | 126 | 37 (42.5) | 89 (24.7) | |
| No | 321 | 50 (57.5) | 271 (75.3) | |
| Time to recurrence, mo | 126 | .02 | ||
| Up to 24 | 61 | 25 (67.5) | 36 (40.4) | |
| 25-36 | 26 | 6 (16.2) | 20 (22.5) | |
| 37-60 | 39 | 6 (16.2) | 33 (37.1) | |
| 5-y overall survival | 447 | <.001 | ||
| Dead | 102 | 33 (37.9) | 69 (19.2) | |
| Alive | 345 | 54 (62.1) | 291 (80.8) | |
Abbreviation: TNBC, triple-negative breast cancer.
Total varies due to missing data.
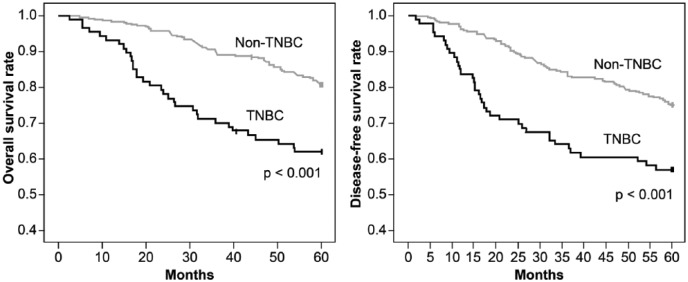
The 5-year overall and disease-free survival rates for the entire patient population were 77% and 72%, respectively. Overall survival was 62% for TNBC and 81% for non-TNBC, and disease-free survival was 57% for TNBC and 75% for non-TNBC (log-rank P < .001; Figure 1). The median survival time was 46 months (95% CI: 42-51) for TNBC versus 55 months (95% CI: 54-57) for non-TNBC, and the median disease-free interval was 43 months (95% CI: 38-48) and 53 months (95% CI: 41-54), respectively.
Figure 1.
Overall and disease-free survival in patients with triple-negative breast cancer (TNBC) and non-TNBC (Juiz de Fora, Brazil, 2003-2005).
Overall survival: TNBC, 62.7% (95% CI: 51.9-71.2); non-TNBC, 81.1% (95% CI: 76.3-84.5); log-rank test P < .001.
Disease-free survival: TNBC, 57.5% (95% CI: 46.4-67.1); non-TNBC, 75.3% (95% CI: 70.5-79.4); log-rank test P < .001.
Univariate Cox regression analysis showed that the risk of recurrences (HR: 2.23; 95% CI: 1.52-3.28) and risk of death (HR: 2.97; 95% CI: 1.96-4.50) were higher in TNBC compared with non-TNBC. In non-TNBC patients, the risk of recurrence was higher among patients aged <40 years, of nonwhite ethnicity, with lower education level, and with tumors larger than 2 cm, of grade III and at stage III. Lymph node involvement, p53, Ki67, and chemotherapy treatment were also associated with higher recurrence in this group. In patients with TNBC, only lymph node involvement and staging were associated with recurrence (Table 2).
Table 2.
The 5-year disease-free survival estimated by univariate Cox analysis for 447 women diagnosed with breast cancer (Juiz de Fora, Brazil, 2003-2005).
| Variable | TNBC |
Non-TNBC |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age at diagnosis, y | ||||||
| <40 vs ⩾40 | 1.15 | 0.28–4.77 | .85 | 1.91 | 1.10-3.34 | .02 |
| Ethnicity | ||||||
| Nonwhite vs white | 1.47 | 0.76–2.88 | .26 | 1.91 | 1.16–3.16 | .01 |
| Education level | ||||||
| Low/moderate vs high | 1.55 | 0.67–3.58 | .3 | 1.92 | 1.19–3.10 | .01 |
| Health services | ||||||
| Public vs private | 1.16 | 0.60–2.24 | .66 | 1.4 | 0.92–2.12 | .12 |
| Chemotherapy | ||||||
| Yes vs no | 0.9 | 0.38–2.17 | .82 | 1.74 | 1.08–2.82 | .02 |
| Tumor size, cm | ||||||
| >2 vs ⩽2 | 1.4 | 0.67–2.90 | .37 | 2.58 | 1.59–4.19 | <.001 |
| Lymph node involvement | ||||||
| Positive vs negative | 2.15 | 1.07–4.31 | .03 | 2.28 | 1.47–3.53 | <.001 |
| Tumor grade | ||||||
| III vs I-II | 0.88 | 0.42–1.82 | .73 | 2.23 | 1.28–3.89 | .01 |
| Staging | ||||||
| III vs I-II | 1.89 | 0.98–3.64 | .05 | 3.89 | 2.56–5.90 | <.001 |
| p53 | ||||||
| Moderate/high vs low | 1.07 | 0.53–2.16 | .86 | 1.88 | 1.16–3.05 | .01 |
| Ki67 | ||||||
| Moderate/high vs low | 0.91 | 0.39–2.11 | .83 | 2.06 | 1.08–3.94 | .03 |
| Multifocality | ||||||
| Yes vs no | 1.53 | 0.63–3.68 | .34 | 1.62 | 0.81–3.22 | .17 |
| Multicentricity | ||||||
| Yes vs no | 2.46 | 0.87–7.01 | .09 | 1.7 | 0.74–3.90 | .21 |
| Lymphocytic infiltration | ||||||
| Positive vs negative | 1.47 | 0.74–2.92 | .27 | 1 | 0.65–1.54 | .99 |
Abbreviations: CI, confidence interval; HR, hazard ratio; TNBC, triple-negative breast cancer.
Overall survival in TNBC was negatively associated with lymph node involvement, stage III, and metastasis during the course of the disease. For the non-TNBC group, poorer survival was observed in patients of nonwhite ethnicity, with lower education level, who were attended in the public health sector, with tumors larger than 2 cm, of grade III and at stage III, lymph node involvement, elevated Ki67, and metastasis during the course of the disease (Table 3).
Table 3.
The 5-year overall survival estimated by univariate Cox analysis for 447 women diagnosed with breast cancer (Juiz de Fora, Brazil, 2003-2005).
| Variable | TNBC |
Non-TNBC |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age at diagnosis, y | ||||||
| <40 vs ⩾40 | 1.06 | 0.25–4.43 | .94 | 1.65 | 0.87–3.15 | .13 |
| Ethnicity | ||||||
| Nonwhite vs white | 1.51 | 0.75–3.04 | .25 | 2.41 | 1.41–4.12 | .001 |
| Education level | ||||||
| Low/moderate vs high | 1.66 | 0.68–4.08 | .27 | 2.05 | 1.17–3.61 | .01 |
| Health services | ||||||
| Public vs private | 1.42 | 0.70–2.89 | .33 | 1.82 | 1.13–2.46 | .01 |
| Chemotherapy | ||||||
| Yes vs no | 0.75 | 0.31–1.81 | .52 | 1.34 | 0.79–2.25 | .27 |
| Tumor size | ||||||
| >2 vs ⩽2 cm | 1.37 | 0.63–2.95 | .43 | 3.29 | 1.82–5.94 | <.001 |
| Lymph node involvement | ||||||
| Positive vs negative | 2.72 | 1.27–5.82 | .01 | 2.25 | 1.36–3.73 | .002 |
| Metastasis | ||||||
| Positive vs negative | 13.5 | 5.36–34.03 | <.001 | 14.75 | 8.42–25.83 | <.001 |
| Tumor grade | ||||||
| III vs I-II | 1.01 | 0.47–2.19 | .98 | 2.87 | 1.55–5.29 | .001 |
| Staging | ||||||
| III vs I-II | 2.18 | 1.09–4.37 | .03 | 3.93 | 2.44–6.32 | <.001 |
| p53 | ||||||
| Moderate/high vs low | 1.06 | 0.50–2.23 | .88 | 1.51 | 0.86–2.63 | .15 |
| Ki67 | ||||||
| Moderate/high vs low | 0.98 | 0.40–2.40 | .97 | 2.12 | 1.00–4.50 | .05 |
| Multifocality | ||||||
| Yes vs no | 1.51 | 0.62–3.68 | .37 | 0.81 | 0.29–2.22 | .68 |
| Multicentricity | ||||||
| Yes vs no | 2.67 | 0.93–7.65 | .07 | 1.79 | 0.72–4.45 | .21 |
| Lymphocytic infiltration | ||||||
| Positive vs negative | 1.57 | 0.75–3.28 | .23 | 1.01 | 0.50–1.35 | .82 |
Abbreviations: CI, confidence interval; HR, hazard ratio; TNBC, triple-negative breast cancer.
The multivariate analysis revealed that lymph node involvement appears to increase the risk of recurrence and mortality in patients with TNBC. Multicentricity was also associated with a higher risk of death in this group. In non-TNBC patients, the risk of recurrence was higher in those with lower education level and lymph node involvement, and survival was worse in nonwhite women and those with advanced-stage tumors (Table 4). For all multivariate models, the proportional hazards assumption was confirmed overall and separately for each variable by the scaled Schoenfeld residual test.
Table 4.
The 5-year disease-free and overall survival estimated by multivariate Cox analysis for 447 women diagnosed with breast cancer (Juiz de Fora, Brazil, 2003-2005).
| Disease-free survival |
Overall survival |
|||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | P value | HR | 95% CI | P value |
| TNBC | ||||||
| Lymph node involvement | ||||||
| Positive vs negative | 2.28 | 1.12–4.57 | .02 | 2.8 | 1.26–6.25 | .012 |
| Multicentricity | ||||||
| Yes vs no | — | — | — | 4.11 | 1.17–14.36 | .027 |
| Non-TNBC | ||||||
| Ethnicity | ||||||
| White vs nonwhite | — | — | — | 2.01 | 1.17–3.45 | .01 |
| Education level | ||||||
| Low/moderate vs high | 1.91 | 1.14–3.22 | .01 | — | — | — |
| Lymph node involvement | ||||||
| Positive vs negative | 1.81 | 1.14–2.90 | .01 | — | — | — |
| Staging II | ||||||
| II vs I | — | — | — | 3.06 | 1.16–8.05 | .02 |
| Staging III | ||||||
| III vs I | — | — | — | 8.36 | 3.27–21.36 | <.001 |
Abbreviations: CI, confidence interval; HR, hazard ratio; TNBC, triple-negative breast cancer.
All models were adjusted for age at diagnosis, in continuous format.
Discussion
This study described the clinical, pathological, and sociodemographic characteristics of patients with TNBC and non-TNBC in a medium-size city in Southeastern Brazil, which is a regional reference for cancer care. The TNBC prevalence in this study is in accordance with the literature, as it accounts for 10% to 20% of all breast cancers.2,5,21 The TNBC was more likely to be found in nonwhite women than non-TNBC, which is expected because TNBC is more common in women of African ancestry or Hispanic ethnicity.22,23 Despite lower incidences of breast cancer in black and Hispanic women, studies have persistently shown that these women are more commonly affected by tumors with increased severity and worse outcomes.22–25
Although TNBC is frequently diagnosed in younger patients,2,5,21 the mean age at diagnosis in our study cohort was 57 years, which may partly reflect late diagnosis or difficult access to health care services. The higher percentage of TNBC in women with lower education level and assisted in the public health sector is consistent with the higher occurrence of TNBC in socially disadvantaged populations.22,26 Other demographic variables such as parity, breastfeeding, reproductive status, and family history did not seem to differ in the 2 groups.
Similar to findings in other studies, the 5-year overall and disease-free survival were worse in women with TNBC.2,4,5,21,22,27 About 38% of patients with TNBC died during the 5-year follow-up, compared with only 19% of non-TNBC patients. Recurrences were observed in 43% of patients with TNBC, compared with 25% in non-TNBC patients. Not only were recurrences more prevalent in the TNBC group but they also occurred earlier in the course of the disease. In the patients with TNBC, 67.5% of recurrences were found within the first 2 years and 83.7% within the first 3 years after diagnosis, whereas in the non-TNBC group, these values were 40.4% and 62.9%, respectively. These recurrence rates were higher than those observed in a Chinese cohort (2016), in which the 5-year recurrence was 27.5% in the patients with TNBC and 13.4% in non-TNBC patients; the 5-year overall survival rates were 88.5% and 95.5%,28 respectively, higher than those found in our study (62.1% in TNBC and 80.8% in non-TNBC). These differences are probably due the fact that 28% of TNBC and 14% of non-TNBC were diagnosed at stage III (advanced stage) in the Chinese cohort, whereas in our study, these rates were 38% and 28%, respectively. In a Taiwan cohort study, worse survival was also observed in the patients with TNBC, but disease-free survival had no difference when compared with non-TNBC patients.29
Survival rates in this study were also similar to those found in another Brazilian cohort, in which TNBC women without lymph node involvement had a survival rate of 69% in 5 years and 61.6% in 10 years. Among non-TNBC patients, the survival was 82.2% and 70.1% in 5 and 10 years, respectively. In that study, skin involvement, histological grade, and Ki67 were identified as prognostic and predictive factors.27 In another Brazilian study, the 5-year survival was 67.8% in TNBC, which was lower compared with non-TNBC subtypes (86.4% for luminal A tumors and 91.4% for luminal B tumors).30
Here, lymph node involvement was a prognostic factor for both mortality and recurrence in the TNBC group, representing a nearly 3 times risk of mortality. The same was observed in an American cohort, in which a 5-year overall survival of 80% was reported for the patients with TNBC without lymph node involvement, compared with 65% in those with up to 3 positive lymph nodes.31 The 5-year survival in this study decreased from 77.3% to 50% (P = .004) in the patients with TNBC in the case of positive lymph node involvement.
Our study confirms recent findings on the prognostic role of multicentricity in TNBC, which has been related to higher cell proliferation indexes and tendency to early axillary involvement.32,33 A recent meta-analysis including 67 557 patients with breast carcinoma identified multifocality or multicentricity in 9.5% of them. On multivariate analysis, multicentricity was associated with worse overall survival (HR: 1.65; P = .02),34 which is similar to the findings of our study. It seems, therefore, that due to its relevance and independent prognostic value, multicentricity should be considered in the individual management and treatment of patients with TNBC.35
In the non-TNBC group, lymph node involvement was associated with more frequent recurrence and advanced-stage tumors were related to worse survival. The mortality in patients with non-TNBC diagnosed at stage III was 8 times higher compared with that at stage I. In addition to biological variables, sociodemographic variables such as ethnicity and education level were associated with poorer outcomes in non-TNBC, which has also been identified in several studies.22–24,26 Those variables possibly did not remain statistically significant on multivariate analysis in TNBC due to the smaller sample size of this group. However, it remains unclear as to what extent the prognosis of TNBC can be attributable to sociodemographic and biologic variables,36 although this study’s findings support the second one.
The small sample size, its retrospective design, and performing this study in only one city were major limitations. However, the standardized method of data collection, the use of stratified multivariate models, and the analysis of data from both private and public sectors probably resulted in less likely bias. The statistical analysis showed that data losses were random, partially overcoming the missing data limitation. Finally, the miscegenation of the Brazilian population needs to be considered when comparing the results with other populations. It is noteworthy that despite this factor, patients with TNBC and non-TNBC patients still presented markedly different characteristics, which were generally similar to other population groups. The heterogeneity of TNBC is inter- and intratumoral, and different population studies have yet to provide a common factor that could be used in the therapy of this disease.
Conclusions
The TNBC exhibited distinct sociodemographic and tumor characteristics, as it was more prevalent in nonwhite and less educated women and was diagnosed at a later stage of the disease. This tumor subtype tended to display a worse clinical course, with earlier and more frequent recurrence and worse 5-year survival, compared with non-TNBC. A more aggressive behavior was notably seen in patients with TNBC with lymph node involvement and multicentricity, important clinical features to be considered in the management of these patients.
Acknowledgments
The authors would like to thank Editage (www.editage.com) for English language editing and Publication Support. Part of this paper was taken from the doctoral thesis of the first author developed in the Post-Graduate Program in Health of the Universidade Federal de Juiz de Fora (UFJF), Juiz de Fora, Minas Gerais, Brazil.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: All authors contributed to the conception of the work; participated in the analysis and interpretation of data; performed a critical review of its content; and approved the final manuscript.
ORCID iD: Homero Gonçalves Jr  https://orcid.org/0000-0001-5960-1482
https://orcid.org/0000-0001-5960-1482
References
- 1. Blows FM, Driver KE, Schmidt MK, et al. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med. 2010;7:e1000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fernandes GS, Calabrich A, Katz A. Câncer de mama triplo-negativo: aspectos clínicos, laboratoriais e terapêuticos. Rev Bras Mastol. 2009;19:76–82. [Google Scholar]
- 3. Anders CK, Abramson V, Tan T, Dent R. The evolution of triple-negative breast cancer: from biology to novel therapeutics. Am Soc Clin Oncol Educ Book. 2016;35:34–42. [DOI] [PubMed] [Google Scholar]
- 4. Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. [DOI] [PubMed] [Google Scholar]
- 5. Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–1948. [DOI] [PubMed] [Google Scholar]
- 6. Lehmann BD, Bauer JA, Chen X, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Prat A, Parker JS, Karginova O, et al. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 2010;12:R68. doi: 10.1186/bcr2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang L, Fang C, Xu X, Li A, Cai Q, Long X. Androgen receptor, EGFR, and BRCA1 as biomarkers in triple-negative breast cancer: a meta-analysis. Biomed Res Int. 2015;2015:357485. doi: 10.1155/2015/357485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Palma G, Frasci G, Chirico A, et al. Triple negative breast cancer: looking for the missing link between biology and treatments. Oncotarget. 2015;6:2560–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barrios CH Buzaid AC Rocha Cruz M et al.. Manual de Oncologia Clínica do Brasil. 10th ed. São Paulo: Dendrix; 2012:1–38. [Google Scholar]
- 11. Esposito A, Criscitiello C, Curigliano G. Highlights from the 14th St Gallen International Breast Cancer Conference 2015 in Vienna: dealing with classification, prognostication, and prediction refinement to personalize the treatment of patients with early breast cancer Ecancermedicalscience. 2015;9:518–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. O’Shaughnessy J, Schwartzberg L, Danso MA, et al. Phase III study of iniparib plus gemcitabine and carboplatin versus gemcitabine and carboplatin in patients with metastatic triple-negative breast cancer. J Clin Oncol. 2014;32:3840–3847. [DOI] [PubMed] [Google Scholar]
- 13. Inwald EC, Klinkhammer-Schalke M, Hofstadter F, et al. Ki-67 is a prognostic parameter in breast cancer patients: results of a large population-based cohort of a cancer registry. Breast Cancer Res Treat. 2013;139:539–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pruneri G, Vingiani A, Bagnardi V, et al. Clinical validity of tumor-infiltrating lymphocytes analysis in patients with triple-negative breast cancer. Ann Oncol. 2016;27:249–256. [DOI] [PubMed] [Google Scholar]
- 15. Denkert C, von Minckwitz G, Darb-Esfahani S, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19:40–50. [DOI] [PubMed] [Google Scholar]
- 16. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–410. [DOI] [PubMed] [Google Scholar]
- 17. Montagna E, Maisonneuve P, Rotmensz N, et al. Heterogeneity of triple-negative breast cancer: histologic subtyping to inform the outcome. Clin Breast Cancer. 2013;13:31–39. [DOI] [PubMed] [Google Scholar]
- 18. Goldhirsch A Wood WC Coates AS et al.. Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gradishar WJ, Anderson BO, Balassanian R, et al. Invasive breast cancer version 1.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016;14:324–354. [DOI] [PubMed] [Google Scholar]
- 20. Hudis CA, Barlow WE, Costantino JP, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol. 2007;25:2127–2131. [DOI] [PubMed] [Google Scholar]
- 21. Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California Cancer Registry. Cancer. 2007;109:1721–1728. [DOI] [PubMed] [Google Scholar]
- 22. Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. [DOI] [PubMed] [Google Scholar]
- 23. Lara-Medina F, Pérez-Sanchez V, Saavedra-Pérez D, et al. Triple-negative breast cancer in Hispanic patients: high prevalence, poor prognosis, and association with menopausal status, body mass index, and parity. Cancer. 2011;117:3658–3669. [DOI] [PubMed] [Google Scholar]
- 24. Patel TA, Colon-Otero G, Hume CB, Copland JA 3rd, Perez EA. Breast cancer in Latinas: gene expression, differential response to treatments, and differential toxicities in Latinas compared with other population groups. Oncologist. 2010;15:466–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lindner R, Sullivan C, Offor O, et al. Molecular phenotypes in triple negative breast cancer from African American patients suggest targets for therapy. PLoS ONE. 2013;8:e71915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vona-Davis L, Rose DP. The influence of socioeconomic disparities on breast cancer tumor biology and prognosis: a review. J Womens Health. 2009;18:883–893. [DOI] [PubMed] [Google Scholar]
- 27. Eisenberg ALA, Pinto IV, Koifman S. Triple-negative breast cancer in Brazilian women without metastasis to axillary lymph nodes: ten-year survival and prognostic factors. Br J Med Med Res. 2013;3:880–896. [Google Scholar]
- 28. Qiu J, Xue X, Hu C, et al. Comparison of clinicopathological features and prognosis in triple-negative and non-triple negative breast cancer. J Cancer. 2016;7:167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lin C, Chien SY, Chen LS, et al. Triple negative breast carcinoma is a prognostic factor in Taiwanese women. BMC Cancer. 2009;9:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cintra JRD, Bustamante-Teixeira MT, Diniz RW, et al. Perfil imuno-histoquímico e variáveis clinicopatológicas no câncer de mama. Rev Assoc Med Bras. 2012;58:178–187.22569612 [Google Scholar]
- 31. Hernandez-Aya LF, Chavez-MacGregor M, Lei X, et al. Nodal status and clinical outcomes in a large cohort of patients with triple-negative breast cancer. J Clin Oncol. 2011;29:2628–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kanumuri P, Hayse B, Killelea BK, et al. Characteristics of multifocal and multicentric breast cancers. Ann Surg Oncol. 2015;22:2475–2482. [DOI] [PubMed] [Google Scholar]
- 33. Moon HG, Han W, Kim JY, et al. Effect of multiple invasive foci on breast cancer outcomes according to the molecular subtypes: a report from the Korean Breast Cancer Society. Ann Oncol. 2013;24:2298–2304. [DOI] [PubMed] [Google Scholar]
- 34. Vera-Badillo FE, Napoleone M, Ocana A, et al. Effect of multifocality and multicentricity on outcome in early stage breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat. 2014;146:235–244. [DOI] [PubMed] [Google Scholar]
- 35. Neri A, Marrelli D, Megha T, et al. Clinical significance of multifocal and multicentric breast cancers and choice of surgical treatment: a retrospective study on a series of 1158 cases. BMC Surg. 2015;15:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kang SP, Martel M, Harris LN. Triple negative breast cancer: current understanding of biology and treatment options. Curr Opin Obstet Gynecol. 2008;20:40–46. [DOI] [PubMed] [Google Scholar]