Abstract
Micelles as colloidal suspension have attracted considerable attention due to their potential use for both cancer diagnosis and therapy. These structures have proven their ability to deliver poorly water-soluble anticancer drugs, improve drug stability, and have good penetration and site-specificity, leading to enhance therapeutic efficacy. Micelles are composed of hydrophobic and hydrophilic components assembled into nanosized spherical, ellipsoid, cylindrical, or unilamellar structures. For their simple formation, they are widely studied, either by using opposite polymers attachment consisting of two or more block copolymers, or by using fatty acid molecules that can modify themselves in a rounded shape. Recently, hybrid and responsive stimuli nanomicelles are formed either by integration with metal nanoparticles such as silver, gold, iron oxide nanoparticles inside micelles or by a combination of lipids and polymers into single composite. Herein, through this special issue, an updated overview of micelles development and their application for cancer therapy will be discussed.
Keywords: micelles, hybrid polymeric and stimuli-responsive nanomicelles, cancer therapy
1. Introduction
1.1. Identification of Micelles
Micelles are assembled colloidal dispersions having a small diameter, normally ranging from 5 to 100 nm [1,2], depending on the type of head groups and length of the alkyl chains [3,4]. Their surfactant molecules can be aggregated either by cationic, anionic, zwitterionic or non-ionic groups [5]. In aqueous solution, the non-polar hydrocarbon chain “tail” can be arranged into the center of a ball like structure “head” to form a micelle, because they are hydrophobic or “water hating” (Scheme 1) [6,7]. They can be formed from a fatty acid, a salt of a fatty acid (soap), phospholipids, or other similar molecules. In this case, micelles made with a lipid might have lower Critical Micelle Concentration (CMC) [8]. Hence, in an amphiphilic copolymer, fatty acyl chains play a valuable role as hydrophobic segments. The structure of Distearoylphosphatidyl ethanolamine (DSPE) has been used as the hydrophobic compound in a di-block copolymer with hydrophilic polyethylene oxide (PEO) to form 22 nm micelles [9]. Due to the limitation of these molecules, amphiphilic copolymers have been developed as alternative amphiphilic materials [10].
Scheme 1.
Assembled micelles structure.
In micelle structure formation, the interaction between the polar head groups and surrounding water might cause separation between hydrophobic and hydrophilic regimens. This results in flexible and porous micelles [11]. In this case, micelles are considered a suitable model for biological applications and drug delivery systems [12], because they can increase drug solubility, reduce toxicity, prolong circulation time, enhance tissue penetration, and have targeting ability.
1.2. Micelles Structure
Micelles are mostly composed of amphiphilic molecules in aqueous solution that self-assemble into a structure containing both hydrophobic and a hydrophilic segments (Scheme 2) [13,14,15]. In the case of concentration reduction, the amphiphiles are present as small units (monomers) in true solution, while at a high concentration, aggregation and self-assembly occur, leading to micelles formation [2]. The critical concentration that is needed to form micelles is called Critical Micelle Concentration (CMC). The micelles formed at above their CMC are being driven by dehydration of the hydrophobic tails, forming a state of entropy. Additionally, the micelles’ core will be formed by Van der Waals bonds recognition [2]. At the final structure, the hydrophilic shell forms hydrogen bond crosslink with water surrounding its out-surface [16]. Micelles can be assembled in different morphologies, such as spheres, rods, tubules, lamellae, and vesicles, depending on the quality of solvent, length of blocker chain, nature of the blocker and temperature [17,18,19]. In previous reports, the structure of micelles had been studied using numerous experimental techniques, such as nuclear magnetic resonance (NMR) [1], diffraction of X-ray (XRD) [20], photon correction spectroscopy (PCS) [21], fluorescence spectroscopy [22], electron spin resonance (ESR) [23], neutron scattering [24] and others [25].
Scheme 2.
Polymer micelle structures.
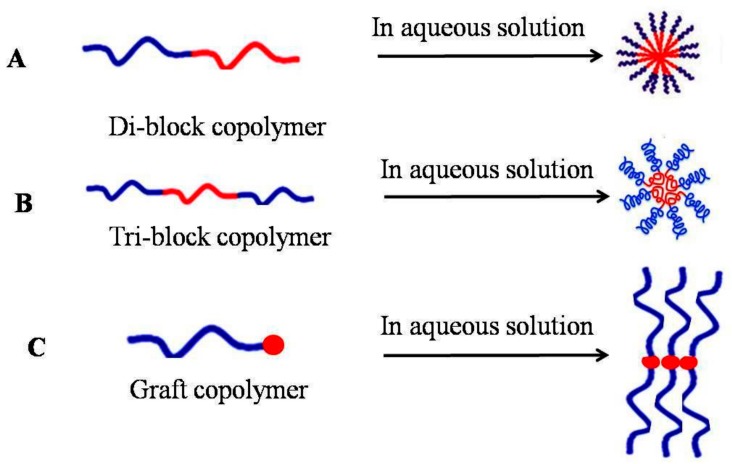
These structures usually observe less CMC compared to low-molecular-weight surfactants. It is found that for the surfactant of low molecular weight, the CMC can be considered as 10−3 to 10−4 M, while it is 10−6 to 10−7 M for polymeric micelles. The stability of micelles’ structure remains valuable at very low polymer concentrations due to the low CMC, which makes them relatively insensitive to dilution, leading to enhance their circulation in the blood stream compared to surfactant micelles [26]. Block copolymers, also known as mosaic copolymer, is a special polymer that linked two or more polymer segments on the main chain directly [27]. Block co-polymers can be classified through the number of blocks and their arrangement [28]. Block copolymers containing two, three and more blockers are called di-blocks (AB type copolymers), tri-blocks (ABA type copolymer), and grafted copolymers respectively. Some topology can be in a linear state, in which the blocks are attached at the both ends, and stars, where blocks are linked across one of their ends at a single connection. Many attachments like brushes, (4-)miktoarm stars, or H-shape are also possible [29]. For instance, di-block co polymer (AB) can be obtained from mono-functional polymers such as photo polymerization of styrene (St) with benzyl N,N-diethyldithiocarbamate (BDC) [30], while triblock co-polymers can be synthesized from di functional polymers (e.g., p-xylylenebis(N,N-diethyldithiocarbamate)) [31]. For instance, poly(ethylene glycol)-block-poly(d,l-lactic acid) (PEG-b-PLLA) micelles have been extensively studied and the in vitro release of the hydrophobic drug quercetin from these micelles was investigated [32].
Grafted polymers are polymers which have branches formed from one hydrophilic backbone and one to multiple hydrophobic polymer side chains, or vice versa [33]. Drugs can be released through cellulose graft polymers. Like this, the cellulose portion can form the hydrophilic part, with any hydrophobic segment conjugated to it, resulting in an amphiphilic graft polymer. Such polymers have biodegradable properties. Hence, prednisone acetate has been delivered by Cellulose-g-poly-l-lactic acid (PLLA). Similarly, camptothecin was also released gradually from graft polymer micelles of pthaloyl chitosan and mPEG-2000 for 96 h. The diameter of amphiphilic block copolymers composites into spherical core-shell micelles reached approximately 10 to 80 nm, consisting of a hydrophobic core for drug loading. Besides, a physical barrier will be formed by a hydrophilic shell at both micelle complication in aqueous solution and to protein attachment and opsonizing state during intravenous administration [34].
Many polymers can be used to fabricate micelles. However, the selection is limited for those polymers having biocompatible and biodegradable properties, and they are either hydrophobic or hydrophilic dissolution. Steric stability of micelles is provided mainly by hydrophilic characterization of the shell, and once properly selected avoids rapid uptake by the reticulo-endothelial system (RES), leading to increase time circulating in the body [26]. Poly(ethylene glycol) (PEG) is hydrophilic polymer, having highly hydrated, an efficient steric protector, and biocompatible, with low toxicity [2,35]. Other hydrophilic polymers also can be used such as poly(N-vinyl pyrrolidone) (PVP) and poly(N-isopropylacrylamide) pNIPAM [36]. While for the hydrophobic core, polyesters, polyethers, and polyaminoacids are commonly used [35,37]. In the case of core-forming structures, many polymers are used, such as poly(propylene oxide) (PPO), poly(d,l-lactic acid) (PDLLA), poly(ε-caprolactone) (PCL), poly(l-aspartate) and poloxamers [38]. Micelles are intrinsically stealth particles when formed with a hydrophilic outer shell, and they are able to avoid engulfment by immune-system cells without further modification. Recently, block copolymers were produced by several techniques with well-defined composition, molecular weight, and structures such as single-electron-transfer living radical polymerization (SET-LRP), fragmentation chain transfer (RAFT), atom transfer radical polymerization (ATRP) and nitroxide-mediated polymerization (NMP) [39,40,41]. These combinations exhibit many positive features, such as the ability to control the composition and molecular weight of the block copolymers prepared through such methodologies.
2. Hybrid Polymeric Micelles
Using polymers for directed assembly with biological molecules such as lipids, proteins, peptides and nucleotides is a very interesting approach, due to the wide range of polymers’ synthesis. Currently, there are various types of polymers available, each one has special properties and responsiveness. The polymers that have charged electrons are very useful because the biological molecules are already charged and their electrostatic interactions are a very convenient approach for assembly. Additionally, this assembly can be characterized according to hydrophobicity of polymers. The assembly of amphiphilic polymers results in formation of Nanomicelles (NMs) with a hydrophobic core and a hydrophilic shell [42]. The core-shell composite of NMs allows them to: (1) encapsulate and carry poorly water-soluble drugs; (2) decrease the bio-fouling of the NMs resulting in long circulation half-life; (3) release drugs at a sustained rate in the optimal range of drug concentration; and (4) be further functionalized with targeting ligands for differential delivery [43]. The attention was taken for micelles formed by conjugation of polyethylene glycol (PEG) and diacyl-lipids [44]. Phospholipid residues attached to PEG moieties represent short, however, extremely hydrophobic blocks due to the presence of two long-chain fatty acyl groups, and effectively form a hydrophobic core of the micelle [45]. Oleic acid (OA), as a mono-unsaturated fatty acid, was attached with chitosan by amide linkage bonds through the EDAC-mediated reaction with various degrees of amino substitution (DS), as described in a previous study [46]. Scientifically, chitosan molecules present no amphiphilic properties and, therefore, cannot form micelles in water. However, modification of chitosan chains was done by oleic acid. Hence in the presence of water-soluble carbodiimide, carboxyl groups of fatty acids were activated and ester intermediates were created (Scheme 3). Consequently, the intermediates can react with primary amine groups of chitosan to create an amide bond. The final product of this assembly is a nano-sized self-aggregation in aqueous media [47]. These rounded shape-like nuclei were furthermore coated by folic acid conjugated with bovine serum albumin (BSA) to target cancer cells and to minimize side effects [48]. Oleic acid modified chitosan caused well dispersion for micelles in aqueous media, raised amide linkage, and formed a denser hydrophobic core [49].
Scheme 3.
Hybrid polymer lipid protein nanocarrier. Step 1: Assembly of chitosan and oleic acid; step 2: Folic acid (FA) conjugation with bovine serum albumin; and step 3: functionalization of chitosan grafted oleic acid surface by using bovine serum albumin (BSA)-FA (Figure adapted with permission from ref. [53]).
The lipid core micelles were first produced by composition of polyethylene glycol-phosphatidylethanolamine (PEG-PE) forming micelles instead of PEGylated liposomes after their concentration exceeded a critical limit [50,51]. It is found that stabilization of the lipid cores can also be measured by their CMC; that is, the concentration at which the copolymer chains start to associate themselves to form micelles [17]. It is observed that many PEG-PE compositions have CMCs in a range of 1025 M, which is at least 100-fold lower than those of conventional detergents [52].
3. Hybrid Nanomicelles with Metal Nanoparticles
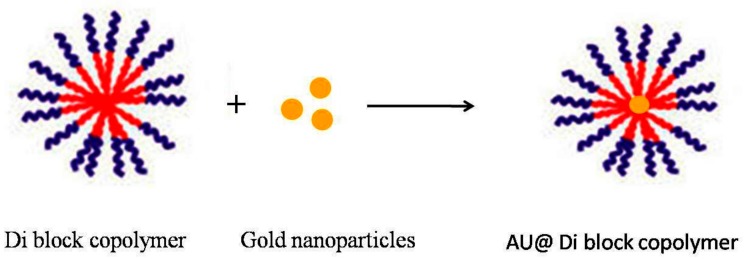
Hybrid composition of nanoparticles can gain many positive features, from both mixing materials and then generating new single composites we are able to meet the requirements in applications such as labeled materials, photonic nano-devices, or chemical sensors [54]. Encapsulation of metal nanoparticles in micellar aggregates can serve many purposes: (1) improving stability [55]; (2) reducing toxicity [56]; (3) easy to be multi-functionalized; (4) improving collective properties; and (5) serving as a template for functional cavity formation. In 2005, the Taton group produced hybrid micelles depending on amphiphilic-polystyrene-block-poly(acrylicacid) (PS-PAA) and gold NPs by using the co-precipitation approach [57]. Similarly, gold nanoparticles (AU NPs) were integrated inside PS-PAA. Since hydrophilic poly(acrylic acid) (PAA) shell can stabilize the hybrid micelles and remains soluble in water, which also can be attached by using cross-linker such as carbodiimide (EDC) that effectively avoids dissociation in organic solvent.
Mantzaridis and Pispas developed a new hybrid polymeric colloidal system, where gold nanoparticles are integrated inside the micelles of a poly(styrene-b-2-vinyl pyridine) (PS-P2VP) di-block copolymer in toluene and then they are encapsulated again in larger micelles of another rdi-block copolymer, namely poly(isoprene-b-styrene) (PIPS). This approach could be useful in circumstances that require different outer coronas of the micelles, rather than the one that is used in the nano-reactor scheme for metal nanoparticle production (Scheme 4) [58].
Scheme 4.
General scheme for the formation of the hybrid compound micelles.
4. Micelles Sensitive to Biological Stimuli
These micelles are formed to respond to biological stimulation, offering a great opportunity for drug delivery as controllable systems in the treatment of cancer therapy. The stimuli systems cause the micelles to answer their effect allowing drugs to be released respecting to specific external or internal stimuli, such as temperature, pH, ultrasound or enzymes, by including thermo- or pH-sensitive components or by attaching specific targeting moieties to the outer hydrophilic surface of polymeric micelles [59]. Among these stimuli, pH-sensitive polymeric micellar systems are developed, and they depend on two main strategies: pH-sensitive polymer-drug conjugates, such as hydrazone, cis-acotinyl, and acetal bonds [60]. These bonds are stable at neutral or alkaline pH but occur hydrolytic cleavage at acid pH [61].
Another strategy is to attach “titratable” groups in the copolymers, such as amines or carboxyl groups, to control micelle formation by producing physical or chemical dissociation. Hovewer pH-sensitive polymers can be protonated at pH, causing polymer rapture and drug release. Many protonated polymers have been published, such as poly(histidine) (polyHis), poly(acrylic acid) and poly-sulfonamides. PolyHis is the common pH-sensitive polymers can be used because it contains an imidazole ring endowing it with pH-dependent amphoteric properties [62]. For example, tri-block copolymer PLA-b-PEG-b-polyHis micelles were designed. Doxorubicin showed 60% release at pH 6.8 and 74% at pH 6.0, while only minimal release observed at pH 7.4 [63]. In an advanced work, new “nano-elastic” micelles have been developed. This property has been showed through the principle dissolution of polygalacturonic acid (PgA), since PgA is not soluble at the acidity of the stomach due to its hydrophobicity, while it is soluble in the pH of the colon. The strategy used is to block the PgA chain with polyacrylic acid (PAA) forming PgA-PAA composition. This property allows micelles to answer to the gastrointestinal tract pH. Therefore, they can swell at alkaline pH and shrink at acidic pH. In this case, PgA-PAA can pass gastrointestinal tract with no dissolution [64].
The modification of micelles is summarized on Table 1.
Table 1.
Micelles modification summary.
| Micelles | Formulation |
|---|---|
| Lipid micelle | Phospholipid or cholesterol |
| Polymeric micelles | Polymers having hydrophobic and hydrophilic properties |
| Hybrid polymeric lipids micelles | Polymers integrated into lipids |
| Hybrid micelles with metal nanoparticles | Micelles assembled with gold, silver or iron oxide nanoparticles |
| Micelles coated by layer by layer technique | Micelles incorporated into calcium carbonate and coated by polymers |
| Stimuli-responsive micelles | Micelles doped with stimuli such as pH-sensitive components |
5. Drug-Loaded Micelles
Drugs can be encapsulated inside micelle moieties either by physical properties or chemical attachments. Drugs loaded by chemical conjugation are released by bulk degradation or surface erosion of the polymer, while drugs loaded by physical entrapment are released by diffusion. Drug release is furthermore affected by the extent of micelle moieties, resulting in slower release, leading to extended release times [65]. Doxorubicin and paclitaxel have been recently approved for clinical trials, and are good examples of micelles used to carry chemotherapies [66]. Several methods are widely used to load drugs inside micelles: (1) oil-in-water (O/W) emulsion techniques [67]; (2) water-in-oil-in-water (W/O/W) emulsion techniques [68]; (3) direct dialysis [69]; (4) co-solvent evaporation [70]; and (5) freeze-drying/lyophilization [71].
Among the abovementioned methods, O/W, direct dialysis, and co-solvent evaporation are well suited for the encapsulation of hydrophobic drugs, whereas W/O/W is often preferred for the encapsulation of more hydrophilic compounds [72]. Application of polymeric micelles for drug delivery depends on the stability of micelles at both thermodynamic and kinetic potential after their intravenous injection and dilution in the vascular compartment. Release of the drug out of its target site can be avoided completely if the structure of micelles is stable enough upon intravenous administration, and they remain as nanoparticles long enough to accumulate in sufficient concentrations at the target site [73].
6. Advantages and Disadvantages of Micelles
The main characteristic of micelles is the core-shell structure. Hence the hydrophobic drugs can be still stable in water, and the corona shell, causing protection for the drug by preventing elimination by the mononuclear phagocyte system (MPS) [74], which enables prolonged their blood circulation [35]. Additionally, micelles have less toxicity and can be removed by renal filtration [75]. Micelles can save and derive the water-insoluble drugs in their hydrophobic core [76,77]. The ideal micelles for delivery of hydrophobic drugs were limited for those characterized by a hydrophilic corona to stabilize and protect the hydrophobic drug. Water solubility of drugs can be increased from 10- to 500-fold if they are encapsulated inside polymeric micelle moieties [78], which enables the intravenous injection of micelle-encapsulating hydrophobic drugs. For example, although paclitaxel is a water-insoluble drug, its water solubility is significantly enhanced when it is encapsulated in a micelle [79].
Concerning disadvantages of micelles, several challenges associated with their use concern the stability of micelles in the blood stream, where critical micelle concentration could be reduced by blood dilution, and the encapsulated drugs can leak out of the polymer assembly, minimizing the drug circulation half-life [80,81]. In order to overcome these issues, physical-chemical properties of micelles were addressed to improve stability and loaded drug efficiency inside micelles, one of the physical interactions between micelle moieties and the drug is a pi-stacking (π-π stacking) interaction. It is applied to be the subject of doxorubicin encapsulation into PEG-b-poly(α,β-aspartic acid) with the poly(α,β-aspartic acid) block co polymers [82]. Free radical polymerization was used upon assembly of modification of PEG-b-PLA with methacrylic acid anhydride, resulting in good stability of micelles’ structure [83]. Another notable strategy is to integrate drug attached polymers into lipids to prevent leakage out of the layer capsules [54]. Recently, micelles entrapped into CaCO3 crystals were coated by alternate adsorption of several layers forming, after core removal, several layers around the micelles’ structure [84].
7. Applications of Micelles in Cancer Therapy
Micelles that were being characterized by their small size have received scientific interest, since their diameters enables them to penetrate the vasculature of tumors effectively [83]. For instance, paclitaxel encapsulated inside moieties of polymeric micelles showed high drug capacity and good efficiency in patients with advanced malignancies [84,85,86], metastatic breast cancer [87] and advanced non-small lung cancer [88]. Similarly, paclitaxel, pluronic polymer-bound doxorubicin (SP1049C) [89], and NK911, a micelle-encapsulated doxorubicin [90], were used extensively for cancer treatment. The hydrophobic core of micelles designed from lipid conjugated PEG can be occupied by several insoluble drugs, such as paclitaxel, tamoxifen, porphyrin [91], camptothecin [92], and vitamin K3 [93]. In aqueous solution, adriamycin–attached into (PEG-P[Asp(ADR)]) exhibits antitumor activity in vivo [94].
Curcumin conjugated with PEG exhibited good cytotoxicity against several human cancer cell lines compared to free curcumin, such as breast [95], colon [96], prostate [97], kidney [98], liver [99] lymphoid and myeloid tissues [100], and melanoma [101]. Curcumin attached with Beta-thioester bonds can be selectively released by glutathione and esterase [102]. Hanafy et al. succeeded in loading glycolysis inhibitor (Bromopyruvic acid) inside chitosan-oleic acid nano-composite. The structure exhibited good cytotoxicity effects on hepatocellular carcinoma (HLF) cell line [53]. Also, micelles containing photosensitizing agent have been used in treatment of murine lewis lung carcinoma [103].
Micelles-foliate used as targeting therapy has shown significant effects against ovarian carcinoma cells compared to non-targeted micelles [104,105]. In clinical trials, NK911 and SP1049C [104] and NK105 and NC6004 are currently passed to Phases I and II [106,107]. The hydrophobic-hydrophilic regimens of micelles may offer several advantages for cancer therapy and drug delivery systems, including: increasing their capacity for water-insoluble drugs, resulting in mostly increased drug solubility; raising their drug accumulation inside the cancer site; prolonging their drug time circulation inside the blood stream; and their corona allowing micelles not react with biological components and then not be recognized by permeability and retention. Micelles can be designed to be used in several medical applications. Various micelle structures have been used as drug delivery system to possible penetrate solid tumors such as N-(2-hydroxypropyl) methacrylamide (HPMA) copolymers covalently conjugating doxorubicin via enzymatically cleavable glycyl–phenylalanyl–leucyl–glycine spacer [108,109]. Similar for this structure, paclitaxel was conjugated poly(glutamic acid) [110,111] and SN-38 was integrated into PEG-b-poly(l-glutamic acid) [112].
Micelles used as carriers for cancer therapies were summarized in Table 2.
Table 2.
Micelles as carriers for cancer therapies (reproduced from ref [81]) with permission, (Copyright Elsevier 2014).
| Name | Drug | Block Copolymer | Drug Loading (%w Drug/w Polymer) | Size (nm) | Company | Indication |
|---|---|---|---|---|---|---|
| NK105 | Paclitaxel | PEG-b-poly(α,β-aspartic acid) | 23 | 85 | Nippon Kayaku, Co. | Gastric cancer/Breast cancer |
| NK012 | SN-38 | PEG-b-poly(l-glutamic acid) | 20 | 20 | Nippon Kayaku, Co. | Triple negative breast cancer |
| NK911 | Doxorubicin | PEG-b-poly(α,β-aspartic acid) | 17 | 40 | Nippon Kayaku, Co. | Various solid tumors |
| NC-6004 | Cisplatin | PEG-b-poly(l-glutamic acid) | 30 | 20 | Nanocarrier, Co. | Pancreatic cancer |
| NC-4016 | Oxaliplatin | PEG-b-poly(l-glutamic acid) | 30 | 30 | Nanocarrier, Co. | Various solid tumors |
| NC-6300 | Epirubicin | PEG-b-poly(aspartate-hydrazone) | 20 | 60 | Nanocarrier, Co. | Various solid tumors |
| siRNA micelles | siRNA | PEG-b-polycations | Various | 40–60 | Nanocarrier, Co. | – |
8. Conclusions
Micelles have received great attention as an interesting drug delivery system, due to their simple fabrication, their capacity to be loaded with a wide variety of insoluble drugs, and the possibility to develop and improve their moieties. Micelles’ formation enables them to be used in wide medical applications. They can be fabricated simply by blocking copolymers, forming various shapes of micelle, or can be trapped by metal nanoparticles, or can be doped by responsive polymers to respond the biological stimuli. Micelles attached by biological molecules, such as lipids or proteins, are considered valuable and applicable to use as drug delivery systems.
Acknowledgments
N.A.N. Hanafy would like to thank president of Kafrelsheikh University and director of Nanoscience and Nanotechnology Institute, Maged El Kemary for his generous help, advice, and support.
Author Contributions
N.A.N.H. designed the paper, generated figures and wrote the manuscript; M.E.-K. revised the manuscript; S.L. supervised and revised the manuscript. All authors read and approved the final manuscript version.
Funding
This work was partially supported by the REA research grant No. PITN-GA-2012-316549 (IT LIVER) from the People Programme (Marie Curie Actions) of the European Union’s Seventh Framework Programme (FP7/2007–2013). Also, it is partially supported by Nanoscience and Nanotechnology Institute, Kafrelsheikh University, Egypt.
Conflicts of Interest
Authors declare no conflict of interest.
References
- 1.Kabanov A.V., Batrakova E.V., Melik-Nubarov N.S., Fedoseev N.A., Dorodnich T.Y., Alakhov V.Y., Chekhonin P., Nazarova I.R., Kabanov V.A. A new class of drug carriers: Micelles poly(oxyethylene)–poly(oxypropylene) block copolymers as microcontainers for drug targeting from blood to brain. J. Control. Release. 1992;22:41–158. doi: 10.1016/0168-3659(92)90199-2. [DOI] [Google Scholar]
- 2.Torchilin V.P. Micellar nanocarriers: Pharmaceutical perspectives. Pharm. Res. 2007;24:1–16. doi: 10.1007/s11095-006-9132-0. [DOI] [PubMed] [Google Scholar]
- 3.Schramm L.L., Stasiuk E.N., Marangoni D.G. Surfactants and their applications. Annu. Rep. Prog. Chem. Sect. C. 2003;99:3–48. doi: 10.1039/B208499F. [DOI] [Google Scholar]
- 4.Kellermann M., Bauer W., Hirsch A., Schade B., Ludwig K., Böttcher C. The first account of a structurally persistent micelle. Angew. Chem. Int. Ed. 2004;43:2959–2962. doi: 10.1002/anie.200353510. [DOI] [PubMed] [Google Scholar]
- 5.Loppinet B., Monteux C. Dynamics of surfactants and polymers at liquid interfaces. Lect. Notes Phys. 2016;917:137–157. [Google Scholar]
- 6.Chen L.G., Strasburg S.H., Bermudez H. Micelle co-assembly in surfactant/ionic liquid mixtures. J. Colloid Interface Sci. 2016;477:40–45. doi: 10.1016/j.jcis.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 7.Jia K., Hu J., Dong J., Li X. Light-responsive multillamellar vesicles in coumaric acid/alkyldimethylamine oxide binary systems: Effects of surfactant and hydrotrope structures. J. Colloid Interface Sci. 2016;477:156–165. doi: 10.1016/j.jcis.2016.05.046. [DOI] [PubMed] [Google Scholar]
- 8.Patil V.K., Gawali I.T., Usmani G.A. Synthesis and Properties of Novel Cationic Triazolium Gemini Surfactants. J. Dispers. Sci. Technol. 2016;37:1630–1637. doi: 10.1080/01932691.2015.1089779. [DOI] [Google Scholar]
- 9.Gaber N.N., Darwis Y., Peh K.K., Tan Y.T. Characterization of polymeric micelles for pulmonary delivery of beclomethasone dipropionate. J. Nanosci. Nanotechnol. 2006;6:3095–3101. doi: 10.1166/jnn.2006.426. [DOI] [PubMed] [Google Scholar]
- 10.Nishiyama N., Kataoka K. Current state, achievements, and future prospects of polymeric micelles as nanocarriers for drug and gene delivery. Pharmacol. Ther. 2006;112:630–648. doi: 10.1016/j.pharmthera.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Magid L.J., Li Z., Butler P.D. Flexibility of elongated sodium dodecyl sulfate micelles in aqueous sodium chloride: A small-angle neutron scattering study. Langmuir. 2000;16:10028–10036. doi: 10.1021/la0006216. [DOI] [Google Scholar]
- 12.Aoun B., Sharma V.K., Pellegrini E., Mitra S., Johnson M., Mukhopadhyay R. Structure and dynamics of ionic micelles: MD simulation and neutron scattering study. J. Phys. Chem. B. 2015;119:5079–5086. doi: 10.1021/acs.jpcb.5b00020. [DOI] [PubMed] [Google Scholar]
- 13.Kabanov A.V., Nazarova I.R., Astafieva I.V., Batrakova E.V., Alakhov V.Y., Yaroslavov A.A, Kabanov V.A. Micelle formation and solubilization of fluorescent probes in poly(oxyethylene-b-oxypropylene-b-oxyethylene) solutions. Macromolecules. 1995;28:2303–2314. doi: 10.1021/ma00111a026. [DOI] [Google Scholar]
- 14.Papaioannou A.I., Papiris S., Papadaki G., Manali E.D., Roussou A., Spathis A., Kostikas K. Surfactant proteins in smoking-related lung disease. Curr. Top. Med. Chem. 2016;16:1574–1581. doi: 10.2174/1568026616666150930120640. [DOI] [PubMed] [Google Scholar]
- 15.Shah A., Shahzad S., Munir A., Nadagouda M.N., Khan G.S., Shams D.F., Rana U.A. Micelles as soil and water decontamination agents. Chem. Rev. 2016;116:6042–6074. doi: 10.1021/acs.chemrev.6b00132. [DOI] [PubMed] [Google Scholar]
- 16.Ferreira A.R.V., Torres C.A.V., Freitas F., Sevrin C., Grandfils C., Reis M.A.M., Coelhoso I.M. Development and characterization of bilayer films of FucoPol and chitosan. Carbohydr. Polym. 2016;147:8–15. doi: 10.1016/j.carbpol.2016.03.089. [DOI] [PubMed] [Google Scholar]
- 17.Jones M.C., Leroux J.C. Polymeric micelles—A new generation of colloidal drug carriers. Eur. J. Pharm. Biopharm. 1999;48:101–111. doi: 10.1016/S0939-6411(99)00039-9. [DOI] [PubMed] [Google Scholar]
- 18.Giorgio G., Colafemmina G., Mavelli F., Murgia S., Palazzo G. The impact of alkanes on the structure of Triton X100 micelles. RSC Adv. 2016;6:825–836. doi: 10.1039/C5RA21691E. [DOI] [Google Scholar]
- 19.Pottage M.J., Greaves T.L., Garvey C.J., Tabor R.F. The effects of alkylammoniumcounterions on the aggregation of fluorinated surfactants and surfactant ionic liquids. J. Colloid Interface Sci. 2016;475:72–81. doi: 10.1016/j.jcis.2016.04.039. [DOI] [PubMed] [Google Scholar]
- 20.Anderson M.T., Martin J.E., Odinek J.G., Newcomer P.P. Effect of Methanol Concentration on CTAB Micellization and on the Formation of Surfactant-Templated Silica (STS) Chem. Mater. 1998;10:1490–1500. doi: 10.1021/cm970240m. [DOI] [Google Scholar]
- 21.Kuperkar K.C., Mata J.P., Bahadur P. Effect of 1-alkanols/salt on the cationic surfactant micellar aqueous solutions-A dynamic light scattering study. Colloids Surf. A Physicochem. Eng. Asp. 2011;380:60–65. doi: 10.1016/j.colsurfa.2011.02.019. [DOI] [Google Scholar]
- 22.Techen A., Hille C., Dosche C., Kumke M.U. Fluorescence study of drug carrier interactions in CTAB/PBS buffer model systems. J. Colloid Interface Sci. 2012;377:251–261. doi: 10.1016/j.jcis.2012.03.063. [DOI] [PubMed] [Google Scholar]
- 23.Li Z.Q., Gong Q.T., Luo L., Zhang L., Zhao S., Yu J.Y. Aggregation of sodium 2,4,5-(tri-n-Alkyl)-benzene sulfonates in aqueous solution: Micellization and microenvironment characteristics studied by electron paramagnetic resonance and steady-state fluorescence quenching. J. Dispers. Sci. Technol. 2011;32:167–173. doi: 10.1080/01932691003656680. [DOI] [Google Scholar]
- 24.Gibhardt H., Haramagatti C.R., Islamov A.K., Ivankov O.I., Kuklin A.I., Eckold G. Universal Behaviour of the structure and dynamics of micelles formed from cationic surfactants. Z. Phys. Chem. 2014;228:769–791. doi: 10.1515/zpch-2014-0524. [DOI] [Google Scholar]
- 25.Helgeson M.E., Hodgdon T.K., Kaler E.W., Wagner N.J. A systematic study of equilibrium structure, thermodynamics, and rheology of aqueous CTAB/NaNO3 wormlike micelles. J. Colloid Interface Sci. 2010;349:1–12. doi: 10.1016/j.jcis.2010.05.045. [DOI] [PubMed] [Google Scholar]
- 26.Adams M.L., Lavasanifar A., Kwon G.S. Amphiphilic block copolymers for drug delivery. J. Pharm. Sci. 2003;92:1343–1355. doi: 10.1002/jps.10397. [DOI] [PubMed] [Google Scholar]
- 27.Ghamkhari A., Massoumi B., Jaymand M. Novel ‘schizophrenic’ di-block copolymer synthesized via RAFT polymerization: Poly (2-succinyloxyethyl methacrylate)-b-poly [(N-4-vinylbenzyl),N,N-diethylamine] Des. Monomers Polym. 2017;20:190–200. doi: 10.1080/15685551.2016.1239165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.IUPAC Glossary of basic terms in polymer science. Pure Appl. Chem. 1996;68:2287–2311. [Google Scholar]
- 29.Iatrou H., Avgeropoulos A., Hadjichristidis N. Synthesis of model super H-shaped block copolymers. Macromolecules. 1994;27:6232–6233. doi: 10.1021/ma00099a047. [DOI] [Google Scholar]
- 30.Otsu T., Yoshida M., Kuriyama A. A model for living radical polymerization. Makromol. Chem. Rapid Commun. 1982;3:133–140. doi: 10.1002/marc.1982.030030209. [DOI] [Google Scholar]
- 31.Otsu T., Kuriyama A. Polymer Design by Iniferter Technique in Radical Polymerization: Synthesis of AB and ABA Block Copolymers Containing Random and Alternating Copolymer Sequences. J. Polym. 1985;17:97. doi: 10.1295/polymj.17.97. [DOI] [Google Scholar]
- 32.Yang X., Zhu B., Dong T., Pan P., Shuai X., Inoue Y. Interactions between an anticancer drug and polymeric micelles based on biodegradable polyesters. Macromol. Biosci. 2008;8:1116–1125. doi: 10.1002/mabi.200800085. [DOI] [PubMed] [Google Scholar]
- 33.Pierri E., Avgoustakis K. Poly(lactide)-poly(ethylene glycol) micelles as a carrier for grise of ulvin. J. Biomed. Mater. Res. A. 2005;75:639–647. doi: 10.1002/jbm.a.30490. [DOI] [PubMed] [Google Scholar]
- 34.Torchilin V.P. Targeted polymeric micelles for delivery of poorly soluble drugs. Cell. Mol. Life Sci. 2004;61:2549–2559. doi: 10.1007/s00018-004-4153-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sutton D., Nasongkla N., Blanco E., Gao J. Functionalized micellar systems for cancer targeted drug delivery. Pharm. Res. 2007;24:1029–1046. doi: 10.1007/s11095-006-9223-y. [DOI] [PubMed] [Google Scholar]
- 36.Chung J.R., Yokoyama M., Okano T. Inner core segment design for drug delivery control of thermo-responsive polymeric micelles. J. Control. Release. 2000;65:93–103. doi: 10.1016/S0168-3659(99)00242-4. [DOI] [PubMed] [Google Scholar]
- 37.Gaucher G., Dufresne N.H., Sant V.P., Maysinger D., Leroux J.C. Block copolymer micelles: Preparation, characterization and application in drug delivery. J. Control. Release. 2005;109:169–188. doi: 10.1016/j.jconrel.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 38.Blanco E., Kessinger C.W., Sumer B.D., Gao J. Multifunctional micellar nanomedicine for cancer therapy. Exp. Biol. Med. 2009;234:123–131. doi: 10.3181/0808-MR-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J.-S., Matyjaszewski K. “Living”/controlled radical polymerization. Transition-metal-catalyzed atom transfer radical polymerization in the presence of a conventional radical initiator. Macromolecules. 1995;28:7572–7573. doi: 10.1021/ma00126a041. [DOI] [Google Scholar]
- 40.Nicolas J., Guillaneuf Y., Lefay C., Bertin D., Gigmes D., Charleux B. Nitroxide-mediated polymerization. Prog. Polym. Sci. 2013;38:63–235. doi: 10.1016/j.progpolymsci.2012.06.002. [DOI] [Google Scholar]
- 41.Orilall M.C., Wiesner U. Block copolymer based composition and morphology control in nanostructured hybrid materials for energy conversion and storage: Solar cells, batteries, and fuel cells. Chem. Soc. Rev. 2011;40:520–535. doi: 10.1039/C0CS00034E. [DOI] [PubMed] [Google Scholar]
- 42.Tong R., Cheng J. Anticancer Polymeric Nanomedicines. Polym. Rev. 2007;47:345–381. doi: 10.1080/15583720701455079. [DOI] [Google Scholar]
- 43.Cheow W.S., Hadinoto K. Factors affecting drug encapsulation and stability of lipid- polymer hybrid nanoparticles. Colloids Surf. B Biointerfaces. 2011;85:214–220. doi: 10.1016/j.colsurfb.2011.02.033. [DOI] [PubMed] [Google Scholar]
- 44.Trubetskoy V.S., Torchilin V.P. Use of polyoxyethylenelipidconjugates as long-circulating carriers for delivery of therapeutic and diagnostic agents. Adv. Drug Deliv. Rev. 1995;16:311–320. doi: 10.1016/0169-409X(95)00032-3. [DOI] [Google Scholar]
- 45.Torchilin V.P, Weissig V. Polymeric micelles for delivery of poorly soluble drugs. Polym. Prepr. 1999;40:320–321. [Google Scholar]
- 46.Zhang J., Chen X.G., Huang L., Han J.T., Zhang X.F. Self-assembled polymeric nanoparticles based on oleic acid-grafted chitosan oligosaccharide: Biocompatibility, protein adsorption and cellular uptake. J. Mater. Sci. Mater. Med. 2012;23:1775–1783. doi: 10.1007/s10856-012-4651-1. [DOI] [PubMed] [Google Scholar]
- 47.Sabharanjak S., Mayor S. Folate receptor endocytosis and trafficking. Adv. Drug Deliv. Rev. 2004;56:1099–1109. doi: 10.1016/j.addr.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 48.Janes K.A., Fresneau M.P., Marazuela A., Fabra A., Alonso M.J. Chitosan nanoparticles as delivery systems for doxorubicin. J. Control. Release. 2001;73:255–267. doi: 10.1016/S0168-3659(01)00294-2. [DOI] [PubMed] [Google Scholar]
- 49.Esquenet C., Terech P., Boue F., Buhler E. Structural and rheological properties of hydrophobically modified polysaccharide associative networks. Langmuir. 2004;20:3583–3592. doi: 10.1021/la036395s. [DOI] [PubMed] [Google Scholar]
- 50.Bedu-Addo F.K., Tang P., Xu Y., Huang L. Effects of polyethyleneglycol chain length and phospholipid acyl chain composition on the interaction of polyethyleneglycol-phospholipid conjugates with phospholipid: Implications in liposomal drug delivery. Pharm. Res. 1996;13:710–717. doi: 10.1023/A:1016091314940. [DOI] [PubMed] [Google Scholar]
- 51.Edwards K., Johnsson M., Karlsson G., Silvander M. Effect of polyethyleneglycol-phospholipids on aggregate structure in preparations of small unilamellar liposomes. J. Biophys. 1997;73:258–266. doi: 10.1016/S0006-3495(97)78066-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosen M.J., Kunjappu J.T. Surfactants and Interfacial Phenomena. 4th ed. John Wiley & Sons; Hoboken, NJ, USA: 2012. [Google Scholar]
- 53.Hanafy N.A., Dini L., Citti C., Cannazza G., Leporatti S. Inhibition of Glycolysis by Using a Micro/Nano-Lipid Bromopyruvic Chitosan Carrier as a Promising Tool to Improve Treatment of Hepatocellular Carcinoma. Nanomaterials. 2018;8:34. doi: 10.3390/nano8010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yuan J.Y., Müller A.H.E. One-dimensional organic-inorganic hybrid nanomaterials. Polymer. 2010;51:4015–4036. doi: 10.1016/j.polymer.2010.06.064. [DOI] [Google Scholar]
- 55.Chen Y., Cho J., Young A., Taton TA. Enhanced stability and bioconjugation of photo-cross-linked polystyrene-shell, Au-core nanoparticles. Langmuir. 2007;23:7491–7497. doi: 10.1021/la700494e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dubertret B., Skourides P., Norris D.J., Noireaux V., Brivanlou A.H., Libchaber A. In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science. 2002;298:1759–1762. doi: 10.1126/science.1077194. [DOI] [PubMed] [Google Scholar]
- 57.Kang Y., Taton T.A. Core/Shell Gold Nanoparticles by Self-Assembly and Crosslinking of Micellar, Block Copolymer Shells. Angew. Chem. Int. Ed. 2005;44:409–412. doi: 10.1002/anie.200461119. [DOI] [PubMed] [Google Scholar]
- 58.Mantzaridis C., Pispas S. Hybrid Compound Block Copolymer Micelles Encapsulating Gold Nanoparticles. Macromol. Rapid Commun. 2008;29:1793–1797. doi: 10.1002/marc.200800402. [DOI] [Google Scholar]
- 59.Liu Z., Torchilin V.P. Stimulus-responsive nano-preparations for tumor targeting. Integr. Biol. 2013;5:96–107. doi: 10.1039/c2ib20135f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oerlemans C., Bult W., Bos M., Storm G., Nijsen J.F.W., Hennink W.E. Polymeric micelles in anticancer therapy: Targeting, imaging and triggered release. Pharm. Res. 2010;27:2569–2589. doi: 10.1007/s11095-010-0233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fleige E., Quadir M.A., Haag R. Stimuli-responsive polymeric nanocarriers for the controlled transport of active compounds: Concepts and applications. Adv. Drug Deliv. Rev. 2012;64:866–884. doi: 10.1016/j.addr.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 62.Duan C., Gao J., Zhang D., Jia L., Liu Y., Zheng D., Liu G., Tian X., Wang F., Zhang Q. Galactose-decorated pH-responsive nanogels for hepatoma-targeted delivery of oridonin. Biomacromolecules. 2011;12:4335–4343. doi: 10.1021/bm201270m. [DOI] [PubMed] [Google Scholar]
- 63.Lee E.S., Bae Y.H. Recent progress in tumor pH targeting nanotechnology. J. Control. Release. 2008;132:164–170. doi: 10.1016/j.jconrel.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hanafy N.A.N., Quarta A., Ferraro M.M., Dini L., Nobile C., De Giorgi M.L., Carallo S., Citti C., Gaballo A., Cannazza G., et al. Polymeric Nano-Micelles as Novel Cargo-Carriers for LY2157299 Liver Cancer Cells Delivery. Int. J. Mol Sci. 2018;19:748. doi: 10.3390/ijms19030748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu D.-Z., Hsieh J.-H., Fan X.-C., Yang J.-D., Chung T.-W. Synthesis, characterization and drug delivery behaviors of new PCP polymeric micelles. Carbohydr. Polym. 2007;8:544–554. doi: 10.1016/j.carbpol.2006.10.019. [DOI] [Google Scholar]
- 66.Kedar U., Phutane P., Shidhaye S., Kadam V. Advances in polymeric micelles for drug delivery and tumor targeting. Nanomedicine. 2010;6:714–729. doi: 10.1016/j.nano.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 67.Chen H., Khemtong C., Yang X., Chang X., Gao J. Nanonization strategies for poorly water-soluble drugs. Drug Discov. Today. 2011;16:354–360. doi: 10.1016/j.drudis.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 68.Sant V.P., Smith D., Leroux J.-C. Enhancement of oral bioavailability of poorly water-soluble drugs by poly(ethylene glycol)-block-poly(alkyl acrylate-co-methacrylic acid) self-assemblies. J. Control. Release. 2005;104:289–300. doi: 10.1016/j.jconrel.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 69.Xie J., Wang C.H. Self-assembled biodegradable nanoparticles developed by direct dialysis for the delivery of paclitaxel. Pharm. Res. 2005;22:2079–2090. doi: 10.1007/s11095-005-7782-y. [DOI] [PubMed] [Google Scholar]
- 70.Meng F.T., Ma G.H., Qiu W., Su Z.G. W/O/W double emulsion technique using ethyl acetate as organic solvent: Effects of its diffusion rate on the characteristics of microparticles. J. Control. Release. 2003;91:407–416. doi: 10.1016/S0168-3659(03)00273-6. [DOI] [PubMed] [Google Scholar]
- 71.Jette K., Law D., Schmitt E., Kwon G. Preparation and drug loading of poly (ethylene glycol)-block-poly(ε-caprolactone) micelles through the evaporation of a cosolvent azeotrope. Pharm. Res. 2004;21:1184–1191. doi: 10.1023/B:PHAM.0000033005.25698.9c. [DOI] [PubMed] [Google Scholar]
- 72.Fournier E., Dufresne M.H., Smith D.C., Ranger M., Leroux J.C. A novel one-step drug- Drug Loading of Polymeric Micelles. Pharm. Res. 2013;30:584–595. doi: 10.1007/s11095-012-0903-5. [DOI] [PubMed] [Google Scholar]
- 73.Kabanov A.V., Alakhov V.Y. Micelles of amphilphilic block copolymers as vehicles for drug delivery. In: Alexandridis P., Lindman B., editors. Amphiphilic Block Copolymers: Self-Assembly and Applications. Elsevier; Amsterdam, The Netherlands: 2000. pp. 347–376. [Google Scholar]
- 74.Yokoyama M. Clinical Applications of Polymeric Micelle Carrier Systems in Chemotherapy and Image Diagnosis of Solid Tumors. J. Exp. Clin. Med. 2011;3:151–158. doi: 10.1016/j.jecm.2011.06.002. [DOI] [Google Scholar]
- 75.Moghimi S.M., Hunter A.C., Murray J.C. Nanomedicine: Current status and future prospects. FASEB J. 2005;19:311–330. doi: 10.1096/fj.04-2747rev. [DOI] [PubMed] [Google Scholar]
- 76.Lee P., Zhang R., Li V., Liu X., Sun R.W., Che C.M., Wong K.K. Enhancement of anticancer efficacy using modified lipophilic nanoparticle drug encapsulation. Int. J. Nanomed. 2012;7:731–737. doi: 10.2147/IJN.S28783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Savić R., Eisenberg A., Maysinger D. Block copolymer micelles as delivery vehicles of hydrophobic drugs: Micelle–cell interactions. J. Drug Target. 2006;14:343–355. doi: 10.1080/10611860600874538. [DOI] [PubMed] [Google Scholar]
- 78.Soga O., van Nostrum C.F., Fens M., Rijcken C.J., Schiffelers R.M., Storm G., Hennink W.E. Thermosensitive and biodegradable polymeric micelles for paclitaxel delivery. J. Control. Release. 2005;103:341–353. doi: 10.1016/j.jconrel.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 79.Shi Y., Lammers T., Storm G., Hennink W.E. Physico-Chemical Strategies to Enhance Stability and Drug Retention of Polymeric Micelles for Tumor-Targeted Drug Delivery. Macromol. Biosci. 2017;17 doi: 10.1002/mabi.201600160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Owen S.C., Chan D.P.Y., Shoichet M.S. Polymeric micelle stability. Nanotoday. 2012;7:53–65. doi: 10.1016/j.nantod.2012.01.002. [DOI] [Google Scholar]
- 81.Cabral H., Kataoka K. Progress of drug-loaded polymeric micelles into clinical studies. J. Control. Release. 2014;190:465–476. doi: 10.1016/j.jconrel.2014.06.042. [DOI] [PubMed] [Google Scholar]
- 82.Iijima M., Nagasaki Y., Okada T., Kato M., Kataoka K. Core-Polymerized Reactive Micelles from Heterotelechelic Amphiphilic Block Copolymers. Macromolecules. 1999;32:1140–1146. doi: 10.1021/ma9815962. [DOI] [Google Scholar]
- 83.Xu L. Sacrificial PSS doped CaCO3 template to prepare chitosan capsules and their deformation under bulk pressure. Polym. Bull. 2013;70:455–465. doi: 10.1007/s00289-012-0811-1. [DOI] [Google Scholar]
- 84.Kim S., Shi Y., Kim J.Y., Park K., Cheng J.X. Overcoming the barriers in micellar drug delivery: Loading efficiency, in vivo stability, and micelle-cell interaction. Expert Opin. Drug Deliv. 2010;7:49–62. doi: 10.1517/17425240903380446. [DOI] [PubMed] [Google Scholar]
- 85.Shi Y., van der Meel R., Theek B., Oude Blenke E., Pieters E.H., Fens M.H., Ehling J., Schiffelers R.M. Storm, G.; van Nostrum, C.F.; et al. Complete Regression of Xenograft Tumors upon Targeted Delivery of Paclitaxel via Π-Π Stacking Stabilized Polymeric Micelles. ACS Nano. 2015;9:3740–3752. doi: 10.1021/acsnano.5b00929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shuai X., Merda T., Schaper A.K., Xi F., Kissel T. Core-cross-linked polymeric micelles as paclitaxel carriers. Bioconjug. Chem. 2004;15:441–448. doi: 10.1021/bc034113u. [DOI] [PubMed] [Google Scholar]
- 87.Kim T.Y., Kim D.W., Chung J.Y., Shin S.G., Kim S.C., Heo D.S., Kim N.K., Bang Y.J. Phase I and pharmacokinetic study of Genexol-PM, a cremophor-free, polymeric micelle-formulated paclitaxel, in patients with advanced malignancies. Clin. Cancer Res. 2004;10:3708–3716. doi: 10.1158/1078-0432.CCR-03-0655. [DOI] [PubMed] [Google Scholar]
- 88.Lee K.S., Chung H.C., Im S.A., Park Y.H., Kim C.S., Kim S.B., Rha S.Y., Lee M.Y., Ro J. Multicenter phase II trial of Genexol-PM, a Cremophor-free, polymeric micelle formulation of paclitaxel, in patients with metastatic breast cancer. Breast Cancer Res. Treat. 2008;108:241–250. doi: 10.1007/s10549-007-9591-y. [DOI] [PubMed] [Google Scholar]
- 89.Kim D.W., Kim S.Y., Kim H.K., Kim S.W., Shin S.W., Kim J.S., Park K., Lee M.Y., Heo D.S. Multicenter phase II trial of Genexol-PM, a novel Cremophor-free, polymeric micelle formulation of paclitaxel, with cisplatin in patients with advanced non-small-cell lung cancer. Ann. Oncol. 2007;18:2009–2014. doi: 10.1093/annonc/mdm374. [DOI] [PubMed] [Google Scholar]
- 90.Danson S., Ferry D., Alakhov V., Margison J., Kerr D., Jowle D., Brampton M., Halbert G., Ranson M. Phase I dose escalation and pharmacokinetic study of pluronic polymer-bound doxorubicin (SP1049C) in patients with advanced cancer. Br. J. Cancer. 2004;90:2085–2091. doi: 10.1038/sj.bjc.6601856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Matsumura Y., Hamaguchi T., Ura T., Muro K., Yamada Y., Shimada Y., Shirao K., Okusaka T., Ueno H., Ikeda M., et al. Phase I clinical trial and pharmacokinetic evaluation of NK911, a micelle-encapsulated doxorubicin. Br. J. Cancer. 2004;91:1775–1781. doi: 10.1038/sj.bjc.6602204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gao Z., Lukyanov A.N., Singhal A., Torchilin V.P. Diacyl lipid-polymer micelles as nanocarriers for poorly soluble anticancer drugs. Nano Lett. 2002;2:979–982. doi: 10.1021/nl025604a. [DOI] [Google Scholar]
- 93.Mu L., Chrastina A., Levchenko T., Torchilin V.P. Micelles from poly(ethylene glycol) phosphatidyl ethanolamine conjugates (PEG-PE) as pharmaceutical nanocarriers for poorly soluble drug camptothecin. J. Biomed. Nanotechnol. 2005;1:190–195. doi: 10.1166/jbn.2005.030. [DOI] [Google Scholar]
- 94.Wang J., Mongayt D.A., Lukyanov A.N., Levchenko T.S., Torchilin V.P. Preparation and in vitro synergistic anticancer effect of vitamin K3 and 1,8-diazabicyclo[5,4,0]undec-7-ene in poly(ethylene glycol)-diacyl lipid micelles. Int. J. Pharm. 2004;272:129–135. doi: 10.1016/j.ijpharm.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 95.Yokoyama M., Okano T., Sakurai Y., Ekimoto H., Shibazaki C., Kataoka K. Toxicity and antitumor activity against solid tumors of micelle-forming polymeric anticancer drug and its extremely long circulation in blood. Cancer Res. 1991;51:3229–3236. [PubMed] [Google Scholar]
- 96.Deeb D., Jiang H., Gao X., Al-Holou S., Danyluk A.L., Dulchavsky S.A., Gautam S.C. Curcumin [1,7-Bis(4-hydroxy-3-methoxyphenyl)-1–6-heptadine-3,5-dione; C21H20O6] sensitizes human prostate cancer cells to tumor necrosis factor-related apoptosis-inducing ligand/Apo2L-induced apoptosis by suppressing nuclear factor-κB via inhibition of the pro survival Akt signaling pathway. J. Pharmacol. Exp. Ther. 2007;321:616–625. doi: 10.1124/jpet.106.117721. [DOI] [PubMed] [Google Scholar]
- 97.Jiang M.C., Yang-Yen H.F., Yen J.J., Lin J.K. Curcumin induces apoptosis in immortalized NIH 3T3 and malignant cancer cell lines. Nutr. Cancer. 1996;26:111–120. doi: 10.1080/01635589609514468. [DOI] [PubMed] [Google Scholar]
- 98.Holy J. Curcumin inhibits cell motility and alters microfilament organization and function in prostate cancer cells. Cell. Motil. Cytoskelet. 2004;58:253–268. doi: 10.1002/cm.20012. [DOI] [PubMed] [Google Scholar]
- 99.Woo J.H., Kim Y.H., Choi Y.J., Kim D.G., Lee K.S., Bae J.H., Min D.S., Chang J.S., Jeong Y.J., Lee Y.H., et al. Molecular mechanisms of curcumin-induced cytotoxicity: Induction of apoptosis through generation of reactive oxygen species, down-regulation of Bcl-XL and IAP, the release of cytochrome C and inhibition of Akt. Carcinogenesis. 2003;24:1199–1208. doi: 10.1093/carcin/bgg082. [DOI] [PubMed] [Google Scholar]
- 100.Yoysungnoen P., Wirachwong P., Bhattarakosol P., Niimi H., Patumraj S. Effects of curcumin on tumor angiogenesis and biomarkers, COX-2 and VEGF, in hepatocellular carcinoma cell-implanted nude mice. Clin. Hemorheol. Microcirc. 2006;34:109–115. [PubMed] [Google Scholar]
- 101.Anuchapreeda S., Limtrakul P., Thanarattanakorn P., Sittipreechacharn S., Chanarat P. Inhibitory effect of curcumin on WT1 gene expression in patient leukemic cells. Arch. Pharm. Res. 2006;29:80–87. doi: 10.1007/BF02977473. [DOI] [PubMed] [Google Scholar]
- 102.Odot J., Albert P., Carlier A., Tarpin M., Devy J., Madoulet C. In vitro and in vivo anti-tumoral effect of curcumin against melanoma cells. Int. J. Cancer. 2004;111:381–387. doi: 10.1002/ijc.20160. [DOI] [PubMed] [Google Scholar]
- 103.Tang H., Murphy C.J., Zhang B., Shen Y., Sui M., Van Kirk E.A., Feng X., Murdoch W.J. Amphiphilic curcumin conjugate-forming nanoparticles as anticancer prodrug and drug carriers: In vitro and in vivo effects. Nanomedicine. 2010;5:855–865. doi: 10.2217/nnm.10.67. [DOI] [PubMed] [Google Scholar]
- 104.Huang Y., Lu J., Gao X., Li J., Zhao W., Sun M., Stolz D.B., Venkataramanan R., Rohan L.C., Li S. PEG-derivatizedembelin as a dual functional carrier for the delivery of paclitaxel. Bioconjug. Chem. 2012;23:1443–1451. doi: 10.1021/bc3000468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Varela-Moreira A., Shi Y., Fens M.H.A.M., Lammers T., Hennink W.E., Schiffelers R.M. Clinical application of polymeric micelles for the treatment of cancer. Mater. Chem. Front. 2017;1:1485–1501. doi: 10.1039/C6QM00289G. [DOI] [Google Scholar]
- 106.Roby A., Erdogan S., Torchilin V.P. Enhanced in vivo antitumor efficacy of poorly soluble PDT agent, meso-tetraphenylporphine, in PEG-PEbased tumortargeted immune-micelles. Cancer Biol. Ther. 2007;6:1136–1142. doi: 10.4161/cbt.6.7.4345. [DOI] [PubMed] [Google Scholar]
- 107.Kim D., Lee E.S., Oh K.T., Gao Z.G., Bae Y.H. Doxorubicin-loaded polymeric micelle overcomes multidrug resistance of cancer by double-targeting folate receptor and early endosomal pH. Small. 2008;4:2043–2050. doi: 10.1002/smll.200701275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Valle J.W., Armstrong A., Newman C., Alakhov V., Pietrzynski G., Brewer J., Campbell S., Corrie P., Rowinsky E.K., Ranson M. A phase 2 study of SP1049C, doxorubicin in P-glycoprotein-targeting pluronics, in patients with advanced adenocarcinoma of the esophagus and gastro-esophageal junction. Investig. New Drugs. 2011;29:1029–1037. doi: 10.1007/s10637-010-9399-1. [DOI] [PubMed] [Google Scholar]
- 109.Duncan R., Cable H.C., Lloyd J.B., Rejmanova P.J. Kopeček, Polymers containing enzymatically degradable bonds. 7. Design of oligopeptide side-chains in poly[n-(2-hydroxypropyl)methacrylamide] copolymers to promote efficient degradation by lysosomal-enzymes, Macromol. Chem. Macromol. Chem. Phys. 1983;184:1997–2008. [Google Scholar]
- 110.Wilson R.H., Plummer R., Adam J., Eatock M.M., Boddy A.V., Griffin M., Miller R., Matsumura Y., Shimizu T., Calvert H. Phase I and pharmacokinetic study of NC-6004, a new platinum entity of cisplatin conjugated polymer forming micelles. J. Clin. Oncol. 2008;26:2573. doi: 10.1200/jco.2008.26.15_suppl.2573. [DOI] [Google Scholar]
- 111.Singer J.W. Paclitaxel poliglumex (XYOTAX, CT-2103): A macromolecular taxane. J. Control. Release. 2005;109:120–126. doi: 10.1016/j.jconrel.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 112.Koizumi F., Kitagawa M., Negishi T., Onda S., Matsumoto S., Hamaguchi T., Matsumura Y. Novel SN-38-incorporating polymeric micelles, NK012, eradicate vascular endothelial growth factor-secreting bulky tumors. Cancer Res. 2006;66:10048–10056. doi: 10.1158/0008-5472.CAN-06-1605. [DOI] [PubMed] [Google Scholar]