Abstract
Idiopathic orbital inflammation (IOI) is a benign inflammatory condition usually confined to the orbit but extraorbital extension can also occur. IOI has been classified into categories including anterior, diffuse, posterior or apical, myositis, and dacryoadenitis. Other rare types of IOI include periscleritis, perineuritis, and focal mass. Diagnosis is based on careful history, clinical findings, computed tomography, and magnetic resonance imaging findings. An orbital biopsy is usually done for accessible orbital lesions such as dacryoadenitis. For other types such as myositis and apical IOI where surgery is difficult or dangerous, orbital biopsy is not initially considered. The mainstay of therapy consists of systemic corticosteroids, but other options including external beam radiotherapy, antimetabolites, alkylating agents, T-cell/calcineurin inhibitors, lymphocyte inhibitors, tumor necrosis factor-α inhibitors, and surgical debulking have also been used.
Keywords: Corticosteroids, dacryoadenitis, idiopathic orbital inflammation, myositis, pseudotumor
Introduction
Idiopathic orbital inflammation (IOI), also known as orbital pseudotumor, nonspecific orbital inflammation, orbital inflammatory syndrome, is a benign, noninfective inflammatory condition of the orbit without any identifiable local or systemic cause. IOI accounts for approximately 8%–10% of all orbital mass lesions.[1,2]
There is no universally accepted classification for IOI. Depending on the orbital site of involvement, IOI can be conveniently divided into categories including anterior, diffuse, apical or posterior, myositis, and dacryoadenitis. Other rare IOI types include periscleritis, perineuritis, and focal mass. This review aims to highlight the clinical features, diagnostic approaches, treatment, and prognosis of IOI and review the recent publications on the subject.
Clinical Features
IOI may present in acute, subacute, or chronic fashion. It is usually unilateral, but bilateral disease, either simultaneously or sequentially, occurs with an incidence of 8%–20%.[3,4] IOI is usually seen in the fifth decade and there is no sex predilection. However, orbital myositis most commonly affects young adults in the third to fourth decade of life and shows female predilection.[5]
IOI may present with a wide range of clinical manifestations. The anterior IOI affects the globe, conjunctiva, eyelids, neural, and adjacent muscular structures. Pain and periorbital swelling are the most frequently encountered presenting features [Figure 1a]. Other common features include conjunctival chemosis and limited ocular motility. Rarely, proptosis, uveitis, papillitis, and exudative retinal detachment can also be seen.[6]
Figure 1.
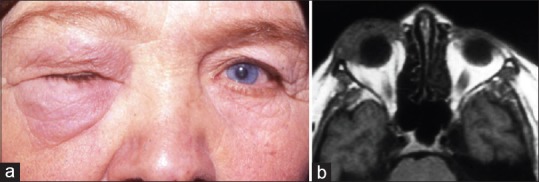
Anterior idiopathic orbital inflammation. (a) A 60-year-old woman presenting with right complete ptosis due to eyelid edema. (b) T1-weighted axial magnetic resonance image shows that inflammation occupying the right anterior orbit has a molded appearance with ill-defined margins and is isointense with respect to extraocular muscles and cerebral gray matter (Reproduced from Gündüz K, Yesiltas YS, Shields CL. Orbital Tumors: A systematic review part II. Expert Rev Ophthalmol 2015;22:485-508)
Patients with diffuse IOI present with features similar to anterior IOI. However, the findings are more severe in diffuse IOI. Furthermore, proptosis is seen more frequently with the diffuse variant compared to anterior IOI [Figure 2a].[6]
Figure 2.
Diffuse idiopathic orbital inflammation. (a) A 43-year-old woman showing marked proptosis of the left eye with downward displacement and upper eyelid swelling. (b) T1-weighted axial magnetic resonance image shows diffusely infiltrating mass with ill-defined borders in the left orbit that is isointense with respect to extraocular muscles and cerebral gray matter. (c) Histopathologic examination shows that the orbital fat is infiltrated by lymphocytes and plasma cells consistent with idiopathic orbital inflammation (H and E, ×200)
Apical or posterior IOI, while less common, is associated with a poorer visual outcome.[7] Clinically, apical IOI presents with orbital pain, restricted eye movement, visual loss, and minimal proptosis.[8] Inflammatory lesions of the orbital apex may extend intracranially through superior orbital fissure, optic canal, and inferior orbital fissure. The cavernous sinus and the middle cranial fossa are the two most common locations for intracranial involvement.[9] In a series of 90 consecutive cases of IOI, 8.8% (8 cases) showed radiological evidence of intracranial extension.[10] Tolosa–Hunt syndrome is a rare clinical condition caused by idiopathic granulomatous inflammation in the region of cavernous sinus and/or superior orbital fissure. Tolosa–Hunt syndrome presents with relapsing/remitting partial/complete ophthalmoplegia, visual loss, and unilateral headache.[11]
Myositis involves single or multiple extraocular muscles (EOMs).[5] Clinically, it presents with unilateral orbital or periorbital pain, diplopia, ocular motility restriction, proptosis, eyelid swelling, and conjunctival injection at the site of tendon insertion [Figure 3a].[12] The most frequently involved muscle is the medial rectus followed by the superior, lateral and inferior rectus muscles.[7] Isolated levator palpebrae muscle involvement has also been seldom reported.[13]
Figure 3.
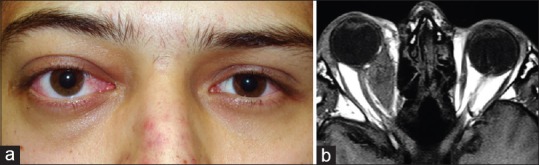
Myositis. (a) A 19-year-old man with myositis of medial rectus muscle in the right eye. In this patient, upper eyelid swelling, proptosis, and conjunctival injection were observed. (b) T1-weighted axial magnetic resonance image demonstrates fusiform enlargement of the right medial rectus. The lesion is isointense with respect to extraocular muscles and cerebral gray matter
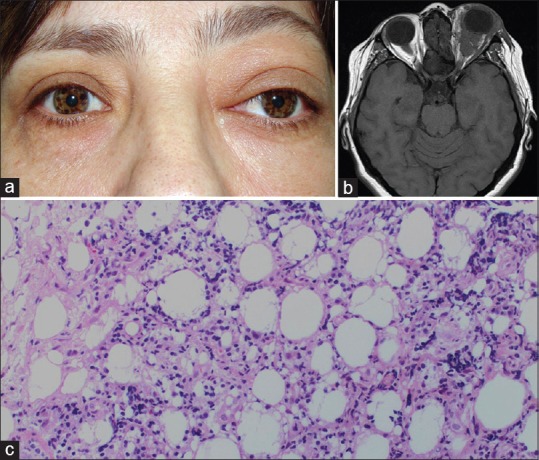
Dacryoadenitis is the most commonly encountered subtype of IOI, accounting for approximately 50% of all IOIs.[7,14] The typical acute presentation of dacryoadenitis includes a painful, firm, erythematous mass with edema in the lateral upper eyelid, and S-shaped ptosis sometimes associated with dry eye [Figure 4a].[4] In 20% of patients, both lacrimal glands are affected, either simultaneously or sequentially.[4] Since the description of IgG4-related disease, a subset of patients with the initial diagnosis of idiopathic dacryoadenitis have been relabeled as IgG4-related dacryoadenitis. Immunoglobulin G4-related ophthalmic disease (IgG4-ROD) most frequently involves lacrimal gland but can also affect the orbital soft tissue, optic nerve, trigeminal nerve branches, especially infraorbital nerve, sclera, choroid, and orbital adnexa.[15]
Figure 4.
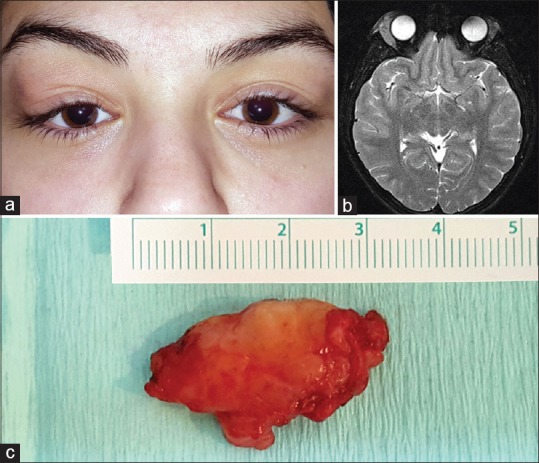
Dacryoadenitis. (a) A 16-year-old girl with right dacryoadenitis presenting with a characteristic S-shaped ptosis. (b) T2-weighted axial magnetic resonance image demonstrates bilateral diffuse enlargement of lacrimal gland which is more pronounced in the right orbit. The tumor has an ill-defined molded appearance and is isointense with respect to extraocular muscles and cerebral gray matter. (c) The patient underwent orbitotomy and surgical debulking. Gross appearance of the excised yellow-reddish lacrimal gland tissue is depicted in the photograph
Periscleritis refers to inflammation involving the sclera, uvea, and/or Tenon's capsule. Clinical features of periscleritis include orbital pain, exophthalmos, eyelid edema, vision decrease, and associated uveitis.[8]
Optic perineuritis also known as perioptic neuritis, is a rare idiopathic orbital inflammatory disease, in which the specific target tissue is the optic nerve sheath, rather than the optic nerve axons as occurs in optic neuritis or optic neuropathy. Optic perineuritis usually presents with pain, swollen optic disc, and arcuate and paracentral visual field defects. Optic perineuritis usually responds well to systemic corticosteroids unlike the other entities in the differential diagnosis.[16]
IOI may also present as a focal inflammatory mass in the orbit. The clinical presentation varies according to the location and extent of the mass, with resulting symptoms related to mass effect and inflammation or infiltration [Figure 5a]. Clinically, a space-occupying or infiltrating focal mass may present with proptosis, motility disturbance, optic nerve compression, and other inflammatory signs including edema and hyperemia.
Figure 5.
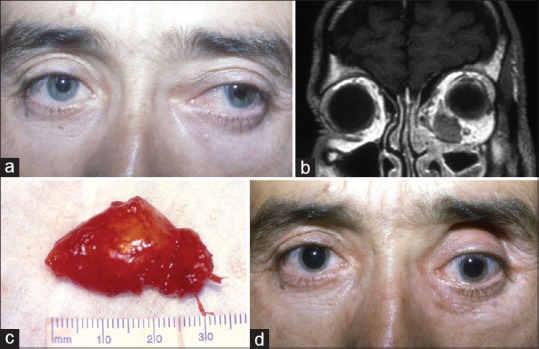
Focal idiopathic orbital inflammation. (a) A 36-year-old man presenting with left proptosis characterized by lateral and inferior displacement of the globe. (b) T1-weighted coronal magnetic resonance image shows an ill-defined, hypointense mass, discrete from adjacent extraocular muscles in the left orbit. (c) Photograph demonstrates gross appearance of the completely excised inflammatory mass. (d) Postoperative facial photograph at 3-month follow-up shows the absence of proptosis with slight hypotropia of the left eye
A small subset of IOI is associated with sclerosing orbital inflammation. Sclerosing orbital inflammation produces serious morbidity with a severe, chronic, progressive disease often characterized by proptosis, mild inflammatory signs, restricted ocular motility, and pain.[17] Due to the progressive nature of the lesion, it may present as diffuse orbital involvement or with extraorbital involvement (intracranial or infratemporal fossa).[17] The relationship between sclerosing orbital inflammation and IgG4-ROD has not yet been elucidated.
IOI is uncommon in the pediatric age group.[18] It is usually characterized by bilateral involvement in children that differ from the adult presentations. Constitutional signs and symptoms such as headache, fever, emesis, anorexia, lethargy, and abdominal pain can be seen in up to 50% of the patients. Optic disc edema, uveitis, and eosinophilia appear to be more common in the pediatric population.[19] Differential diagnosis of pediatric IOI may include orbital cellulitis, rhabdomyosarcoma, thyroid orbitopathy, leukemia, orbital trauma with retained foreign body, ruptured dermoid cyst, lymphangioma, neuroblastoma, Langerhans cell histiocytosis, and secondary orbital retinoblastoma.[18]
Imaging Features
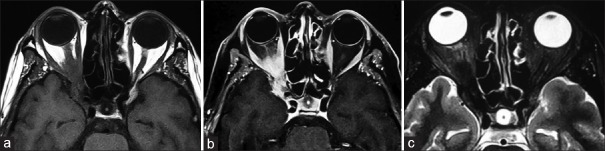
In anterior IOI, the characteristic computed tomography (CT) and magnetic resonance imaging (MRI) findings include an ill-defined anterior orbital mass demonstrating a molded configuration, sometimes with uveoscleral thickening [Figure 1b]. IOI is hypointense to isointense with respect to EOM on T1-weighted images and hypointense to isointense on T2-weighted (T2-W) images [Figures 1b, 2b, 3b, 4b, 5b, 6a, and 6c]. The sclerosing type of inflammation shows definite hypointensity on T2-W images.[6] After gadolinium injection, enhancement is variable depending on the stage of inflammation. In the acute stages of the disease, enhancement is marked [Figures 6b and 7a]. In chronic and sclerosing types of inflammation, minimal-to-moderate enhancement is seen.
Figure 6.
Apical or posterior idiopathic orbital inflammation. (a) T1-weighted axial magnetic resonance image reveals the right lesion in the orbital apex that is isointense with respect to extraocular muscles and cerebral gray matter. (b) The lesion demonstrates marked enhancement after contrast injection. (c) T2-weighted axial magnetic resonance image demonstrates that the lesion is hypointense to isointense with respect to extraocular muscles and cerebral gray matter
Figure 7.
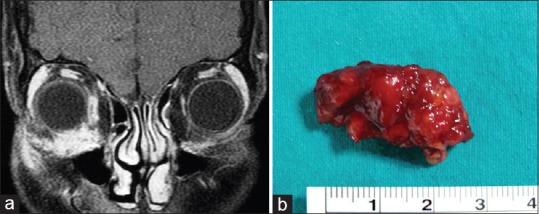
Granulomatous orbital inflammation. (a) Contrast-enhanced T1-weighted coronal magnetic resonance image shows ill-defined mass in the inferonasal right orbit with marked enhancement. (b) Gross photograph shows the excised reddish-colored orbital mass with irregular surface. Histopathologic examination revealed granulomatous inflammation
The CT and MRI findings in diffuse IOI are similar to anterior IOI. When there is diffuse infiltration of the orbital fat and EOM, the imaging finding is referred to as the “casting sign.” The orbital lymphoproliferative disease can cause similar imaging findings. Diffusion-weighted (DW) imaging is perhaps the most reliable technique to distinguish lymphoproliferative disease and cellulitis from inflammation based on the apparent diffusion coefficient.[20]
The signs of intracranial involvement that can be seen in apical IOI on CT and MRI include abnormal soft tissue in the superior orbital fissure extending into the middle cranial fossa, expansion of the ipsilateral cavernous sinus, and thickening and/or enhancement of the meninges contiguous with the orbital inflammation.[10]
Myositis is characterized by a unilateral thickening of one or more EOMs often involving the myotendinous junction with a fusiform configuration on CT and MRI [Figure 3b]. There may be ill-defined infiltrates throughout the surrounding orbital fat.[6] These are important features in distinguishing myositis from thyroid orbitopathy, which affects EOM bilaterally and spares the myotendinous junction with an increase in orbital fat volume.
Dacryoadenitis demonstrates a diffusely enlarged lacrimal gland with ill-defined margins conforming to the shape of the globe and orbital bone on CT and MRI [Figure 4b].[6] Lymphoma in the lacrimal gland region may also mimic dacryoadenitis and as described earlier may be distinguished from IOI using DW MRI and definitely by orbital biopsy.[20]
In periscleritis, ultrasonography, CT, and MRI show heterogeneous thickening of the sclera and/or uvea. This is often associated with edema fluid extending into Tenon's space yielding a lucent area or the so-called “ring sign.”[21] The blurring of the sclera margins may be seen.
In perineuritis, MRI is typical with a characteristic circumferential enhancement around optic nerve sheath, a “doughnut sign” on coronal views and “tram-track” sign on axial views. There may be streaky densities in the contiguous orbital fat.[22] In contrast, optic neuritis tends to show enhancement of optic nerves itself, with or without white matter lesions.
Imaging features of focal IOI are similar to other subtypes and may include hypointensity or isointensity on T1 and T2 images, and contrast enhancement [Figure 5b].
The Role of Orbital Biopsy in Idiopathic Orbital Inflammation
A major of debate in the management of IOI is the need for orbital biopsy. Typical IOI cases can be treated with systemic corticosteroids without a biopsy. Given the concern for surgical morbidity, myositis and apical IOI can be managed with systemic corticosteroids initially. Orbital biopsy is required in cases that do not respond appropriately, demonstrate progression or recurrence despite corticosteroid treatment.[23]
Differential Diagnosis
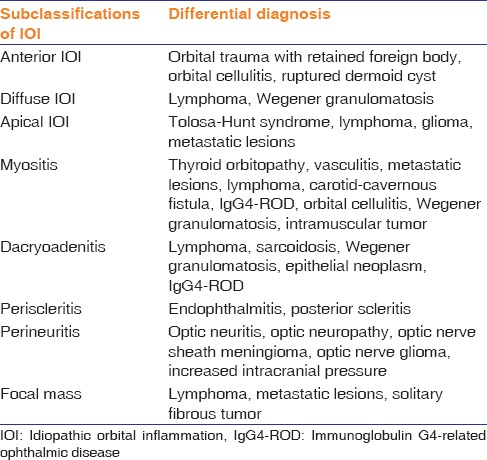
There are many diseases that can mimic IOI. Differential diagnosis according to subclassifications of IOI is given in Table 1.
Table 1.
Differential diagnosis of idiopathic orbital inflammation by subclassification
Pathogenesis
The etiology and pathogenesis of IOI are currently unknown. Infectious process, autoimmune disorder, and aberrant wound healing have all been implicated in the pathogenesis. Molecular mimicry where a foreign antigen shares structural similarities with self-antigens has been suggested as an explanation for IOI after an acute infection. Infectious process such as upper respiratory tract infections and viral illness can be temporally linked to the onset of orbital myositis. Streptococcal infection, Lyme disease, varicella-zoster disease also have been implicated in the development of orbital myositis.[24,25,26]
Concurrent autoimmune disease such as Crohn disease, ankylosing spondylitis, and psoriasis, has been shown to be present in 10% of patients with IOI, which suggest immunologic dysregulation being at least one component underlying IOI.[27] An autoimmune process has also been suggested based on the presence of circulating autobodies against EOM antigens in patients with orbital myositis.[28]
Mombaerts et al. proposed aberrant immune-mediated production of fibrogenic cytokines leading to aberrant wound healing as the ocular mechanism underlying the process of fibrosis in sclerosing orbital inflammation.[29] In light of this theory, the development of IOI can be considered as aberrant wound healing with cytokine-driven fibroblast proliferation and collagen synthesis as a final common pathway, provoked by infection or autoimmune disease.
Imbalance of inflammatory cytokines has also been documented in IOI. Recently, Wladis et al. have reported high levels of interleukin (IL)-2, IL-8, IL-10, IL-12, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α cytokines in IOI histopathological specimens and inferred an important pathogenetic role of a T helper 1-related disorder in the pathogenesis of IOI.[30] Another immunohistochemical study has documented that CD20 and CD25 are strongly expressed in IOI, suggesting that rituximab (RTX) which targets CD20 and denileukin diftitox (ONTAK) which targets CD25 may be beneficial in IOI.[31]
Recently, gene expression profiling methods have been used to investigate phenotypic variability in IOI. Genomic upregulation involving immunoglobulin, CXCR4, YKL-40, CXCL9, SLAM Family 7, IL-7 receptor and genomic downregulation involving alcohol dehydrogenase 1B, perilipin 1, adiponectin, leptin receptor, and C1Q have been documented in patients with IOI by gene expression array methods.[32] Genotypic analysis will definitely shed more light on the phenotypic variability observed among IOI cases. Consequently, targeted subset treatment of IOI cases may become possible in the future.
Histopathology
Histologically, IOI has the features of chronic inflammatory infiltrate, composed mainly of small mature lymphocytes (predominantly T-cells), plasma cells, neutrophils, eosinophils, and occasionally with histiocytes and macrophages [Figure 2c]. The infiltrate can be focally organized in lymphoid follicles with reactive germinal centers. Stromal changes may include edema, proliferative fibrosis, and sclerosis. Vascular changes may include perivasculitis and/or angiocentric lymphocytic cuffing. Macroscopically, excised IOI tissue appears as a yellow-gray-pink firm, rubbery lesion [Figures 4c and 5c].[29]
Sclerosing IOI is a distinct subtype of IOI characterized by the replacement of orbital tissues by dense, fibrous tissue with sparse inflammatory cell infiltrate.[33] Historically, this has been thought to be a sequelae to long-standing IOI, but it is now thought that this condition might represent a specific subtype and separate entity and may even be related to IgG4-ROD although this is at present not proven.
The presence of granulomatous inflammation in IOI is relatively uncommon [Figures 7a and 7b]. Granulomatous orbital inflammation is characterized by histiocytic infiltration and sometimes with well-formed noncaseating granulomas, and is not associated with systemic vasculitic or granulomatous diseases.[34]
Treatment and Prognosis
Corticosteroids
Corticosteroids have both anti-inflammatory and immunosuppressive effects. The anti-inflammatory effects occur through inhibition of phospholipase A2 and cyclooxygenase pathways. The immunosuppressive effects are due to the inhibition of IL and IFN synthesis, inhibition of major histocompatibility antigen expression, and cytotoxic effect on T lymphocytes. Systemic corticosteroids are currently considered first-line therapy for IOI. Over 75% of patients show dramatic improvement in all symptoms and findings initially.[35] A starting dose of 1 mg/kg/day of prednisone is typically used with a slow taper over 6–8 weeks. There are well-established undesirable systemic side effects of long-term systemic corticosteroids. These include hyperglycemia, hypertension, cushingoid features, osteoporosis, adrenal suppression, weight gain, avascular bone necrosis, and growth retardation in children. Ocular side effects include glaucoma, cataract, and herpetic corneal infection.[36,37]
In a study by Mombaerts et al. that reviewed the treatment of 32 patients with IOI, 27 patients initially received systemic corticosteroids. Of these 27 patients, 21 (78%) showed an initial response and 10 (37%) obtained a definitive cure. After the initial response, 11 (52%) of the 21 patients recurred.[38] The authors concluded that systemic corticosteroids offered little help in the treatment of IOI other than IOI-related optic neuropathy. Yuen and Rubin reported complete resolution without recurrence in 63% of patients with IOI, 20% of whom required additional immunosuppressive agents.[7] Intraorbital triamcinolone acetonide injection has also been found to be an effective treatment for IOI.[39] Therefore, it appears that systemic corticosteroids may be effective as sole treatment in at least 40% patients with IOI and when combined with other immunosuppressants may show effect in about 60% of patients. Further, there may be a role for intraorbital corticosteroids either alone in combination with systemic corticosteroids.
Nonsteroidal Anti-Inflammatory Drugs
Nonsteroidal anti-inflammatory drugs (NSAIDs) possess anti-inflammatory properties by virtue of their ability to inhibit prostaglandin synthesis through the cyclooxygenase pathway. Oral NSAIDs have been used in mild cases of IOI. Noble et al. have reported successful treatment with indomethacin 150 mg daily in one patient with orbital myositis who had developed adverse effects from corticosteroids.[40] Compared with systemic corticosteroids, oral NSAIDs have fewer side effects. The side effects depend on the specific drug but include an increased risk of gastrointestinal ulcers and bleeds, heart attack, and kidney disease.
External Beam Radiotherapy
External beam radiotherapy (EBRT) is commonly used as an adjuvant or even an alternative to steroids when symptoms recur during tapering or when the steroids are ineffective or contraindicated. EBRT reduces dependence on corticosteroids with complete cessation of corticosteroid therapy in 56.3% of patients and reduction of corticosteroid dose in 25% of patients.[41]
An average dose is 1000–3000 cGy delivered over 2–3 weeks.[42,43,44] Success rates vary from 50% to 75%, depending on the citing paper.[43,44] Side effects of radiation include dry eye, keratitis, cataract, retinopathy, optic neuropathy, and periocular dermatitis.[45]
Antimetabolites
Methotrexate
Methotrexate is a folic acid antagonist that inhibits dihydrofolate reductase in folic acid synthesis which is an enzyme needed for synthesis of DNA and RNA, resulting in the inhibition of rapidly proliferating cells, suppressing both B-cell and T-cell function. Adverse effects of methotrexate include fatigue, hair loss, gastrointestinal disturbances, neutropenia, and hepatotoxicity.
Smith and Rosenbaum treated seven IOI patients with methotrexate as a steroid-sparing drug at a dose of 15–25 mg/week. Of these seven patients, four (57%) demonstrated clinical benefit, one (14.3%) had no response, and the remaining two patients preferred to stop treatment.[46] Shah et al. reported better findings, with 5 (83%) of 6 IOI patients showing clinical benefit with the use of methotrexate.[47]
Azathioprine
Azathioprine is a purine analog that interferes with DNA synthesis and inhibits the proliferation of quickly proliferating cells, especially cells of the immune system. There have been several case reports of therapeutic success with azathioprine in IOI.[48] Rootman et al. found azathioprine useful in one patient in conjunction with systemic corticosteroids.[49] Azathioprine is associated with side effects including bone marrow suppression, gastrointestinal irritation, and induction of malignancy.
Mycophenolate mofetil
Mycophenolate mofetil is an antimetabolite that has a similar mechanism of action to azathioprine and inhibits purine synthesis, preventing B-cell and T-cell replication. Adverse effects include gastrointestinal disturbances and immunosuppression. Mycophenolate mofetil therapy resulted in complete resolution of inflammation in four patients with refractory IOI and in three of four patients, complete tapering of corticosteroids was possible.[50]
T-Cell/calcineurin Inhibitors
Cyclosporine-A
Cyclosporine-A (CsA) is an immunomodulatory agent that inhibits IL-1 and IL-2, decreasing the activation of T-lymphocytes. Serious adverse effects include renal dysfunction, hypertension, liver toxicity, and hematologic and dermatologic malignancies.[51]
Several cases have shown the efficacy of the CsA in cases of uncontrolled IOI, including myositis.[52,53] Zacharopoulos et al. treated a patient with IOI using CsA, at a starting dose of 4 mg/kg/day tapered to 2 mg/kg/day and continued for 18 months. No recurrence was noted at the end of 5 years' follow-up.[54]
Alkylating Agents
Cyclophosphamide
Cyclophosphamide is an alkylating agent that damages proliferating cells by cross linking DNA. Adverse drug reactions include myelosuppression, gastrointestinal disorders, hemorrhagic cystitis, and induction of secondary malignancy.[55]
There are only a few case reports in the literature of IOI treated with cyclophosphamide in combination with other therapies. Eagle et al. have reported successful treatment result in one patient with IOI using cyclophosphamide 100 mg daily after corticosteroids, radiotherapy, and azathioprine have been tried and found to be ineffective. The patient has remained in remission up to 3 years of follow-up.[55]
Chlorambucil
Chlorambucil has a mechanism of action similar to cyclophosphamide and alkylates and cross-links DNA during all phases of the cell cycle. Significant adverse drug reactions are dose-related bone marrow depression, gastrointestinal distress, infertility, and central nervous system manifestations including seizure, ataxia, and tremor.
There are only limited case reports of chlorambucil used in IOI. Paris et al. reported good results with 5-day pulsed chemotherapy consisting of prednisone (100 mg/day) combined with either cyclophosphamide (100 mg/day) or chlorambucil (10 mg/day) in five patients with IOI. This 5-day regimen was well tolerated by all patients without unfavorable side effects.[56]
Lymphocyte Inhibitors
Rituximab (B-lymphocyte inhibitor)
RTX is a chimeric mouse-human monoclonal antibody against CD20, a cell-surface phosphoprotein on B-cells. Potential side effects of RTX include infusion reactions, pulmonary toxicity, bowel obstruction, cardiac toxicity, and immunosuppression.
There are a few case reports in which RTX treatment of IOI achieved a reduction in disease activity improving edema, discomfort, redness, and diplopia.[57,58] In one report, RTX and CyberKnife radiosurgery resulted in symptomatic resolution of refractory IOI, and the effect was maintained up to 18 months' follow-up.[59] Savino et al. reported successful treatment in three patients with IOI with intraorbital injections of RTX at a dose of 10 mg once a week for 1 month. Of these patients, two patients required 2 months of RTX and one required 1 month of RTX treatment at 17.6 months' follow-up.[60]
Daclizumab (T-lymphocyte inhibitor)
Daclizumab is a humanized monoclonal antibody directed against IL-2 receptors (CD25) on T cells. It was initially used to prevent rejection in liver and heart transplants. Side effects include rash, liver complications, and infections.
There is limited clinical experience in IOI with daclizumab. A single case with orbital myositis has been reported in which daclizumab treatment improved chemosis, exophthalmos, pain, and diplopia and remission of the inflammatory signs was maintained up to 1-year follow-up.[61]
Tumor Necrosis Factor-α Inhibitors
Infliximab
Infliximab is a chimeric monoclonal antibody acting against TNF-α. It was initially used in Crohn's disease and subsequently in rheumatoid arthritis. This agent is variably effective in ocular inflammatory disease including uveitis, scleritis, and IOI. Side effects include reactivation of latent tuberculosis, rash, headache, hypotension, lupus-like reactions, infusion reactions, elevation of antinuclear autoantibodies, and possible risk of lymphoma. It may have synergistic effects with methotrexate, therefore, dose reduction may be possible.
Favorable response to infliximab has been reported in several patients with chronic and refractory IOI previously treated with corticosteroids, chemotherapy, and radiotherapy. The dose of infliximab is 3-5 mg/kg intravenously at weeks 0, 2, 6, and every 4-8 weeks thereafter until the desired effect is achieved. A recent report of 7 patients with chronic and recalcitrant orbital myositis documented clinical response in all patients to infliximab therapy. It was also noted that only the IOI patients without systemic disease were able to discontinue all therapies.[62] Wilson et al. reported success in the treatment of a pediatric patient with bilateral refractory IOI who has remained symptom-free and off corticosteroids 2 years after initial diagnosis.[63]
Adalimumab
Adalimumab is a fully humanized IgG1 monoclonal antibody targeting TNF-α. Because adalimumab is fully humanized, there is less risk of developing autoantibodies and allergic reactions; otherwise, the side effect profile is similar to infliximab. Two pediatric cases with refractory, steroid dependent, recurrent nonspecific orbital myositis have been treated with adalimumab therapy, showing improvement in clinical signs.[64]
Surgery
Surgical resection may be used in focal mass type IOI, in some anterior/diffuse IOI cases, and in dacryoadenitis [Figures 4c, 5a, 5b, 5c, 5d, and 7b]. Obtaining a generous biopsy or debulking of the orbital lobe of the lacrimal gland has been found to be effective in 80% of patients with idiopathic dacryoadenitis as initial treatment. Although these patients did not require medication, 8% of them developed recurrence during follow-up requiring systemic anti-inflammatory treatment. This type of debulking procedure has not been found to be associated with dry eye development as long as the palpebral lobe was left intact.[4]
Other Treatments
Treating IOI with intravenous immunoglobulin (IVIG) and plasmapheresis have been attempted. The mechanism of both IVIG and plasmapheresis is removal of autoantibodies either by neutralization or by filtration. These modalities may be considered in recalcitrant IOI cases that have failed all other treatments.[65]
Conclusions
IOI represents a diagnostic and therapeutic challenge for ophthalmologists. The condition may involve a number of structures in the orbit, the clinical presentations, and imaging findings of which are variable and overlapping. As IOI is a diagnosis of exclusion, other pathological conditions affecting the orbit must be ruled out. The mainstay of therapy is systemic corticosteroids. The need for orbital biopsy is judicial. Some prefer to obtain a biopsy in many accessible cases while others reserve biopsy for nonresponding or refractory cases. A range of other therapeutic modalities including EBRT, immunosuppressive/immunomodulatory agents, lymphocyte inhibitors, tumor necrosis factor-α inhibitors, and surgical debulking or resection can be used in patients that do not respond to systemic corticosteroids. Future studies should target the use of new immunomodulatory medications to assess their efficacy and safety.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Shields JA, Shields CL, Scartozzi R. Survey of 1264 patients with orbital tumors and simulating lesions: The 2002 montgomery lecture, part 1. Ophthalmology. 2004;111:997–1008. doi: 10.1016/j.ophtha.2003.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Wilson MW, Grossniklaus HE. Orbital disease in North America. Ophthalmol Clin North Am. 1996;9:539–47. [Google Scholar]
- 3.Swamy BN, McCluskey P, Nemet A, Crouch R, Martin P, Benger R, et al. Idiopathic orbital inflammatory syndrome: Clinical features and treatment outcomes. Br J Ophthalmol. 2007;91:1667–70. doi: 10.1136/bjo.2007.124156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mombaerts I, Cameron JD, Chanlalit W, Garrity JA. Surgical debulking for idiopathic dacryoadenitis: A diagnosis and a cure. Ophthalmology. 2014;121:603–9. doi: 10.1016/j.ophtha.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Yan J, Wu P. Idiopathic orbital myositis. J Craniofac Surg. 2014;25:884–7. doi: 10.1097/SCS.0000000000000510. [DOI] [PubMed] [Google Scholar]
- 6.Pakdaman MN, Sepahdari AR, Elkhamary SM. Orbital inflammatory disease: Pictorial review and differential diagnosis. World J Radiol. 2014;6:106–15. doi: 10.4329/wjr.v6.i4.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuen SJ, Rubin PA. Idiopathic orbital inflammation: Distribution, clinical features, and treatment outcome. Arch Ophthalmol. 2003;121:491–9. doi: 10.1001/archopht.121.4.491. [DOI] [PubMed] [Google Scholar]
- 8.Ding ZX, Lip G, Chong V. Idiopathic orbital pseudotumour. Clin Radiol. 2011;66:886–92. doi: 10.1016/j.crad.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 9.Lee EJ, Jung SL, Kim BS, Ahn KJ, Kim YJ, Jung AK, et al. MR imaging of orbital inflammatory pseudotumors with extraorbital extension. Korean J Radiol. 2005;6:82–8. doi: 10.3348/kjr.2005.6.2.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clifton AG, Borgstein RL, Moseley IF, Kendall BE, Shaw PJ. Intracranial extension of orbital pseudotumour. Clin Radiol. 1992;45:23–6. doi: 10.1016/s0009-9260(05)81462-x. [DOI] [PubMed] [Google Scholar]
- 11.Mullen E, Green M, Hersh E, Iloreta AM, Bederson J, Shrivastava R. Tolosa-Hunt Syndrome: Appraising the ICHD-3 beta diagnostic criteria. Cephalalgia. 2017 doi: 10.1177/0333102417745271. 1:333102417745271. doi: 10.1177/0333102417745271. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Montagnese F, Wenninger S, Schoser B. “Orbiting around” the orbital myositis: Clinical features, differential diagnosis and therapy. J Neurol. 2016;263:631–40. doi: 10.1007/s00415-015-7926-x. [DOI] [PubMed] [Google Scholar]
- 13.Almekhlafi MA, Fletcher WA. Levator palpebrae myositis. Neurology. 2008;71:1202. doi: 10.1212/01.wnl.0000327565.42954.7a. [DOI] [PubMed] [Google Scholar]
- 14.Mombaerts I. The many facets of dacryoadenitis. Curr Opin Ophthalmol. 2015;26:399–407. doi: 10.1097/ICU.0000000000000183. [DOI] [PubMed] [Google Scholar]
- 15.Derzko-Dzulynsky L. IgG4-related disease in the eye and ocular adnexa. Curr Opin Ophthalmol. 2017;28:617–22. doi: 10.1097/ICU.0000000000000427. [DOI] [PubMed] [Google Scholar]
- 16.Purvin V, Kawasaki A, Jacobson DM. Optic perineuritis: Clinical and radiographic features. Arch Ophthalmol. 2001;119:1299–306. doi: 10.1001/archopht.119.9.1299. [DOI] [PubMed] [Google Scholar]
- 17.Zakir R, Manners RM, Ellison D, Barker S, Crick M. Idiopathic sclerosing inflammation of the orbit: A new finding of calcification. Br J Ophthalmol. 2000;84:1322–4. doi: 10.1136/bjo.84.11.1318f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spindle J, Tang SX, Davies B, Wladis EJ, Piozzi E, Pellegrini M, et al. Pediatric idiopathic orbital inflammation: Clinical features of 30 cases. Ophthalmic Plast Reconstr Surg. 2016;32:270–4. doi: 10.1097/IOP.0000000000000494. [DOI] [PubMed] [Google Scholar]
- 19.Belanger C, Zhang KS, Reddy AK, Yen MT, Yen KG. Inflammatory disorders of the orbit in childhood: A case series. Am J Ophthalmol. 2010;150:460–3. doi: 10.1016/j.ajo.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 20.Sepahdari AR, Aakalu VK, Setabutr P, Shiehmorteza M, Naheedy JH, Mafee MF, et al. Indeterminate orbital masses: Restricted diffusion at MR imaging with echo-planar diffusion-weighted imaging predicts malignancy. Radiology. 2010;256:554–64. doi: 10.1148/radiol.10091956. [DOI] [PubMed] [Google Scholar]
- 21.Trokel SL, Hilal SK. Submillimeter resolution CT scanning of orbital diseases. Ophthalmology. 1980;87:412–7. doi: 10.1016/s0161-6420(80)35223-8. [DOI] [PubMed] [Google Scholar]
- 22.Szatmáry G. Imaging of the orbit. Neurol Clin. 2009;27:251–84. doi: 10.1016/j.ncl.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Mombaerts I, Rose GE, Garrity JA. Orbital inflammation: Biopsy first. Surv Ophthalmol. 2016;61:664–9. doi: 10.1016/j.survophthal.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Alshaikh M, Kakakios AM, Kemp AS. Orbital myositis following streptococcal pharyngitis. J Paediatr Child Health. 2008;44:233–4. doi: 10.1111/j.1440-1754.2008.01288.x. [DOI] [PubMed] [Google Scholar]
- 25.Nieto JC, Kim N, Lucarelli MJ. Dacryoadenitis and orbital myositis associated with lyme disease. Arch Ophthalmol. 2008;126:1165–6. doi: 10.1001/archopht.126.8.1165. [DOI] [PubMed] [Google Scholar]
- 26.Kawasaki A, Borruat FX. An unusual presentation of herpes zoster ophthalmicus: Orbital myositis preceding vesicular eruption. Am J Ophthalmol. 2003;136:574–5. doi: 10.1016/s0002-9394(03)00323-4. [DOI] [PubMed] [Google Scholar]
- 27.Mombaerts I, Koornneef L. Current status in the treatment of orbital myositis. Ophthalmology. 1997;104:402–8. doi: 10.1016/s0161-6420(97)30301-7. [DOI] [PubMed] [Google Scholar]
- 28.Atabay C, Tyutyunikov A, Scalise D, Stolarski C, Hayes MB, Kennerdell JS, et al. Serum antibodies reactive with eye muscle membrane antigens are detected in patients with nonspecific orbital inflammation. Ophthalmology. 1995;102:145–53. doi: 10.1016/s0161-6420(95)31066-4. [DOI] [PubMed] [Google Scholar]
- 29.Mombaerts I, Goldschmeding R, Schlingemann RO, Koornneef L. What is orbital pseudotumor? Surv Ophthalmol. 1996;41:66–78. doi: 10.1016/s0039-6257(97)81996-0. [DOI] [PubMed] [Google Scholar]
- 30.Wladis EJ, Iglesias BV, Gosselin EJ. Characterization of the molecular biologic milieu of idiopathic orbital inflammation. Ophthalmic Plast Reconstr Surg. 2011;27:251–4. doi: 10.1097/IOP.0b013e31820768f7. [DOI] [PubMed] [Google Scholar]
- 31.Ho VH, Chevez-Barrios P, Jorgensen JL, Silkiss RZ, Esmaeli B. Receptor expression in orbital inflammatory syndromes and implications for targeted therapy. Tissue Antigens. 2007;70:105–9. doi: 10.1111/j.1399-0039.2007.00863.x. [DOI] [PubMed] [Google Scholar]
- 32.Rosenbaum J, Choin D, Harrington C, Harris G, Czyz C, White V, et al. Identifying and classifying nonspecific orbital inflammation (NSOI) by gene expression array. Invest Ophthalmol Vis Sci. 2013;54:2035. [Google Scholar]
- 33.Lokdarshi G, Pushker N, Bajaj MS. Sclerosing lesions of the orbit: A Review. Middle East Afr J Ophthalmol. 2015;22:447–51. doi: 10.4103/0974-9233.167807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mombaerts I, Schlingemann RO, Goldschmeding R, Koornneef L. Idiopathic granulomatous orbital inflammation. Ophthalmology. 1996;103:2135–41. doi: 10.1016/s0161-6420(96)30378-3. [DOI] [PubMed] [Google Scholar]
- 35.Harris GJ. Idiopathic orbital inflammation: A pathogenetic construct and treatment strategy: The 2005 ASOPRS foundation lecture. Ophthalmic Plast Reconstr Surg. 2006;22:79–86. doi: 10.1097/01.iop.0000203734.52333.93. [DOI] [PubMed] [Google Scholar]
- 36.Le Moli R, Baldeschi L, Saeed P, Regensburg N, Mourits MP, Wiersinga WM, et al. Determinants of liver damage associated with intravenous methylprednisolone pulse therapy in Graves' ophthalmopathy. Thyroid. 2007;17:357–62. doi: 10.1089/thy.2006.0267. [DOI] [PubMed] [Google Scholar]
- 37.Ciriaco M, Ventrice P, Russo G, Scicchitano M, Mazzitello G, Scicchitano F, et al. Corticosteroid-related central nervous system side effects. J Pharmacol Pharmacother. 2013;4:S94–8. doi: 10.4103/0976-500X.120975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mombaerts I, Schlingemann RO, Goldschmeding R, Koornneef L. Are systemic corticosteroids useful in the management of orbital pseudotumors? Ophthalmology. 1996;103:521–8. doi: 10.1016/s0161-6420(96)30663-5. [DOI] [PubMed] [Google Scholar]
- 39.Reggie S, Neimkin M, Holds J. Intralesional corticosteroid injections as treatment for non-infectious orbital inflammation. Orbit. 2018;37:41–7. doi: 10.1080/01676830.2017.1353110. [DOI] [PubMed] [Google Scholar]
- 40.Noble AG, Tripathi RC, Levine RA. Indomethacin for the treatment of idiopathic orbital myositis. Am J Ophthalmol. 1989;108:336–8. doi: 10.1016/0002-9394(89)90135-9. [DOI] [PubMed] [Google Scholar]
- 41.Matthiesen C, Bogardus C, Jr, Thompson JS, Farris B, Hildebrand L, Wilkes B, et al. The efficacy of radiotherapy in the treatment of orbital pseudotumor. Int J Radiat Oncol Biol Phys. 2011;79:1496–502. doi: 10.1016/j.ijrobp.2009.12.071. [DOI] [PubMed] [Google Scholar]
- 42.Kennerdell JS, Johnson BL, Deutsch M. Radiation treatment of orbital lymphoid hyperplasia. Ophthalmology. 1979;86:942–7. doi: 10.1016/s0161-6420(79)35439-2. [DOI] [PubMed] [Google Scholar]
- 43.Sergott RC, Glaser JS, Charyulu K. Radiotherapy for idiopathic inflammatory orbital pseudotumor. Indications and results. Arch Ophthalmol. 1981;99:853–6. doi: 10.1001/archopht.1981.03930010853013. [DOI] [PubMed] [Google Scholar]
- 44.Orcutt JC, Garner A, Henk JM, Wright JE. Treatment of idiopathic inflammatory orbital pseudotumours by radiotherapy. Br J Ophthalmol. 1983;67:570–4. doi: 10.1136/bjo.67.9.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prabhu RS, Kandula S, Liebman L, Wojno TH, Hayek B, Hall WA, et al. Association of clinical response and long-term outcome among patients with biopsied orbital pseudotumor receiving modern radiation therapy. Int J Radiat Oncol Biol Phys. 2013;85:643–9. doi: 10.1016/j.ijrobp.2012.06.033. [DOI] [PubMed] [Google Scholar]
- 46.Smith JR, Rosenbaum JT. A role for methotrexate in the management of non-infectious orbital inflammatory disease. Br J Ophthalmol. 2001;85:1220–4. doi: 10.1136/bjo.85.10.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shah SS, Lowder CY, Schmitt MA, Wilke WS, Kosmorsky GS, Meisler DM, et al. Low-dose methotrexate therapy for ocular inflammatory disease. Ophthalmology. 1992;99:1419–23. doi: 10.1016/s0161-6420(92)31790-7. [DOI] [PubMed] [Google Scholar]
- 48.Chiu CS, Rubin PA. Pharmacotherapies and nonpharmacotherapies for orbital inflammatory diseases. Int Ophthalmol Clin. 2004;44:165–85. doi: 10.1097/00004397-200404430-00015. [DOI] [PubMed] [Google Scholar]
- 49.Rootman J, McCarthy M, White V, Harris G, Kennerdell J. Idiopathic sclerosing inflammation of the orbit. A distinct clinicopathologic entity. Ophthalmology. 1994;101:570–84. doi: 10.1016/s0161-6420(94)31298-x. [DOI] [PubMed] [Google Scholar]
- 50.Hatton MP, Rubin PA, Foster CS. Successful treatment of idiopathic orbital inflammation with mycophenolate mofetil. Am J Ophthalmol. 2005;140:916–8. doi: 10.1016/j.ajo.2005.04.053. [DOI] [PubMed] [Google Scholar]
- 51.Buell C, Koo J. Long-term safety of mycophenolate mofetil and cyclosporine: A review. J Drugs Dermatol. 2008;7:741–8. [PubMed] [Google Scholar]
- 52.Diaz-Llopis M, Menezo JL. Idiopathic inflammatory orbital pseudotumor and low-dose cyclosporine. Am J Ophthalmol. 1989;107:547–8. doi: 10.1016/0002-9394(89)90503-5. [DOI] [PubMed] [Google Scholar]
- 53.Sánchez-Román J, Varela-Aguilar JM, Bravo-Ferrer J, Sequeiros Madueño E, Fernández de Bobadilla M. Idiopathic orbital myositis: Treatment with cyclosporin. Ann Rheum Dis. 1993;52:84–5. doi: 10.1136/ard.52.1.84-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zacharopoulos IP, Papadaki T, Manor RS, Briscoe D. Treatment of idiopathic orbital inflammatory disease with cyclosporine-A: A case presentation. Semin Ophthalmol. 2009;24:260–1. doi: 10.3109/08820530903392639. [DOI] [PubMed] [Google Scholar]
- 55.Eagle K, King A, Fisher C, Souhami R. Cyclophosphamide induced remission in relapsed, progressive idiopathic orbital inflammation ('pseudotumour') Clin Oncol (R Coll Radiol) 1995;7:402–4. doi: 10.1016/s0936-6555(05)80017-2. [DOI] [PubMed] [Google Scholar]
- 56.Paris GL, Waltuch GF, Egbert PR. Treatment of refractory orbital pseudotumors with pulsed chemotherapy. Ophthalmic Plast Reconstr Surg. 1990;6:96–101. doi: 10.1097/00002341-199006000-00003. [DOI] [PubMed] [Google Scholar]
- 57.Schafranski MD. Idiopathic orbital inflammatory disease successfully treated with rituximab. Clin Rheumatol. 2009;28:225–6. doi: 10.1007/s10067-008-1040-8. [DOI] [PubMed] [Google Scholar]
- 58.Ibrahim I, Barton A, Ibrahim A, Ho P. Idiopathic orbital inflammation successfully treated using rituximab in a patient with rheumatoid arthritis. J Rheumatol. 2012;39:1485–6. doi: 10.3899/jrheum.111230. [DOI] [PubMed] [Google Scholar]
- 59.On AV, Hirschbein MJ, Williams HJ, Karesh JW. CyberKnife radiosurgery and rituximab in the successful management of sclerosing idiopathic orbital inflammatory disease. Ophthalmic Plast Reconstr Surg. 2006;22:395–7. doi: 10.1097/01.iop.0000231549.24125.7a. [DOI] [PubMed] [Google Scholar]
- 60.Savino G, Battendieri R, Siniscalco A, Mandarà E, Mulè A, Petrone G, et al. Intraorbital injection of rituximab in idiopathic orbital inflammatory syndrome: Case reports. Rheumatol Int. 2015;35:183–8. doi: 10.1007/s00296-014-3054-7. [DOI] [PubMed] [Google Scholar]
- 61.Garcia-Pous M, Hernández-Garfella ML, Díaz-Llopis M. Treatment of chronic orbital myositis with daclizumab. Can J Ophthalmol. 2007;42:156–7. [PubMed] [Google Scholar]
- 62.Garrity JA, Coleman AW, Matteson EL, Eggenberger ER, Waitzman DM. Treatment of recalcitrant idiopathic orbital inflammation (chronic orbital myositis) with infliximab. Am J Ophthalmol. 2004;138:925–30. doi: 10.1016/j.ajo.2004.06.077. [DOI] [PubMed] [Google Scholar]
- 63.Wilson MW, Shergy WJ, Haik BG. Infliximab in the treatment of recalcitrant idiopathic orbital inflammation. Ophthalmic Plast Reconstr Surg. 2004;20:381–3. doi: 10.1097/01.iop.0000139521.38345.ba. [DOI] [PubMed] [Google Scholar]
- 64.Adams AB, Kazim M, Lehman TJ. Treatment of orbital myositis with adalimumab (Humira) J Rheumatol. 2005;32:1374–5. [PubMed] [Google Scholar]
- 65.Symon Z, Schneebaum N, Eyal A, Tal S, Rozen N, Shoenfeld Y, et al. Successful intravenous immunoglobulin therapy for resistant inflammatory pseudotumor of the orbit. Thyroid. 2005;15:398–9. doi: 10.1089/thy.2005.15.398. [DOI] [PubMed] [Google Scholar]