ABSTRACT
Objectives: To investigate differences in pressure pain thresholds (PPTs) and longitudinal mechanosensitivity of the greater occipital nerve (GON) between patients with side-dominant head and neck pain (SDHNP) and healthy controls. Evaluation of neural sensitivity is not a standard procedure in the physical examination of headache patients but may influence treatment decisions.
Methods: Two blinded investigators evaluated PPTs on two different locations bilaterally over the GON as well as the occipitalis longsitting-slump (OLSS) in subjects with SDHNP (n = 38)) and healthy controls (n = 38).
Results: Pressure pain sensitivity of the GON was lower at the occiput in patients compared to controls (p = 0.001). Differences in pressure sensitivity of the GON at the nucheal line, or between the dominant headache side and the non-dominant side were not found (p > 0.05). The OLSS showed significant higher pain intensity in SDHNP (p < 0.001). In comparison to the non-dominant side, the dominant side was significantly more sensitive (p = 0.004).
Discussion: Palpation of the GON at the occiput and the OLSS may be potentially relevant tests in SDHNP. One explanation for an increased bilateral sensitivity may be sensitization mechanisms. Future research should investigate the efficacy of neurodynamic techniques directed at the GON.
Level of Evidence: 3b.
KEYWORDS: Greater occipital nerve, mechanosensivity, neurodynamic, pressure pain threshold, migraine, cervicogenic headache
Introduction
Localization of symptoms, especially unilateral localization is an essential component for classification of headaches [1,2]. Across all types of headaches, almost one-fifth are described as side-dominant [3,4]. Subjects with migraine describe a side-dominant localization in 26% of all cases [5]. Cervicogenic headache (CGH) is unilateral without side change by definition [2,4]. Not only CGH but also subjects with migraine complain of headache-associated neck pain [6–9]. Neck pain in migraine is more common than nausea or vomiting, but not included in current migraine criteria [10]. Thus, side-dominant headache and neck pain (SDHNP) is found in a relevant subgroup of migraine and all CGH.
Migraine is described as a complex neurological condition with interactions between the central and the peripheral nervous system [11]. Pain mechanisms of CGH are based on the convergence of cervical and trigeminal afferents. This allows trigeminal and cervical afferents to refer pain into parietal, frontal and orbital regions [12]. All anatomical nociceptive structures innervated by the first three cervical nerves can be potential sources of pain [13–15]. The greater occipital nerve (GON) is identified as one of the major nociceptives sources [16]. Systematic reviews show that an anesthetic infiltration of the GON can be an effective treatment method for various types of headaches [17–22]. Recent randomized-controlled trials show a marked effect of GON blocks in chronic migraine [23–26]. Ambrossini and Schoenen highlighted that a good effect with GON blocks can be expected in subjects with a side-dominant migraine without a side change [17]. As an alternative for the invasive GON block, pericranial GON stimulation is described as an effective treatment [27–29]. Furthermore, massaging the GON has been reported as pain relieving by patients with migraine [30].
For the examination of cervical contributing factors in headache, a distinction is made between the articular, the muscular, and the neural system [31]. Recently published systematic reviews summarize physical examination test for subjects with CHG [32,33]. These do not include neural signs. However, a survey focussing on non-responsive patients with CGH showed that increased neural sensitivity may be one of the falsely neglected features [34]. To investigate neuronal mechanosensitivity in subjects with headache, nerve palpation of the occipital nerves[31,35,36] and neurodynamic tests are recommended [16,37,38]. In diagnostic headache research, pressure pain thresholds (PPT) are the most frequently used test to determine neural pressure sensitivity [24,39–45]. A range of studies reported controversial results for the GON between SDHNP and a control group [46–49]. Recommended neurodynamic tests in subjects with headaches are the cervical slump and the occipital slump [16,38]. Altered neurodynamic response, using these tests, was reported for patients with headaches compared to controls [49–51]. During maintained cervical slump position, headache symptoms were reproduced in patients with primary headaches [51]. Furthermore, in children with primary and secondary headaches, a decreased mobility and a higher sensory response were described [50]. In another study, however, no difference was found between CGH, migraine and a control group [49]. There is no scientific consensus for the diagnostic examination of the GON in side-dominant headaches. The aim of this study was to evaluate whether patients with SDHNP, compared to headache-free control participants, show altered mechanosensitivity of the GON, using PPTs at two different locations over the GON and the occipitalis longsitting-slump (OLSS).
Methods
Design and setting
This study is an assessor-blinded diagnostic case-control study. The study was previously registered in the German Register for Clinical Studies (DRKS00010163) and approved by the ethics committee of the University of Osnabrück (WISO_MA_ELP_SS16_2/2). This study fulfills the methodical requirements of the Strengthening the Reporting of Observational studies in Epidemiology [52]. All examinations were carried out at the University Clinic Hamburg.
Participants
Participants were recruited by various physicians and physiotherapists in Hamburg, Germany. Potentially eligible patients and controls were interviewed during telephone calls. If the eligibility criteria were fulfilled, an examination date was agreed. An international expert in headache and musculoskeletal examination and treatment (postdoc research fellow) examined all subjects to confirm eligility criteria again. Included were subjects between 18 and 70 years with SDHNP for a minimum of 3 months; a minimum of two headache days per month; a minimum intensity of 2/10 (numerical rating scale). Ninety percent of the primary dominant headache had to be unilateral without switching sides and headache episodes had to be associated with neck pain. Furthermore, patients had to be classified according to the diagnostic criteria for CGH[53], episodic migraine (without aura) or chronic migraine [54].
The criteria of Cervicogenic Headache International Study Group (CHISG) ‘diffuse shoulder pain’ and ‘diffuse arm pain’ were not considered in the inclusion criteria, because they point toward other pathologies [55]. Therefore, five main criteria led to the diagnosis CGH[56]: ‘unilaterality without side change’; a described pain pattern ‘starting posteriorly- ending up anteriorly’; ‘cervical range of motion deficit’ (assessed using flexion-rotation test). Flexion-rotation test (FRT) was considered to be positive when less than 30° rotation is reached in maximum cervical flexion [57]. ‘Provocation, externally’ was examined by unilateral posteroanterior passive accessory intervertebral motions from C1 to C3. ‘Provocation, unphysiological neck position’ is confirmed by the upper quadrant test [58]. Migraine without aura was diagnosed as a recurring headache disease in at least five attacks of 4–72 h duration [54]. At least two of the following four typical characteristics were required: unilateral location, pulsating quality, moderate-to-severe pain intensity and aggravation by or causing avoidance of routine physical activity. During headache, at least one of these two accompanying symptoms should occur: nausea and/or vomiting or photophobia and phonophobia. Chronic migraine is described as a tension-type and/or migraine-like headache on 15 or more days per month for more than 3 months.
Subjects with cervical trauma, rheumatoid disease or other relevant pathologies were excluded [16]. To exclude medication-overuse headache, patients were also excluded if pain or headache medication on more than 10 days per month was required [59]. Furthermore, the main focus of this study is on the side-dominant headache and not on face and jaw pain. Therefore, patients with temporomandibular disorders and chronic dysfunctional facial pain were excluded. A dysfunctional chronic facial pain is considered to the orofacial version of the Graded Chronic Pain Scale [60,61]. Excellent clinometric properties have been demonstrated before [62–65]. A relevant temporomandibular disorder is scored at six points or higher of the Jaw Pain and Function questionnaire [66]. A sensitivity of 95.8% and a specificity of 100% were shown for differentiation of temporomandibular disorders and healthy subjects [67,68]. Healthy subjects between 18 and 70 years of age with less than three recurring headaches suffering from primary or secondary headache according to the criteria of the International Classification of Headache Disorders [54] or CHISG [69] were included as control participants. The same exclusion criteria as described for patients were applied.
Outcomes
Pressure pain thresholds (PPTs)
The amount of pressure required for the sensation of pressure to change to a sensation of pain was dete-cted with a mechanical pressure algometer (Wagner Instruments, Greenwich, UK) on two different points over the GON on each side (Figure 1). Pressure was applied using a 1 cm2 probe with measurable values from 0 to 6kg/cm2 in 0.1 intervals. Participants were instructed to say ‘stop’ as soon as pressure changed into pain. In previous in vitro studies, the GON was located at an average distance of 4 cm (range 1.5–7.5) lateral to a horizontal line through the occipital protuberance (OP) [70,71]. Another superficial localization has been described at the nucheal line (NL) with an average horizontal distance to the mid point of 4.36 cm [72]. In order to find the most sensitive location, horizontal lines were palpated through the OP and along the NL from 1.5 to 7.5 cm on each side. After palpation, the PPT was determined three times with a resting period of 30 s on both sides and both locations in prone position. The increase in pressure was standardized to 250 g per second. Therefore, a chronometer was used to consistently increase pressure: 1 kilogram in 4 s. The reliability of this procedure has been found to be high for the GON [48,73].
Figure 1.
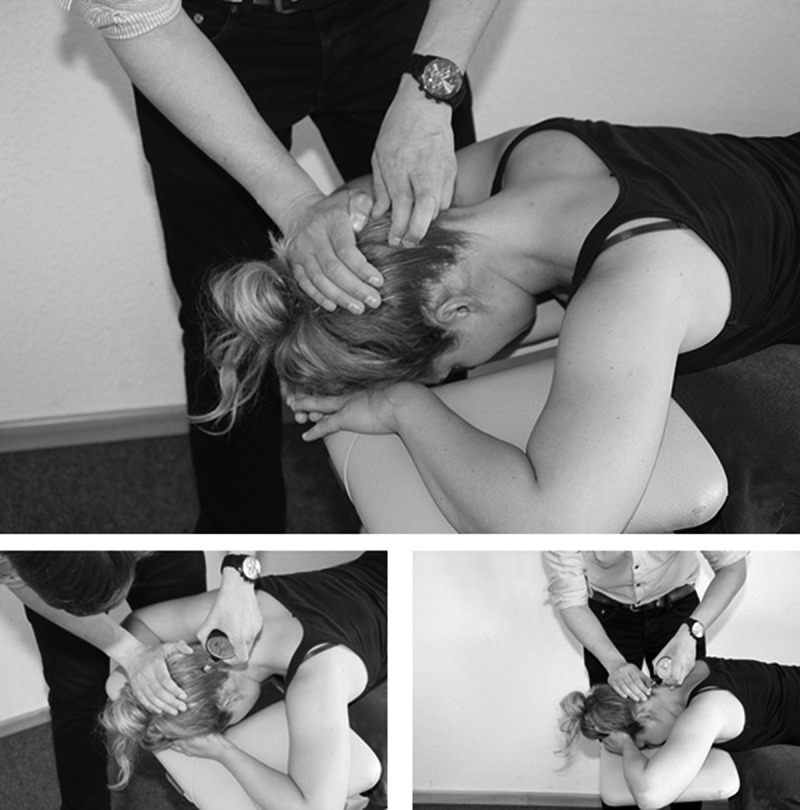
Pressure pain thresholds (PPTs) of the greater occipital nerve (GON). GON localization at the occipital protuberance (OP) (above), pressure pain thresholds (PPT) at OP (lower left) and PPT evaluation at linea nuchae (LN) (lower left).
Occipitalis longsitting-slump (OLSS)
The OLSS was performed bilaterally with a standardized test procedure [16]. During this procedure, legs were parallel, knees were fixed in extension with a belt and soles of the feet kept in complete contact with the wall to maintain the ankle in a neutral position. Thereafter, the subject was asked to bend the trunk as far as possible. The therapist initiated a cervical flexion focussing on the upper cervical spine. In addition, approximately 15° lateral flexion and a cervical ipsilateral rotation of 30° were attempted. The upper body was held in this position. The examiner subsequently grasp into the hair region above the GON to move the scalp longitudinally which may load the GON. Immediately after this procedure, the patient was asked to identify the pain localization in face, head, cervical spine, thoracic spine, lumbar spine and/or legs. For any location above C7, subsequently the belt was released and the knees flexed. Participants were asked to indicate their pain on the visual analogue scale (VAS) and asked if symptoms were reduced by knee flexion. The OLSS is considered to be positive if symptoms are indicated above C7 and a reduction in intensity occurs due to knee flexion (Figure 2).
Figure 2.
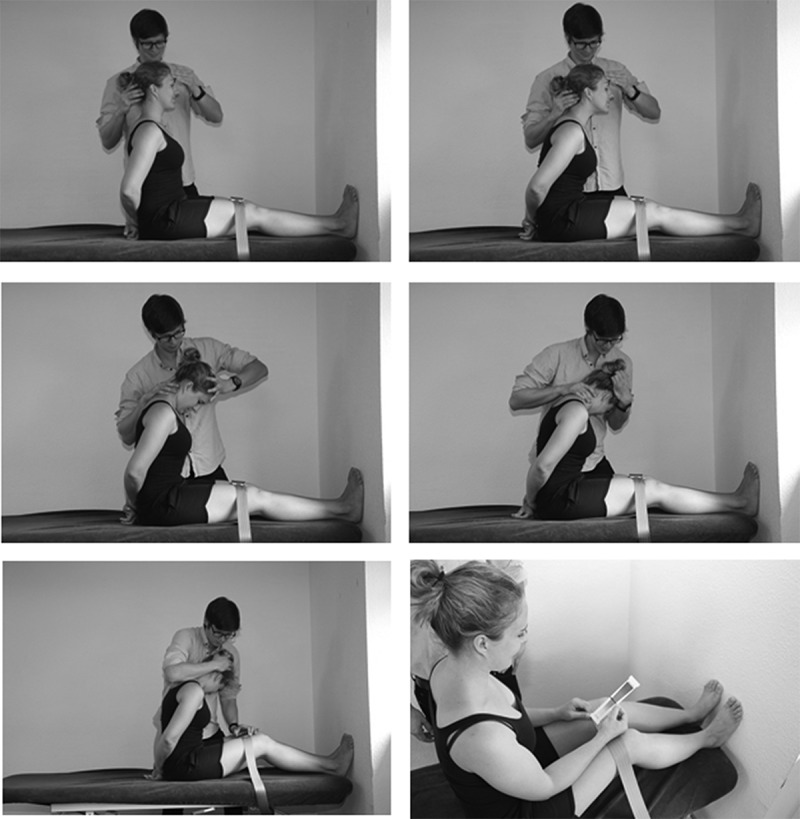
Standardized procedure of the occipitalis longsitting-slump (OLSS). Starting position (top left), slump position with neutral cervical spine (top right), cervical flexion (center left), lateral flexion and ipsilateral rotation (center right), release belt (lower left) and evaluation with visual analogue scale (lower right).
Examiner and training
The two assessors were blinded toward the headache status (patient or control) and diagnosis (migraine or CGH). Both researchers are postgraduate physiotherapists and manual therapist with at least 5 years experience in the assessment and treatment of musculoskeletal disorders. On three different days, all examiners completed a comprehensive multi-hour training, 2 h each day, of the examination techniques described above. They were supervised several times before the data collection. In addition, videos and scripts were distributed. For detecting inter-rater and intra-rater reliability before study start, both assessors examined two subjects with SDHNP (chronic migraine, CGH) three times in a random order. According to PPTs and VAS intra-class correlation for intra-rater reliability (ICC 3,3) and inter-rater reliability (ICC 2,3) was used. Cohens Kappa was calculated for localization while OLSS. Reliability testing revealed a good inter-rater (ICC 0.79; 95% CI 0.67–0.93) and intra-rater (ICC 0.90; 95% CI 0.83–0.95) reliability for PPT measurements. For OLSS (VAS), good values were obtained for inter-rater (ICC 0.72; 95% CI 0.61–0.89) and intra-rater (ICC 0.83; 95% CI 0, 60–0.93) reliabilty. For the localization during the OLSS, values of Kappa 0.79 and 0.82 could be shown.
Subjective outcomes
Head and neck pain symptoms, such as pain intensity, localization, duration and frequency were recorded on a questionnaire. Furthermore, the Migraine Disability Assessment for headache-related impairment[74], the Neck Dissability Index for neck pain-associated impairment[75] and the Patient Health Questionnaire[76], an assessment for depression, were evaluated. Headache patients were asked to draw their typical symptoms of the head and neck pain into a body chart.
Sample size calculation
The sample size was determined using data from a previous study [46]. Pressure pain thresholds were evaluated on different points including GON in subjects with CGH, migraine, tension-type headache and a healthy control group. For the group differences of subjects with CGH and migraine in comparison to healthy controls, an effect size of 0.67 was shown. Since data for a linear regression were not present, the sample calculation was performed on the basis of t-tests for independent samples. For an alpha of 0.05 and a power of 80%, a group size of n = 36, total n = 72, was determined.
Statistical analysis
Data were analyzed using SPSS statistical package (24.0 Version). To investigate significant group differences for sociodemographic data, unpaired t-tests for independent samples and chi2-tests were used depending on the type of data. In a further procedure, a backwards elimination confounder analysis with a binary logistic regression was applied for age, sex, height, and weight or sports frequency. Whether potential confounders have a relevant influence was tested with a stepwise linear regression. If the regression coefficients change by more than 10%, analysis was adjusted for the relevant confounders [77]. Pain localizations during OLSS were examined with chi2-test. In addition, sensitivity, specificity, positive and negative likelihood ratios and predictive values were calculated for OLSS. For subgroup differences, chi2-tests, Kruskal–Wallis test and analysis of variance were performed. Scheffé post hoc tests were applied. Whether a significant difference between sides existed was explored by paired t-tests. All parametric data were tested for normal distribution with the Kolmogorov–Smirnov test and for homoscedasticity with Levene’s test. A p value <.05 was considered statistically significant.
Results
A total of 67 headache patients were contacted. During telephone preselection, 24 were excluded because they did not fulfill the eligibility criteria. The remaining 43 subjects were invited for an examination appointment. After the examination, a further 5 participants were excluded because of chronic dysfunctional orofacial facial pain (n = 2), craniomandibular disorders (n = 2) and pain medication on more than 10 days per month (n = 1). The remaining 38 headache patients were diagnosed as CGH (n = 13), episodic migraine (n = 5) and chronic migraine (n = 20). Additionally, 38 asymptomatic control participants were included. Regarding demographic data (age, sex, height, sports frequency and sports type), no significantly differences were shown (Table 1). However, a significant difference in weight was detected between the headache group 72.9 kg (SD 12.8) and the control group 67.8 kg (SD 9.0) (p = 0.047). Furthermore, weight (p = 0.011) and gender (p = 0.053) were identified by logistic regression as potentially relevant confounders (R 2 = 0.137).
Table 1.
Sociodemographic and headache characteristics.
| SDHNP (n = 38) | CG (n = 38) | P value | ||
|---|---|---|---|---|
| Age | Years x̅ (SD) | 41.8 (12.2) | 38.0 (12.1) | 0.18 |
| Sex | m:f | 6:32 | 8: 30 | 0.70 |
| Height | cm x̅ (SD) | 171.1 (8.1) | 172.8 (SD 7.8) | 0.56 |
| Weight | Kg x̅ (SD) | 72.9 (12.8) | 67.8 (SD 9.0) | 0.047* |
| Sport frequency | Per week | 1.3 (1.1) | 1.6 (SD 0.8) | 0.15 |
| Sport | No sport Ball sports Endurance Gymnastics Strength training |
10 4 12 6 6 |
5 5 12 7 9 |
0.63 |
| Headache at examination date | 11 | 0 | ||
| Headache duration | Years x̅ (SD) | 11.9 (10.8) | ||
| Headache intensity | NRS (IQR) | 6.5 (1) | ||
| Headache frequencies | Days per month | 14 (8.1) | ||
| Neck pain intensity | NRS (IQR) | 4 (2.4) | ||
| Midas | Median (IQR) | 27 (26) | ||
| NDI | Median (IQR) | 13 (7.75) | ||
| PHQ-9 | Median (IQR) | 7 (6) | ||
SDHNP: side-dominant head and neck pain, CG: control group, Midas: migraine disability assessment, NDI: neck disability index, PHQ-9: patient health questionnaire, x̅ mean, SD: standard deviation, NRS: numeric rating scale, IQR: interquartile range, * <0.05.
Primary analysis
In the control group, no statistically significant difference was observed for PPT between left and right side at OP (p = 0.974) and NL (p = 0.953). Therefore, for all further analyses, mean values from both sides were used for healthy controls. In GON PPT measurement at the level of the OP, a significant difference was shown between subjects with SDHNP and healthy controls: the pain-dominant side (mean = 1.89 Kg/cm2 (SD 0.84)) showed a significant difference (p = 0.001) compared to the control group (mean = 2.56 Kg/cm2 (SD 0.76)). In addition, the non-domaint side, when compared to the control group, also showed a significant difference (p = 0.005) (Figure 3). There was no difference between the dominant and the non-dominant side in subjects with SDHNP (p = 0.156). An adjustment of confounders was not necessary. In the PPT measurement at NL, no significant difference was detected between the SDHNP and the control group. Furthermore, no difference was shown between dominant and non-dominant side (p = 0.115) (Table 2). A confounder adjustment by sex and weight was required but did not influence the results.
Figure 3.
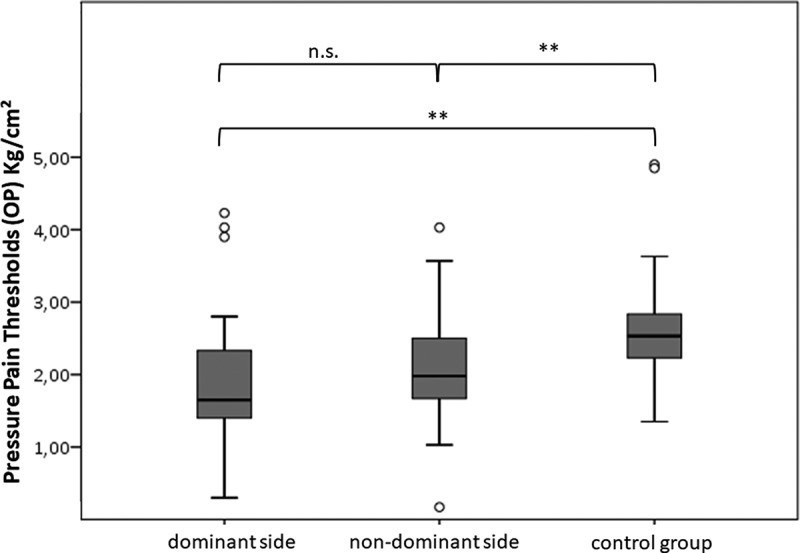
Greater occipital nerve (GON) pressure pain thresholds (PPTs) horizontal to occipital protuberance (OP) at the dominant side, non-dominant side and a control group.
Table 2.
Greater occipital nerve tests in subjects with side-dominant head and neck pain and a healthy control group.
| Side | SDHNPx̅ (SD; SEM) |
CG x̅ (SD; SEM) | Mean difference | 95% Confidence interval of the difference |
p value | |
|---|---|---|---|---|---|---|
| PPT OP (Kg/cm2) | Pain-dominant | 1.89 (0.84; 0.14) | −0.66 | −1.03; −0.30 | 0.001** | |
| Non-dominant | 2.07 (0.72; 0.12) | −0.48 | −0.82; −0.15 | 0.005** | ||
| Mean | 1.98 (0.78; 0.13) | 2.56 (0.76; 0.1) | −0.57 | −0.90; −0.25 | 0.001** | |
| PPT NL (Kg/cm2) | Pain-dominant | 2.07 (0.99; 0.16) | −0.29 | −0.72; 0.15 | 0.296 (ad) | |
| Non-Dominant | 2.25 (1.03; 0.16) | −0.11 | −0.55; 0.33 | 0.805 (ad | ||
| Mean | 2.16 (1.00; 0.6) | 2.36 (0.90; 0.17) | −0.20 | −0.62; 0.23 | 0.406 (ad) | |
| OLSS (VAS) | Pain-dominant | 4.81 (2.56; 0.43) | 2.44 | 1.45; 3.44 | <0.001*** | |
| Non-dominant | 3.98 (2.01; 0.34) | 1.61 | 0.77; 2.46 | <0.001*** | ||
| Mean | 4.27 (2.17; 0.39) | 2.37 (1.68; 0.39) | 1.90 | 1.03; 2.78 | <0.001*** | |
| OLSS (VAS) above C7 | Pain-dominant | 5.73 (2.26; 0.49) n = 21 |
2.62 | 1.32; 3.93 | <0.001*** | |
| Non-dominant | 4.56 (1.75; 0.39) n = 20 |
1.45 | 0.34; 2.57 | 0.001** | ||
| Mean | 5.00 (2.03; 0.44) n = 26 |
3.11 (1.56; 0.44) n = 17 |
1.89 | 0.72; 3.06 | 0.012 *(ad)) |
SDHNP: side-dominant head and neck pain, CG: control group, PPTs: pressure pain thresholds, OP: occipital protuberance, NL: nucheal line, OLSS: Occiput Longsitting-slump, OLSS above C7 localizations during OLSS: face, occiput, cervical spine, x̅ mean, SD standard deviation, SEM standard error of the mean, ad adjustment using Confounder variables gender and weight, * <0.05, ** <0.01, ***<0.001.
For the OLSS, no statistically significant difference between sides was shown in the control group. As for the PPTs, mean values across sides were used for further analyses (Figure 4). Reported VAS during OLSS showed a statistically significant difference for all comparisons between the headache and the control group: the pain-dominant and the non-dominant side were significantly different from the control group (p < 0.001) (Table 2). A significant difference was found between the dominant and non-dominant side for the OLSS (p = 0.004) and the OLSS with symptom localization above C7 (p = 0.045). A confounder adjustment was necessary for OLSS above C7. This again did not alter the interpretation of the significance. There was no side-to-side difference within the headache or control group for pain localizations, face, occiput, cervical spine, thoracic spine, lumbar spine or legs, during OLSS (p > 0.05). Within the SDHNP group, 23 subjects showed pain localizations above C7 on the pain-dominant side during OLSS. A relief by knee flexion was shown on the dominant side in 18 subjects. This results in a sensitivity of 47% and an average specificity of 75% (left 76%, right 75%) and a positive and negative likelihood ratio of 0.63 and 0.70 and an average positive and negative predictive value of 0.65 and 0.58, respectively.
Figure 4.
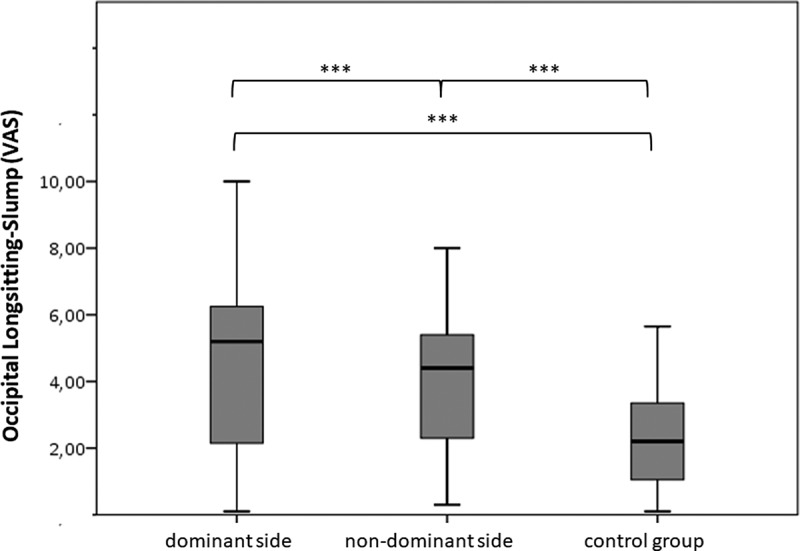
Visual analogue scale (VAS) during the occipitalis longsitting-slump (OLSS) at the dominant side, non-dominant side and a control group.
Secondary analyses
Subjects with SDHNP which suffered from headaches at the day of examination (n = 11) did not differ from those who did not have headaches (n = 27) in terms of pressure sensitivity (PPT) neither at the OP, nor at the NL at both sides (p > 0.05). A difference between both groups for dominant (p = 0.016) and non-dominant side (p = 0.017) was shown in OLSS. Subjects who indicated their typical headache in the sensory area of the GON (n = 14) differed in PPTs at the OP with those who indicated their headache elsewhere (n = 24) on the pain-dominant (p = 0.025) and non-dominant side (p = 0.02). There was no difference in the pressure sensitivity of the PPT at the NL or the OLSS (p > 0.05). For subgroup analysis, a statistically significant difference was found for the GON pressure sensitivity at OP between subjects with CGH and a control group (p = 0.004). No differences were identified at the NL or for other headache subgroups. For OLSS, the difference between the CHG and controls (p = 0.001) as well as between CM and controls was shown for VAS (p = 0.001).
Discussion
Both sides, pain-dominant and non-dominant, in subjects with SDHNP showed a lower pressure-mechanosensitivity of the GON at the OP compared to a healthy control group, but not at the NL. During OLSS, significantly higher VAS values were reported for all comparisons between SDHNP and healthy controls.
The present study confirms the results of the very limited available studies on cranial nerve mechanosensitivity: an increased pressure sensitivity over supra- and infraorbital nerves was previously shown in various types of headaches [39,41,42]. The GON was evaluated in several studies in subjects with headaches compared to control groups [46–49]. The results for PPT shown in the present study were consistent with Bovim et al [46]. In another study, GON PPTs were compared between subjects with CGH and subjects with neck pain [47]. Differences were shown between sides within the CGH group, but not for between-group comparison. However, investigators were not blinded[46,47] and with regard to a large variance of reported GON localizations[78], identification of the GON was not or only insufficiently reported [46–49].
Neurodynamic tests in subjects with headaches were previously investigated [49–51]. Results of this current study were similar to Piekartz et al. [50], who described an intense sensory response during the longsitting-slump in children with headache. A reproduction of headaches during a neurodynamic test was previously shown in subjects with migraine [51]. This could not be reproduced in this present study. Regarding the presence of headache symptoms on the examination day, no significant difference was recorded for the PPTs over the GON. However, SDHNP subjects with current headache were more sensitive during OLSS than SDHNP subjects without current headache symptoms. This is comparable to a previous publication on the FRT [79].
In neurodynamic testing, sensitizing movements are carried out as far away as possible from the location of pain. Theoretically, mechanosensitivity of the nervous system is influenced by movements in the periphery [80]. For OLSS, however, compared to a control group, pain relief during sensitizing movements was not observed in many participants and low diagnostic accurancy was shown. One possible explanation might be that not only nerves, but also extraneural structures are stressed during the testing procedure. The OLSS is based primarily on a biomechanical in vitro study [81]. Vital described during a cervical flexion and rotation manoeuvre an increase in tension of the GON. This has never been confirmed in imaging studies. An explanation for increased mechanosensivity could be a bridge of connective tissue between the rectus capitis posterior minor muscle and the dorsal dura mater [82]. It is possible that during the OLSS procedure, a mechanical stimulus is transmitted to the dura [83]. Yuan et al. showed in a cross-sectional study an association between the hypertrophy of rectus capitis posterior minor with chronic headaches [84].
This current study showed no difference between the dominant and the non-dominant headache side regarding the pressure sensitivity. This is best explained by central sensitization. Various mechanisms could be involved [42]: Antidromal discharges of the central nervous system can sensitize peripheral nerves. The nociceptive fibers of the nervi nervorum could be sensitized [80,85]. Alternatively, neurons in the posterior horn could be depolarized during central sensitizations, which can also lead to an increased pain perception in other areas [86,87]. Palpation of asymptomatic nerves can lead to allodynia due to a reduced inhibition and an overactive central nervous system [88]. Central sensitization may also be an explanation for the similarity of some of the symptoms in CGH and Migraine [16,53]. However, more research is required to distinguish the effect of central sensitization between the various types of headaches.
Although different pathomechanisms exist, clinical signs of migraine and CGH can be similar [53,89]. It has also been hypothesized that migraine and CGH can co-exist [90]. One study reported that in 44% of all headaches, more than one headache diagnosis was attributed [91] and over 30% of subjects with CHG also met the criteria for migraine [92]. Other studies showed that 42% of subjects with CGHs indicated a simultaneous migraine [56]. Hall et al. suggest that migraine, CGH may be a continuum across different headache forms [31].
Limitations
It might be questioned whether the applied procedure was precise enough to identify the GON by locating the most sensitive site. Ducic et al. described considerable variations of the GON [78]. In the occipital area, other structures could be highly sensitive, e.g. epicranial structures. However, the GON is the main sensory nerve of the occiput [93] and accordingly, the sensitivity to palpation appears to be high. Another method of localizing the GON would be via diagnostic ultrasound [94–96]. Nevertheless, a high reliability of this procedure has been found previously [48,73].
There are two commonly used classification systems for CGH [54,69]: The diagnostic criteria of the CHISG were chosen since the criteria of the International Classification of Headache Disorders (ICHD-3 beta) appeared to be not feasible for this study. It has been shown that findings in imaging methods such as x-ray [97], MRI [98,99] or discrimitative, laboratory-based evidence [89] are not related to CGH. Likewise, in subjects with CGH, effectiveness of anesthetic blocks can neither be verified nor dismissed [19,21,22,100,101].. The remaining criteria of the ICHD-3 and the corresponding comments are comparable to the criteria of the CHISG. In general, CHISG provides more practical and specific criteria for the clinical diagnosis of CGH [102].
Conclusion
The pressure sensitivity over the GON at the occipital area was shown to be lower in subjects with SDHNP than in a healthy control group. This was also shown for the subgroup of CGH. A difference in the pressure sensitivity of the GON at the NL could not be shown. There were no differences between the dominant and the non-dominant headache side. During OLSS, significantly higher pain intensity was reported by participants with SDHNP compared to the control group. The pain-dominant side was significantly more sensitive compared to the non-dominant side. The OLSS showed a low diagnostic accuracy, but has a potential relevance for the clinical reasoning regarding the decision on a potentially effective treatment strategy in the individual headache patient.
Supplementary Material
Appendix 1.
STROBE Statement – Checklist of items that should be included in reports of case-control studies
| Item No | Recommendation | Reported | ||
|---|---|---|---|---|
| Title and abstract | 1 | (a) Indicate the study’s design with a commonly used term in the title or the abstract | Yes | Title page |
| (b) Provide in the abstract an informative and balanced summary of what was done and what was found | Yes | Abstract | ||
| Introduction | Page | |||
| Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported | Yes | 1–2 |
| Objectives | 3 | State specific objectives, including any prespecified hypotheses | Yes | 2 |
| Methods | ||||
| Study design | 4 | Present key elements of study design early in the paper | Yes | 3 |
| Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up and data collection | Yes | 3 |
| Participants | 6 | (a) Give the eligibility criteria, and the sources and methods of case ascertainment and control selection. Give the rationale for the choice of cases and controls | Yes | 3–5 |
| (b) For matched studies, give matching criteria and the number of controls per case | Not applicable | |||
| Variables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders and effect modifiers. Give diagnostic criteria, if applicable | Yes | 4–6 |
| Data sources/measurement | 8* | For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group | Yes | 4–6 |
| Bias | 9 | Describe any efforts to address potential sources of bias | Yes | 6–7 |
| Study size | 10 | Explain how the study size was arrived at | Yes | 7–8 |
| Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why | Yes | 8 |
| Statistical methods | 12 | (a) Describe all statistical methods, including those used to control for confounding | Yes | 8 |
| (b) Describe any methods used to examine subgroups and interactions | Yes | 8 | ||
| (c) Explain how missing data were addressed | Not applicable | |||
| (d) If applicable, explain how matching of cases and controls was addressed | Not applicable | |||
| (e) Describe any sensitivity analyses | Not applicable | |||
| Results | ||||
| Participants | 13* | (a) Report numbers of individuals at each stage of study – e.g. numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up and analyzed | Yes | 8–9 |
| (b) Give reasons for non-participation at each stage | Not applicable | |||
| (c) Consider use of a flow diagram | Not applicable | |||
| Descriptive data | 14* | (a) Give characteristics of study participants (e.g. demographic, clinical, social) and information on exposures and potential confounders | Yes | 9 |
| (b) Indicate number of participants with missing data for each variable of interest | Not applicable | |||
| Outcome data | 15* | Report numbers in each exposure category or summary measures of exposure | Yes | 9–10 |
| Main results | 16 | (a) Give unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (e.g., 95% confidence interval). Make clear which confounders were adjusted for and why they were included | Yes | 9–10 |
| (b) Report category boundaries when continuous variables were categorized | Yes | 9–10 | ||
| (c) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period | Not applicable | |||
| Other analyses | 17 | Report other analyses done – e.g. analyses of subgroups and interactions, and sensitivity analyses | Yes | 10–11 |
| Discussion | ||||
| Key results | 18 | Summarise key results with reference to study objectives | Yes | 11 |
| Limitations | 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias | Yes | 13–14 |
| Interpretation | 20 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies and other relevant evidence | Yes | 11–12 |
| Generalisability | 21 | Discuss the generalizability (external validity) of the study results | Yes | 11–13 |
| Other information | ||||
| Funding | 22 | Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based | Yes | Title page |
Funding Statement
International Maitland Teacher Association within the Geoff Maitland Research Grant Foundation.
Acknowledgments
We thank the International Maitland Teacher Association (IMTA) for a supporting fund or realizing the logistic part of this study.
Disclosure statement
We certify that we do not have any conflicts of interest in the study.
References
- [1]. D’Amico D, Leone M, Bussone G.. Side-locked unilaterality and pain localization in long-lasting headaches: migraine, tension-type headache, and cervicogenic headache. Headache. 1994;34(9):526–530. [DOI] [PubMed] [Google Scholar]
- [2]. Prakash S, Rathore C. Side-locked headaches: an algorithm-based approach. J Headache Pain. 2016;17(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Prakash S, Rathore C, Makwana P, et al. Study in patients with side-locked unilateral headache and facial pain. Headache. 2016;56(7):1183–1193. [DOI] [PubMed] [Google Scholar]
- [4]. Ramon C, Mauri G, Vega J, et al. Diagnostic distribution of 100 unilateral, side-locked headaches consulting a specialized clinic. Eur Neurol. 2013;69(5):289–291. [DOI] [PubMed] [Google Scholar]
- [5]. Da Silva AN, Tepper SJ, Evans RW. Side-locked and side shifting primary headaches. Headache. 2012;52(7):1178–1183. [DOI] [PubMed] [Google Scholar]
- [6]. Ashina S, Bendtsen L, Lyngberg AC, et al. Prevalence of neck pain in migraine and tension-type headache: a population study. Cephalalgia. 2015;35(3):211–219. [DOI] [PubMed] [Google Scholar]
- [7]. Fernandez-de-Las-Penas C, Cuadrado ML, Arendt-Nielsen L, et al. Side-to-side differences in pressure pain thresholds and pericranial muscle tenderness in strictly unilateral migraine. Eur J Neurol. 2008;15(2):162–168. [DOI] [PubMed] [Google Scholar]
- [8]. Laimi K, Salminen JJ, Metsahonkala L, et al. Characteristics of neck pain associated with adolescent headache. Cephalalgia. 2007;27(11):1244–1254. [DOI] [PubMed] [Google Scholar]
- [9]. Oksanen A, Poyhonen T, Ylinen JJ, et al. Force production and EMG activity of neck muscles in adolescent headache. Disabil Rehabil. 2008;30(3):231–239. [DOI] [PubMed] [Google Scholar]
- [10]. Calhoun AH, Ford S, Pruitt AP. Presence of neck pain may delay migraine treatment. Postgrad Med. 2011;123(2):163–168. [DOI] [PubMed] [Google Scholar]
- [11]. Pietrobon D, Moskowitz MA. Pathophysiology of migraine. Annu Rev Physiol. 2013;75:365–391. [DOI] [PubMed] [Google Scholar]
- [12]. Bogduk N, Govind J. Cervicogenic headache: an assessment of the evidence on clinical diagnosis, invasive tests, and treatment. Lancet Neurol. 2009;8(10):959–968. [DOI] [PubMed] [Google Scholar]
- [13]. Cervicogenic Headache: BW. Evidence that the neck is a pain generator. Headache. 2010;40(4):699–705. [DOI] [PubMed] [Google Scholar]
- [14]. Frese A, Schilgen M, Husstedt I-W ES. Pathophysiology and clinical manifestation of cervicogenic headache. Schmerz. 2003;17(2):125–130. [DOI] [PubMed] [Google Scholar]
- [15]. Jensen S. Neck related causes of headache. Aust Fam Physician. 2005;34(8):635–639. [PubMed] [Google Scholar]
- [16]. HJMv P, Andreotti D, Arendt-Nielsen L, et al. Gesichts- und zervikalregion: neuromuskuloskeletale untersuchung, therapie und management. 2nd ed. stuttgart. New York: Georg Thieme Verlag; 2015. [Google Scholar]
- [17]. Ambrosini A, Schoenen J. Invasive pericranial nerve interventions. Cephalalgia. 2016. [DOI] [PubMed] [Google Scholar]
- [18]. Baron EP, Cherian N, Tepper SJ. Role of greater occipital nerve blocks and trigger point injections for patients with dizziness and headache. Neurologist. 2011;17(6):312–317. [DOI] [PubMed] [Google Scholar]
- [19]. Gross A, Peloso P, Galway E, et al Suppl.4; Physician-delivered injection therapies for mechanical neck disorders: a systematic review update (non-oral, non-intravenous pharmacological interventions for neck pain). Open J Orthop.2013;(7):562–581. 7, 562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Lauretti GR, Correa SWRO, Mattos AL. Efficacy of the greater occipital nerve block for cervicogenic headache: comparing classical and subcompartmental techniques. Pain Pract. 2015;15(7):654–661. [DOI] [PubMed] [Google Scholar]
- [21]. Tobin J, Flitman S. Occipital nerve blocks: when and what to inject? Headache. 2009;49(10):1521–1533. [DOI] [PubMed] [Google Scholar]
- [22]. Voigt CL, Murphy MO. Occipital nerve blocks in the treatment of headaches: safety and efficacy. J Emerg Med. 2015;48(1):115–129. [DOI] [PubMed] [Google Scholar]
- [23]. Cuadrado ML, Aledo-Serrano A, Lopez-Ruiz P, et al. Greater occipital nerve block for the acute treatment of prolonged or persistent migraine aura. Cephalalgia. 2016;37(8):812–818. [DOI] [PubMed] [Google Scholar]
- [24]. Cuadrado ML, Aledo-Serrano A, Navarro P, et al. Short-term effects of greater occipital nerve blocks in chronic migraine: a double-blind, randomised, placebo-controlled clinical trial. Cephalalgia. 2016;37(9):864–872. [DOI] [PubMed] [Google Scholar]
- [25]. Gul HL, Ozon AO, Karadas O, et al. The efficacy of greater occipital nerve blockade in chronic migraine: a placebo-controlled study. Acta Neurol Scand. 2016;136(2):138–144. [DOI] [PubMed] [Google Scholar]
- [26]. Okmen K, Dagistan Y, Dagistan E, et al. Efficacy of the greater occipital nerve block in recurrent migraine type headaches. Neurol Neurochir Pol. 2016;50(3):151–154. [DOI] [PubMed] [Google Scholar]
- [27]. D’Ostilio K, Invasive MD. Non-invasive electrical pericranial nerve stimulation for the treatment of chronic primary headaches. Curr Pain Headache Rep. 2016;20(11):61. [DOI] [PubMed] [Google Scholar]
- [28]. Yang Y, Song M, Fan Y, et al. Occipital nerve stimulation for migraine: a systematic review. Pain Pract. 2016;16(4):509–517. [DOI] [PubMed] [Google Scholar]
- [29]. Zhou L, Ashkenazi A, Smith JW, et al. Outcome of peripheral nerve stimulation for chronic headache and complication prevention. Anesth Pain Med. 2016;6(4):e35983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Piovesan EJ, Di Stani F, Kowacs PA, et al. Massaging over the greater occipital nerve reduces the intensity of migraine attacks: evidence for inhibitory trigemino-cervical convergence mechanisms. Arq. Neuro-Psiquiatr. 2007;65(3a):599–604. [DOI] [PubMed] [Google Scholar]
- [31]. Hall T, Briffa K, Hopper D. Clinical evaluation of cervicogenic headache a clinical perspective. J Man Manip Ther. 2008;16(2):73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Howard PD, Behrns W, Di Martino M, et al. Manual examination in the diagnosis of cervicogenic headache: a systematic literature review. J Man Manip Ther. 2015;23(4):210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Rubio-Ochoa J, Benitez-Martinez J, Lluch E, et al. Physical examination tests for screening and diagnosis of cervicogenic headache: a systematic review. Man Ther. 2016;21:35–40. [DOI] [PubMed] [Google Scholar]
- [34]. Liebert A, Rebbeck T, Elias S, et al. Musculoskeletal physiotherapists’ perceptions of non-responsiveness to treatment for cervicogenic headache. Physiother Theory Pract. 2013;29(8):616–629. [DOI] [PubMed] [Google Scholar]
- [35]. Von PH, Moog E. Palpation peripherer Nerven: auf den Nerv gefühlt. Physiopraxis. 2006;4(07/08):22–26. [Google Scholar]
- [36]. Vanderweeen L, Oostendorp RA, Vaes P, et al. Pressure algometry in manual therapy. Man Ther. 1996;1(5):258–265. [DOI] [PubMed] [Google Scholar]
- [37]. Fernández-de-las-Peñas C, Arendt-Nielsen L, Gerwin R. Tension-type and cervicogenic headache: pathophysiology, diagnosis, and management. Sudbury, Mass: Jones and Bartlett Publishers; 2010. [Google Scholar]
- [38]. Shacklock MO. Clinical neurodynamics: a new system of musculoskeletal treatment. Edinburgh, New York: Elsevier Butterworth-Heinemann; 2005. [Google Scholar]
- [39]. Fernandez-de-Las-Penas C, Arendt-Nielsen L, Cuadrado ML, et al. Generalized mechanical pain sensitivity over nerve tissues in patients with strictly unilateral migraine. Clin J Pain. 2009;25(5):401–406. [DOI] [PubMed] [Google Scholar]
- [40]. Fernandez-de-Las-Penas C, Fernandez-Mayoralas DM, Ortega-Santiago R, et al. Bilateral, wide-spread, mechanical pain sensitivity in children with frequent episodic tension-type headache suggesting impairment in central nociceptive processing. Cephalalgia. 2010;30(9):1049–1055. [DOI] [PubMed] [Google Scholar]
- [41]. Fernandez-de-Las-Penas C, Ortega-Santiago R, Cuadrado ML, et al. Bilateral widespread mechanical pain hypersensitivity as sign of central sensitization in patients with cluster headache. Headache. 2011;51(3):384–391. [DOI] [PubMed] [Google Scholar]
- [42]. Fernandez-de-Las-Penas C, Coppieters MW, Cuadrado ML, et al. Patients with chronic tension-type headache demonstrate increased mechano-sensitivity of the supra-orbital nerve. Headache. 2008;48(4):570–577. [DOI] [PubMed] [Google Scholar]
- [43]. Fernandez-de-Las-Penas C, Cuadrado ML. Physical therapy for headaches. Cephalalgia. 2015;36(12):1134–1142. [DOI] [PubMed] [Google Scholar]
- [44]. Scott D, Jull G, Sterling M. Widespread sensory hypersensitivity is a feature of chronic whiplash-associated disorder but not chronic idiopathic neck pain. Clin J Pain. 2005;21(2):175–181. [DOI] [PubMed] [Google Scholar]
- [45]. Sterling M, Treleaven J, Jull G. Responses to a clinical test of mechanical provocation of nerve tissue in whiplash associated disorder. Man Ther. 2002;7(2):89–94. [DOI] [PubMed] [Google Scholar]
- [46]. Bovim G. Cervicogenic headache, migraine, and tension-type headache. Pressure-Pain Threshold Measurements. Pain. 1992;51(2):169–173. [DOI] [PubMed] [Google Scholar]
- [47]. Chua NHL, Van Suijlekom HA, Vissers KC, et al. Differences in sensory processing between chronic cervical zygapophysial joint pain patients with and without cervicogenic headache. Cephalalgia. 2011;31(8):953–963. [DOI] [PubMed] [Google Scholar]
- [48]. Sand T, Zwart JA, Helde G, et al. The reproducibility of cephalic pain pressure thresholds in control subjects and headache patients. Cephalalgia. 1997;17(7):748–755. [DOI] [PubMed] [Google Scholar]
- [49]. Zito G, Jull G, Story I. Clinical tests of musculoskeletal dysfunction in the diagnosis of cervicogenic headache. Man Ther. 2006;11(2):118–129. [DOI] [PubMed] [Google Scholar]
- [50]. Von PH, Schouten S, Aufdemkampe G. Neurodynamic responses in children with migraine or cervicogenic headache versus a control group. A comparative study. Man Ther. 2007;12(2):153–160. [DOI] [PubMed] [Google Scholar]
- [51]. Rankin G. Are the neuromeningeal tissue a potential source of headache?: an investigation into effective testing. Manipulative Physiotherapist. 1993;25:156–167. [Google Scholar]
- [52]. Von EE, Dg A, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573–577. [DOI] [PubMed] [Google Scholar]
- [53]. Sjaastad O. Cervicogenic headache: comparison with migraine without aura; Vaga study. Cephalalgia. 2008;28(Suppl 1):18–20. [DOI] [PubMed] [Google Scholar]
- [54]. Headache Classification Committee of the International Headache Society (IHS) The International Classification Of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629–808. [DOI] [PubMed] [Google Scholar]
- [55]. Persson LCG, Carlsson JY, Anderberg L. Headache in patients with cervical radiculopathy: a prospective study with selective nerve root blocks in 275 patients. Eur Spine J. 2007;16(7):953–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56]. Knackstedt H, Bansevicius D, Aaseth K, et al. Cervicogenic headache in the general population: the Akershus study of chronic headache. Cephalalgia. 2010;30(12):1468–1476. [DOI] [PubMed] [Google Scholar]
- [57]. Hall TM, Briffa K, Hopper D, et al. Comparative analysis and diagnostic accuracy of the cervical flexion-rotation test. J Headache Pain. 2010;11(5):391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58]. Maitland GD. Manipulation der Wirbelsäule. 4th ed. Heidelberg: Springer; 2008. [Google Scholar]
- [59]. Limmroth V, Katsarava Z, Fritsche G, et al. Features of medication overuse headache following overuse of different acute headache drugs. Neurology. 2002;59(7):1011–1014. [DOI] [PubMed] [Google Scholar]
- [60]. Türp J, Nilges P. Diagnostik von Patienten mit chronischen orofazialen Schmerzen: die deutsche version des “Graded Chronic Pain Status”. Quintessenz. 2000;51(7):721–727. [Google Scholar]
- [61]. Hawker GA, Mian S, Kendzerska T, et al. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res (Hoboken). 2011;63(Suppl 11):S240–52. [DOI] [PubMed] [Google Scholar]
- [62]. Von KM, Ormel J, Fj K, et al. Grading the severity of chronic pain. Pain. 1992;50(2):133–149. [DOI] [PubMed] [Google Scholar]
- [63]. Penny KI, Purves AM, Smith BH, et al. Relationship between the chronic pain grade and measures of physical, social and psychological well-being. Pain. 1999;79(2–3):275–279. [DOI] [PubMed] [Google Scholar]
- [64]. Salaffi F, Stancati A, Grassi W. Reliability and validity of the Italian version of the Chronic Pain Grade questionnaire in patients with musculoskeletal disorders. Clin Rheumatol. 2006;25(5):619–631. [DOI] [PubMed] [Google Scholar]
- [65]. Smith BH, Penny KI, Purves AM, et al. The Chronic Pain Grade questionnaire: validation and reliability in postal research. Pain. 1997;71(2):141–147. [DOI] [PubMed] [Google Scholar]
- [66]. Clark GT, Seligman DA, Solberg WK, et al. Guidelines for the examination and diagnosis of temporomandibular disorders. J Craniomandib Disord. 1989;3(1):7–14. [PubMed] [Google Scholar]
- [67]. Gerstner G, Clark G, Goulet J. Validity of a brief questionnaire in screening asymptomatic subjects from subjects with tension-type headaches or temporomandibular disorders. Community Dent Oral Epidemiol. 1994;22(4):235. [DOI] [PubMed] [Google Scholar]
- [68]. Undt G, Murakami K-I, Clark GT, et al. Cross-cultural adaptation of the JPF-questionnaire for German-speaking patients with functional temporomandibular joint disorders. J Craniomaxillofac Surg. 2006;34(4):226–233. [DOI] [PubMed] [Google Scholar]
- [69]. Sjaastad O, Fredriksen TA, Pfaffenrath V. Cervicogenic headache: diagnostic criteria. The Cervicogenic Headache International Study Group. Headache. 1998;38(6):442–445. [DOI] [PubMed] [Google Scholar]
- [70]. Loukas M, El-Sedfy A, Tubbs R, et al. Identification of greater occipital nerve landmarks for the treatment of occipital neuralgia. Folia Morphol. 2006;4(65):337–342. [PubMed] [Google Scholar]
- [71]. Tubbs RS, Salter EG, Wellons JC, et al. Landmarks for the identification of the cutaneous nerves of the occiput and nuchal regions. Clin Anat. 2007;20(3):235–238. [DOI] [PubMed] [Google Scholar]
- [72]. Janis JE, Hatef DA, Ducic I, et al. The anatomy of the greater occipital nerve: part II. Compression Point Topography Plast Reconstr Surg. 2010;126(5):1563–1572. [DOI] [PubMed] [Google Scholar]
- [73]. Antonaci F, Sand T, Lucas GA. Pressure algometry in healthy subjects: inter-examiner variability. Scand J Rehabil Med. 1998;30(1):3–8. [DOI] [PubMed] [Google Scholar]
- [74]. Stewart WF, Lipton RB, Kolodner K, et al. Reliability of the migraine disability assessment score in a population-based sample of headache sufferers. Cephalalgia. 1999;19(2):107. [DOI] [PubMed] [Google Scholar]
- [75]. Swanenburg J, Humphreys K, Langenfeld A, et al. Validity and reliability of a German version of the Neck Disability Index (NDI-G). Man Ther. 2014;19(1):52–58. [DOI] [PubMed] [Google Scholar]
- [76]. Lowe B, Grafe K, Zipfel S, et al. Detecting panic disorder in medical and psychosomatic outpatients: comparative validation of the Hospital Anxiety and Depression Scale, the patient health questionnaire, a screening question, and physicians’ diagnosis. J Psychosom Res. 2003;55(6):515–519. [DOI] [PubMed] [Google Scholar]
- [77]. Stoddard G. Biostatistics and epidemiology using stata: a course manual: [unpublished manuscript] University of Utah School of Medicine; 2010. [Google Scholar]
- [78]. Ducic I, Moriarty M, Al-Attar A. Anatomical variations of the occipital nerves: implications for the treatment of chronic headaches. Plast Reconstr Surg. 2009;123(3): 859–863. discussion 864. [DOI] [PubMed] [Google Scholar]
- [79]. Hall TM, Briffa K, Hopper D, et al. The relationship between cervicogenic headache and impairment determined by the flexion-rotation test. J Manipulative Physiol Ther. 2010;33(9):666–671. [DOI] [PubMed] [Google Scholar]
- [80]. Butler DS. The neurodynamic techniques: a definitive guide from the Noigroup team. 1st ed. [Adelaide City West, S. Australia], Minneapolis Minn.: [Published by Noigroup Publications for NOI Australasia]; Distributed by OPTP; 2005. [Google Scholar]
- [81]. Vital J, Grenier F, Dautheribes M, et al. An anatomic and dynamic study of the greater occipital nerve (n. of Arnold). Surg Radiol Anat. 1989;11:205–210. [DOI] [PubMed] [Google Scholar]
- [82]. Hack GD, Koritzer RT, Robinson WL, et al. Anatomic relation between the rectus capitis posterior minor muscle and the dura mater. Spine. 1995;20(23):2484–2486. [DOI] [PubMed] [Google Scholar]
- [83]. Alix ME, Bates DK. A proposed etiology of cervicogenic headache: the neurophysiologic basis and anatomic relationship between the dura mater and the rectus posterior capitis minor muscle. J Manipulative Physiol Ther. 1999;22(8):534–539. [DOI] [PubMed] [Google Scholar]
- [84]. Yuan X-Y, Yu S-B, Liu C, et al. Correlation between chronic headaches and the rectus capitis posterior minor muscle: a comparative analysis of cross-sectional trail. Cephalalgia. 2017;37(11):1051–1056. [DOI] [PubMed] [Google Scholar]
- [85]. Bove GM, Light A. The nervi nervorum: missing link for neuropathic pain? Pain Forum. 1997;6(3):181–190. [Google Scholar]
- [86]. Hoheisel U, Mense S, Simons DG, et al. Appearance of new receptive fields in rat dorsal horn neurons following noxious stimulation of skeletal muscle: a model for referral of muscle pain? Neurosci Lett. 1993;153(1):9–12. [DOI] [PubMed] [Google Scholar]
- [87]. Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44(3):293–299. [DOI] [PubMed] [Google Scholar]
- [88]. Pielsticker A, Haag G, Zaudig M, et al. Impairment of pain inhibition in chronic tension-type headache. Pain. 2005;118(1–2):215–223. [DOI] [PubMed] [Google Scholar]
- [89]. Frese A, Evers S. Biological markers of cervicogenic headache. Cephalalgia. 2008;28(Suppl 1):21–23. [DOI] [PubMed] [Google Scholar]
- [90]. Sjaastad O, Fredriksen T, Pareja JA, et al. Coexistence of cervicogenic headache and migraine without aura (?). Funct Neurol. 1999;14(4):209–218. [PubMed] [Google Scholar]
- [91]. Fishbain DA, Cutler R, Cole B, et al. International Headache Society headache diagnostic patterns in pain facility patients. Clin J Pain. 2001;17(1):78–93. [DOI] [PubMed] [Google Scholar]
- [92]. Vincent MB, Luna RA. Cervicogenic headache: a comparison with migraine and tension-type headache. Cephalalgia. 1999;19(25 suppl):11–16. [DOI] [PubMed] [Google Scholar]
- [93]. Bogduk N. The clinical anatomy of the cervical dorsal rami. Spine. 1982;7(4):319–330. [DOI] [PubMed] [Google Scholar]
- [94]. Cho J, Haun D, Kettner N. Sonographic evaluation of the greater occipital nerve in unilateral occipital neuralgia. J Ultrasound Med. 2012;31(1):37–42. [DOI] [PubMed] [Google Scholar]
- [95]. Greher M, Moriggl B, Curatolo M, et al. Sonographic visualization and ultrasound-guided blockade of the greater occipital nerve: a comparison of two selective techniques confirmed by anatomical dissection. Br J Anaesth. 2010;104(5):637–642. [DOI] [PubMed] [Google Scholar]
- [96]. Shim JH, Ko SY, Bang MR, et al. Ultrasound-guided greater occipital nerve block for patients with occipital headache and short term follow up. Korean J Anesthesiol. 2011;61(1):50–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97]. Pfaffenrath V, Dandekar R, Mayer E, et al. Cervicogenic headache: results of computer-basedmeasurements of cervical spine mobility in 15 patients. Cephalalgia. 1988;8:45–48. [DOI] [PubMed] [Google Scholar]
- [98]. Coskun O, Ucler S, Karakurum B, et al. Magnetic resonance imaging of patients with cervicogenic headache. Cephalalgia. 2003;8(23):842–845. [DOI] [PubMed] [Google Scholar]
- [99]. Knackstedt H, Krakenes J, Bansevicius D, et al. Magnetic resonance imaging of craniovertebral structures: clinical significance in cervicogenic headaches. J Headache Pain. 2012;13(1):39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100]. Nagar V, Birthi P, Grider J, et al. Systematic review of radiofrequency ablation and pulsed radiofrequency for management of cervicogenic headache. Pain Physician. 2015;18:109–130. [PubMed] [Google Scholar]
- [101]. Ng A, Wang D. Cervical facet injections in the management of cervicogenic headaches. Curr Pain Headache Rep. 2015;19(5):484. [DOI] [PubMed] [Google Scholar]
- [102]. Fredriksen TA, Antonaci F, Sjaastad O. Cervicogenic headache: too important to be left un-diagnosed. J Headache Pain. 2015;16:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


