Abstract
Aim and Objectives:
Oral surgical procedures can cause spread of infections in the clinics through visually imperceptible, splattered, and aerosolized blood. The aim of this study was to evaluate visually imperceptible blood contamination of clinical surfaces and personal protective equipment (PPE) in an oral surgery clinic using luminol.
Materials and Methods:
Following ethical approval, oral surgical procedures were performed under local anesthesia in a disinfected clinic, and PPE was used by the oral surgeon, dental assistant, and patients. After the procedure, clinical surfaces and PPE were evaluated for traces of visually imperceptible blood contamination using luminol. Data regarding blood contamination and the duration of the procedure were collected. Nonparametric tests, with 95% significance level (Epi Info, Stat Calc 7, CDC, Atlanta, USA), were used to identify statistical interactions between the duration of the procedure and the frequency of blood contamination.
Results:
Blood contamination was detected in flooring below surgical field (86.67%), instrument tray, operating light, dental chair, and suction unit (100%). Except head caps and shoe covers, blood contamination was detected in all the PPE used by the clinical personnel, and the eyewear and chest drapes used by patients. An increase in the surgical time beyond 40 min significantly increased the risk of blood contamination in the handcuffs of the clinical personnel (P < 0.01).
Discussion and Conclusion:
Visually imperceptible blood contamination of the clinical surfaces and PPE is associated with minor oral surgical procedures. This mandates the cleaning and disinfection of all clinical surfaces before and after minor oral surgical procedures and PPE for clinicians and patients during every procedure.
Keywords: Aerosols, blood contamination, luminol, oral surgery, splatter
INTRODUCTION
Oral surgical procedures involve clinical interventions which can cause spread of infections in the dental clinics through direct splatter as well as aerosolized blood, saliva, and body fluids.[1,2,3] The potential sources of infection in the oral surgery clinic could, therefore, not only be direct contact with the body fluids of a patient but also through contact with airborne aerosolized infectious particles either directly or indirectly from surfaces contaminated with aerosols.[4] Failure of infection control protocols could lead to infection being transmitted to either the patients or the clinical staff members.[5,6]
Dental and oral surgical procedures involving the use of high-speed rotary instruments results in considerable amounts of respirable aerosols in the dental clinic.[3] However, the use of high-volume suction devices and evacuators close to the field of operation of the rotary instruments have been known to significantly reduce the amount of aerosols released into the dental clinic environment.[7] Most of the minor oral surgical procedures require the use of a combination of hand instruments and rotary instruments under saline irrigation for bone removal and sectioning of teeth. While oral surgical procedures contraindicate the use of high-speed air-driven rotary handpieces due to the risk of emphysema, electric handpieces operating at 30,000–50,000 RPM are routinely used.[4] Moreover, surgical procedures involving exposure of soft tissues and bone, dictate the use of low-volume suction evacuators leading to an increased potential for aerosolized blood and body fluids being released into the clinic setting.[4]
This combination of hand instruments and rotary instruments with low-volume evacuators also increases the risk of blood splatter outside the oral surgical field.[1,3] Since most of the aerosolized blood and splattered blood are visually imperceptible, there is a significant risk of spread of infection from the patients to the clinicians and vice versa.[1,4] In addition, blood splattered onto clinical surfaces might lead to cross-contamination from one patient to another. The Centers for Disease Control and Prevention (CDC) of the United States of America has published global recommendations for infection prevention in the dental care setting.[8] The CDC recommendations mandate infection control procedures for the entire dental clinical environment including instruments, surfaces, and personal protective equipment (PPE)[8] which includes cleaning and sterilization of all instruments, disinfection of clinical surfaces and usage of suitable disposable PPE. Although sterilization eliminates the risk of cross-infection through instruments, visually imperceptible aerosolized and splattered blood droplets on clinical surfaces could easily escape disinfection and potentially lead to spread of infection.[3]
It has been reported in the literature that visually imperceptible traces of blood could be detected with the help of forensic luminol (5-amino-2, 3-dihydro-1, 4-phthalazinedione).[9] The luminol reagent exhibits chemiluminescence on contact with blood in the presence of a suitable oxidizing agent (Hydrogen peroxide and Sodium hydroxide). Interestingly, it is the iron in hemoglobin which catalyzes the reaction, and hence, even trace quantities of blood could be detected without any false-positive reactions with other body fluids.[9] This property of luminol could be used to evaluate the efficiency of disinfection in oral surgery clinics from contamination by visually imperceptible aerosolized and splattered blood droplets. The aim of the present study was to identify the extent of visually imperceptible blood contamination of the different surfaces of the oral surgery clinic and the PPE used therein, using forensic luminol.
MATERIALS AND METHODS
Following ethical approval from the Ethical Committee at the College of Dentistry Research Center, King Saud University, (CDRC approval #FR 0186), a cross-sectional study was conducted from January 2017 to March 2017. An estimated sample size of 24, based on a statistical power of 0.80, confidence level of 95% (α = 0.05), and 5% confidence interval (Epi Info, Stat Calc 7, CDC, Atlanta, USA) was utilized for our research. The final sample size (n = 30) was arrived after 25% overestimation. The sampling frame included adult patients reporting to the oral surgical clinic for surgical removal of impacted mandibular third molar teeth. The patients with history of uncontrolled systemic illnesses and the patients with history of allergy or hypersensitivity were excluded from the final sample. The sample size was achieved prospectively by enrolling the patients who volunteered to take part in the study and signed a consent form.
CLINIC PREPARATION
One of the oral surgery outpatient clinics was specifically designated for the present study and was isolated from all ambient outdoor light sources. The entire clinic area, including the dental chair unit, was subdivided into fifteen subsites for identifying contamination with aerosolized and splattered blood at each site [Figure 1]. All the clinical subsites were cleaned and disinfected thoroughly before each oral surgical procedure followed by spraying of luminol reagent (luminol Blood Detection Reagent, TRITECH Forensics, Southport, North Carolina, USA), under darkness, to confirm the absence of traces of blood contamination. This was followed by a second round of cleaning and disinfection using commercially available hospital disinfecting solutions.
Figure 1.
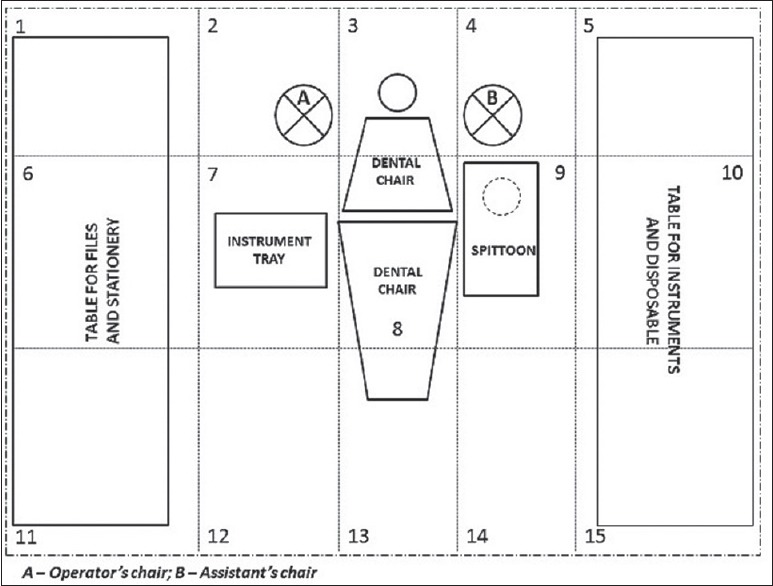
Subsites in the dental clinic identified for detecting blood contamination through aerosols and splatter. (tabletop for files and stationery - 1, 6, 11; table for instruments and disposable – 5, 10, 15; flooring behind the dental chair (including the operator's and assistant's chairs) - 2, 3, 4; instrument tray and handpiece unit - 7; operating light and dental chair armrests - 8; cuspidor and suction unit - 9; and flooring in front of dental chair - 12, 13, 14)
ORAL SURGICAL PROCEDURES
The patients, who consented to participate in the study, underwent minor oral surgical procedures under local anesthesia for removal of either one or both of their impacted mandibular third molar teeth. All procedures were done by the same oral surgeon following strict aseptic surgical protocols. Bone removal and sectioning of the teeth were carried out using rotary handpiece along with sterile saline irrigation, and fluids from the oral cavity were evacuated using a low-volume vacuum suction. Disposable PPE was used by the oral surgeon, the dental assistant (DA), and was also provided for the patient. This included sterile gloves, face masks, eyewear, surgical gown, head cap, and shoe cover for the surgeon, and the DA, and head cap, eyewear, and chest drape for the patient.
DETECTING BLOOD CONTAMINATION
Contamination by visually imperceptible blood droplets occurring as a result of aerosolization and splatter were evaluated in all the clinical subsites and the PPE. On completion of the oral surgical procedure, the patients were discharged, and the clinic was isolated for evaluation. The oral surgeon and the DA were requested to leave the clinic and all the instruments were removed, leaving behind the PPE. Two independently calibrated observers who were trained in identifying chemiluminescence arising as a result of the reaction between blood and luminol reagent began the process of detecting blood contamination. As mentioned previously, the room was completely isolated from all light sources and with the help of a black light, the observers sprayed all the clinical subsites and the PPE with the luminol reagent. Clinical subsites and PPE which exhibited chemiluminescence were identified and marked after the agreement between the observers.
DATA COLLECTION AND STATISTICAL ANALYSIS
The presence of blood contamination in each clinical subsite, and PPE was marked by the observers in a specially designed data collection form. In addition, the duration of each surgical procedure, excluding the time taken for local anesthesia, was recorded to identify its potential confounding effect in causing blood contamination due to aerosols and splatter. The collected data were entered in a spreadsheet software (Microsoft EXCEL 2010), and were further exported to a statistical software package (SPSS Version 21, IBM, Armonk, NewYork, USA). Descriptive statistical analysis was done to identify frequency of contamination for each subsite and PPE used by the oral surgeon, DA, and patient. Nonparametric Mann–Whitney U-test was used to identify any statistically significant interaction between the duration of the procedure and the frequency of blood contamination in any particular clinical subsite or PPE. A 95% significance level (P < 0.05) was assumed for statistical analysis.
RESULTS
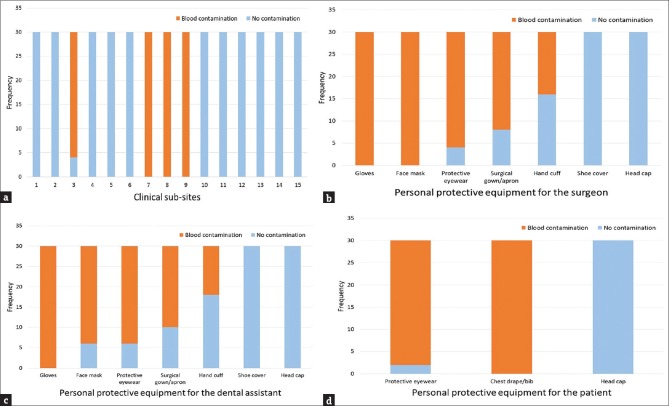
A total of 30 minor oral surgical procedures were done as part of the study. The nature and outcome of the surgical procedures were unremarkable, and there were no reported complications. The median duration of the surgical procedures was 40 min (Mean - 40 min; standard deviation 7.88; range 25 - 60 min; 20 surgical procedures with duration ≤40 min). Among the clinical subsites, blood contamination was detected using luminol in only four subsites, namely, subsite 3 (flooring below the patient's headrest - 26 out of 30 cases; 86.67%), subsite 7 (instrument tray and handpiece unit - all cases; 100%), subsite 8 (operating light and dental chair armrests - all cases; 100%), and subsite 9 (cuspidor and suction unit - all cases; 100%) [Figure 2a].
Figure 2.
Bar graph showing the frequency of blood contamination in: (a) Clinical subsites, (b) Personal protective equipment used by the oral surgeon, (c) Personal protective equipment used by the dental assistant and (d) Personal protective equipment used by the patient
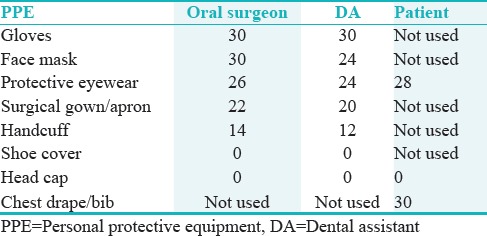
Blood contamination was detected in all the PPE used by the oral surgeons, DA, and patients except the head caps and shoe covers [Table 1]. Among the PPE used by the oral surgeons, there was 100% contamination of the gloves and the face masks. While protective eyewear (n = 26/30; 86.68%) and the surgical gowns (n = 22/30; 73.33%) were contaminated with blood in the most cases, the handcuffs of the aprons were contaminated in only 14 of the 30 cases (46.67%) [Figure 2b]. Similarly, 100% blood contamination was observed in the gloves used by the DAs. Furthermore, the face masks (n = 24/30; 80%), protective eyewear (n = 24/30; 80%), surgical gowns (n = 20/30; 66.67%), and the handcuffs of the aprons (n = 12/30; 40%) showed evidence of blood contamination [Figure 2c]. While the chest drapes used by the patients showed 100% blood contamination, the protective eyewear was observed to be contaminated in 28 of the 30 cases (93.33%) [Figure 2d]. Mann–Whitney U-test revealed a statistically significant interaction between surgical procedure time and the frequency of blood contamination in the handcuffs of the aprons of the oral surgeon and the DA (P < 0.01). An increase in the surgical procedure time beyond 40 min significantly increased the risk of blood contamination in the handcuffs of the aprons for both the oral surgeon and the DA.
Table 1.
Frequency of blood contamination in the different personal protective equipment used by the oral surgeon, dental assistant, and the patient (n=30)
DISCUSSION
In this study, forensic luminol was used to identify visually imperceptible/undetectable blood contamination in the oral surgical clinic following minor oral surgical procedures. Recently, luminol has been used to evaluate the effectiveness of disinfection in the healthcare setting.[10] Based on an earlier study to detect blood contamination during laparoscopic surgery, Englehardt et al.[11] reported that aerosolized and splattered blood were capable of traveling considerable distances from the surgical field. Moreover, they reported the ability of luminol to detect visually undetectable blood traces in all the specimen boards that were placed around the surgical field for collection of aerosolized and splattered blood.[11] Although minor oral surgical procedures involve a limited surgical field similar to laparoscopic procedures, the risk of aerosols and splatter is compounded by the use of rotary handpieces and instruments to cut hard tissue.
Internationally, acceptable policies and procedures dictate standardized infection control protocols to avoid the risk of transmission of infections in the dental clinic.[6,8,12] Nevertheless, the high incidence of aerosolized and splattered blood droplets potentially increases the risk of transmission of blood-borne infections during minor oral surgical procedures.[1,4] Ishihama et al.[1] reported that oral surgical procedures involving the use of rotary instruments resulted in aerosolized and splattered blood contamination in almost 90% of the cases. They also observed that more than 50% of the blood contamination escapes visual detection and could be identified only by indirect blood detection techniques.[1] The most common techniques described in the literature to detect visually imperceptible blood contamination include the use of luminol and leukomalachite green staining.[1,2,4,9,10] Luminol acts through a catalytic pathway involving peroxidase-like activity on the hemoglobin present in the blood and the outcome is chemiluminescence in the form of emitted light at a wavelength of around 428 nm (blue in the visible spectrum).[10] A relatively dark working environment is required to detect the chemiluminescence arising as a result of the reaction between luminol and blood.[9] In the present study, this was simulated in the oral surgical clinic by blocking out all sources of visible light in the clinic.
OPERATING SITES AT INCREASED RISK OF CONTAMINATION
Among the clinical subsites evaluated for blood contamination in this study, the clinical surfaces adjoining the instrument tray, suction apparatus, cuspidor, and dental chair armrests were found to be contaminated in all the cases. This is in accordance with universal infection control protocols prescribed for the dental and oral surgical clinic, which mandate routine disinfection of the above mentioned clinical surfaces.[6,8,12] Surprisingly, the clinical surface directly beneath the dental chair headrest and apparently below the surgical field showed blood contamination in 86.7% of the cases. This might be a possible area that could be overlooked during cleaning and disinfection of the oral surgical clinic. Similar results have been reported by Bortoluzzi et al.[9] based on their study to evaluate blood contamination using luminol in the different clinical surfaces of a dental school clinic.
VULNERABILITY IN DENTAL OPERATORY/MODES OF DISEASE TRANSMISSION
It has been reported that approximately 200 diseases could potentially be transmitted as a result of blood contamination in the dental clinic.[9] Majority of the studies reported in the literature have regarded the oral surgeon and the DA to be at the greatest risk of disease transmission due to aerosolized and splattered blood.[1,3,4,13] In the present study, it was found that almost all the PPE used by the oral surgeon and the DA were contaminated with blood, except the head caps and shoe covers. Critical PPE, that are directly related to the risk of disease transmission when not used,[14] such as gloves, face masks, and protective eyewear were either contaminated with blood in all the cases or at least more than 75% of the cases. Considering the fact that these critical PPE were contaminated in majority of the cases irrespective of the duration of the surgical procedure, it is imperative that these PPE are used by the oral surgeons and the DA for all minor oral surgical procedures and disposed safely thereafter.[15]
The results of the present study reinstated that the frequency of facial blood contamination was highest among the oral surgeons (100%) followed by the assistants (80%). While the face masks showed greater frequency of blood contamination than protective eyewear among the surgeons, the frequencies were equal among DA. This is in accordance with a multicenter study of 600 cases reported by Endo et al.,[2] wherein the risk of facial splatter and subsequent blood contamination during different surgical procedures, were found to be as high as 66%. In addition, they reported that the mask region of the face was predominantly contaminated with blood (57%) followed by the paraorbital (37.8%) and orbital regions (36.6%). Similarly, the surgeon (83.5%) and the first assistant (68.5%) were at risk of greatest contamination due to splattered blood on their faces. All the above observations are in correlation with previously reported studies which dictate the use of a visor face mask for all minor oral surgical procedures and especially while using rotary surgical handpieces.[1]
Visually imperceptible blood contamination of the surgical gowns was detected in 73.3% of the cases among oral surgeons and in 66.7% of the cases among DAs. This indicates the need for routine use of disposable gowns/aprons for all minor oral surgical procedures as part of infection control policies and procedures.[1,8] Interestingly, handcuffs of the surgical gowns were found to be contaminated with visually imperceptible blood in 46.7% of the cases among oral surgeons and in 40% of the cases among DAs. Moreover, there was a statistically significant association between blood contaminations of the handcuffs with increasing duration of surgery greater than 40 min. It has been reported that the handcuff region of surgical gowns forms the weakest link in the gown-glove interface, potentially placing at risk the patients and the clinicians.[16] It might be alluring to hypothesize that with increasing duration of surgery, there are increasing chances for the beaded end of the gloves slipping off from the cuff region of the gown, thereby leading to contamination of the handcuffs of the gowns. While modifications to the handcuffs of the gowns have been suggested,[16] it is again imperative that standard gloving and gowning procedures are adhered to before all minor oral surgical procedures.[15,17,18]
Although PPE are primarily indicated for reducing the risk of disease transmission from the patients to the clinicians and vice versa, it is also essential that the patients are provided with PPE for their safety as well. Based on a survey, it was found that the patients were comfortable being treated by clinicians using PPE such as gloves, face masks, gowns, and protective eyewear.[19] Furthermore, they were satisfied when they were actively involved in the infection control practices by providing them with protective eyewear and drapes. In the present study, blood contamination was detected in all cases in the patient drapes and in 93.3% of the cases in the protective eyewear used by patients. While protective eyewear could prevent iatrogenic eye injuries and ocular infections among patients,[20] drapes and bibs would avoid the risk of blood-borne disease transmission through the patients’ clothing. The above facts indicate the need for PPE in all the patients undergoing minor oral surgical procedures.
CONCLUSION
Based on the results of the present study, it could be concluded that visually imperceptible blood contamination as a result of aerosolization and splatter is often associated with minor oral surgical procedures. In addition to the critical clinical surfaces which are routinely disinfected, even the flooring beneath the surgical field was found to be contaminated. More importantly, the PPE used by the oral surgeon, DA and the patient showed evidence of blood contamination. This indicates the need for cleaning and disinfection of all clinical surfaces before and after minor oral surgical procedures, using PPE, and disposing them safely thereafter. Furthermore, visor face masks, proper gowning and gloving techniques, and protective eyewear and drapes for the patients must be insisted for all minor oral surgical procedures. A limitation of the present study was the inability to quantify the extent of visually imperceptible blood contamination. Further long-term studies should be conducted to identify the same in all dental clinical settings.
FINANCIAL SUPPORT AND SPONSORSHIP
Nil.
CONFLICTS OF INTEREST
There are no conflicts of interest.
Acknowledgment
The authors would like to thank the College of Dentistry Research Center, King Saud University, Riyadh, Saudi Arabia (Research Approval #FR 0186) for their support.
REFERENCES
- 1.Ishihama K, Iida S, Koizumi H, Wada T, Adachi T, Isomura-Tanaka E, et al. High incidence of blood exposure due to imperceptible contaminated splatters during oral surgery. J Oral Maxillofac Surg. 2008;66:704–10. doi: 10.1016/j.joms.2007.06.663. [DOI] [PubMed] [Google Scholar]
- 2.Endo S, Kanemitsu K, Ishii H, Narita M, Nemoto T, Yaginuma G, et al. Risk of facial splashes in four major surgical specialties in a multicentre study. J Hosp Infect. 2007;67:56–61. doi: 10.1016/j.jhin.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 3.Zemouri C, de Soet H, Crielaard W, Laheij A. A scoping review on bio-aerosols in healthcare and the dental environment. PLoS One. 2017;12:e0178007. doi: 10.1371/journal.pone.0178007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishihama K, Koizumi H, Wada T, Iida S, Tanaka S, Yamanishi T, et al. Evidence of aerosolised floating blood mist during oral surgery. J Hosp Infect. 2009;71:359–64. doi: 10.1016/j.jhin.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Laheij AM, Kistler JO, Belibasakis GN, Välimaa H, de Soet JJ. European Oral Microbiology Workshop (EOMW) 2011. Healthcare-associated viral and bacterial infections in dentistry. J Oral Microbiol. 2012:4. doi: 10.3402/jom.v4i0.17659. doi: 10.3402/jom.v4i0.17659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rahman B, Abraham SB, Alsalami AM, Alkhaja FE, Najem SI. Attitudes and practices of infection control among senior dental students at college of dentistry, university of Sharjah in the United Arab Emirates. Eur J Dent. 2013;7:S15–9. doi: 10.4103/1305-7456.119058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desarda H, Gurav A, Dharmadhikari C, Shete A, Gaikwad S. Efficacy of high-volume evacuator in aerosol reduction: Truth or myth? A clinical and microbiological study. J Dent Res Dent Clin Dent Prospects. 2014;8:176–9. doi: 10.5681/joddd.2014.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cleveland JL, Gray SK, Harte JA, Robison VA, Moorman AC, Gooch BF, et al. Transmission of blood-borne pathogens in US dental health care settings: 2016 update. J Am Dent Assoc. 2016;147:729–38. doi: 10.1016/j.adaj.2016.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bortoluzzi MC, Cadore P, Gallon A, Imanishi SA. Forensic luminol blood test for preventing cross-contamination in dentistry: An evaluation of a dental school clinic. Int J Prev Med. 2014;5:1343–6. [PMC free article] [PubMed] [Google Scholar]
- 10.Bergervoet PW, van Riessen N, Sebens FW, van der Zwet WC. Application of the forensic luminol for blood in infection control. J Hosp Infect. 2008;68:329–33. doi: 10.1016/j.jhin.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 11.Englehardt RK, Nowak BM, Seger MV, Duperier FD. Contamination resulting from aerosolized fluid during laparoscopic surgery. JSLS. 2014;18:e201400361. doi: 10.4293/JSLS.2014.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rider CA. Infection control within the oral surgeon's office. Compend Contin Educ Dent. 2004;25:529–33. [PubMed] [Google Scholar]
- 13.Graetz C, Bielfeldt J, Tillner A, Plaumann A, Dörfer CE. Spatter contamination in dental practices – How can it be prevented? Rev Med Chir Soc Med Nat Iasi. 2014;118:1122–34. [PubMed] [Google Scholar]
- 14.Ganczak M, Szych Z. Surgical nurses and compliance with personal protective equipment. J Hosp Infect. 2007;66:346–51. doi: 10.1016/j.jhin.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 15.de Amorim-Finzi MB, Cury MV, Costa CR, Dos Santos AC, de Melo GB. Rate of compliance with hand hygiene by dental healthcare personnel (DHCP) within a dentistry healthcare first aid facility. Eur J Dent. 2010;4:233–7. [PMC free article] [PubMed] [Google Scholar]
- 16.Fernández M, Del Castillo JL, Nieto MJ. Surgical gown's cuff modification to prevent surgical contamination. J Maxillofac Oral Surg. 2015;14:474–5. doi: 10.1007/s12663-013-0607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pirie S. Surgical gowning and gloving. J Perioper Pract. 2010;20:207–9. doi: 10.1177/175045891002000603. [DOI] [PubMed] [Google Scholar]
- 18.Thivichon-Prince B, Barsotti O, Girard R, Morrier JJ. Hand hygiene practices in a dental teaching center: Measures and improve. Eur J Dent. 2014;8:481–6. doi: 10.4103/1305-7456.143629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bârlean L, Săveanu I, Balcoş C. Dental patients’ attitudes towards infection control. Rev Med Chir Soc Med Nat Iasi. 2014;118:524–7. [PubMed] [Google Scholar]
- 20.Barbeau J. Lawsuit against a dentist related to serious ocular infection possibly linked to water from a dental handpiece. J Can Dent Assoc. 2007;73:618–22. [PubMed] [Google Scholar]