Abstract
PURPOSE:
The purpose of this study is to evaluate patients with uveal metastasis based on primary tumor site.
METHODS:
Retrospective analysis from Wills Eye Hospital, Philadelphia, PA, USA, for uveal metastasis clinical features and outcomes based on the primary tumor site.
RESULTS:
There were 2214 uveal metastases diagnosed in 1111 consecutive patients. The demographics included mean age of 60 years (median 61 years), Caucasian race (88%), and female gender (64%). The tumor was unilateral (82%) and primary site was established before uveal metastasis (67%). The primary tumor originated in the breast (37%), lung (26%), kidney (4%), gastrointestinal (GI) tract (4%), cutaneous melanoma (2%), lung carcinoid (2%), prostate (2%), thyroid (1%), pancreas (1%), other sites (3%), and unknown (16%). Comparative analysis of the 5 most common primary sites (breast, lung, kidney, GI tract, and cutaneous melanoma), revealed metastasis at mean age (57, 62, 66, 61, 59 years), as unilateral tumor (74%, 86%, 85%, 93%, 85%), with mean number of metastasis/eye (1.9, 1.7, 1.0, 1.1, 2.0), and in females (99%, 46%, 26%, 25%, 30%). Choroidal metastases measured mean base (9.3, 10.2, 9.1, 11.0, 7.3 mm), mean thickness (2.4, 3.6, 4.4, 4.0, 2.9 mm), and demonstrated predominant color yellow (94%, 91%, 56%, 97%, 36%). Of the 769 patients with documented follow-up, mean patient survival was poor (22.2, 11.5, 8.6, 12.4, 11.4 months) and Kaplan–Meier analysis revealed 3-year survival (33%, 19%, 0%, 14%, 21%) and 5-year survival (24%, 13%, 0%, 14%, 21%). The worst survival was found in patients with pancreatic metastasis (mean 4.2 months) and best survival with lung carcinoid (92% at 5 years).
CONCLUSION:
In a tertiary referral service, uveal metastasis originates from cancer in the breast, lung, kidney, GI tract, cutaneous melanoma, or others. Overall prognosis is poor with 5-year survival at 23% and worst survival with pancreatic metastasis whereas best survival with lung carcinoid metastasis.
Keywords: Choroid, ciliary body, eye, iris, metastasis, uvea
Introduction
The uveal tract is the most common ophthalmic site for hematogenously dissemination of metastatic tumors from remote sites, predominantly related to the luxurious blood flow within the choroidal tissue.[1,2,3,4,5,6,7,8,9] Less common ophthalmic sites for hematogenous metastasis include the orbit, eyelid, conjunctiva, retina, and vitreous.
The most comprehensive publication on uveal metastatic disease, an analysis of 520 eyes, revealed 88% involving the choroid, typically appearing as a solitary (72%) yellow mass (94%) with associated subretinal fluid (73%).[1] In that analysis, detailed description of clinical features, tumor location, laterality, and size were presented based on primary tumor origin. In the 420 affected patients, the primary cancer arose in breast (47%), lung (21%), gastrointestinal (GI) tract (4%), kidney (2%), and others. When comparing features (breast vs. lung primary site) of the 100 patients with bilateral tumors (64% vs. 18%) and the 320 patients with unilateral tumor (41% vs. 22%), breast cancer was more common.[1] Regarding mean tumor thickness, tumors arising from primary site in the GI tract (4 mm) or kidney (4 mm) were substantially thicker at diagnosis than those from breast (2 mm) or cutaneous melanoma (1 mm). In that cohort, there was no evaluation of survival outcome.
There are few published reports on systemic survival in patients with uveal metastases. In 1987, Freedman and Folk evaluated 112 patients with metastasis to the eye and orbit and found differences in the median survival time following metastasis from cancer of the breast (n = 666 days) versus lung (n = 276 days).[4] Regarding breast cancer metastases alone, those with choroidal involvement showed shorter median survival time (n = 314 days) compared to those with orbital metastasis (n = 794 days).[4] Demirci et al. evaluated 264 patients with choroidal metastasis from breast cancer alone and found Kaplan–Meier survival at 1 year in 65% and at 5 years in 24%.[10]
Herein, we analyze our 44-year experience with 1111 patients with uveal metastases. In this analysis, we assess the demographic and clinical features of the malignancy based on primary tumor site. In addition, we provide Kaplan–Meier analysis for survival outcomes based on the primary tumor origin.
Methods
A retrospective review of all patients diagnosed and managed by the Ocular Oncology Service, Wills Eye Hospital, Philadelphia, PA, USA, with uveal metastasis between January 1, 1974, and June 1, 2017, was performed. Patients with lymphoproliferative disorders such as lymphoma, leukemia, and other hematologic malignancies were excluded from the study.
The data included information on patient demographics, primary cancer site, uveal metastasis clinical features and management, and survival outcome. The demographic data included patient age at the time of ocular diagnosis, gender, and race. The primary cancer site information included organ of primary malignancy (breast, lung, kidney, GI tract, skin, prostate, thyroid, pancreas, others, and unknown), date of primary cancer diagnosis, and interval between primary cancer and uveal metastasis.
The clinical ocular features included patient symptoms, involved eye (right, left), laterality (unilateral, bilateral), visual acuity, and intraocular pressure. The clinical tumor features included total number of metastases per eye, anatomic location (iris, ciliary body, and choroid), meridional location of epicenter (superior, nasal, inferior, temporal, macula [choroid only]), basal diameter (mm), thickness (mm), and color (yellow, orange, brown). For those with multiple uveal metastasis, data were recorded for the largest tumor per uveal tissue (iris, ciliary body, choroid). For choroidal metastasis, tumor anteroposterior epicenter (macula, macula to equator, equator to ora serrata), distance to foveola (mm) and optic nerve (mm), configuration (plateau, dome, mushroom, flat), related subretinal fluid, and ultrasound characteristics (dense, hollow) were recorded.
The initial management of the uveal metastasis was recorded (external beam radiotherapy, plaque radiotherapy, systemic chemotherapy, photodynamic therapy [PDT], enucleation, observation, and others). The time interval between the date of ocular metastasis and patient death was listed.
All data were tabulated in Microsoft Excel 2016 and measures of central tendencies (mean, median, range) and Kaplan–Meier analyses were performed using its built-in functions. Log-rank testing was performed for statistical significance (P < 0.05) of Kaplan–Meier survival estimates between primary tumor types.
Results
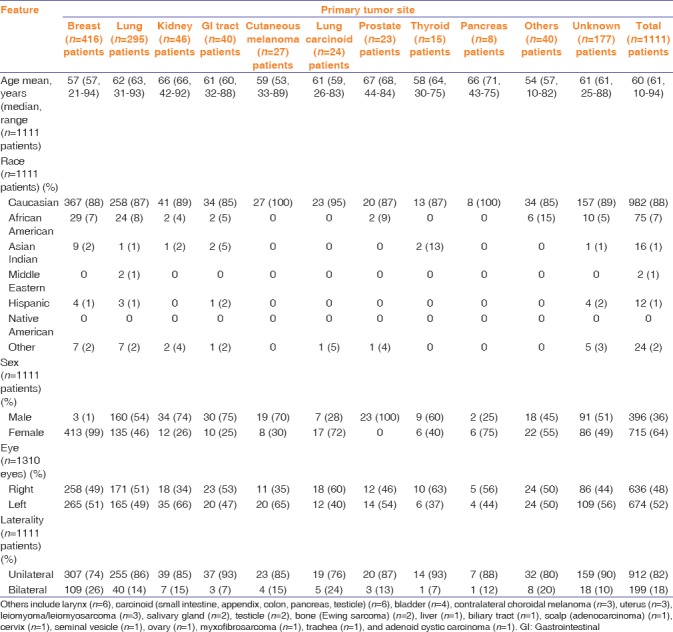
There was a total of 2214 uveal metastases in 1310 eyes of 1111 patients. Patient demographics are listed in Table 1. Overall, patient mean age at ocular diagnosis was 60 years (median 61, range 10–94 years). Some primary sites demonstrated younger mean age including breast (57 years), cutaneous melanoma (59 years), and thyroid (58 years). The youngest patient with uveal metastasis was a 10-year-old female with malignant myxofibrosarcoma of the leg to the ciliary body. Regarding race, most patients were Caucasian (88%) or African American (7%). All patients with cutaneous melanoma (n = 27, 100%) and pancreatic carcinoma (n = 8, 100%) were Caucasian. Regarding gender, uveal metastasis occurred more commonly in females (n = 715, 64%), which is likely attributable to the frequent occurrence of breast metastasis to the uvea, embodying 416 patients (37%) overall with the majority of cases in females (n = 413, 99%). The metastasis affected the right (48%) or left (52%) eye and was unilateral (82%) in most cases.
Table 1.
Uveal metastases in 2214 cases of 1310 eyes in 1111 patients. Demographics based on the 10 most common primary tumor sites
The primary tumor site is listed in Table 1. The primary tumor originated in the breast (n = 416, 37%), lung (n = 295, 27%), kidney (n = 46, 4%), GI tract (n = 40, 4%), cutaneous melanoma (n = 27, 2%), lung carcinoid (n = 24, 2%), prostate (n = 23, 2%), thyroid (n = 15, 1%), pancreas (n = 8, 1%), and other sites (n = 40, 3%). The primary tumor site was unknown in 177 (16%) [Figures 1, 2, 3].
Figure 1.
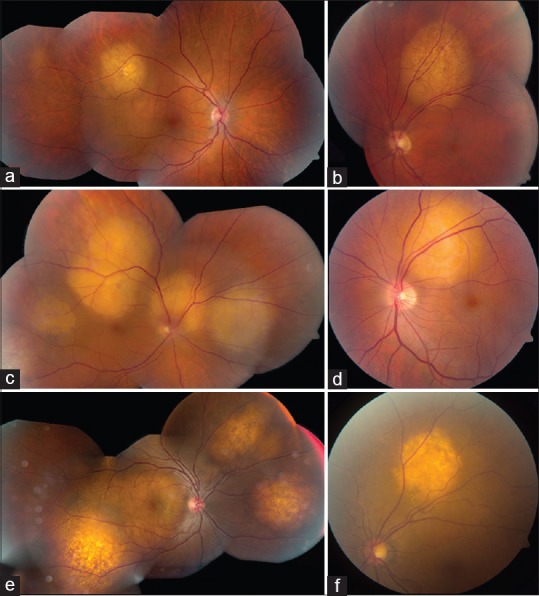
Yellow-colored choroidal metastases. Amelanotic (yellow) choroidal metastases from various primary malignancies including (a) breast cancer, (b) lung cancer, (c) kidney cancer, (d) esophageal cancer, (e) prostate cancer, and (f) thyroid cancer
Figure 2.
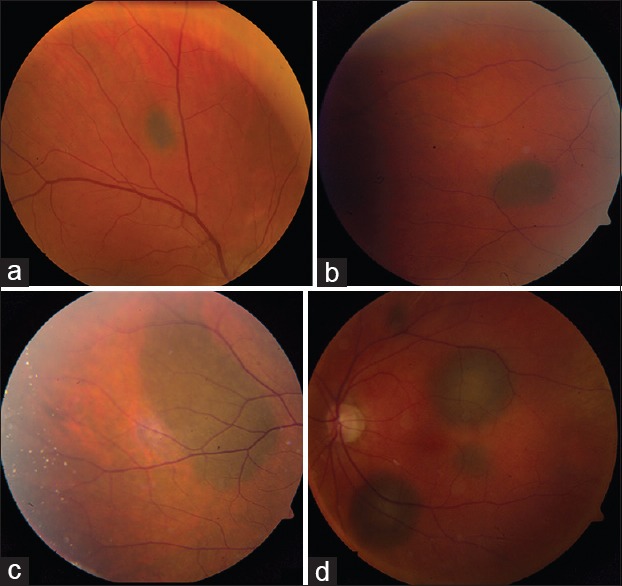
Brown-colored choroidal metastases. Pigmented (brown) choroidal metastases. From (a-c) cutaneous melanoma in 3 patients with known systemic melanoma metastasis. Multifocal brown choroidal metastases from (d) choroidal melanoma in the fellow eye
Figure 3.
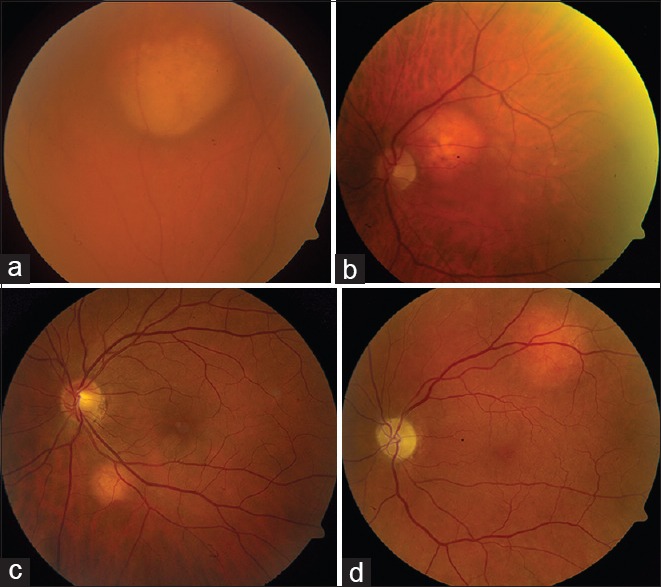
Orange-colored choroidal metastasis. Solitary orange-colored choroidal metastasis from (a) lung carcinoid tumor, (b) kidney cancer, (c) lung cancer, and (d) from the unknown tumor, presumed to represent cryptic carcinoid tumor
The clinical features of the uveal metastases are listed in Table 2. The uveal location included choroid (90%), iris (8%), or ciliary body (2%), and the mean number of metastases per eye was 1.7. Regarding iris metastases (n = 100), mean basal diameter was 6.6 mm and mean thickness was 2.6 mm, tumor color was yellow (54%), and hyphema was noted (17%). Regarding ciliary body metastases (n = 28), mean basal diameter was 11.6 mm and mean thickness was 5.3 mm, and tumor most often originated from breast cancer (36%). Regarding choroidal metastases (n = 1213), mean basal diameter was 9.5 mm and mean thickness was 3.2 mm, and tumor color was yellow (86%), orange (8%), or brown (4%), subretinal fluid was present (72%), and ultrasound confirmed echodensity (80%) [Figure 4].
Table 2.
Uveal metastases in 2214 cases of 1310 eyes in 1111 patients. Clinical features based on the 10 most common primary tumor sites
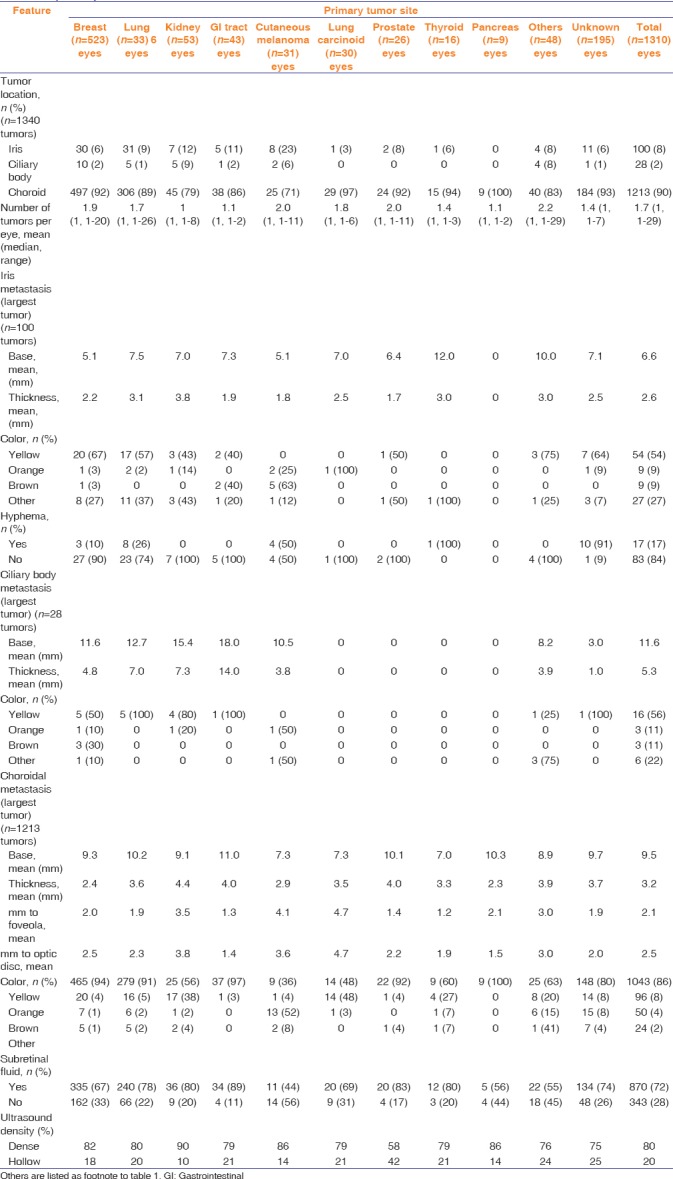
Figure 4.
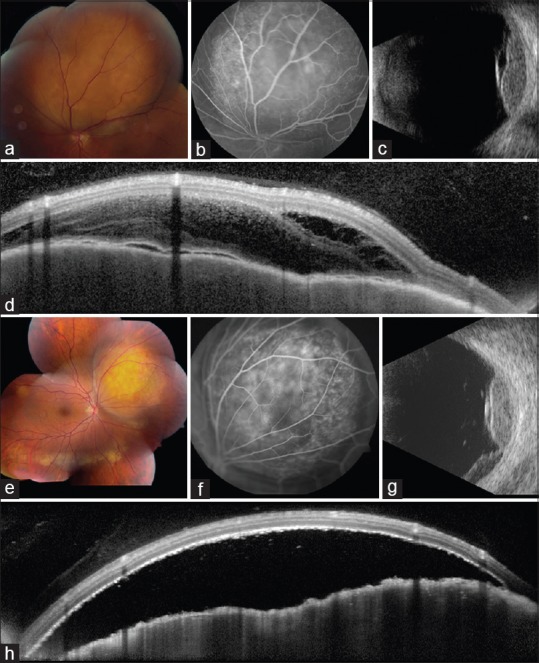
Multimodal imaging in breast and lung cancer metastases. (a-d) Yellow choroidal metastasis (a) from breast cancer, abutting the superior aspect of the optic disc, (b) showing late hyperfluorescence on fluorescein angiography, (c) echodensity with overlying subretinal fluid on ultrasonography, and (d) "lumpy-bumpy" surface with overlying fluid on optical coherence tomography. Yellow choroidal metastasis (e) from lung cancer, (f) showing mottled hyperfluorescence on angiography, (g) echodensity with dependent subretinal fluid on ultrasonography, and (h) "lumpy-bumpy" surface with overlying fluid on OCT
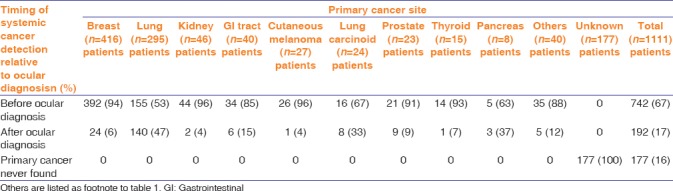
The relationship of primary cancer to uveal metastasis is listed in Table 3. Overall, the primary cancer was detected before (67%) or after (18%) the uveal tumor. Those with predominant detection before uveal metastasis include breast (94%), kidney (96%), GI tract (85%), cutaneous melanoma (96%), and thyroid (93%). Those with late detection after the uveal metastasis include primaries of lung carcinoma (47%), lung carcinoid (33%), and pancreatic cancer (37%).
Table 3.
Uveal metastases in 2214 cases of 1310 eyes in 1111 patients. The timing of systemic cancer detection based on the 10 most common primary tumor sites
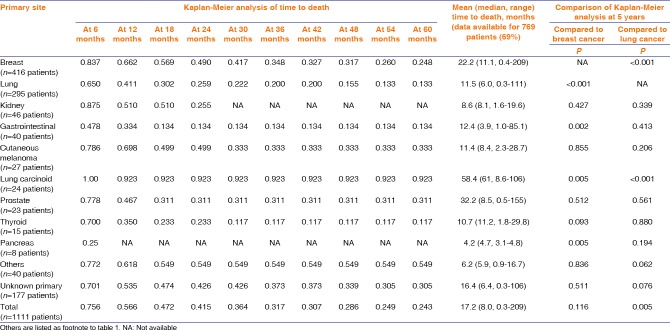
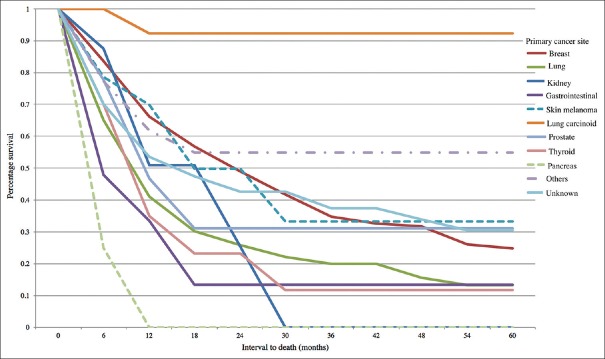
The overall individual primary tumor site survival rates are listed in Table 4. Of the 769 patients with documented follow-up, the patient survival was generally poor. Overall Kaplan–Meier survival was 57% at 1 year, 42% at 2 years, 32% at 3 years, 29% at 4 years, and 24% at 5 years, with mean survival time of 17.2 months [Figure 5]. At 5 years, survival with primary tumor in the breast (25%), prostate (31%), and cutaneous melanoma (33%) was better than lung (13%), GI tract (13%), and thyroid (12%), whereas lung carcinoid displayed the most favorable 5-year survival (92%) [Figure 6]. By comparison, relative to breast cancer, 5-year survival was significantly worse with lung cancer (P < 0.001), GI cancer (P = 0.002), and pancreatic cancer (P = 0.005), and significantly better with lung carcinoid (P = 0.005). By comparison, relative to lung cancer, 5-year survival was significantly better with breast cancer (P < 0.001), lung carcinoid (P < 0.001), and with the entire cohort (P = 0.005).
Table 4.
Uveal metastases in 1111 patients. Analysis of time to death based on the 10 most common primary tumor sites
Figure 5.
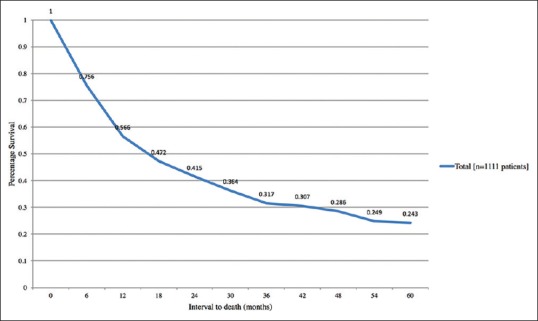
Kaplan–Meier analysis of time to death in 1111 patients with choroidal metastases based on total cohort
Figure 6.
Kaplan–Meier analysis of time to death in 1111 patients with choroidal metastases based on primary site
Discussion
Cancer metastatic to the eye and particularly the uveal tissue is a serious finding and is often suggestive of poor life prognosis.[9] There have been a few series published on the topic of patient survival following detection of ocular metastases.[4,10,11,12,13,14,15] Freedman and Folk studied 141 eyes of 112 patients with metastatic disease to the eye and orbit, arising from primary sites in the breast (49%), lung (14%), cutaneous melanoma (4%), prostate (4%), colon (4%), and others.[4] They found that metastases from lung cancer demonstrated shorter mean survival time compared to those from breast cancer (188 vs. 666 days, respectively), and noted that patients with breast cancer metastasis to the ocular region showed longer survival if older than younger (P = 0.009).[4]
Despite excellent local control of uveal metastasis with methods of teletherapy, brachytherapy, chemotherapy, and PDT, often with favorable visual outcomes, systemic prognosis remains guarded.[7,8,9,16,17,18,19] Large cohort data analysis on systemic prognosis following detection of uveal metastasis, specific to primary tumor site, has rarely been studied, generally only with regard to breast cancer and lung cancer.[10,11] In 2003, we reviewed prognosis in 264 patients with breast cancer metastases to the uvea and noted Kaplan–Meier survival rates of 65% at 1 year, 34% at 3 years, and 24% at 5 years.[10] Others have noted that breast cancer survival largely depended on systemic status, whether classified as stage 1 or 2 versus stage 3 or 4 at the time of ocular diagnosis (873 vs. 139 days).[4] Regarding prognosis of lung cancer with ocular metastasis, a collaborative analysis from two referral centers at Wills Eye Hospital and Emory University revealed 194 patients with lung cancer metastatic to the uvea and found 54% mortality at mean 12-month follow-up.[11] The authors noted that 44% of patients denied knowledge of lung cancer at the time of uveal metastasis and the primary malignancy was discovered after uveal metastasis.[11]
Other series on prognosis comprise small cohort analysis with mean (median) outcomes.[12,13,14,15] A review of 5 patients with pancreatic cancer metastatic to the choroid revealed the primary malignancy preceded the metastasis by a mean interval of 34 months in 4 cases and was detected at autopsy in the 5th case.[12] Average survival time was 6 months in 3 cases, with 2 remaining alive at mean 17 months' follow-up.[12] A review on 10 patients with cutaneous melanoma metastatic to the choroid (n = 6), iris (n = 3), or retina (n = 1) revealed Kaplan–Meier median survival time of 72 days.[13] Further review of the literature on 28 cases of cutaneous melanoma selectively metastatic to the retina and vitreous revealed mean 22 months survival.[14] By comparison, analysis of 9 patients with carcinoid tumor metastatic to the uvea found mortality in 5 patients at mean interval of 34 months with 4 patients alive at mean follow-up of 34 months.[15]
In 1997, we evaluated 420 consecutive patients with uveal metastasis to assess clinical features of the various types of malignancies. In that analysis, tumor location was most commonly in the choroid (88%), with a mean of 2 metastases per eye, and mean choroidal tumor size of 9 mm in base and 3 mm in thickness. Most tumors originated from breast (47%), lung (21%), or GI tract (4%). In 17% of cases, the primary site was not established despite systemic evaluation by medical physician and/or medical oncologist. Prognostic information was not provided in that analysis.
In this current series of 1111 patients with uveal metastasis, from a single center over 44 years, long-term clinical and prognostic information was available. We found the primary tumor originated in the breast (37%), lung (27%), kidney (4%), GI tract (4%), cutaneous melanoma (2%), lung carcinoid (2%), prostate (2%), thyroid (1%), pancreas (1%), other sites (3%), and unknown primary tumor site (16%). There were significant differences in survival depending on primary tumor site. Compared to breast cancer, 5-year survival was worse with lung cancer (P < 0.001), GI cancer (P = 0.002), and pancreatic cancer (P = 0.005), and better with lung carcinoid (P = 0.005). Compared to lung cancer, 5-year survival was better with breast cancer (P < 0.001), lung carcinoid (P < 0.001), and with the entire cohort (P = 0.005). The 5-year survival was more favorable for malignancies originating in the breast (25%), prostate (31%), and cutaneous melanoma (33%), compared to those from the lung (13%), GI tract (13%), and thyroid (12%). Survival for malignancies from the kidney at 2 years (26%) and pancreas at 6 months (25%) yielded no survivors at 5 years. Notably, lung carcinoid displayed the most favorable 5-year survival (92%).
There are limitations to this analysis in that follow-up was available for only 769 (69%) of the 1111 patients for many reasons including illness, transportation issues, preference for follow-up near home, death, and others. This could have impacted outcomes analysis, but these data still qualifies as the largest published cohort on uveal metastases. All 1111 patients were available for data collection regarding clinical features and therapy at initial evaluation. Another limitation is related to referral pattern as patients with systemic cancer are managed by medical oncologists and only referred to the ophthalmologist or ocular oncologist if visual symptoms occur, thus those patients with asymptomatic metastasis could have been overlooked. In addition, our department is often referred patients with solitary metastasis and no known systemic cancer to rule out primary uveal melanoma, and this could bias our data toward that phenotype. Finally, we realize that cancer screening for “at-risk” individuals and specific systemic therapies used over this long-term 40-year period could have evolved leading to change in cancer incidence, severity, and prognosis.
Conclusion
We report a comprehensive series of 1111 patients with uveal metastasis and document clinical features and prognostic outcomes. These data can assist the ophthalmologist in decision-making based on anticipated patient survival.
Financial support and sponsorship
Support provided by the Eye Tumor Research Foundation, Philadelphia, PA (CLS). The funders had no role in the design and conduct of the study, in the collection, analysis, and interpretation of the data, or in the preparation, review or approval of the manuscript. Carol Shields, M. D. has had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Shields CL, Shields JA, Gross NE, Schwartz GP, Lally SE. Survey of 520 eyes with uveal metastases. Ophthalmology. 1997;104:1265–76. doi: 10.1016/s0161-6420(97)30148-1. [DOI] [PubMed] [Google Scholar]
- 2.Ferry AP, Font RL. Carcinoma metastatic to the eye and orbit. I. A clinicopathologic study of 227 cases. Arch Ophthalmol. 1974;104:276, 86. doi: 10.1001/archopht.1974.01010010286003. [DOI] [PubMed] [Google Scholar]
- 3.Stephens RF, Shields JA. Diagnosis and management of cancer metastatic to the uvea: A study of 70 cases. Ophthalmology. 1979;86:1336–49. doi: 10.1016/s0161-6420(79)35393-3. [DOI] [PubMed] [Google Scholar]
- 4.Freedman MI, Folk JC. Metastatic tumors to the eye and orbit. Patient survival and clinical characteristics. Arch Ophthalmol. 1987;105:1215–9. doi: 10.1001/archopht.1987.01060090073031. [DOI] [PubMed] [Google Scholar]
- 5.Shields JA, Shields CL, Kiratli H, de Potter P. Metastatic tumors to the iris in 40 patients. Am J Ophthalmol. 1995;119:422–30. doi: 10.1016/s0002-9394(14)71227-9. [DOI] [PubMed] [Google Scholar]
- 6.Shields CL, Kaliki S, Crabtree GS, Peshtani A, Morton S, Anand RA, et al. Iris metastasis from systemic cancer in 104 patients: The 2014 Jerry A. Shields Lecture. Cornea. 2015;34:42–8. doi: 10.1097/ICO.0000000000000285. [DOI] [PubMed] [Google Scholar]
- 7.Arepalli S, Kaliki S, Shields CL. Choroidal metastases: Origin, features, and therapy. Indian J Ophthalmol. 2015;63:122–7. doi: 10.4103/0301-4738.154380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Konstantinidis L, Damato B. Intraocular metastases – A review. Asia Pac J Ophthalmol (Phila) 2017;6:208–14. doi: 10.22608/APO.201712. [DOI] [PubMed] [Google Scholar]
- 9.Shields JA, Shields CL. An Atlas and Textbook. 3rd ed. Philadelphia: Lippincott, Wolters Kluwer; 2016. Intraocular Tumors; pp. 213–45. [Google Scholar]
- 10.Demirci H, Shields CL, Chao AN, Shields JA. Uveal metastasis from breast cancer in 264 patients. Am J Ophthalmol. 2003;136:264–71. doi: 10.1016/s0002-9394(03)00192-2. [DOI] [PubMed] [Google Scholar]
- 11.Shah SU, Mashayekhi A, Shields CL, Walia HS, Hubbard GB, 3RD, Zhang J, et al. Uveal metastasis from lung cancer: Clinical features, treatment, and outcome in 194 patients. Ophthalmology. 2014;121:352–7. doi: 10.1016/j.ophtha.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 12.Shah SU, Shields CL, Bianciotto CG, Shields JA. Pancreatic cancer metastasis to choroid. Ophthalmology. 2011;118:1483.e4. doi: 10.1016/j.ophtha.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 13.de Bustros S, Augsburger JJ, Shields JA, Shakin EP, Pryor CC., 2nd Intraocular metastases from cutaneous malignant melanoma. Arch Ophthalmol. 1985;103:937–40. doi: 10.1001/archopht.1985.01050070063031. [DOI] [PubMed] [Google Scholar]
- 14.Zografos L, Mirimanoff RO, Angeletti CA, Frosini R, Beati D, Schalenbourg A, et al. Systemic melanoma metastatic to the retina and vitreous. Ophthalmologica. 2004;218:424–33. doi: 10.1159/000080948. [DOI] [PubMed] [Google Scholar]
- 15.Harbour JW, De Potter P, Shields CL, Shields JA. Uveal metastasis from carcinoid tumor. Clinical observations in nine cases. Ophthalmology. 1994;101:1084–90. doi: 10.1016/s0161-6420(94)38030-4. [DOI] [PubMed] [Google Scholar]
- 16.Rudoler SB, Corn BW, Shields CL, De Potter P, Hyslop T, Shields JA, et al. External beam irradiation for choroid metastases: Identification of factors predisposing to long-term sequelae. Int J Radiat Oncol Biol Phys. 1997;38:251–6. doi: 10.1016/s0360-3016(97)00050-3. [DOI] [PubMed] [Google Scholar]
- 17.Rudoler SB, Shields CL, Corn BW, De Potter P, Hyslop T, Curran WJ, Jr, et al. Functional vision is improved in the majority of patients treated with external-beam radiotherapy for choroid metastases: A multivariate analysis of 188 patients. J Clin Oncol. 1997;15:1244–51. doi: 10.1200/JCO.1997.15.3.1244. [DOI] [PubMed] [Google Scholar]
- 18.Shields CL, Shields JA, De Potter P, Quaranta M, Freire J, Brady LW, et al. Plaque radiotherapy for the management of uveal metastasis. Arch Ophthalmol. 1997;115:203–9. doi: 10.1001/archopht.1997.01100150205010. [DOI] [PubMed] [Google Scholar]
- 19.Kaliki S, Shields CL, Al-Dahmash SA, Mashayekhi A, Shields JA. Photodynamic therapy for choroidal metastasis in 8 cases. Ophthalmology. 2012;119:1218–22. doi: 10.1016/j.ophtha.2011.12.024. [DOI] [PubMed] [Google Scholar]