Abstract
Phosphorylation is the most extensively studied posttranslational modification of proteins. There are approximately 500 kinases known in the human genome. The kinase-activated pathways regulate almost every aspect of cell function and a deregulated kinase cascade leads to impaired cellular function. Impaired regulation of several kinase cascades, including the epidermal growth factor receptor (EGFR) pathway, leading to tumor pathogenesis, is well documented. Thus, a phosphospecific test with prognostic or predictive value was expected in oncology. However, no phosphospecific IHC test is used in oncology clinics. Human topoisomerase I (topoI) inhibitors, camptothecin and its analogues (CPT), are used extensively to treat various solid tumors. Depending on tumor type, the response rate is only 13–32%. We have demonstrated that the deregulated kinase cascade is at the core of CPT resistance. DNA-PKcs, a kinase central to the DNA–double-strand break (DSB) response pathway, phosphorylates topoI at serine 10 (topoI-pS10), and cells with higher basal levels of topoI-pS10 degrade topoI rapidly and are resistant to this class of drug. The higher basal level of topoI phosphorylation is due to continual activation of DNA-PKcs, and one potential mechanism of this pathway activation is failure of upstream effector phosphatases such as phosphatase and tensin homolog (PTEN). Based on this understanding, we have developed an IHC-based test (P-topoIDx) that can stratify the responder and non-responder patient population.
Keywords: phosphospecific IHC, predictive biomarker, topoI-pS10 IHC
Introduction
In recent years, immunohistochemical assays have been rapidly and prolifically incorporated into everyday surgical pathology.1 Urgently needed progress in personalized medicine, particularly in oncology, has provided a strong impetus for adopting novel, IHC-based diagnostic, prognostic, and predictive tests. However, there are unique challenges to validating IHC assays. The challenges are even more critical when it comes to the optimization of IHC tests for phosphospecific antibodies. It is important to emphasize that posttranslational modifications of proteins form the very basis of cell signaling that regulates various cellular processes. There are hundreds of phosphorylation state specific antibodies (PSSA) that now make the study of protein phosphorylation in situ possible, and are opening many exciting opportunities in investigative and diagnostic pathology.2 However, despite some success, many studies have failed to demonstrate the added value of PSSAs over general antibody IHC. Moreover, there is still a large degree of uncertainty about the interpretation of complex and heterogeneous staining patterns in tissue samples and their relationship to the actual phosphorylation states in vivo.3
Human DNA-topoisomerase I (topoI) is an essential and ubiquitous enzyme that is involved in the release of torsional forces generated during DNA transcription and replication.4,5 The identification of topoI as the target of the anticancer drug camptothecin and its analogues (CPT) led to the elucidation of topoI structure and function in the context of cancer therapy.6,7 Two CPT analogues, topotecan and irinotecan, are in clinical use as first-line therapy for metastatic colon cancer (CRC) and as second- or third-line therapy in several other solid tumors, including small cell lung cancer (SCLC), ovarian, pancreatic, gastric, and non-small cell lung cancer (NSCLC). However, only 13–32% of patients respond to these drugs, and the mechanism of resistance is not understood.8 One of the most remarkable cellular phenomena observed in response to CPT is the ubiquitin proteasomal pathway (UPP)-mediated degradation of topoI. Importantly, cells that degrade topoI rapidly are resistant to CPT.9 Although the mechanism of UPP-mediated topoI degradation was not understood, our work has elucidated the molecular mechanism of topoI degradation by UPP. We have demonstrated that DNA-PKcs-dependent higher basal level of topoI serine 10 phosphorylation (topoI-pS10) ensures rapid degradation of topoI and CPT resistance.10 Based on this understanding, we have developed an IHC-based test that will identify the patients who will likely respond to CPT-based therapy.
Here, we demonstrate how we generated topoI-pS10 antibody, determined the critically needed specificity, developed standard operating procedure (SOP) for a semi-automated IHC platform, and transferred the protocol to a fully automated IHC platform. We have also optimized the digital pathology quantitative analysis of topoI-pS10 level in immunohistochemically stained tumor tissue.
Materials and Methods
Developing TopoI-pS10 Mouse Monoclonal Antibody (MAb)
Immunization
In total, 4 Balb/c mice were immunized with a synthetic 14 amino acid peptide containing phosphorylated serine 10 of topoI (p-topoI peptide). TopoI peptide was separately conjugated to keyhole limpet hemocyanin (KLH) and BSA carrier proteins. KLH-peptide was used for immunizations and to exclude anti-KLH antibodies; BSA-peptide was used during antibody screening. Primary immunization was performed with 100 µg of peptide mixed with Freund’s Complete Adjuvant, and then mice were boosted 2 more times, every 2 weeks, with 50 μg of peptide mixed with Freund’s Incomplete Adjuvant. A total of 10 days after the third injection, serum samples were collected to determine the immune response. Anti-sera were tested on p-topoI peptide and counter-screened on non-phosphorylated topoI (np-topoI) peptide by indirect ELISA. After resting for 3 weeks, mice with the best immune responses were boosted with p-topoI peptide intravenously without any adjuvant, and spleens were harvested 4 days after the final boost.
Fusion and Subcloning
Two polyethylene glycol (PEG)-assisted fusions were performed with spleens from mice #3 (M#3) and #4 (M#4) separately. Briefly, single cell suspensions were prepared from whole spleens and then mixed with mouse myeloma cell line, SP2/0, at 2:1 ratio. After performing PEG-assisted fusions, cells were seeded in 96-well plates in hypoxanthine-aminopterin-thymidine (HAT) selection medium. After 14 days, hybridoma supernatants were screened on p-topoI-coated 96-well microtiter plates by indirect ELISA. Briefly, BSA-conjugated p-topoI peptide was immobilized on the microtiter ELISA plates by incubating them with the BSA-peptide in PBS overnight at 4C. Test samples (anti-sera from immunized mice, culture supernatants from hybridomas, or purified antibodies) were added to the plates, and then the assay was developed using goat anti-mouse secondary antibody conjugated to alkaline phosphatase. A total of 45 parental hybridomas were scaled up to 24-well plates, and then retested on p-topoI, np-topoI, and irrelevant phosphopeptide to determine their specificity. A total of 4 parental clones (3 from M#3 and 1 from M#4) were selected for subcloning. Subcloning was performed by limiting dilutions. Out of 4 selected parental clones, 3 were subcloned to stability. Subclone screening was performed by indirect ELISA on p-topoI peptide, as mentioned earlier. The resulting positive clones were expanded and subcloned further to stability as required. Supernatants from all stable clones were tested for IHC.
Early Screen of Hybridoma Clones for IHC Specificity
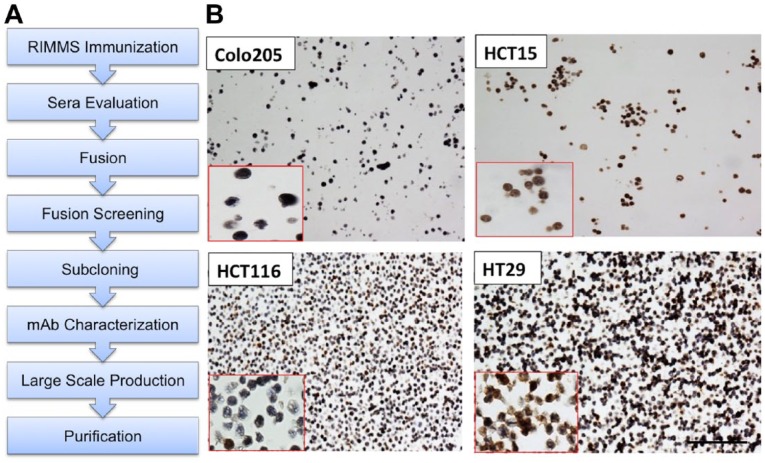
TopoI is degraded rapidly in HCT15 cells while in Colo205, no topoI degradation is observed in response to CPT. We have also demonstrated that serine 10 phosphorylation of topoI by DNA-PKcs is critical for topoI ubiquitination and degradation.10 We asked if HCT15 cells have a higher topoI-pS10 level compared with Colo205. Hybridoma culture supernatants from 9 stable clones were used to determine the possible differential immunostaining of these two colon cancer cell lines. A total of 34 formalin-fixed paraffin embedded (FFPE) slides of Colo205 and HCT15 were immunostained with the culture supernatants. Based on the results of both ELISA and IHC data, 357.3.1C1.H5.H7 clone was selected for further investigation and hereafter will be referred to as 1C1.H5.H7.
Antibody Production and Purification
Anti-topoI-pS10 mAb was purified from 1C1.H5.H7 hybridoma culture supernatant. Briefly, 1C1.H5.H7 hybridoma was cultured in DMEM medium (Gibco), to 1 L, and it was exhausted for 7 days to let the antibody accumulate in the supernatant. Culture was then harvested, and supernatant was clarified by passing it through a 0.45 µm filter. The clarified culture supernatant was purified by affinity chromatography. To further characterize the antibody, sequencing of the variable regions of the light and heavy chains was performed (Gene Script; Piscataway, NJ). Immunizations and hybridoma development including fusions, subcloning, antibody production, and purification were performed at the Monoclonal Antibody Core (MAC) facility of Dana-Farber Cancer Institute, Boston, MA.
IHC Assay Development
An indirect IHC assay using Intellipath auto-stainer (Biocare Medical; Pacheco, CA) was developed for immunostaining tissue with anti-topoI-pS10. All the reagents used to develop this assay were also procured from Biocare Medical. Antigen retrieval (AR) was performed at 85C for 30 min and at 75C for 10 min in acidic pH citrate buffer (pH 6.0). The tissue was washed, and peroxidase blocking was performed for 10 min followed by universal blocking (Biocare Medical sniper block reagent) for 30 min. It was then incubated with anti-topoI-pS10 for 2 hr at room temperature and mouse secondary antibody for 15 min at room temperature. Tissue was then washed with a universal horseradish peroxidase (HRP) reagent, and incubated with diaminobenzidine tetrahydrochloride (DAB) chromagen reagents for 5 min. Immunostained slides were counterstained with hematoxylin, dehydrated, and mounted with permanent mounting media (Vector Labs; Burlingame, CA). Details of reagents used are provided in Table 1.
Table 1.
Details of Reagents Used for TopoI-pS10 IHC.
| Component | Vendor Reference | Part Number | Description |
|---|---|---|---|
| Peroxidazed 1 | Biocare Medical | PX968 H, M, MM | Buffered hydrogen peroxide, plus stabilizer |
| Background Sniper | Biocare Medical | BS966 H, JJ, L, M, MM | Purified casein combination of proteins in modified PBS with preservative and surfactant |
| MACH 4 Universal HRP-Polymer | Biocare Medical | M4U534 G | Biotin-free Detection |
| DAB Chromagen Kit | Biocare Medical | IPK 5010 G80 | intelliPATH FLX DAN Chromagen (IPC5008G3) intelliPATH FLX DAB Buffet (IPBF5009G20) 4 × 20 mL |
| Target Retrieval Solution Low pH | Dako North America Inc. | S2369 | Citric acid, pH 6.0 |
| Antibody Diluent | Pierce (Thermo Fisher) | 37571 | Pierce Protein-free T20 (TBS) blocking buffer-proprietary formulation in TBS with surfactant and stabilizing agent |
| Hemotoxylin | Biocare Medical | CATHE | Counterstain |
Abbreviations: topoI-pS10, topoisomerase I phosphorylates at serine 10; DAB, diaminobenzidine tetrahydrochloride; HRP, horseradish peroxidase.
Optimization of AR
Heat-induced epitope retrieval (HIER) in the Decloaking chamber was performed in a citrate buffer, pH 6.0 either from Biocare or DAKO (Agilent Dako; Santa Clara, CA). AR temperature was optimized using topoI-pS10 positive serial section at different temperatures, ranging from 75C to 110C. The AR time was fixed for 30 min.
Optimal Antibody Titer
To determine the optimal antibody concentration for topoI-pS10 immunohistochemical assay, a serial dilution of antibody (40 μg/ml to 1 μg/ml) was used in both positively and negatively staining tissues. Species-specific immunoglobulin isotype-matched control (IgG1 Kappa; BD Biosciences) was used in place of the primary antibody to evaluate the presence of unintended, nonspecific immunoreactivity. The antibody was diluted with protein-free diluent (Fisher Scientific; Waltham, MA). Experiments were reproduced 3 times with 6 slides each.
Assay Validation
Our IHC assay validation was performed in 3 phases. In the first phase, FFPE slides of cell pellets were used to develop the IHC-specific antibody. FFPE slides from two colorectal cancer cell lines, Colo205 and HCT15, were used to screen hybridoma clones. A total of 34 slides were immunostained using hybridoma culture supernatant to screen specific clones. Several cell lines (17 cell lines from CRC, SCLC, triple-negative breast cancer [TNBC], gastric, prostate, and renal cancer) were also immunohistochemically tested to determine topI-pS10 level. A total of 250 cell pellet slides were immunohistochemically stained with anti-topoI-pS10. In the second phase, 3 tumor microarray slides, representing colorectal cancer (Cat# BC05011), ovarian cancer (Cat#BC11109), and breast cancer (Cat# BC08014), were immunostained. The tissue microarrays (TMA) were procured from US Biomax (Rockville, MD). In the third phase, FFPE slides from CRC, breast, and gastric cancer patients were screened to identify topoI-pS10 positive and negative tissue. Once identified, slides from the FFPE tissue blocks were immunostained for validation studies and SOP development. A total of 200 human tumor tissue slides were used for this optimization phase of our assay.
Reproducibility
Inter-run, intra-run, inter-operator, intra-operator, and inter-instrument analyses were performed to determine the repeatability, reproducibility, and robustness of topoI-pS10 IHC staining. The staining was performed at 2 different IHC automated platforms, Intellipath from Biocare Medical (at Boston University Medical School; Department of Medicine Core facility), and Benchmark Ultra IHC system from Ventana (at StrataDx; Lexington, MA). On the Biocare Medical, Intellipath IHC platform, over a period of 6 years, 3 technicians used our antibody to immunostain cell pellets and tumor tissues.
Specificity
To determine the specificity, 3 sets of immunostaining experiments were performed: immunostaining with isotype-specific IgG, absorption assays, and phosphatase treatment of the tissue. Our sequencing data have shown that anti-topoI-pS10 is IgG1K. We used isotype-specific IgG1K immunoglobulin to determine the specificity. A total of 12 slides were immunostained with an isotype-specific antibody. Absorption IHC assays with phosphopeptide and non-phosphopeptide were performed in the presence of anti-topoI-pS10. This assay was repeated 3 times using serial sections of colon and gastric cancer tissues. Different concentrations of peptides were used, and a total of 12 slides were immunostained to determine the epitope specificity. In a series of experiments, serial sections of topoI-pS10 positive tumor tissue were treated with calf intestinal phosphatase (CIP) before primary antibody incubation. The experiments were repeated 3 times using 2 slides in each experiment.
Quantitative Analysis
TopoI-pS10 positive and negative slides were immunostained with anti-topoI-pS10 and quantitatively analyzed by Aperio AT2 high-resolution digital pathology scanner. Percent positive nuclei and score of staining intensity were determined by Aperio Image scope software package using V9 nuclear algorithm.
Human Tumor Tissue Procurement
We received tumor tissues from university biobanks and also from commercial biobanks. Under scientific collaboration and Material Transfer Agreement (MTA), we received tissue from University of Massachusetts, Worcester, MA; Kyushu University, Japan; Shizuoka Cancer Center, Japan; and Boston University Medical School. The tissue was collected retrospectively under approved Institutional Review Board (IRB) protocol (Boston University IRB study number: H-32486). We also purchased tissue from commercial sources, US Biomax (Rockville, MD), iSpecimen (Lexington, MA), Sapien Biosciences (Hyderabad, India), and Indivumed (Lewisburg, PA).
Results
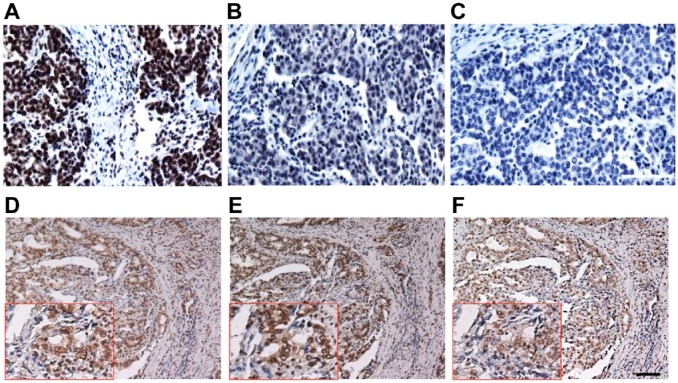
TopoI-pS10 specific antibody development, large-scale antibody production, and purification: We have successfully developed a mouse monoclonal phosphospecific antibody against topoI-pS10 at the MAC facility, Dana-Farber Cancer Institute, Boston (Fig. 1A). Characterization of clones by IHC assay was performed at the IHC core facility, Boston University School of Medicine. Based on our previous findings, we selected two colon cancer cell lines HCT15 (CPT resistant) and Colo205 (CPT sensitive) to determine (1) if there is any differential staining and (2) if this can be used to identify a hybridoma clone that specifically immunostains topoI-pS10. A comparative study on CPT response in 7 CRC cells has shown a strong differential response. KM12 and HCT15 are most resistant while Colo205 and HCT116 are most sensitive, and HT29 occupies the middle of the resistance spectrum.11 We immunostained FFPE slides of Colo205, HCT15, HCT116, and HT20 with clone anti-topoI-pS10. The results demonstrate that the highest percentage of topoI-pS10 positive nuclei were HCT 15 cells while in Colo205, very few cells showed nuclear immunostaining. HCT116 has approximately 20% positive nuclei while HT29 has more than 50% positive nuclei (Fig. 1B).
- Developing SOP for IHC assay to specifically immunostain phosphorylated topoI-pS10: FFPE slides from colorectal, gastric, and breast cancer patients were immunostained to identify topoI-pS10 positive and negative tissue. Once identified, 100 positive and 50 negative slides were acquired to develop SOP for immunostaining.
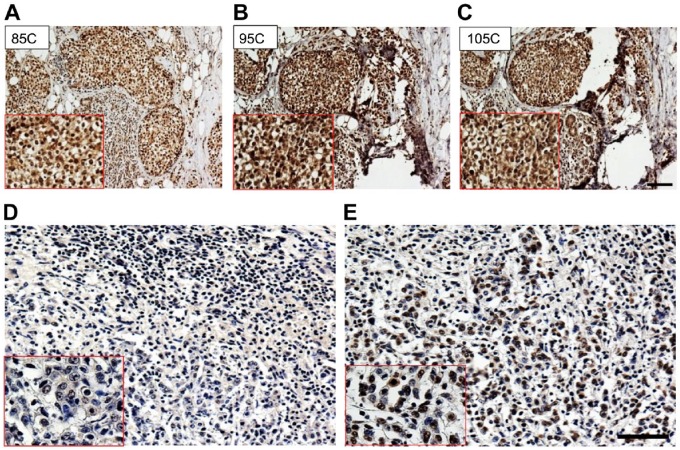
- AR optimization: The assay was reproduced 3 times, and a total of 14 colon and 7 gastric cancer tissues were immunostained with anti-topoI-pS10. In every run, 6 topoI-pS10 positive and 1 negative tissue were immunostained. TopoI-pS10 staining was positive at both a lower temperature of 75C and a higher temperature of 110C (Fig. 2A, B, and C). However, the strongest DAB positive nuclei were observed when AR was performed between 95C to 105C. Because we observed positive staining in a wide range of temperature, we asked if HIER is required to immunohistochemically stain topoI-pS10. The slides subjected to high temperature AR showed topoI-pS10 positive nuclei (Fig. 2E). In contrast, same tissue without AR showed no immunostaining (Fig. 2D).
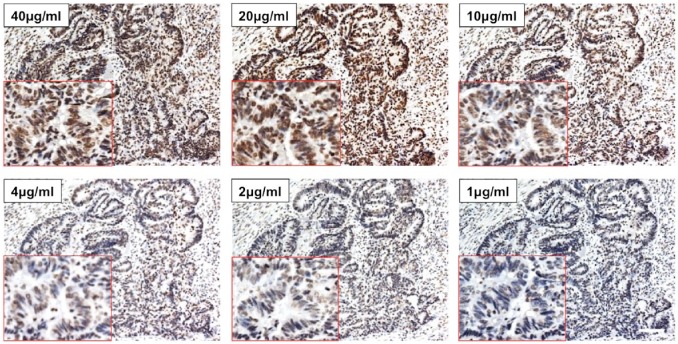
- Antibody titration for specific topoI-pS10 immunostaining: Serial section from topoI-pS10 positive slides were immunostained, representative images clearly demonstrated a very strong staining at 40, 20, and 10 μg/ml antibody concentration. However, unspecific immunostaining of non-tumor cells, including connective tissue, suggested that the staining is not specific at higher concentration of 40 and 20 μg/ml concentration (Fig. 3). In contrast, a significant loss of DAB positive nuclear signal was seen at 2 μg/ml and 1 μg/ml. Antibody concentration between 4 μg/ml and 10 μg/ml appears to be specific (Fig. 3).
- Antibody specificity determination.
- Peptide competition assays: 250 nm and 500 nm of phosphopeptide and non-phosphopeptide were incubated with anti-topoI-pS10. While in the presence of 250 nm phosphopeptide, a significant loss of DAB signal was observed (Fig. 5B), and a complete loss of DAB positive nuclei was observed with 500 nm phosphopeptide (Fig. 5C). In contrast, no loss of DAB positive nuclei was observed with either at 250 nm (Fig. 5E) or 500 nm (Fig. 5F) non-phosphopeptide. No peptide was used in control immunostaining (Fig. 5D).
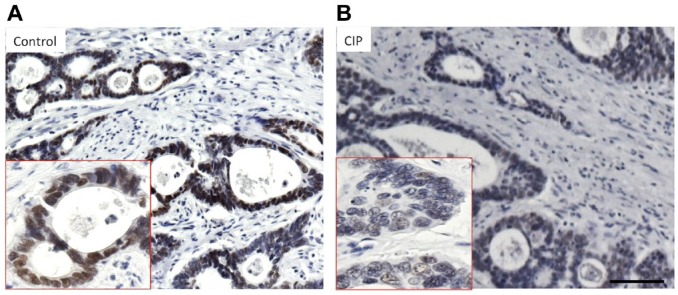
- Phosphatase treatment: Post-antigen retrieved tissues were treated with 1000 U of CIP for 60 min in a phosphatase buffer. The enzyme was washed, and the tissues were incubated with anti-topoI-pS10. The CIP treatment clearly reduced the nuclear DAB signal (Fig. 6).
Cross-platform validation: Biocare Medical and Ventana systems showed similar staining patterns of topoI-pS10 (Fig. 7A and B).
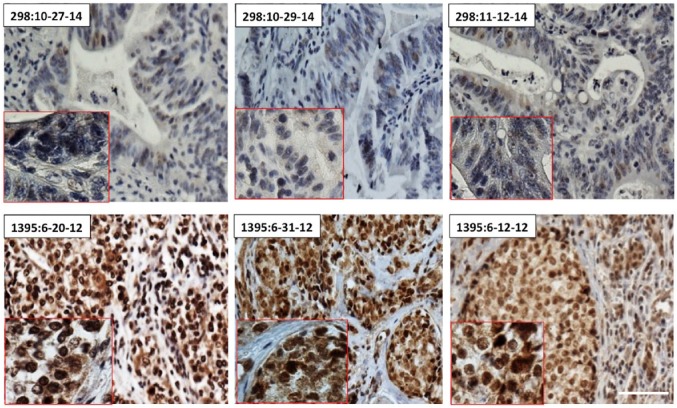
Reproducibility and repeatability: Inter-operator and intra-operator; inter-day and intra-day IHC of both topoI-pS10 positive and negative tissue showed similar results (Fig. 8).
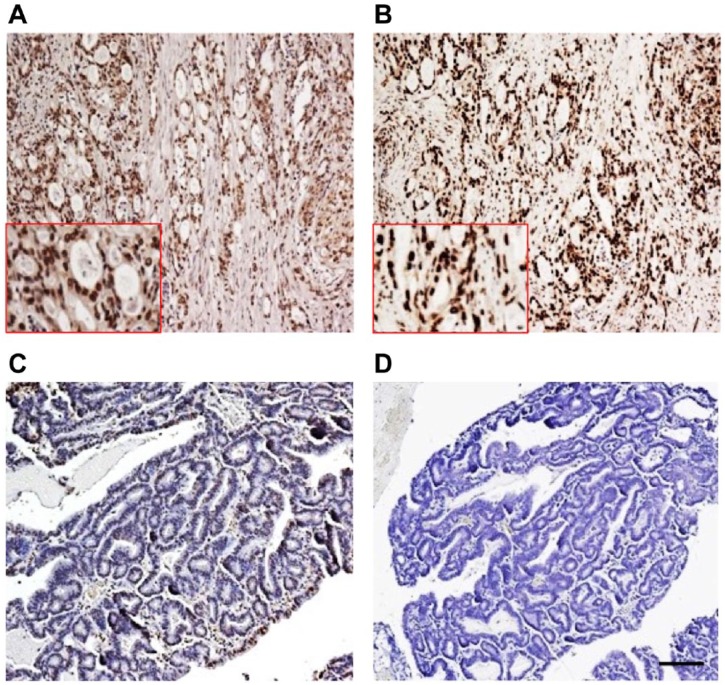
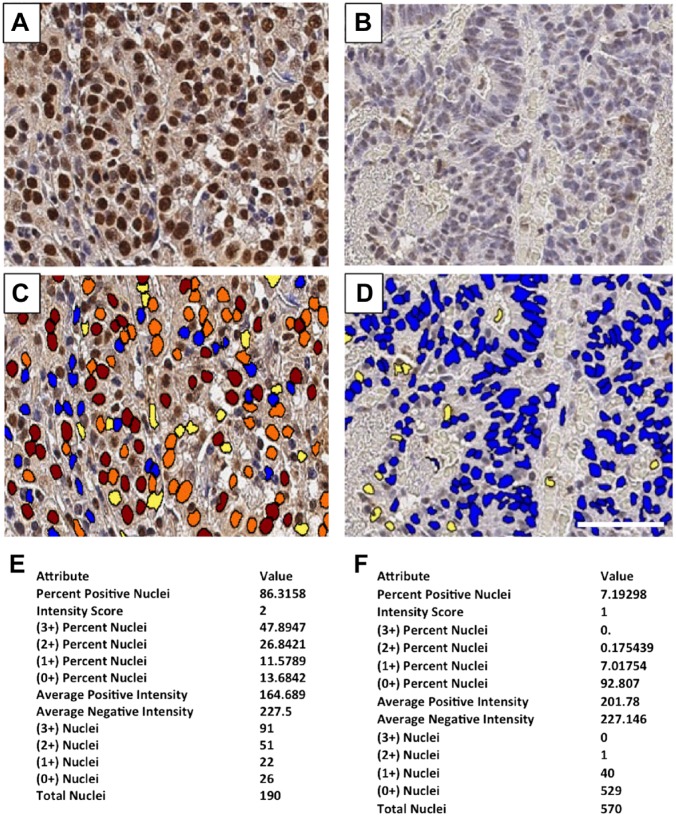
TopoI-pS10 quantitative analysis: FFPE slide from CPT non-responder gastric cancer patient (Fig. 9A) and CPT responder colon cancer patient tissue (Fig. 9B) were immunostained with anti-topoI-pS10 and quantitatively analyzed. DAB positive nuclei with highest, +3 intensity were coded red, +2 intensity with orange, +1 intensity with yellow, and +0 intensity with blue (Fig. 9C and D). Percent positive nuclei were also determined. In total, 86.3% positive nuclei with intensity score of 2 were observed in irinotecan non-responder patient (Fig. 9E). In contrast, only 7.9% positive nuclei with intensity score of 1 were observed in the responder patient tumor tissue (Fig. 9F).
Figure 1.
Developing monoclonal antibody against topoI-pS10: (A) Schematics representing various steps to develop mouse monoclonal antibody specific to phosphorylated topoI-S10. (B) FFPE slides of colon cancer cell lines were immunostained with hybridoma cell culture media to identify the antigen-specific clones. Scale bar = 100 µm. Abbreviations: topoI-pS10, topoisomerase I phosphorylates at serine 10; MAb, monoclonal antibody; RIMMS, Repetitive Immunizations Multiple Sites; FFPE, formalin-fixed paraffin embedded.
Figure 2.
AR optimization. (A, B, and C): Serial sections of breast cancer FFPE slides were subjected to AR at different temperatures for immunostaining with anti-topoI-pS10. (D) CRC FFPE slides were also subjected to immunostaining with anti-topoI-pS10 without heat-induced AR. (E) The same tissue was immunostained with anti-topoI-pS10 after heat-induced AR at 100C. The red box is an enlarged image of the positive area. Scale bar = 100 µm. Abbreviations: AR, antigen retrieval; FFPE, formalin-fixed paraffin embedded; topoI-pS10, topoisomerase I phosphorylates at serine 10; CRC, colon cancer.
Figure 3.
Antibody titration to determine optimal antibody concentration for topoI-pS10 IHC. Serial sections of gastric cancer FFPE slides were immunostained with different concentrations of anti-topoI-pS10 ranging from 40 μg/ml to 1 μg/ml. The red box indicates the enlarged image of positive area. Scale bar = 100 µm. Abbreviations: topoI-pS10, topoisomerase I phosphorylates at serine 10; FFPE, formalin-fixed paraffin embedded.
Figure 4.
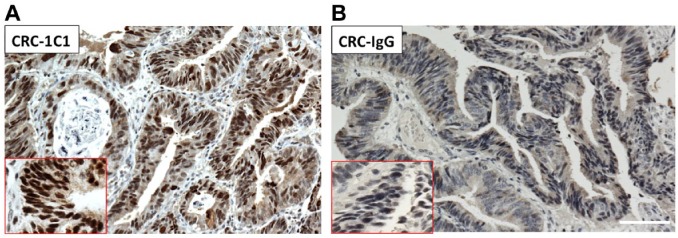
Anti-topoI-pS10 specifically immunohistochemically stains phosphorylated topoI: Serial sections of colon cancer FFPE slides were immunohistochemically stained with anti-topoI-pS10 [clone 1C1.H5.H7]. (A) Slides were also immunostained with mIgG1K. (B) The red box indicates the enlarged image of positive area. Scale bar = 100 µm. Abbreviations: topoI-pS10, topoisomerase I phosphorylates at serine 10; FFPE, formalin-fixed paraffin embedded.
Figure 5.
Antibody specificity determination by peptide competition: (A) Serial sections of CRC-FFPE slides were immunostained with anti-topoI-pS10; (B and C) Slides from the tissue were immunostained with anti-topoI-pS10 in the presence of a phosphopeptide against which the antibody was raised. Two different concentrations: 250 and 500 nm phosphopeptide (Fig. B and C, respectively) were used for this absorption assay. (D) Serial section of gastric cancer FFPE slides were immunostained with anti-topoI-pS10. (E and F) Tissues were also immunostained with anti-topoI-pS10 in the presence of topoI-non-phosphopeptide peptide. Two different concentrations: 250 and 500 nm (Fig. E and F, respectively) were used for the IHC absorption assay. Scale bar = 100 µm. Abbreviations: topoI-pS10, topoisomerase I phosphorylates at serine 10; CRC, colon cancer; FFPE, formalin-fixed paraffin embedded.
Figure 6.
Antibody epitope specificity determination after CIP treatment. (A) Serial sections of gastric cancer FFPE tissue were immunostained with anti-topoI-pS10. (B) After AR, tissues were subjected to CIP treatment for 1 hr at 37C and immunostaining was then performed with anti-topoI-pS10. Scale bar = 100 µm. Abbreviations: CIP, calf intestinal phosphatase; FFPE, formalin-fixed paraffin embedded; AR, antigen retrieval; topoI-pS10, topoisomerase I phosphorylates at serine 10.
Figure 7.
Reproducibility of topoI-pS10 immunohistochemical staining: Serial sections of topoI-pS10 negative and positive immunostaining FFPE slides were immunostained on Biocare Medical instrument (A and B) and Ventana, Benchmark Ultra automated IHC system (C and D). Scale bar = 100 µm. Abbreviations: topoI-pS10, topoisomerase I phosphorylates at serine 10; FFPE, formalin-fixed paraffin embedded.
Figure 8.
Reproducibility and repeatability of topoI-pS10 immunohistochemical staining assay: FFPE serial sections from topoI-pS10 negative CRC tissue (tissue ID 298) and topoI-pS10 positive breast cancer (tissue ID 1395) were immunostained on different dates by 2 different operators using the SOP on Intellipath IHC platform. Scale bar = 100 µm. Abbreviations: topoI-pS10, topoisomerase I phosphorylates at serine 10; CRC, colon cancer; SOP, standard operating procedure.
Figure 9.
Quantitative analysis of topoI-pS10 level in human tumor tissue: FFPE tissue from gastric cancer non-responder (Fig. A and C) and responder (B and D) were immunostained with anti-topoI-pS10. The slides were analyzed by high-resolution whole slides Aperio AT2 scanner, and Aperio Image Scope using V9 nuclear algorithm. The quantitative results are shown as output of topoI-pS10 positive slides (Fig. E) and negative slide (Fig. F). Scale bar = 100 µm. Abbreviations: topoI-pS10, topoisomerase I phosphorylates at serine 10; FFPE, formalin-fixed paraffin embedded.
Discussion
The immunohistochemical detection and quantification of phosphoprotein levels can provide valuable information regarding activation of cell signaling pathways within tumor samples, with potential implications for prognosis and treatment selection. Phosphorylation-dependent activation of a growth pathway leads to tumor pathogenesis and progression, such as overexpression and activation of EGFR in NSCLC. However, IHC analysis indicated that neither total EGFR nor phosphospecific EGFR had any prognostics information.12 Similarly, phosphospecific human epidermal growth factor receptor 2 (HER2), which indicates pathway activation, had shown no added information compared with HER2 level determination by IHC.13 However, higher phospho AKT (Ser473) is a strong negative prognostic factor in oropharyngeal squamous cancer.14 Similarly, quantitative analysis of c-Met IHC data have shown some clinical correlation with patient outcomes.15 These findings clearly demonstrate the potential of IHC assays to be developed as prognostic or predictive biomarkers. There are three critical parameters: antibody validation, epitope-specific lability, and implementation of methods of standardization, to use the PSSAs in translational research applications.3
The five suggested elements for antibody validation include validation by Western blotting; discussion on nature of IHC positive and negative control; pre-immunized serum or isotype-specific immunoglobulin immunostaining; use of genetically engineered controls, and absorption controls.16 Our antibody was raised using a 14 amino acid peptide representing topoI amino terminus with phosphorylated serine 10. Blast analysis of the peptide indicated a highly conserved topoI sequence across phyla from bacteria to human. Importantly, the peptide also contained an SQ motif, a known conserved sequence in DNA-PKcs substrates. However, the remainder of the peptide sequence is unique and bears no significant homology with other proteins. Antibody specificity was also determined by ELISA assays, and in the third phase, a selected number of culture supernatants from different clones during antibody development were characterized by immunohistochemical staining. And, based on the specificity in IHC assays, clone 1C1.H5.H7 was selected. The Western blot validation studies indicated lack of specificity of our antibody in immunoblot analysis.
The second element, discussion on positive and negative control selection, remained a strong point of our IHC assays development. Cellular resistance to CPT is not understood. Three proposed mechanisms of CPT resistance are (1) lack of drug accumulation due to efflux by the multidrug resistant (MDR)/ABC transporter, (2) topoI gene mutations, and (3) UPP-mediated degradation of topoI in response to CPT. However, recent studies have shown that CPTs are not MDR substrates, and topoI mutations are rare and do not account for drug resistance in the patient population, leaving the degradation of topoI as a major potential mechanism of drug resistance. We have demonstrated that the deregulated kinase cascade is at the core of CPT resistance. DNA-PKcs, a kinase central to the DNA-DSB response pathway, phosphorylates topoI at serine 10, and cells with higher basal levels of topoI-pS10 degrade topoI rapidly and are resistant to this class of drug. The higher basal level of topoI phosphorylation is due to continual activation of DNA-PKcs.10 These findings led us to develop positive and negative controls; first in FFPE slides of cell palette from CPT-resistant and sensitive colon cancer cell lines and then from various tumor tissue including colon, breast, gastric, SCLC, and renal cancer. Importantly, the cells that are most resistant to CPT have almost 100% cells that have DAB-positive nuclei, indicating a higher basal level of topoI-pS10. Cells that are sensitive have minimal or no DAB-positive nuclei. Interestingly, our finding indicates a potential correlation between the CPT IC50 and percentage of DAB-positive nuclei in CRC cell lines.
The third element, isotype-specific immunostaining using IgG1K in topoI-pS10 positive tissue or cells, resulted in negative immunostaining. FFPE slides of cell lines and tissues that are positive to topoI-pS10 were negative, indicating that our assay is specific. Hewitt et al. have also suggested genetically engineered controls, including overexpression or silencing of the gene as the fourth element.16 It is important to mention that topoI is an essential gene and is very tightly regulated in cells. Either overexpressing or silencing the topoI gene results in cell death. However, our studies with cancer cell lines indicated that the cells that have similar topoI protein levels have significantly different topoI-pS10 levels. In two CRC cells, Colo205 and HCT, topoI protein level is similar, determined by immunoblotting and IHC assay. However, the topoI-pS10 level is significantly higher in HCT15 cells compared with Colo205.10 The fifth element, absorption controls, strongly validates our IHC assay for translational research.
Epitope-specific lability is another critical parameter that determines the translational research applications.3 First, we determined the epitope specificity of our antibody by dephosphorylating a topoI-pS10 positive FFPE slide by treating the tissue with CIP. The data clearly demonstrate a significant loss of DAB intensity in CIP-treated tissue indicating antibody epitope specificity. In addition, we performed IHC assays on an HCT15 cell pellet after changing the pre-analytical variants, including incubating the cell pellet on ice or room temperature for 1 hr before fixation. The results indicated that there was no change in level of topoI-pS10. This finding is in concert with our work demonstrating an activated kinase cascade with higher topoI-pS10 in CPT-resistant cells. The method of standardization of the P-TopoIDx IHC assay has been optimized in two different IHC platforms, and SOP is validated. The first phase of this method of standardization is robust and reproducible and when applied to additional patient tissues, the test may have predictive value for topoI inhibitors.
Acknowledgments
We thank the members of monoclonal antibody core and molecular biology core facility of Dana-Farber Cancer Institute, Boston; and IHC core facility of Department of Medicine, Boston University Medical School, Boston.
Footnotes
Competing Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AKB has financial interest in Predictus Biosciences Inc. AKB’s interests were reviewed by Boston University Medical School in accordance with conflict of interest policies.
Author Contributions: All authors have contributed to this article as follows: KA, AKS, YHT, and AKB conceived the project. KA, YHT, and AKB designed most experiments. KA, AKS, JT-P, YHT, MB, AS, and YH performed experiments. KA and AKB helped with data analysis. EO helped with discussion. KA, MB, and AKB wrote the manuscript. AKB supervised the project, and all authors have read and approved the manuscript as submitted.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: AKB was supported by Boston University Medical School, Department of Medicine Pilot grant, and launch award from Boston University Technology Development. KA was supported by the Uehara Memorial Foundation Research Fellowship (Japan).
Contributor Information
Koji Ando, Division of Hematology Oncology, Department of Medicine, Boston University School of Medicine, Boston, Massachusetts; Department of Surgery and Science, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan.
Yara Hamade Tohme, Division of Hematology Oncology, Department of Medicine, Boston University School of Medicine, Boston, Massachusetts.
Adithi Srinivasiah, Division of Hematology Oncology, Department of Medicine, Boston University School of Medicine, Boston, Massachusetts.
Julian Taylor-Parker, Division of Hematology Oncology, Department of Medicine, Boston University School of Medicine, Boston, Massachusetts.
Yevgeniya Harrington, Division of Hematology Oncology, Department of Medicine, Boston University School of Medicine, Boston, Massachusetts.
Ankur K. Shah, Division of Hematology Oncology, Department of Medicine, Boston University School of Medicine, Boston, Massachusetts
Eiji Oki, Department of Surgery and Science, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan.
Mohan Brahmandam, Monoclonal Antibody Core Facility, Dana-Farber Cancer Institute, Boston, Massachusetts.
Ajit K. Bharti, Division of Hematology Oncology, Department of Medicine, Boston University School of Medicine, Boston, Massachusetts.
Literature Cited
- 1. Hardy LB, Fitzgibbons PL, Goldsmith JD, Eisen RN, Beasley MB, Souers RJ, Nakhleh RE. Immunohistochemistry validation procedures and practices: a College of American Pathologists survey of 727 laboratories. Arch Pathol Lab Med. 2013;137(1):19–25. doi: 10.5858/arpa.2011-0676-CP. [DOI] [PubMed] [Google Scholar]
- 2. Mandell JW. Phosphorylation state-specific antibodies: applications in investigative and diagnostic pathology. Am J Pathol. 2003;163(5):1687–79. doi: 10.1016/S0002-9440(10)63525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mandell JW. Immunohistochemical assessment of protein phosphorylation state: the dream and the reality. Histochem Cell Biol. 2008;130(3):465–71. doi: 10.1007/s00418-008-0474-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu LF, Desai SD, Li TK, Mao Y, Sun M, Sim SP. Mechanism of action of camptothecin. Ann N Y Acad Sci. 2000;922:1–10. [DOI] [PubMed] [Google Scholar]
- 5. Wang JC. Cellular roles of DNA topoisomerases: a molecular perspective. Nat Rev Mol Cell Biol. 2002;3(6):430–40. doi: 10.1038/nrm831. [DOI] [PubMed] [Google Scholar]
- 6. Hsiang YH, Liu LF. Identification of mammalian DNA topoisomerase I as an intracellular target of the anticancer drug camptothecin. Cancer Res. 1988;48(7):1722–6. [PubMed] [Google Scholar]
- 7. Pommier Y. DNA topoisomerase I inhibitors: chemistry, biology, and interfacial inhibition. Chem Rev. 2009;109(7):2894–902. doi: 10.1021/cr900097c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ewesuedo RB, Ratain MJ. Topoisomerase I inhibitors. Oncologist. 1997;2(6):359–64. [PubMed] [Google Scholar]
- 9. Desai SD, Li TK, Rodriguez-Bauman A, Rubin EH, Liu LF. Ubiquitin/26S proteasome-mediated degradation of topoisomerase I as a resistance mechanism to camptothecin in tumor cells. Cancer Res. 2001;61(15):5926–63. [PubMed] [Google Scholar]
- 10. Ando K, Shah AK, Sachdev V, Kleinstiver BP, Taylor-Parker J, Welch MM, Hu Y, Salgia R, White FM, Parvin JD, Ozonoff A, Rameh LE, Joung JK, Bharti AK. Camptothecin resistance is determined by the regulation of topoisomerase I degradation mediated by ubiquitin proteasome pathway. Oncotarget. 2017;8(27):43733–51. doi: 10.18632/oncotarget.16376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goldwasser F, Bae I, Valenti M, Torres K, Pommier Y. Topoisomerase I-related parameters and camptothecin activity in the colon carcinoma cell lines from the National Cancer Institute anticancer screen. Cancer Res. 1995;55(10):2116–62. [PubMed] [Google Scholar]
- 12. Cortas T, Eisenberg R, Fu P, Kern J, Patrick L, Dowlati A. Activation state EGFR and STAT-3 as prognostic markers in resected non-small cell lung cancer. Lung Cancer. 2007;55(3):349–55. doi: 10.1016/j.lungcan.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 13. DiGiovanna MP, Stern DF, Edgerton S, Broadwater G, Dressler LG, Budman DR, Henderson IC, Norton L, Liu ET, Muss HB, Berry DA, Hayes DF, Thor AD. Influence of activation state of ErbB-2 (HER-2) on response to adjuvant cyclophosphamide, doxorubicin, and fluorouracil for stage II, node-positive breast cancer: study 8541 from the Cancer and Leukemia Group B. J Clin Oncol. 2008;26(14):2364–47. doi: 10.1200/JCO.2007.13.6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yu Z, Weinberger PM, Sasaki C, Egleston BL, Speier WF, 4th, Haffty B, Kowalski D, Camp R, Rimm D, Vairaktaris E, Burtness B, Psyrri A. Phosphorylation of Akt (Ser473) predicts poor clinical outcome in oropharyngeal squamous cell cancer. Cancer Epidemiol Biomarkers Prev. 2007;16(3):553–8. doi: 10.1158/1055-9965.EPI-06-0121. [DOI] [PubMed] [Google Scholar]
- 15. Pozner-Moulis S, Cregger M, Camp RL, Rimm DL. Antibody validation by quantitative analysis of protein expression using expression of Met in breast cancer as a model. Lab Invest. 2007;87(3):251–60. doi: 10.1038/labinvest.3700515. [DOI] [PubMed] [Google Scholar]
- 16. Hewitt SM, Baskin DG, Frevert CW, Stahl WL, Rosa-Molinar E. Controls for immunohistochemistry: the Histochemical Society’s standards of practice for validation of immunohistochemical assays. J Histochem Cytochem. 2014;62(10):693–7. doi: 10.1369/0022155414545224. [DOI] [PMC free article] [PubMed] [Google Scholar]