Abstract
Aim and Objectives:
The objective of the present study was to estimate the serum and salivary lactate dehydrogenase (LDH) levels in patients with oral submucous fibrosis (OSMF) and to study the association between serum and salivary LDH levels and mouth opening, frequency of habit, and duration of habit in patients with OSMF.
Materials and Methods:
Participants were divided into two groups: Group I – case group diagnosed with OSMF and Group II – the control group. Unstimulated whole saliva was collected from all the participants. The International Federation of Clinical Chemistry method was carried out with the help of LDH (P-L) kit (Crest Biosystems, Goa). Collection of blood sample was done under aseptic precautions and processed for LDH. The data were analyzed using SPSS software 2010 (version 19, IBM, Armonk, NY, USA). Descriptive statistics including mean, range, standard deviation, and percentage were used along with unpaired t-test and Pearson's correlation test.
Results:
All the participants were male and were in the age range of 18–60 years, with a mean age of 28.63 ± 10.39 years. The mean salivary LDH levels in patients with OSMF were 1057.30 ± 640.12 μg/dl and in the control group were 668.25 ± 498.45 μg/dl. The mean serum LDH level in patients with OSMF was 408.35 ± 158.35 μg/dl as compared to the control group was 313.05 ± 82.69 μg/dl. The Pearson's correlation coefficient between serum LDH and frequency of habit, duration of habit, and mouth opening was found to be 0.55, 0.53, and 0.69, respectively. The Pearson's correlation coefficient between salivary LDH and frequency of habit, duration of habit, and mouth opening was found to be 0.33, 0.04, and 0.13, respectively. The Pearson's correlation coefficient between salivary LDH and serum LDH was found to be −0.18.
Conclusions:
There was a significant increase in the serum and salivary LDH in OSMF patients as compared to controls; the salivary LDH did not seem to correlate with frequency of the habit, duration of the habit, or mouth opening of OSMF patients. However, serum LDH was found to correlate directly with frequency of the habit and mouth opening in OSMF patients. Hence, based on the results of the present study, it can be hypothesized that serum LDH is a better biological marker than salivary LDH in the evaluation of OSMF.
Keywords: Lactate dehydrogenase, oral submucous fibrosis, serum and saliva
INTRODUCTION
Oral submucous fibrosis (OSMF) is a chronic insidious scarring disease affecting any part of oral mucosa and/or pharynx, sometimes preceded by vesicle formation, always associated with juxtaepithelial inflammation followed by fibroelastic changes in the lamina propria and epithelial atrophy, leading to stiffness of the oral mucosa with progressive inability to open the mouth and inability to eat.[1] It was first reported by Schwartz in 1952, which designated the term “atropica idiopathica mucosae oris” to this condition. In 1953, Joshi described this condition as “submucous fibrosis.”[2]
OSMF is preceded by symptoms such as burning sensation of the oral mucosa, ulceration, and pain.[3] The characteristic features of OSMF are reduced movement and depapillation of tongue, blanching and leathery texture of oral mucosa, loss of pigmentation of oral mucosa, and progressive reduction of mouth opening.[4] OSMF occurs at any age but is most commonly seen in adolescents and adults, especially between 16 and 35 years. The reasons for the rapid increase in the prevalence are due to an upsurge in the popularity of commercially prepared areca nut and tobacco preparations – gutkha, pan masala, mawa, flavored supari, etc.[3] The etiology of OSMF is multifactorial, but areca nut chewing is the main causative agent.[3] Nutritional deficiency (deficiency of vitamins and iron), use of chillies, immunological processes, and betel nut chewing have been implicated, with a high incidence of the disease associated with consumption of betel quid (containing areca nut, tobacco, slaked lime, and other spices). A genetic predisposition has also been suggested in the development of OSMF which explains why not all of those using betel quid develop the disease.[5]
Lactate dehydrogenase (LDH) is a hydrogen transfer enzyme and plays a role in the final step in the metabolic chain of anaerobic glycolysis. Within the cell, glucose is used principally for the production of pyruvate in the glycolysis pathway.[6] Under aerobic conditions, pyruvate enters the mitochondrial matrix, where it is oxidized by the action of pyruvate dehydrogenase, being transformed into acetyl-CoA which, still under aerobic conditions, subsequently enters the citric acid cycle. In an anaerobic medium, pyruvate is reduced to lactate in a reversible reaction catalyzed by LDH, which uses nicotinamide adenine dinucleotide as a coenzyme.
The LDH in the whole saliva within the oral cavity originates from various sources as it is a combination of secretions from both major and minor salivary glands, diffusion of fluids through the oral epithelium and gingiva, material originating from gastrointestinal reflux, and cellular and other debris. Some studies found out that whole saliva LDH is nonglandular in origin and probably oral epithelium is the major source for this nonglandular LDH.[7] It might be because of the pathological alternations of oral epithelium such as dysplasia or cancer which may result in alternation of LDH levels in the saliva. Therefore, salivary LDH may be analyzed for possible oral mucosal pathologies.[8]
It has been proven that saliva diagnostics have the potential capabilities in the diagnosis and screening of oral precancer and cancer.[9] The diagnostic capacity is based on the close contact between saliva and the mucosa where cancer evolves. Analysis of saliva for diagnostics has many benefits in comparison to serum, as it is a simple, easy, noninvasive collection procedure and carries less chances of cross-contamination than blood. Hence, the aim of the present study was to estimate the serum and salivary LDH levels in patients with OSMF and to study the association between serum and salivary LDH levels and mouth opening, frequency of habit, and duration of habit in patients with OSMF.
MATERIALS AND METHODS
This study was conducted in the Department of Oral Medicine and Radiology, Thai Moogambigai Dental College and Hospital, Chennai, Tamil Nadu. The study sample was taken from the patients attending the Outpatient Department of Oral Medicine and Radiology. Prior written consent was taken from the patients voluntarily willing to participate in the study. Further, an Institutional Ethical Committee approval (Letter no: MGRERI/15/2012) was obtained before the start of the study.
Participants were divided into two groups.
Group I (Case group): 20 male patients with the clinical diagnosis of OSMF which constituted the case group
Group II (Control group): 20 male patients with no clinical evidence of OSMF, who were matched for age constituted the Group II.
INCLUSION CRITERIA FOR PATIENT SELECTION
Only adult patients were included in the study (aged above 18 years)
Patients consuming gutkha and/or areca nut chewing habit for Group I
Clinically diagnosed OSMF (blanching of oral mucosa, presence of fibrous bands in the oral mucosa) with or without restricted mouth opening.
EXCLUSION CRITERIA FOR CASE GROUP
Patients who have undergone treatment for OSMF
Patients who have received dental treatment 48 h before the study such as extractions and scaling, which might affect the integrity of oral mucosa
Patients with a history of previous malignancy
Patient with other types of mucosal lesions.
COLLECTION OF SAMPLES
Unstimulated whole saliva was collected from all the participants. Participants were asked to refrain from eating, drinking, or smoking for an hour before the collection of saliva. Each participant was asked to accumulate saliva in the mouth for about 2 min and then was asked to spit the accumulated saliva in a sterile plastic container. The unstimulated saliva was then collected, refrigerated, and processed within 12 h.
CLINICAL EXAMINATION
Patients were made to sit comfortably on a dental chair. Clinical examination was carried out by an experienced oral medicine specialist trained in diagnosing oral mucosal lesions and conditions. Patient examination was carried out wearing sterile hand gloves and mouth mask under artificial illumination. A detailed history was recorded regarding symptoms such as burning sensation to normal or spicy food, difficulty in swallowing and speech, and increased or decreased salivation with altered taste sensation. Group II patients’ habit history was recorded with reference to the type of agents used, presence or absence of tobacco, and frequency, duration, and pattern of chewing. The inter-incisal mouth opening was measured using a divider and a scale from the mesio-incisal angle of upper central incisor to the mesio-incisal angle of lower central incisor and recorded in millimeters. Intraorally, different sites were examined for blanching of the mucosa, consistency, presence of fibrous bands, and presence of other lesions.
BLOOD SAMPLE COLLECTION
The patients’ arm was rested on the working table comfortably. The antecubital fossa was exposed and the tourniquet was applied about 1½ to 2 inches above the antecubital fossa. The area was rendered aseptic with 70% alcohol, and using vacutainer needles, 2 ml of blood was drawn. The tourniquet was relieved and the needle was removed; simultaneously, alcohol-soaked cotton was placed on the needle puncture sites and instructions were given to apply finger pressure for 5 min and dispose the cotton. The collected blood was used for routine hematological investigation. The blood was allowed to clot and the serum obtained was stored in a refrigerator.
After the blood coagulated, the test tube along with the blood was subjected to centrifugation for about 4–5 min at 2000 rpm. Later, the test tube was removed from the centrifugation machine, and with the help of pipette, the serum was separated in a vial and stored in refrigerator. The above procedure was repeated for all the patients.
EVALUATION OF LACTATE DEHYDROGENASE IN SERUM/SALIVA
The International Federation of Clinical Chemistry method was carried out with the help of LDH (P-L) kit (Crest Biosystems, Goa). Collection of blood was done under aseptic precautions and processed for LDH. All the measurements were performed on Royato 9200 Chemistry Semi Auto Analyzer present at A.C.S Medical College and Hospital, Clinical Biochemistry Laboratory.
The data were then entered into the Microsoft Excel spread sheet and analyzed using SPSS software 2010 (version 19; IBM, USA). Descriptive statistics including mean, range, standard deviation, and percentage were used. For all the tests, P < 0.05 was considered statistically significant. Pearson's correlation test was used to correlate serum LDH with mouth opening, duration of habit, and frequency of habit across the study group. Pearson's correlation test was used to correlate salivary LDH with mouth opening, duration of habit, and frequency of habit across the study group. Unpaired t-test was used for comparison of statistical significant difference between the groups.
OBSERVATION AND RESULTS
The study group included a total of 20 patients who were clinically diagnosed as OSMF and 20 apparently healthy controls who did not chew tobacco in any form.
AGE DISTRIBUTION
All the participants were males and were in the age range of 18–60 years, with a mean age of 28.63 ± 10.39 years. Among the control groups, all the participants were in the range of 21–44 years with a mean age of 26.20 ± 7.04 years. There was no statistical significant difference between the two groups (t – 0.87; P – 0.38) [Table 1 and Figure 1].
Table 1.
Age distribution
Figure 1.
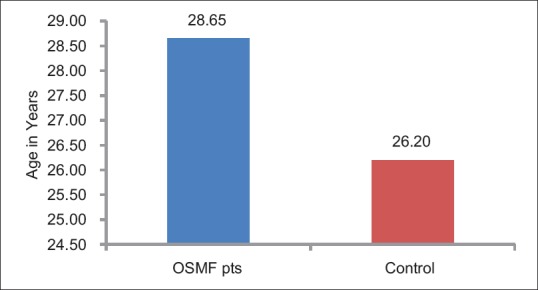
Mean age of patients with oral submucous fibrosis and controls
DISTRIBUTION OF HABIT PATTERN
Twenty study subjects had habit of areca nut chewing out of which 4 (25%) chewed gutkha, 4 (25%) chewed mawa, 3 (15%) chewed khaini, 8 (40%) chewed panparag and 1 (5%) chewed both panparag and mawa [Figure 2].
Figure 2.
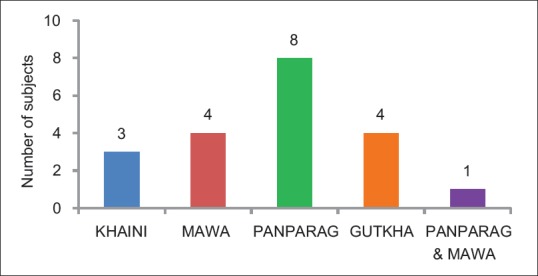
Distribution of study participants based on type of tobacco product used
SALIVARY LACTATE DEHYDROGENASE LEVELS IN PATIENTS WITH ORAL SUBMUCOUS FIBROSIS AND HEALTHY CONTROLS
The mean salivary LDH levels in patients with OSMF were 1057.30 ± 640.12 μg/dl and in the control group were 668.25 ± 498.45 μg/dl. This difference in the mean salivary LDH level between the two groups was statistically significant (t – 2.14; P – 0.04) [Table 2 and Figure 3].
Table 2.
Salivary lactate dehydrogenase levels in patients with oral submucous fibrosis and healthy controls
Figure 3.
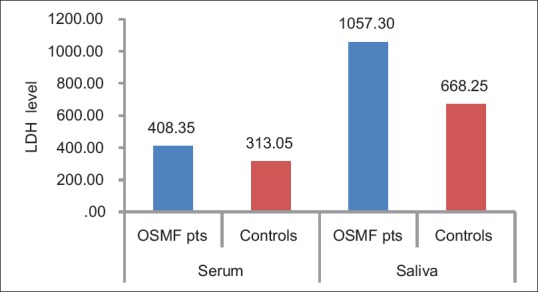
Comparison of serum/salivary lactate dehydrogenase level in patients oral submucous fibrosis and controls
SERUM LACTATE DEHYDROGENASE LEVELS IN PATIENTS WITH ORAL SUBMUCOUS FIBROSIS AND HEALTHY CONTROLS
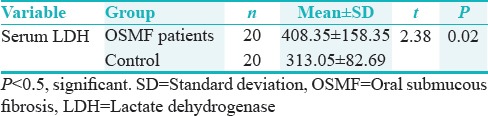
The mean serum LDH level in patients with OSMF was 408.35 ± 158.35 μg/dl as compared to the control group was 313.05 ± 82.69 μg/dl. The mean difference between the two groups was statistically significant (t – 2.38; P – 0.02) [Table 3 and Figure 3].
Table 3.
Serum lactate dehydrogenase levels in patients with oral submucous fibrosis and healthy controls
CORRELATION OF SERUM LACTATE DEHYDROGENASE LEVELS WITH MOUTH OPENING, DURATION OF HABIT, AND FREQUENCY OF HABIT IN THE STUDY GROUP
The Pearson's correlation coefficient between serum LDH and frequency of habit was found to be 0.55 (P = 0.01), which was statistically significant. The Pearson's correlation coefficient between serum LDH and duration of habit was found to be 0.53 (P = 0.15), which was not statistically significant. The Pearson's correlation coefficient between serum LDH and mouth opening was found to be 0.69 (P = 0.001), which was statistically significant [Table 4 and Figures 4–6].
Table 4.
Correlation between serum lactate dehydrogenase and salivary lactate dehydrogenase levels and mouth opening, duration of habit, and frequency of habit
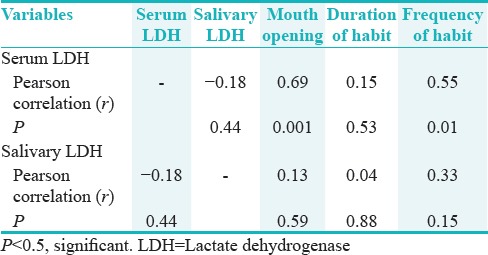
Figure 4.
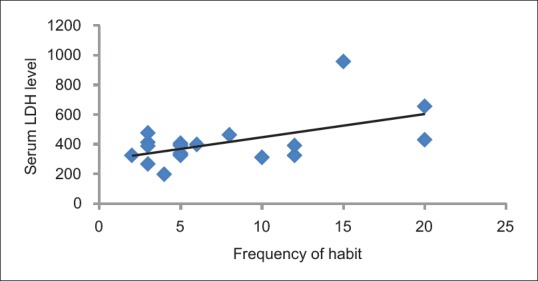
Correlation between serum lactate dehydrogenase level and frequency of habit
Figure 6.
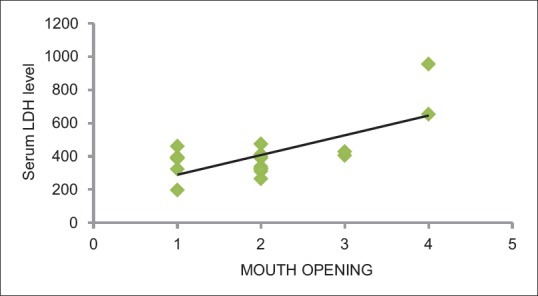
Correlation of serum lactate dehydrogenase level with mouth opening
Figure 5.
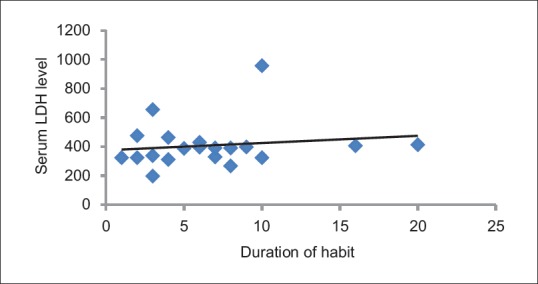
Correlation of serum lactate dehydrogenase level with duration of habit
CORRELATION OF SALIVARY LACTATE DEHYDROGENASE LEVELS WITH MOUTH OPENING, DURATION OF HABIT, AND FREQUENCY OF HABIT IN THE STUDY GROUP
The Pearson's correlation coefficient between salivary LDH and frequency of habit was found to be 0.33 (P = 0.15), which was not statistically significant. The Pearson's correlation coefficient between salivary LDH and duration of habit was found to be 0.04 (P = 0.88), which was not statistically significant. The Pearson's correlation coefficient between salivary LDH with mouth opening was found to be 0.13 (P = 0.59), which was not statistically significant [Table 4].
CORRELATION OF SERUM LACTATE DEHYDROGENASE LEVELS WITH SALIVARY LACTATE DEHYDROGENASE
The Pearson's correlation coefficient between salivary LDH and serum LDH was found to be −0.18 (P = 0.44), which was not statistically significant [Table 4 and Figure 7].
Figure 7.
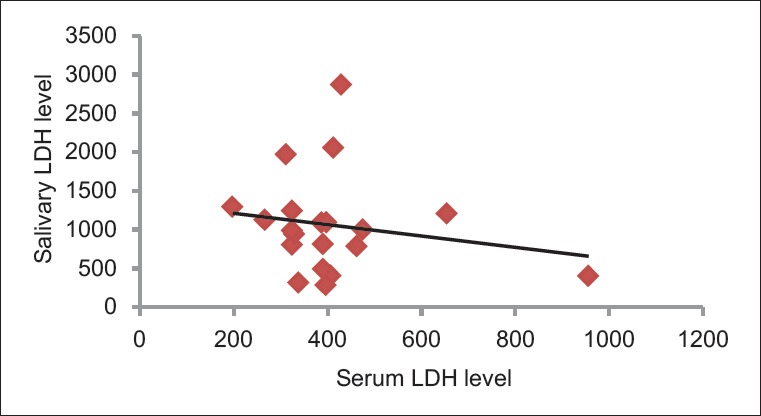
Correlation of salivary lactate dehydrogenase level with serum lactate dehydrogenase level
DISCUSSION
OSMF is a potentially malignant condition that occurs predominantly among Indians. The prevalence in India ranges from 0.2% to 1.2%[10] and malignant transformation rate of 3%–7.6%[11] with varying sex ratios. This disease is one of the most poorly understood and unsatisfactorily treated. This is mainly due to fact that the etiopathogenesis of the disease is not fully understood and the disease is irreversible. Several factors such as chilli consumption,[1] areca nut chewing,[12] nutritional deficiency states,[13] genetic susceptibility,[14] autoimmunity,[15] and collagen disorder[16] have been suggested to be involved in the pathogenesis of the condition. Based on clinical, epidemiological, and in vitro studies, areca nut chewing is considered as an important predisposing factor.
LDH is an enzyme detectable in the cytoplasm of almost every cell in the human body, which becomes extracellular upon cell death. Transformation of normal tissue to premalignant lesion and further to oral cancer results in alteration in glycolytic pathway which manifests as a shift from aerobic to anaerobic glycolysis;[6] with the increase in the glycolytic activity, the concomitant increase in LDH enzyme may be reflected in certain tissues.[17]
In the present study, Group I consists of patients with OSMF and they were in the age range of 18–60 years with a mean age of 28.65 ± 10.39 years. Among the study participants, control group patients were in the age range of 21–44 years with a mean age of 26.20 ± 7.04 years. The mean difference of age was not statistically significant (0.38) between the two groups. The study group can be compared to a report in which OSMF patients were between the age group of 20–65 years with a mean age of 28 years suggests that youngsters are easily habituated to gutkha which make them susceptible to OSMF because of easy accessibility, low cost, and popularity of gutkha.[18]
All the 20 study subjects and 20 controls were male patients. Male predominance in the study can be due to easy accessibility for males to use gutkha more frequently than females in our society. The male predominance is seen in many studies with male-to-female ratio 8:1.[19] Female predominance has been reported in few other study as 1.4:1.[20]
The mean serum LDH level in OSMF patients was 408.35 ± 158.35 μg/dl and healthy control group was 313.05 ± 82.69 μg/dl. There was a statistically significant difference in serum LDH levels between cases and controls (P = 0.02). Using unpaired Student's t-test, comparison between the two study groups was done which indicates that there is an increase in the level of serum LDH in OSMF patients. This might be due to increased mitotic index and more lactic acid production by dysplastic cells due to breakdown of glycoproteins[6] in oral premalignant condition. The result of our study was similar to Joshi et al,[18] who correlated clinical and experimental activity of serum LDH and its iso-enzymes in oral cancer and noted its increased levels. They also evaluated major immunoglobulin status and LDH iso-enzymes profile in oral potentially malignant disorders and malignancy and found elevated LDH levels in potentially malignant disorders and malignancy.[18] Muralidhar M et al,[21] reported a definite rise of serum LDH levels from normal in premalignant and malignant cases.
The mean salivary LDH level in OSMF patients was 1057.30 ± 640.12 μg/dl and that of healthy control group was 668.25 ± 498.45 μg/dl. There was a statistically significant difference in serum LDH levels between cases and controls (P = 0.04). Salivary LDH is predominantly of extraglandular in origin.[7] The source of salivary LDH is probably the oral epithelial shedding. The LDH concentration in the saliva as an expression of cellular necrosis might be a specific indicator for oral lesions affecting the integrity of the oral mucosa. Therefore, increase in salivary LDH levels may be evaluated for possible oral mucosal pathologies.[22] This study can be compared with the study by Langvad et al.[23] that showed the mean isoenzyme ratio in OSMF had altered as compared to oral leukoplakia (P < 0.01). Shpitzer et al.[24] in 2007 analyzed saliva in oral cancer and found the total salivary LDH (88%, P = 0.002) to be elevated in comparison to normal healthy individuals. The present study was not in accordance with the study done by Rajendran,[25] which concluded significant depression of the LDH isoenzyme ratio (LDH IV/LUH 11) at the tissue level in OSMF.
In the present study, correlation between serum LDH and salivary LDH levels and mouth opening, duration of habit, and frequency of habit was done. It was seen that as the severity of OSMF increased, the serum LDH level increases (r – 0.69, P – 0.001). However, a slight correlation was found between salivary LDH and mouth opening of OSMF (r – 0.13, P – 0.59). There was no correlation found between duration of habit and serum LDH (r – 0.15, P – 0.53) and salivary LDH, (r – 0.04, P – 0.88). As the frequency of habit in OSMF increased, the serum LDH level also increased, (r – 0.55, P – 0.01). However, such a correlation was not found between salivary LDH and frequency of the habit with OSMF (r – 0.33, P – 0.15). No studies were found in the available literature in which a similar correlation between serum LDH and salivary LDH levels with mouth opening, duration of habit, and frequency of habit was done.
Rao et al.[26] in their study on serum and salivary LDH levels as biomarkers of tissue damage among cigarette smokers found that cigarette smoking led to an increase in serum as well as salivary LDH levels, which proved to be an indicator for tissue damage in the oral cavity. They also concluded that saliva acts as a better test medium than serum in the determination of LDH levels. Mishra II and Verma BN[27] in their study on estimation of serum LDH levels in OSMF patients and tobacco chewers concluded that elevated levels of LDH were present in patients diagnosed with OSMF. This was in accordance to the present study. Serum LDH estimation was also carried out in patients diagnosed with oral lichen planus; a study done by Nalin et al.[28] did not find any significant difference in serum levels of LDH among patients diagnosed with lichen planus and control group.
The present study was performed using a small sample size (20 subjects in each group) which was the biggest limitation of our study. Further, no histological confirmation of clinical OSMF severity was established in the present study. The present study could provide a baseline data for future studies with a large sample size to get a reliable and authentic data. In the present study, salivary LDH level did not show any correlation with mouth opening, frequency, and duration of the habit in OSMF patients. Further refinements in sample collection, transport, and analysis could yield better results. The present study was conducted only on male patients, so female patients must be included in upcoming studies to assess the gender-related variation as the study done by Shetty et al.[29] found that the salivary LDH levels were significantly higher (P = 0.01) in males compared to females.
CONCLUSIONS
The present study showed clear association of increase in levels of salivary and serum LDH in OSMF patients with good significance when compared to controls. Further, as the severity of OSMF in terms of mouth opening increases, the serum LDH level also increases. However, no correlation was found between salivary LDH and mouth opening of OSMF. There was no correlation found between duration of habit and serum LDH and salivary LDH. As the frequency of habit in OSMF increases, the serum LDH level also increases. However, such a correlation was not found between salivary LDH and frequency of the habit with OSMF. There was a slight negative correlation found between serum LDH and salivary LDH which was not statistically significant.
Hence, in the present study, we have attempted to correlate mouth opening, frequency of the habit, and duration of the habit with levels of serum and salivary LDH in OSMF patients. Although there was a significant increase in the serum and salivary LDH in OSMF patients as compared to controls, the salivary LDH did not seem to correlate with frequency of the habit, duration of the habit, or mouth opening of OSMF patients. However, serum LDH was found to correlate directly with the frequency of the habit and mouth opening in OSMF patients. Hence, based on the results of the present study, it can be hypothesized that serum LDH is a better biological marker than salivary LDH in the evaluation of OSMF.
FINANCIAL SUPPORT AND SPONSORSHIP
Nil.
CONFLICTS OF INTEREST
There are no conflicts of interest.
REFERENCES
- 1.Pindborg JJ, Sirsat SM. Oral submucous fibrosis. Oral Surg Oral Med Oral Pathol. 1966;22:764–79. doi: 10.1016/0030-4220(66)90367-7. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad MS, Ali SA, Ali AS, Chaubey KK. Epidemiological and etiological study of oral submucous fibrosis among gutkha chewers of Patna, Bihar, India. J Indian Soc Pedod Prev Dent. 2006;24:84–9. doi: 10.4103/0970-4388.26022. [DOI] [PubMed] [Google Scholar]
- 3.More CB, Das S, Patel H, Adalja C, Kamatchi V, Venkatesh R, et al. Proposed clinical classification for oral submucous fibrosis. Oral Oncol. 2012;48:200–2. doi: 10.1016/j.oraloncology.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Lee CK, Tsai MT, Lee HC, Chen HM, Chiang CP, Wang YM, et al. Diagnosis of oral submucous fibrosis with optical coherence tomography. J Biomed Opt. 2009;14:054008. doi: 10.1117/1.3233653. [DOI] [PubMed] [Google Scholar]
- 5.Cox SC, Walker DM. Oral submucous fibrosis. A review. Aust Dent J. 1996;41:294–9. doi: 10.1111/j.1834-7819.1996.tb03136.x. [DOI] [PubMed] [Google Scholar]
- 6.Bigler LR, Streckfus CF, Dubinsky WP. Salivary biomarkers for the detection of malignant tumors that are remote from the oral cavity. Clin Lab Med. 2009;29:71–85. doi: 10.1016/j.cll.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Denny P, Ho CM, Montemagno C, Shi W, Qi F, et al. The oral fluid MEMS/NEMS chip (OFMNC): Diagnostic and translational applications. Adv Dent Res. 2005;18:3–5. doi: 10.1177/154407370501800102. [DOI] [PubMed] [Google Scholar]
- 8.Nagler RM, Lischinsky S, Diamond E, Klein I, Reznick AZ. New insights into salivary lactate dehydrogenase of human subjects. J Lab Clin Med. 2001;137:363–9. doi: 10.1067/mlc.2001.114710. [DOI] [PubMed] [Google Scholar]
- 9.Boyle JO, Mao L, Brennan JA, Koch WM, Eisele DW, Saunders JR, et al. Gene mutations in saliva as molecular markers for head and neck squamous cell carcinomas. Am J Surg. 1994;168:429–32. doi: 10.1016/s0002-9610(05)80092-3. [DOI] [PubMed] [Google Scholar]
- 10.Pindborg JJ, Mehta FS, Gupta PC, Daftary DK. Prevalence of oral submucous fibrosis among 50,915 Indian villagers. Br J Cancer. 1968;22:646–54. doi: 10.1038/bjc.1968.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pindborg JJ, Bhonsle RB, Murti PR, Gupta PC, Daftary DK, Mehta FS, et al. Incidence and early forms of oral submucous fibrosis. Oral Surg Oral Med Oral Pathol. 1980;50:40–4. doi: 10.1016/0030-4220(80)90329-1. [DOI] [PubMed] [Google Scholar]
- 12.Seedat HA, van Wyk CW. Submucous fibrosis (SF) in ex-betel nut chewers: A report of 14 cases. J Oral Pathol. 1988;17:226–9. doi: 10.1111/j.1600-0714.1988.tb01529.x. [DOI] [PubMed] [Google Scholar]
- 13.Rajendran R, Vasudevan DM, Vijayakumar T. Serum levels of iron and proteins in oral submucous fibrosis (OSMF) Ann Dent. 1990;49:23–5. 45. [PubMed] [Google Scholar]
- 14.Chiang CP, Hsieh RP, Chen TH, Chang YF, Liu BY, Wang JT, et al. High incidence of autoantibodies in Taiwanese patients with oral submucous fibrosis. J Oral Pathol Med. 2002;31:402–9. doi: 10.1034/j.1600-0714.2002.00117.x. [DOI] [PubMed] [Google Scholar]
- 15.Shin YN, Liu CJ, Chang KW, Lee YJ, Liu HF. Association of CTLA-4 gene polymorphism with oral submucous fibrosis in Taiwan. J Oral Pathol Med. 2004;33:200–3. doi: 10.1111/j.0904-2512.2004.00081.x. [DOI] [PubMed] [Google Scholar]
- 16.Rajalalitha P, Vali S. Molecular pathogenesis of oral submucous fibrosis – A collagen metabolic disorder. J Oral Pathol Med. 2005;34:321–8. doi: 10.1111/j.1600-0714.2005.00325.x. [DOI] [PubMed] [Google Scholar]
- 17.Zondag HA, Klein F. Clinical applications of lactate dehydrogenase isozymes: Alterations in malignancy. Ann N Y Acad Sci. 1968;151:578–86. doi: 10.1111/j.1749-6632.1968.tb11917.x. [DOI] [PubMed] [Google Scholar]
- 18.Joshi PS, Chougule M, Dudanakar M, Golgire S. Comparison Between Salivary and Serum Lactate Dehydrogenase Levels in Patients with Oral Leukoplakia and Oral Squamous Cell Carcinoma-A Pilot Study. International Journal of Oral and Maxillofacial Pathology. 2012;3:1–6. [Google Scholar]
- 19.Phatak A. Serum proteins and immunoglobulins in oral submucous fibrosis. Indian J Otolaryngol Head Neck Surg. 1978;30:1–4. [Google Scholar]
- 20.Maher R, Lee AJ, Warnakulasuriya KA, Lewis JA, Johnson NW. Role of areca nut in the causation of oral submucous fibrosis: A case-control study in Pakistan. J Oral Pathol Med. 1994;23:65–9. doi: 10.1111/j.1600-0714.1994.tb00258.x. [DOI] [PubMed] [Google Scholar]
- 21.Muralidhar M, Raghavan MR, Bailoor DN, Kamath VV. Evaluation of serum lactate dehydrogenase (LDH) in oral premalignant and malignant lesions. Ann Dent. 1988;47:11–5. [PubMed] [Google Scholar]
- 22.Lingen MW, Kalmar JR, Karrison T, Speight PM. Critical evaluation of diagnostic aids for the detection of oral cancer. Oral Oncol. 2008;44:10–22. doi: 10.1016/j.oraloncology.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Langvad E, Zachariah J, Pindborg JJ. Lactate dehydrogenase isoenzyme patterns in leukoplakia, submucous fibrosis and carcinoma of the oral mucosa in South Indians. Acta Pathol Microbiol Scand A. 1970;78:509–15. doi: 10.1111/j.1699-0463.1970.tb02533.x. [DOI] [PubMed] [Google Scholar]
- 24.Shpitzer T, Bahar G, Feinmesser R, Nagler RM. A comprehensive salivary analysis for oral cancer diagnosis. J Cancer Res Clin Oncol. 2007;133:613–7. doi: 10.1007/s00432-007-0207-z. [DOI] [PubMed] [Google Scholar]
- 25.Rajendran R. Oral submucous fibrosis. J Oral Maxillofac Pathol. 2003;7:1. doi: 10.4103/0973-029X.86678. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Rao K, Babu SG, Shetty UA, Castelino RL, Shetty SR. Serum and salivary lactate dehydrogenase levels as biomarkers of tissue damage among cigarette smokers. A biochemical study. Stomatologija. 2017;19:91–6. [PubMed] [Google Scholar]
- 27.Mishra II, Verma BN. Estimation of serum lactate dehydrogenase levels in oral submucosal fibrosis patients, and tobacco chewers without any potentially malignant disorders. Int J Innov Res Dent Sci. 2017;2:117–20. [Google Scholar]
- 28.Nalin AS, Rajeev R, George GB, Padiath S. Level of serum lactate dehydrogenase enzyme in oral lichen planus – A biochemical study. IOSR J Dent Med Sci. 2018;17:20–6. [Google Scholar]
- 29.Shetty SR, Chadha R, Babu S, Kumari S, Bhat S, Achalli S, et al. Salivary lactate dehydrogenase levels in oral leukoplakia and oral squamous cell carcinoma: A biochemical and clinicopathological study. J Cancer Res Ther. 2012;8(Suppl 1):S123–5. doi: 10.4103/0973-1482.92226. [DOI] [PubMed] [Google Scholar]


