Abstract
Background
Recent guidelines recommend a tighter control of systolic blood pressure (SBP) in type 2 diabetes mellitus (T2DM). However, it is unclear whether intensive SBP lowering increases the incidence of chronic kidney disease (CKD) in T2DM.
Methods
Systolic Blood Pressure Intervention Trial (SPRINT) tested the effects of SBP goal < 120 vs. < 140 mm Hg in persons without diabetes. Action to Control Cardiovascular Risk in Diabetes (ACCORD) BP trial tested a similar SBP intervention in T2DM. In participants without CKD at baseline (N = 4305 in ACCORD BP; N = 6677 in SPRINT), we related SBP interventions to incident CKD (defined as a >30% decrease in eGFR to <60 ml/min/1.73 m2).
Findings
The average difference in SBP between the two treatment groups was 13.9, 95% CI 13.4 to 14.4 mm Hg in ACCORD BP and 15.2, 95% CI 14.8 to 15.6 mm Hg in SPRINT. At three-years, cumulative incidences of CKD in ACCORD BP intensive and standard SBP groups were 10.0% and 4.1%, respectively (absolute risk difference: 5.9%, 95% CI 4.3% to 7.5%); corresponding values in SPRINT were 3.5% and 1.0% (absolute risk difference 2.5%, 95% CI 1.8% to 3.2%). The absolute risk difference was significantly higher in ACCORD BP (interaction p-value <0.001).
Interpretation
Intensive SBP lowering increased the risk of incident CKD in persons with and without T2DM; however, the absolute risk of incident CKD was higher in persons with T2DM.
Funding
NIH
Keywords: Hypertension, systolic blood pressure, chronic kidney disease, type 2 diabetes
Introduction
Hypertension is strongly associated with stroke, heart failure, sudden death, end-stage renal disease, and death from all causes(1–5). Recently, the Systolic Blood Pressure Intervention Trial (SPRINT) demonstrated that intensive SBP lowering (SBP target < 120 versus < 140 mm Hg) reduced the risk of death and major cardiovascular events in persons without diabetes but at high cardiovascular risk(6,7). However, the SPRINT Research Group also reported that the intensive SBP lowering group in SPRINT experienced a 3.5-fold higher hazard of incident chronic kidney disease (CKD)(6,8), defined a priori in the protocol as a reduction in estimated glomerular filtration rate (eGFR) ≥30% with a second confirmed eGFR below 60 mL/min/1.73m2.
The Action to Control Cardiovascular Risk in Diabetes Blood Pressure (ACCORD BP) trial in persons with type 2 diabetes mellitus (T2DM) tested the same SBP intervention as in SPRINT in addition to intensive versus standard glycemic control (glycated hemoglobin < 6% [42 mmol/mol] versus 7.0 to 7.9% [53 to 64 mmol/mol]) in a 2 ×2 factorial design(9). The ACCORD BP trial authors(9,10)(reported that compared to the standard SBP group, participants in the intensive SBP group had lower mean eGFR at the final study visit (74.8 ± 25.0 vs. 80.6 ± 24.8 ml/min/1.73 m2) with a similar incidence of a pre-specified primary microvascular outcome composite of renal failure and retinopathy (11.4% vs. 10.9%) and end-stage renal disease (ESRD) (2.5% vs. 2.4%).
To our knowledge, a detailed analysis of the effects of intensive SBP lowering in T2DM on incident CKD has not been published. Examination of the magnitudes of the effects of SBP lowering on kidney outcomes in persons with T2DM and without CKD is highly relevant, as the majority of persons with T2DM do not have CKD, particularly in the early years of their condition.
We herein examined the effects of intensive SBP control on incident CKD in ACCORD BP and compared the magnitude of these effects with those observed in the SPRINT.
Methods
The current study is a secondary analysis of limited-access ACCORD BP(11) and SPRINT(12) BioLINCC datasets obtained from the NIH. Details of study population, interventions and study procedures for ACCORD BP(9,13) and SPRINT(6,14)are provided published elsewhere(15,16). In brief, 4733 participants with T2DM with the use of a two-by-two factorial design were randomly assigned in ACCORD BP to intensive glycemic therapy (hemoglobin A1c target <6.0% [42 mmol/mol]) or standard therapy (HbA1c target 7.0 to 7.9% [53 to 64 mmol/mol]) and intensive SBP therapy (goal <120 mm Hg) or standard therapy (goal <140 mm Hg). SPRINT randomized 9,361 participants without T2DM to a similar SBP intervention. Both studies used a similar protocol to achieve the SBP intervention(15,16).
Definition of renal outcomes
SPRINT protocol pre-specified incident CKD (based on the 4-variable Modification of Diet in Renal Disease (MDRD) study equation to estimate GFR) in participants without CKD at their baseline visit (MDRD eGFR ≥ 60 ml/min/1.73m2), as a >30% decrease in MDRD eGFR from the baseline value with an end value of <60 ml/min/1.73m2, confirmed at the next available SPRINT blood draw. In an earlier report, we observed that the effects of intensive SBP lowering on incident CKD defined either with the MDRD equation or the CKD-EPI equation were similar(8). In the current analysis, we used the SPRINT protocol definition of incident CKD in both SPRINT and ACCORD BP studies.
Statistical methods
Additional details of statistical methods are provided in the supplement.
We used intent to treat analyses for all randomized comparisons between intensive and standard SBP groups in both SPRINT and ACCORD BP (Supplemental Figure 1). We censored follow-up time for incident CKD at the time of final serum creatinine measurement. We used separate Cox regression analyses in the two studies to provide estimates of the hazard ratios for the intensive vs. standard SBP interventions for incident CKD. We compared the effects of the intensive SBP interventions, expressed as relative reduction in the hazards between the intensive and standard SBP groups between the ACCORD BP and SPRINT studies by comparing the difference between the estimated log-transformed hazard ratios in two studies to the standard error of this difference. We tested Schoenfeld residuals and there was no evidence of non-proportionality.
Kaplan-Meier curves depicted the absolute cumulative risk of incident CKD by SBP group within each study. We estimated the absolute risk reductions in these outcomes at 3 years between the intensive and the standard BP groups using a generalized linear model (GLM) using an identity link with a robust variance estimate, and pseudo-survival probabilities as the outcome(17,18). We compared the absolute risk reductions between ACCORD BP and SPRINT by comparing the difference in the estimated risk reductions in the two studies to the standard error of this difference.
We also examined whether the effects of intensive SBP lowering on incident CKD were modified by the glycemia intervention in ACCORD BP by repeating the above analyses in the two glycemia arms in ACCORD BP.
We performed the following sensitivity analyses for incident CKD: 1. In ACCORD BP, we defined incident CKD as 40% decline in eGFR to < 60 ml/min/1.73 m2, with confirmation 2. In ACCORD BP as serum creatinine was measured only annually after month 12, we assumed participants with a 30% decrease in eGFR to a value < 60 ml/min/1.73 m2 but missing a confirmatory value due to death or censoring before the next creatinine measurement to have incident CKD 3. In ACCORD BP, we defined incident CKD as 30% decline in eGFR estimated with CKD-EPI equation to < 60 ml/min/1.73 m2, with confirmation 4. In SPRINT, we performed additional Cox regressions excluding participants with a baseline fasting glucose >6.9 mmol/L 5. In both ACCORD BP and SPRINT, we examined cumulative incidence of incident-CKD (defined as 30% decline in eGFR to < 60 ml/min/1.73 m2, with confirmation) in a competing risk framework with death treated as a competing risk(19) and 6. In both ACCORD BP and SPRINT, we examined the absolute risk differences in the incidence of CKD with intensive SBP lowering stratified by the level of baseline eGFR (≥ 90, 80–89, 70–79 and 60 to 69 ml/min/1.73 m2).
We also explored the incidence of ESRD events as defined in the respective protocols with intensive SBP lowering in SPRINT and ACCORD BP non-CKD participants.
Role of funding source
We used limited access SPRINT and ACCORD BP data obtained from BioLINCC. NIH had no role in the current analysis. SB, TG, RB, GW and GS had access to data. The corresponding author (SB) had full access to all of the data and the final responsibility to submit for publication.
Results
The current analysis included 4311 ACCORD BP participants and 6715 SPRINT participants with baseline eGFR ≥ 60 mL/min/1.73 m2 (Supplemental Figure S1). Baseline demographic, clinical and laboratory characteristics were similar in the intensive and standard SBP groups within ACCORD BP and within SPRINT (Table 1). However, compared to the SPRINT non-CKD population, the ACCORD BP non-CKD population was younger, more likely female and had higher body mass index (BMI), eGFR and albuminuria (Supplemental Table 1). Baseline blood pressures and the number of anti-hypertensive medications were similar in both studies.
Table 1.
Baseline characteristics by study and blood pressure intervention in participants with baseline eGFR ≥ 60 ml/min/1.73 m2
| ACCORD BP | SPRINT | |||
|---|---|---|---|---|
| Standard (N=2162) |
Intensive (N=2149) |
Standard (N=3367) |
Intensive (N=3348) |
|
| Age, (year) | 62.4 ± 6.6 | 62.3 ± 6.5 | 66.4 ± 9.0 | 66.3 ± 9.0 |
| Female sex, (%) | 1007 (47) | 992 (46) | 1127 (33) | 1147 (34) |
| White race, (%) | 1224 (57) | 1280 (60) | 1808 (54) | 1813 (54) |
| Never smoked, (%) | 938 (43) | 925 (43) | 1471 (44) | 1444 (43) |
| Clinical atherosclerotic disease# (%) | 707 (33) | 716 (33) | 503 (15) | 503 (15) |
| Antihypertensive agents, (no./patient) | 1.6 ± 1.1 | 1.7 ± 1.1 | 1.7 ± 1.0 | 1.7 ± 1.0 |
| Systolic blood pressure, (mmHg) | 139 ± 15 | 139 ± 16 | 140 ± 15 | 140 ± 16 |
| Diastolic blood pressure, (mmHg) | 76 ± 10 | 76 ± 10 | 79 ± 12 | 79 ± 12 |
| Duration of diabetes | 11 ± 8 | 11 ± 8 | N/A | N/A |
| Glycated hemoglobin% [mmol/mol] | 8.3 ± 1.0 [67 ± 11] | 8.4 ± 1.0 [68 ± 11] | Not reported | Not reported |
| Fasting plasma glucose, mmol/L | 9.6 ± 3.0 | 9.8 ± 3.0 | 5.5 ± 0.8* | 5.5 ± 0.8* |
| Body-mass index, (kg/m2) | 32.1 ± 5.3 | 32.1 ± 5.6 | 30.0 ± 5.7 | 30.1 ± 5.8 |
| Estimated GFR$, (ml/min/1.73 m2) | 94.0 ± 20.8 | 94.2 ± 20.7 | 81.1 ± 15.5 | 81.3 ± 15.5 |
| Urine albumin creatinine ratio, (mg/mmol) | 1.6(0.8,5.1) | 1.7(0.8,5.0) | 1.0(0.6,1.9) | 1.0 (0.6,1.9) |
Results are presented as a percent (for binary variables) or as mean ± SD (for continuous variables other than ACR) or as median with interquartile range (for ACR).
Clinical atherosclerotic disease was defined in ACCORD as one or more of myocardial infarction, stroke, angina, CABG, PTCI, or other revascularization procedure. Clinical atherosclerotic disease was defined in SPRINT as one or more of MI, ACS, coronary revascularization, carotid revascularization, PAD with revascularization, >50% stenosis of coronary/carotid/lower extremity artery; or AAA ≥5 mm
There were 113 participants in the SPRINT standard arm and 112 participants in the SPRINT intensive arm with a fasting plasma glucose > 6.9 mmol/Lat baseline
Estimated by 4-variable MDRD equation
The intervention effectively lowered SBP in both studies but the average difference in SBP between the two treatment groups was lower in ACCORD BP than in SPRINT (13.9, 95% CI 13.4 to 14.4 mm Hg versus 15.2, 95% CI 14.8 to 15.6 mm Hg, p < 0.001) (Supplemental Figure S2). The mean number of medications used in the intensive versus standard SBP group in ACCORD BP was 2.8 ± 1.5 versus 1.9 ± 1.2 (p < 0.001) and in SPRINT was 2.8 ± 1.1 versus 1.8 ± 1.1 (p < 0.001). The number of participants who were either lost to follow-up or withdrew consent were similar in the intensive and standard SBP groups in ACCORD BP (5.2% versus 4.9%, p = 0.57) and in SPRINT (5.7% versus 5.5%, p = 0.64).
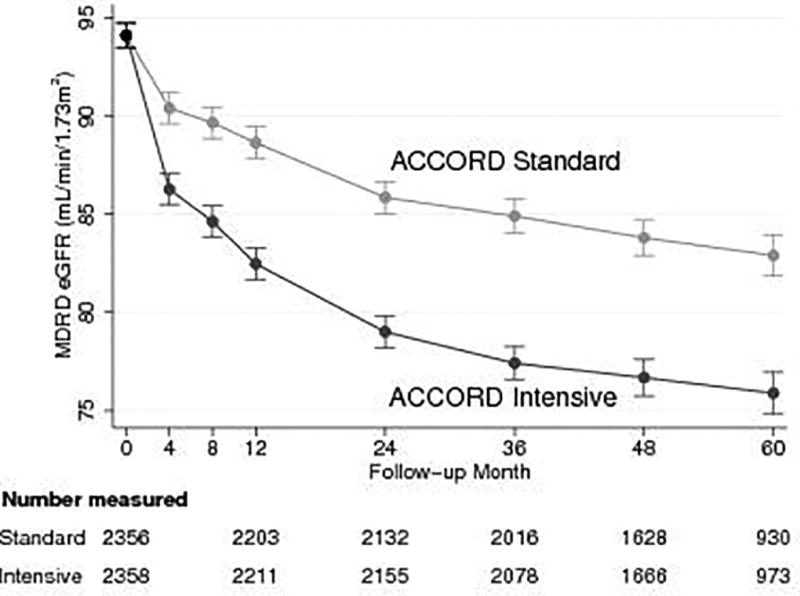
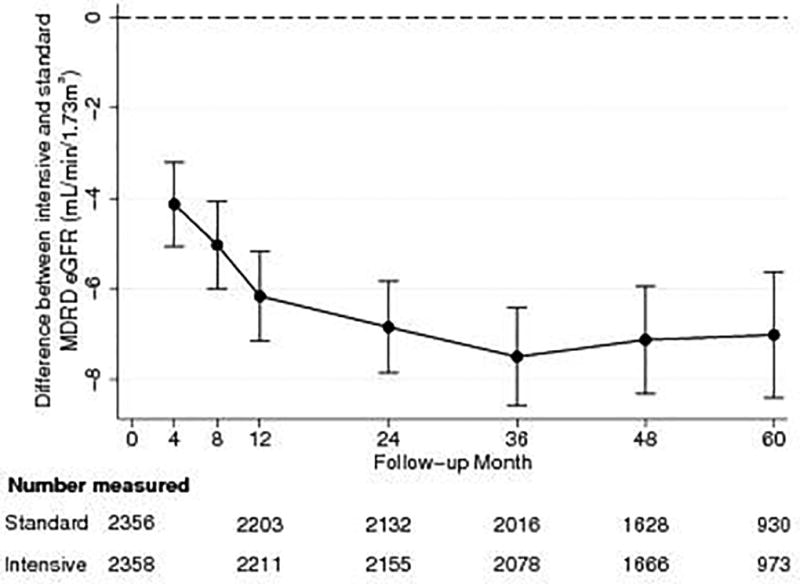
There was an early steep decline in eGFR during the first 12 months in both the standard and intensive SBP groups in ACCORD BP (Figure 1, panels A and B) but the decline was more pronounced in the intensive versus the standard SBP group (first 12 months mean change −11.6, 95% CI −12.4 to −10.9 versus −5.5, 95% CI −6.5 to −4.7 ml/min/1.73 m2, p <0.001). The mean changes in eGFR over the 2-year interval between months 12 and 36 in the intensive versus standard SBP groups were −5.1, 95% CI −5.8 to −4.4 versus −3.7, 95% CI −4.4 to −3.0 ml/min/1.73 m2 (p = 0.009), respectively. After 36 months, the changes in mean eGFR over the subsequent 2-year interval to month 60 did not differ significantly between the treatment groups (−1.5, 95% CI −2.4 to −0.7 versus −2.0, 95% CI −2.8 to −1.2 ml/min/1.73 m2, p =0.43). Similar data for eGFR were not available in the SPRINT limited access public dataset obtained from BioLINCC.
Figure 1.
Panel A: Estimated means of follow-up eGFR by intensive and standard SBP groups in ACCORD BP participants with baseline eGFR ≥ 60 ml/min/1.73 m2
Panel B: Differences in eGFR between intensive and standard SBP groups in ACCORD BP participants with baseline eGFR ≥ 60 ml/min/1.73 m2
Results for both panels were obtained using maximum likelihood estimation under a longitudinal model with an unstructured covariance matrix and common baseline means in each treatment group. Presented are means and 95% confidence intervals.
In ACCORD BP, 333 of the 2149 participants (15.5%) in the intensive SBP group and 160 of the 2157 participants (7.4%) in the standard SBP group experienced an incident CKD event over the duration of the study (mean follow-up 4.6 ± 1.4 years). Rates of incident CKD events were lower in both SBP groups in SPRINT than in ACCORD BP, as 127 of 3348 (3.8%) and 37 of 3367 (1.1%) experienced incident CKD in the intensive and standard SBP groups of SPRINT over a mean follow-up of 3.1 ± 0.9 years.
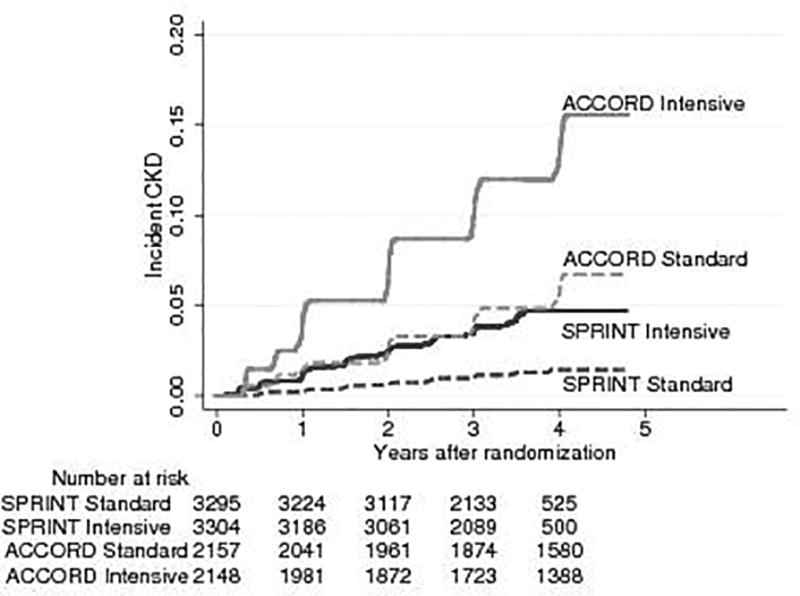
The cumulative incidence of CKD was consistently higher throughout the follow-up period in both of the SBP groups for ACCORD BP than in SPRINT (Figure 2). At three years, the cumulative incidences of CKD in the ACCORD BP intensive and standard SBP groups were 10.0% and 4.1%, respectively and in SPRINT 3.5% and 1.0%, respectively (Figure 3, panel A). The absolute risk difference of 5.9% (95% CI 4.3% to 7.5%) in ACCORD BP was higher than the absolute risk difference of 2.5% (95% CI 1.8% to 3.2%) in SPRINT with an interaction p-value of 0.001 (Figure 3, panel B).
Figure 2.
Cumulative incidence plots of CKD by intensive and standard SBP groups in ACCORD BP and SPRINT participants with baseline eGFR ≥ 60 ml/min/1.73 m2
Figure 3.
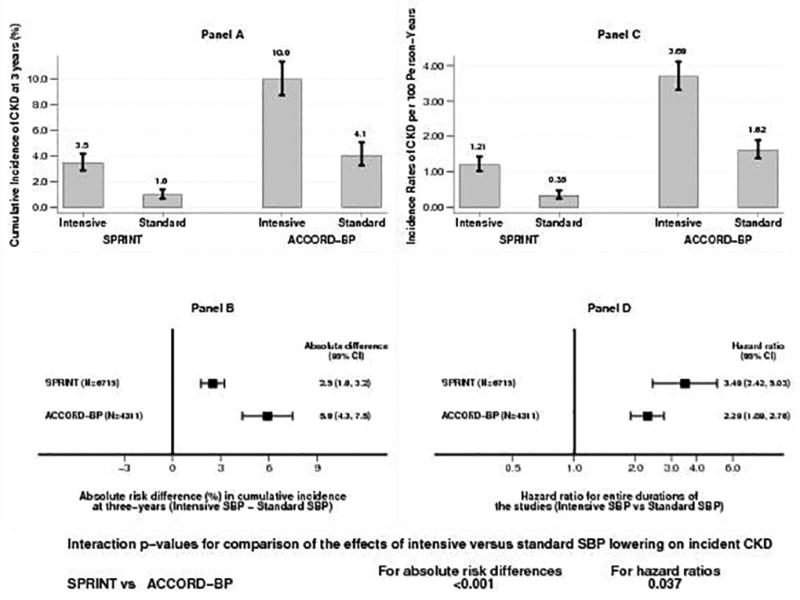
Panel A: Cumulative incidence of CKD at three-years by SBP groups in SPRINT and ACCORD BP
Panel B: Absolute risk differences (intensive SBP minus standard SBP) in incident CKD at three-years in SPRINT and ACCORD BP
Panel C: Incidence rates of CKD per 100 person-years of follow-up for the entire duration of the study in the intensive and standard SBP groups in SPRINT and ACCORD BP
Panel D: Hazard ratios for incident CKD (intensive versus standard SBP group) in SPRINT and ACCORD BP
Incidence rates of CKD per 100 person-years of follow-up for the entire duration of the study in the intensive and standard SBP groups ACCORD BP were 3.69% and 1.62%, respectively and in SPRINT 1.21% and 0.35%, respectively (Figure 3, panel C). Even though incidence rates of CKD were much higher in ACCORD BP compared to SPRINT, increase in the hazard ratio (HR) for incident CKD was significantly more pronounced in SPRINT (HR 3.49, 95% CI 2.42, 5.03) than in ACCORD BP (hazard ratio [HR] 2.29, 95% CI 1.89, 2.76) with an interaction p-value of 0.037 (Figure 3, panel D).
The above patterns were even stronger in participants with baseline urinary ACR ≥ 3.4 mg/mmol as shown in Figures 4 and 5. Compared to SPRINT participants with urinary ACR < 3.4 mg/mmol, ACCORD BP participants with urinary ACR ≥ 30 mg/g had several fold higher incidence of CKD in both the intensive and standard SBP groups (Figure 4, panel A and Figure 5, panel A). The absolute risk difference of 2.1 (95% CI 1.4 to 2.9) in SPRINT participants with urinary ACR < 3.4 mg/mmol was significantly lower (p = 0.005) than that (7.0, 95% CI 3.7 to 10.4) in ACCORD BP participants with urinary ACR ≥ 3.4 mg/mmol (Figure 4, panel B). In contrast, on hazard ratio scale, the hazard ratio (HR 3.65, 95% CI 2.34 to 5.68) for incident CKD with intensive SBP lowering in SPRINT participants with urinary ACR < 3.4 mg/mmol was significantly higher (p = 0.015) that of the corresponding hazard ratio (HR 1.90, 95% CI 1.44 to 2.51) in ACCORD BP participants with urinary ACR ≥ 30 mg/mmol (Figure 5, panel B).
Figure 4.
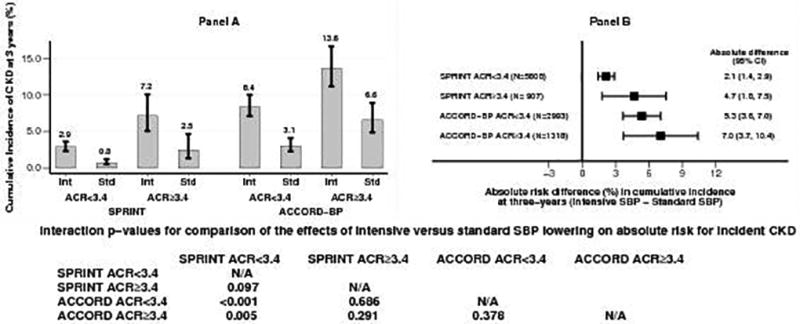
Panel A: Cumulative incidence of CKD at three-years by SBP groups in SPRINT and ACCORD BP participants with urinary ACR < 3.4 mg/mmol or ≥ 3.4 mg/mmol
Panel B: Absolute risk differences (intensive SBP minus standard SBP) in incident CKD at three-years in SPRINT and ACCORD BP participants with urinary ACR < 3.4 mg/mmol or ≥ 3.4 mg/mmol
Figure 5.
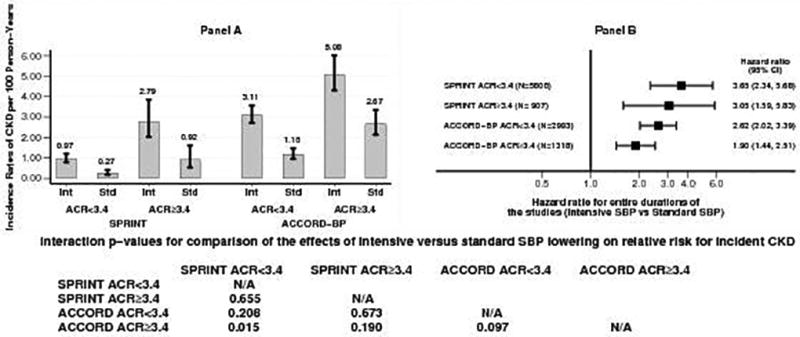
Panel A: Incidence rates of CKD per 100 person-years of follow-up for the entire duration of the study in the intensive and standard SBP groups in SPRINT and ACCORD BP participants with urinary ACR < 3.4 mg/mmol or ≥ 3.4 mg/mmol
Panel B: Hazard ratios for incident CKD (intensive versus standard SBP group) in SPRINT and ACCORD BP participants with urinary ACR < 3.4 mg/mmol or ≥ 3.4 mg/mmol
Results of sensitivity analyses using alternative definitions for incident CKD in ACCORD BP and excluding participants with baseline fasting blood sugars > 6.9 mmol/L in SPRINT (Supplemental Tables 2, 3 and 4) were similar to those of the main analyses presented above. When all-cause death was considered as a competing risk for incident CKD, we still noted that the incidence of CKD was the highest in ACCORD BP participants in the intensive SBP group and the lowest in SPRINT participants in the standard SBP group (Supplemental Figure S3).
In the ACCORD BP participants without CKD at baseline, intensive SBP control resulted in similar increases in the risk of incident CKD within intensive and standard glycemia groups (Supplemental Table 5).
In analyses stratified by the baseline eGFR levels, compared to the standard SBP group, the intensive SBP group had greater incidence of CKD in both SPRINT and ACCORD BP and the absolute risk differences were higher in ACCORD BP (Supplemental Table 6).
There were no incident ESRD events in SPRINT non-CKD cohort. In ACCORD-BP non-CKD cohort, 49 (2.3%) in the standard arm and 53 (2.5%) in the intensive arm developed ACCORD BP protocol defined renal failure outcome (ESRD or serum creatinine ≥ 290 umol/L).
Discussion
The most important finding of our analyses is that intensive SBP lowering increased the risk of incident CKD in persons with and without T2DM. Furthermore, for a clinically similar level of SBP lowering, the absolute risk increase for incident CKD was higher in ACCORD BP participants with T2DM compared to SPRINT participants without T2DM.
Incident CKD was one of the pre-specified secondary outcomes in SPRINT but not in ACCORD BP. However in T2DM, CKD as evidenced by lower estimated GFR is a very strong risk factor for CVD events and death(20). Furthermore, persons with CKD predominantly account for the excess mortality observed in T2DM(21). Hence, it is of public health importance to understand the impact of SBP lowering on incident CKD in persons with T2DM.
The intensive SBP intervention also led to a larger early eGFR decline over the first 12 months compared to the standard SBP intervention in both studies (a mean difference of −6.1 ml/min/1.73m2 in ACCORD BP and a reported difference of −4.4 ml/min/1.73m2 in SPRINT)(8). This suggests that the effect of a given change in SBP on eGFR decline was ~50% greater in the ACCORD BP participants with T2DM than in SPRINT participants without diabetes.
In addition to the differences in early eGFR decline in comparisons between the randomized groups, the early eGFR decline was also notably steeper in ACCORD BP compared to SPRINT within both the intensive SBP groups (a mean change of −11.6 vs. −4.8 ml/min/1.73m2) and the standard SBP groups (−5.5 vs. −0.4 ml/min/1.73m2) of the two trials. The cause of the faster early decline in ACCORD BP compared to SPRINT is unclear.
Nonetheless, results from the current analysis by randomized SBP goals in two large studies suggest caution is warranted in extrapolating SPRINT findings to persons with T2DM. While the CVD and all-cause mortality benefits in SPRINT appear to outweigh the potential effects of the SPRINT intervention on incident CKD(8), the ACCORD BP intervention substantially increased the risk of incident CKD in the current study.
A previous ACCORD BP analysis reported that intensive SBP lowering resulted in a non-significant decrease of cardiovascular disease (CVD) events and a non-significant increase in all-cause mortality(9). It has been suggested that ACCORD BP was underpowered to detect true differences in CVD outcomes(22). A recent participant-level pooled meta-analysis of SPRINT and ACCORD-BP participants suggested that in the combined cohort, intensive SBP lowering decreased the risk of CVD events(23). Another post-hoc analysis of ACCORD BP suggested that when SPRINT selection criteria were applied to the standard glycemia arm of ACCORD BP, intensive SBP lowering was associated with lower risk of CVD outcomes.(24) These studies might support the recent practice guideline recommendation of a SBP goal of < 130 mm Hg in all persons with T2DM(25) or T2DM patients at high risk for CVD(26).
Apart from T2DM, there are other differences in inclusion/ exclusion criteria in the two studies. However, higher baseline eGFR (potentially as a result of hyperfiltration(27)), BMI and greater degree of albuminuria observed in ACCORD BP might reflect the nature of T2DM rather than an artifact induced by other inclusion/ exclusion criteria.
A methodological observation in the current study is that while intensive SBP lowering resulted in much higher absolute risk of incident CKD in persons with T2DM than those without, the relative risk (hazard ratio) was significantly higher in the latter. This is even more pronounced when comparing the effects of intensive SBP lowering on incident CKD in persons without diabetes and with low urinary ACR versus persons with T2DM and urinary ACR ≥ 30 mg/g. This is because relative risks are much more pronounced in populations at lower risk of events than in populations at higher risk of events(28). Thus, the lower hazard ratio for incident CKD with intensive SBP lowering in T2DM with albuminuria than in persons without diabetes and albuminuria should not be interpreted as intensive SBP lowering confers a lower risk of incident CKD in persons with T2DM and albuminuria, rather as a reflection of the higher baseline hazard of incident CKD in this population.
The strengths of the current analysis include the use of data from two large randomized controlled trials that examined the impact of targeting the same intensive SBP goal in persons with and without T2DM respectively. While randomization was not stratified by the presence of CKD at baseline in either study, the non-CKD subgroup represents > 70% of the SPRINT cohort and > 90% of the ACCORD BP cohort. Given the large size of the non-CKD subgroup and that the baseline characteristics between the intensive and standard SBP groups within ACCORD BP and within SPRINT are similar, the comparisons between the intensive and standard BP groups can be inferred to represent the randomized controlled trial designs of each study. The limitations of our analyses include the relatively short duration of follow-up in each study. The long-term implications of higher risk of incident CKD with intensive SBP control in persons with and without diabetes are unclear. Longer term follow-up is required to determine whether CKD induced by more intensive SBP lowering carries the same downstream risk of CKD induced by, or associated with, other conditions.
In conclusion, intensive SBP lowering resulted in higher risk of incident CKD in persons with and without T2DM. However, for a clinically similar level of intensive SBP lowering, the risk of incident CKD appears much higher in persons with T2DM. The early steeper decline in eGFR with intensive SBP lowering suggests a greater susceptibility to hemodynamic effects in T2DM. Further studies are warranted to determine the long-term effects of incident CKD with intensive SBP lowering in persons with and without T2DM.
Supplementary Material
Research in context.
Evidence before this study
The recent ACC/AHA blood pressure guidelines based on systematic review and meta-analysis, recommended a systolic blood pressure (SBP) goal of less than 130 mm Hg in persons with and without diabetes. The Systolic Blood Pressure Intervention Trial (SPRINT) in persons without diabetes demonstrated a lower risk of cardiovascular disease events and all-cause mortality but a higher risk of incident CKD with intensive SBP lowering (SBP goal < 120 mm Hg) compared to standard SBP control (SBP goal < 140 mm Hg). Whether the magnitude of incidence of CKD with intensive SBP lowering is higher in type 2 diabetes compared to those without diabetes is not known.
Added value of this study
Action to Control Cardiovascular Risk in Diabetes (ACCORD) BP trial tested a SBP intervention similar to SPRINT intervention in type 2 diabetes mellitus. In the current analysis, despite clinically similar reduction in SBP in both studies, at three-years, absolute risk differences between intensive and standard SBP groups for incident CKD in ACCORD BP and SPRINT were 5.9% (95% CI 4.3% to 7.5%) and 2.5% (95% CI 1.8% to 3.2%), respectively with an interaction p-value <0.001.
Implications of all available evidence
Despite clinically similar reduction in SBP, the risk of incident CKD was higher in persons with type 2 diabetes mellitus. CKD is known to be a risk factor for future cardiovascular events. However, it is unclear whether incident CKD due to intensive SBP lowering increases the risk of future cardiovascular events. Further studies are warranted to determine whether the higher risk of incident CKD with intensive SBP lowering is outweighed by the cardiovascular disease and all-cause mortality benefits in type 2 diabetes mellitus in long-term.
Acknowledgments
Statistical analyses and preparation of this manuscript are supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK091437 and R21 DK106574) and the University of Utah Study Design and Biostatistics Center (funded in part from the Public Health Services research grant numbers UL1-RR025764 and C06-RR11234 from the National Center for Research Resources).
This manuscript was prepared using ACCORD Research Materials obtained from the NHLBI Biologic Specimen and Data Repository Information Coordinating Center and does not necessarily reflect the opinions or views of the ACCORD or the NHLBI.
This manuscript was prepared using SPRINT Research Materials obtained from the NHLBI Biologic Specimen and Data Repository Information Coordinating Center. The views expressed in this paper are those of the authors and do not represent the official position of the National Institutes of Health (NIH), the Department of Veterans Affairs, the U.S. Government, or the SPRINT Research Group. This paper was approved by the SPRINT Publications and Presentations Committee.
The Systolic Blood Pressure Intervention Trial is funded with Federal funds from the National Institutes of Health (NIH), including the National Heart, Lung, and Blood Institute (NHLBI), the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the National Institute on Aging (NIA), and the National Institute of Neurological Disorders and Stroke (NINDS), under Contract Numbers HHSN268200900040C, HHSN268200900046C, HHSN268200900047C, HHSN268200900048C, HHSN268200900049C, and Inter-Agency Agreement Number A-HL-13-002-001. It was also supported in part with resources and use of facilities through the Department of Veterans Affairs. The SPRINT investigators acknowledge the contribution of study medications (azilsartan and azilsartan combined with chlorthalidone) from Takeda Pharmaceuticals International, Inc. All components of the SPRINT study protocol were designed and implemented by the investigators. The investigative team collected, analyzed, and interpreted the data. All aspects of manuscript writing and revision were carried out by the coauthors. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, the U.S. Department of Veterans Affairs, or the United States Government. For a full list of contributors to SPRINT, please see the supplementary acknowledgement list:
We also acknowledge the support from the following CTSAs funded by NCATS:
CWRU: UL1TR000439, OSU: UL1RR025755, U Penn: UL1RR024134& UL1TR000003, Boston: UL1RR025771, Stanford: UL1TR000093, Tufts: UL1RR025752, UL1TR000073 & UL1TR001064, University of Illinois: UL1TR000050, University of Pittsburgh: UL1TR000005, UT Southwestern: 9U54TR000017-06, University of Utah: UL1TR000105-05, Vanderbilt University: UL1 TR000445, George Washington University: UL1TR000075, University of CA, Davis: UL1 TR000002, University of Florida: UL1 TR000064, University of Michigan: UL1TR000433, Tulane University: P30GM103337 COBRE Award NIGMS.
Footnotes
Trial Registration: NCT01206062, NCT00000620
Statement of competing financial interests: There are no conflicts of interest for any of the authors.
Contributors section:
Conception and Study design: SB, TG, WCC, AKC, PKW and GMC
Analysis of the data: TG, RB, GW, GS
Interpretation of data: All authors
Drafting of the manuscript: SB, TG, RB, GW, PKW, GMC
Critical revision of the article for important intellectual content: All authors
Final approval of the article: All authors
Provision of study materials or patients: SB, WCC, JI, MC, HK, AKC, GMC
Statistical expertise: TG, RB, GW and GS
Obtaining of funding: SB, TG, WCC, JI, MC, HK, AKC, GMC
Administrative, technical, or logistic support: SB, TG
SB, TG and GMC take responsibility for all aspects of the manuscript and each of the authors take responsibility for their contributions.
References
- 1.Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, et al. Global Disparities of Hypertension Prevalence and Control: A Systematic Analysis of Population-Based Studies From 90 Countries. Circulation. 2016;134(6):441–50. doi: 10.1161/CIRCULATIONAHA.115.018912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311(5):507–20. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 3.Hsu CY, McCulloch CE, Darbinian J, Go AS, Iribarren C. Elevated blood pressure and risk of end-stage renal disease in subjects without baseline kidney disease. Arch Intern Med. 2005;165(8):923–8. doi: 10.1001/archinte.165.8.923. [DOI] [PubMed] [Google Scholar]
- 4.Rapsomaniki E, Timmis A, George J, Pujades-Rodriguez M, Shah AD, Denaxas S, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1.25 million people. Lancet. 2014;383(9932):1899–911. doi: 10.1016/S0140-6736(14)60685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–13. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 6.SPRINT Research Group. Wright JT, Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373(22):2103–16. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williamson JD, Supiano MA, Applegate WB, Berlowitz DR, Campbell RC, Chertow GM, et al. Intensive vs Standard Blood Pressure Control and Cardiovascular Disease Outcomes in Adults Aged >/=75 Years: A Randomized Clinical Trial. Jama. 2016;315(24):2673–82. doi: 10.1001/jama.2016.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beddhu S, Rocco MV, Toto R, Craven TE, Greene T, Bhatt U, et al. Effects of Intensive Systolic Blood Pressure Control on Kidney and Cardiovascular Outcomes in Persons Without Kidney Disease: A Secondary Analysis of a Randomized Trial. Ann Intern Med. 2017 doi: 10.7326/M16-2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ACCORD Study Group. Cushman WC, Evans GW, Byington RP, Goff DC, Jr, Grimm RH, Jr, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1575–85. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ismail-Beigi F, Craven TE, O'Connor PJ, Karl D, Calles-Escandon J, Hramiak I, et al. Combined intensive blood pressure and glycemic control does not produce an additive benefit on microvascular outcomes in type 2 diabetic patients. Kidney Int. 2012;81(6):586–94. doi: 10.1038/ki.2011.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Heart, Lung and Blood Institute. Action to Control Cardiovascular Risk in Diabetes (ACCORD) dataset. [Available from: https://biolincc.nhlbi.nih.gov/studies/accord/
- 12.National Heart, Lung and Blood Institute. Systolic Blood Pressure Intervention Trial Primary Outcome Paper (SPRINT-POP) Data. [Available from: https://biolincc.nhlbi.nih.gov/studies/sprint_pop/
- 13.Action to Control Cardiovascular Risk in Diabetes Study G. Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–59. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ambrosius WT, Sink KM, Foy CG, Berlowitz DR, Cheung AK, Cushman WC, et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT) Clinical trials. 2014;11(5):532–46. doi: 10.1177/1740774514537404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Heart, Lung and Blood Institute. Systolic Blood Pressure Intervention Protocol Version 4.0. [Available from: https://www.sprinttrial.org/public/Protocol_Current.pdf.
- 16.National Heart, Lung and Blood Institute. Action to Control Cardiovascular Risk in Diabetes (ACCORD) protocol. [Available from: https://biolincc.nhlbi.nih.gov/static/studies/accord/Protocol.pdf?link_time=2017-08-22_13:28:23.544891.
- 17.Andersen PK, Klein JP, Rosthøj S. Generalised linear models for correlated pseudo-observations, with applications to multi-state models. Biometrika. 2003;90(1):15–27. [Google Scholar]
- 18.Parner ET, Andersen PK. Regression analysis of censored data using pseudo-observations. The Stata Journal. 10(3):408–22. [Google Scholar]
- 19.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94(446):496–509. [Google Scholar]
- 20.Tancredi M, Rosengren A, Svensson A-M, Kosiborod M, Pivodic A, Gudbjörnsdottir S, et al. Excess Mortality among Persons with Type 2 Diabetes. New England Journal of Medicine. 2015;373(18):1720–32. doi: 10.1056/NEJMoa1504347. [DOI] [PubMed] [Google Scholar]
- 21.Afkarian M, Sachs MC, Kestenbaum B, Hirsch IB, Tuttle KR, Himmelfarb J, et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol. 2013;24(2):302–8. doi: 10.1681/ASN.2012070718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perkovic V, Rodgers A. Redefining Blood-Pressure Targets — SPRINT Starts the Marathon. New England Journal of Medicine. 2015;373(22):2175–8. doi: 10.1056/NEJMe1513301. [DOI] [PubMed] [Google Scholar]
- 23.Brouwer TF, Vehmeijer JT, Kalkman DN, Berger WR, van den Born B-JH, Peters RJ, et al. Intensive Blood Pressure Lowering in Patients With and Patients Without Type 2 Diabetes: A Pooled Analysis From Two Randomized Trials. Diabetes Care. 2017 doi: 10.2337/dc17-1722. [DOI] [PubMed] [Google Scholar]
- 24.Buckley LF, Dixon DL, Wohlford GFt, Wijesinghe DS, Baker WL, Van Tassell BW. Intensive Versus Standard Blood Pressure Control in SPRINT-Eligible Participants of ACCORD-BP. Diabetes Care. 2017;40(12):1733–8. doi: 10.2337/dc17-1366. [DOI] [PubMed] [Google Scholar]
- 25.Whelton PK, Carey RM, Aronow WS, Casey DE, Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017 [Google Scholar]
- 26.de Boer IH, Bangalore S, Benetos A, Davis AM, Michos ED, Muntner P, et al. Diabetes and Hypertension: A Position Statement by the American Diabetes Association. Diabetes Care. 2017;40(9):1273. doi: 10.2337/dci17-0026. [DOI] [PubMed] [Google Scholar]
- 27.Cherney DZ, Scholey JW, Miller JA. Insights into the regulation of renal hemodynamic function in diabetic mellitus. Curr Diabetes Rev. 2008;4(4):280–90. doi: 10.2174/157339908786241151. [DOI] [PubMed] [Google Scholar]
- 28.Barratt A, Wyer PC, Hatala R, McGinn T, Dans AL, Keitz S, et al. Tips for learners of evidence-based medicine: 1. Relative risk reduction, absolute risk reduction and number needed to treat. Cmaj. 2004;171(4):353–8. doi: 10.1503/cmaj.1021197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


