Abstract
Aims and Objectives:
Temporomandibular joint disorder (TMD) is an umbrella term for a number of conditions in the area of the joint, temple, and masticatory system. Many of those with TMD also suffer from headaches and anxiety. The aims and objectives of this study were to determine if there exists an association between the Diagnostic Criteria of TMD (DC/TMD) symptom questionnaire and report of headaches as well as stress.
Materials and Methods:
A cross-sectional study was implemented via the use of the DC/TMD symptom questionnaire and the perceived stress scale (PSS). This was distributed conveniently among dental students in a multi-dental school setting in Riyadh, Saudi Arabia, and was completed by 152 dental students. Odds ratios, Chi-square, and their corresponding 95% confidence intervals are reported. Statistical significance was set at P ≤ 0.05.
Results:
Those who ticked yes for pain on the TMD pain screener for pain with chewing hard or tough food, pain on mouth opening and lateral excursion, and pain with jaw habits as parafunction were at an odds risk from twice to thrice as likely to experience headaches. Similarly, those with poor coping skills on the PSS were more likely to have headaches (P = 0.002). Likewise, positive answers on the screener and symptom questionnaire were relevant with higher stress scores on the PSS.
Conclusion:
This study clarifies and reiterates the intertwined power of both stress and headaches; the former being a role player in TMD progression and the latter its product. TMD is unfortunately on the rise; it should not be brushed off as a nuisance. A multidisciplinary approach in diagnosis and treatment of both TMD and headaches by a team of orofacial pain specialist, neurologist, psychiatrist and/or psychologist, and physical therapists to untangle the deceiving presentation of both conditions would not only provide a more favorable prognosis but also improve cost and time expenditures.
Keywords: Dental students, Diagnostic Criteria for Temporomandibular Joint Disorder, headaches, perceived stress scale, temporomandibular joint disorders
INTRODUCTION
Temporomandibular joint disorder (TMD) is a term guided by a cascade of circumstances afflicting the orofacial region and its neighboring masticatory muscles, temporomandibular joint (TMJ), and associated structures.[1]
TMD has a multifactorial etiology where several factors have been implicated by a recent systematic review to play a role in inducing, perpetuating, as well as aggravating TMD. The authors asserted the relevance of early identification of possible etiological factors as well as the degree of independent involvement of each factor in hopes of inhibiting the downhill direction that untreated and unaddressed cases of TMD would ultimately lead to. This entails and is not limited to a worse prognosis in terms of pain, psychological discomfort, physical disability, and limitation of mandibular movement.[2]
Among the most common TMD signs and symptoms are those related to muscle sensitivity through palpation, restricted mouth opening, asymmetric mandibular movements, joint sounds, muscle, and TMJ pain.[1]
The incidence of signs and symptoms in TMD is higher in the general population (20%–75%) than the proportion of the population who presents for treatment (2%–4%). It is four times as common in women (8%–15%) than in men (3%–10%), and the age range of presentation varies from the second to the fourth decade.[3]
It is of interest that the peak of TMD symptom development is at its highest in the second to the fourth decade, which coincides with the childbearing years.[4,5] This reflects on the progress of the condition where sleep bruxism (a major contributor in the etiology and severity of TMD) decreases with age similar to the strength and tonicity of orofacial musculature which also decline with maturing age. Hence, the complications of tonic and phasic bursts of clenching masseter and temporalis muscles dissipate in direct relation to the age of individuals as documented by several studies. This is fascinating that it coincides with the peak of TMD symptoms.[6,7]
Clinicians are challenged in the diagnosis of TMD due to its tricky and confusing presentation that often resembles other conditions; mimicking other disease processes. This is mainly due to the unique anatomic location of the TMJ and its proximity to major structures in the head and neck that in turn have a lower pain threshold than other structures in the body.[3]
There are many ways to diagnose TMD, from epidemiological indices, radiographs, and electronic and clinical diagnostic tests.
Nevertheless, identification and the use of a uniform tool that is both valid and reliable to its diagnosis are crucial. There is the highly acclaimed and popular Research Diagnostic Criteria for TMDs (RDC/TMD), which no doubt is commonly used, however lacks the target sensitivity and specificity. Thus, in 2013, the International RDC/TMD Consortium Network modified RDC/TMD to the most recent Diagnostic Criteria for TMD (DC/TMD) via an international consensus providing a higher sensitivity and specificity than its predecessor.[8]
The novel DC/TMD contains an accurate screener that detects any pain-related TMD as well as valid diagnostic criteria that identify majority of pain-related TMD conditions. In our research, we focused on the TMD screening questions popularized by DC/TMD.[8]
Parafunctional habits including and not limited to clenching and bruxing are one of the most imperative aggravating factors in the etiology of TMD.[2,3] Not everyone is uniformly vulnerable to the development of oral parafunctions, which coexist with TMD. Genetic factors may also play a role; polymorphism of receptor gene 5HT2 and more importantly the presence of allele C rs6313 have attracted attention in the role they play in bruxism.[9]
TMD is highly linked with psychosocial issues due to its effect on the dopaminergic system, wherein stress and cortisol play an initiating factor in daytime bruxism and may perpetuate nocturnal bruxism.[10,11,12] There is a powerful role played by stress and psychological factors. Each person is subjective in his or her perception of the world and environment around him or her and hence responds differently to external stressors. Therefore, stress should be identified and dealt with appropriately.[13] Furthermore, psychological factors have been proven to play a strategic role in chronic TMD both in its onset and development.[14] Most individuals who have anxiety disorders relieve their stress by activating their stomatognathic system, including and not limited to clenching their teeth, masticatory muscle contraction, and teeth grinding among others.[15]
TMD-afflicted persons as well as those with longstanding TMD are more likely to be predisposed to suffer from headaches. Headaches and TMD occur hand in hand in majority of the cases.[16] Tension-type headaches (TTHAs), the most common of all primary headaches, influence the functional and psychosocial quality of life of a patient.[4] Thus, it is interesting to study the screening criteria of TMD and incidence of headaches.[17] Comorbid incidences of both headaches and TMD could be due to the pathophysiology of the disease, but numerous studies have been shown that patients with headache have the same symptoms as patients with TMD, but the actual relationship between headache and TMD is yet to be determined.[4] The quality of sleep is affected by both these conditions, both disturbed and loss of sleep, which pose as an integral trigger and aggravator of TTHAs.
Specifically, a more acceptable hypothesis is the “central sensitization” theory where the trigeminal nucleus is modulated into amplifying the pain in the area, that is, chronic unremitting pain in the area causes increased sensitization of the nociceptive receptors, which in turn affect the feedback of afferent nerve fibers. This leads to hyperexcitability of the dorsal horn neurons in the spinal horn, which concomitantly causes a change at a spinal or supraspinal level modulating the way pain is perceived. The descending inhibitory system ceases to function optimally, which renders widespread pain and further promotes allodynia and hyperalgesia.[18,19,20]
A Brazilian study on headache impact on university students by Souza-e-Silva and Rocha-Filho found a severe impact of headaches on quality of life. TTHA prevalence was 42.4% and overall headache prevalence in n = 344 was 87.2%, with a statistically significant relationship between absenteeism, discipline failure, and degree of headache severity.[21] The prevalence of headache and TTHA ranges from 8% to 12%, and 3.1% to 9.5%, respectively, in Saudi Arabia, targeting more of the female and younger age group.[22]
The aim of this study was to find an association between the DC/TMD symptom questionnaire and report of headaches as well as stress from the PSS.
MATERIALS AND METHODS
STUDY DESIGN
The cross-sectional study was conducted at Riyadh Colleges of Dentistry and Pharmacy, King Saud University, Princess Noura University, and Al-Farabi Dental School from March to May 2017.
INCLUSION/EXCLUSION CRITERIA
The study targeted dental students. All faculty, staff, and patients at the dental school were excluded from participation.
ETHICAL STATEMENT
Ethical approval for this questionnaire-style study was attained out of courtesy and respect to the participants. The only link that could relate the participants to the study was via consent form, which was then coded to ensure their privacy and rights. The study followed the guidelines of the Declaration of Helsinki and was approved by the Institutional Review Board of Riyadh Colleges of Dentistry and Pharmacy with approval no. RC/IRB/2016/340.
DATA COLLECTION
The DC/TMD symptom questionnaire[8] as well as the perceived stress scale (PSS) was used and both were distributed among dental students in a multicenter cross-sectional study in Riyadh.
Participants were conveniently recruited and given the consent form agreeing to participate in the study.
Paper-based questionnaires were distributed from March to May 2017. Of the total 200 questionnaires distributed, 152 were completed and returned conferring a compliance rate of 76%.
INSTRUMENTS
The DC/TMD symptom questionnaire included a 3-item TMD pain screener, 4-item pain symptom questionnaire, and 10-item headache and jaw screener.
The PSS was recorded on a 5-point ordinal scale code: never = 0, almost never = 1, sometimes = 2, fairly often = 3, and very often = 4. The PSS was developed by Lazarus and Folkman evaluating a subjects’ scenario with stress, whereby the amount of stimulus is evaluated to the degree to which it is perceived as a threat that exceeds subject coping skills.[23]
DATA ANALYSIS
The data were analyzed using Statistical Package for the Social Sciences (SPSS, Version 21, and SPSS Inc., Chicago, IL, USA) software for Windows to analyze and attain the Fisher's exact test, odds ratios (ORs), and their corresponding 95% confidence intervals (CIs). Statistical significance was set at P ≤ 0.05.
RESULTS
The power of the sample was 0.80 (n = 152).
DEMOGRAPHICS
More than three quarters, 83.6% (n = 127), of the participants were female, and 16.4% (n = 25) were male. Majority were single (n = 120, 78.9%) and aged between 21 and 25 years (n = 120, 78.9%).
TEMPOROMANDIBULAR JOINT DISORDER PAIN SCREENER
Table 1 shows that in the last 30 days, majority had no pain in jaw or temple area on either side (n = 95, 62.5%) and no pain or stiffness in their jaws on awakening (n = 113, 74.3%).
Table 1.
Temporomandibular joint disorders pain screener

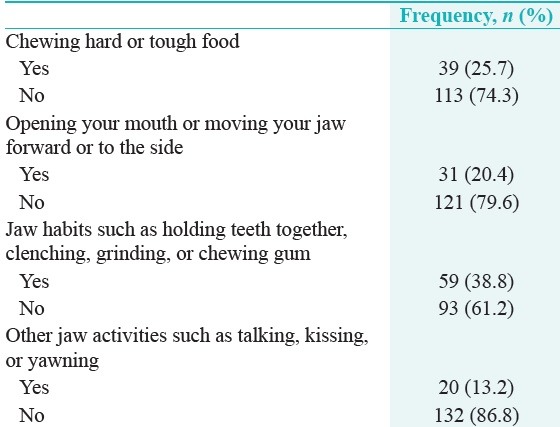
Table 2 shows that nearly 40% (n = 59) of the students reported jaw habits, 20% (n = 31) had pain on opening, and 25.7% (n = 39) had pain on chewing hard or tough food.
Table 2.
Temporomandibular joint disorders pain screener
HEADACHE
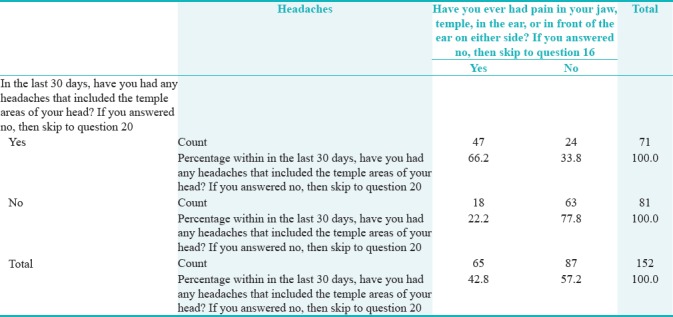
From Figure 1, it is observed that 46.7% (n = 71) of the participants had headaches and 53.3% (n = 81) had no headache that included the temple areas of their head.
Figure 1.
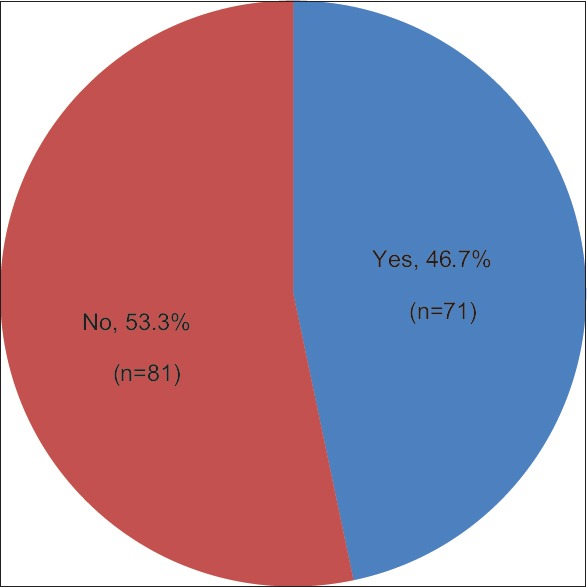
In the last 30 days, have you had any headaches that included the temple areas of your head?
TEMPOROMANDIBULAR JOINT DISORDER PAIN SCREENER AND HEADACHES
Table 3 shows that those who reported any headaches that included the temple areas were more likely to have had pain or stiffness in the jaw on awakening. Fisher's exact test showed that this relation was statistically significant (P = 0.001) (OR = 2.57, CI = 1.41–4.68).
Table 3.
Relationship between temporomandibular joint disorders screener and any headaches
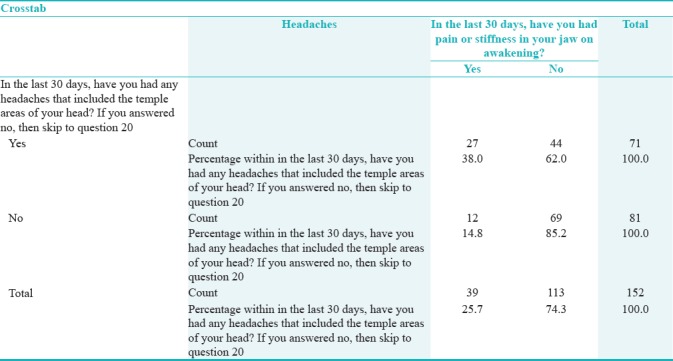
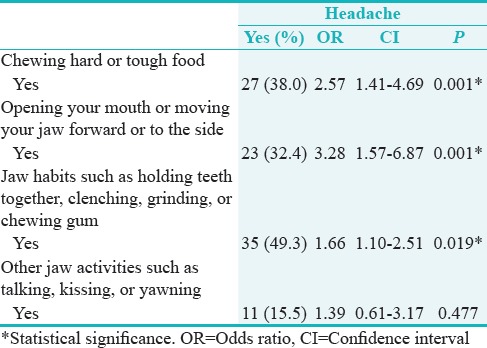
Table 4 shows that there was a statistically significant relationship between any headaches that included the temple areas and chewing hard or tough food (P = 0.001) (OR = 2.57, CI = 1.41–4.69), opening mouth or moving jaw forward or to the side (OR = 3.28, CI = 1.57–6.87), and jaw habits such as holding teeth together, clenching, grinding, or chewing gum (OR = 1.66, CI = 1.10–2.51) (P < 0.05). That is, those reporting pain on chewing hard or tough food were more than twice as likely to have headaches. Those reporting pain upon opening the mouth or moving the jaw forward or side to side were more than thrice as likely to experience headaches. Finally, those who had jaw habits of holding their teeth tighter, clenching, grinding, or chewing gum were more than 1.66 times likely to get a headache.
Table 4.
Relationship between temporomandibular joint disorders pain screener and headaches
TEMPOROMANDIBULAR JOINT DISORDER SYMPTOM QUESTIONNAIRE AND HEADACHES
Table 5 shows that those who have had any headaches that included the temple area were more likely to have pain in their jaw, temple, ear, or in front of the ear on either side. Fisher's exact test showed that this relation was statistically significant (P = 0.000) (OR = 2.98, CI = 1.92–4.63).
Table 5.
Relationship between symptom questionnaire and headache
HEADACHES AND PERCEIVED STRESS SCALE
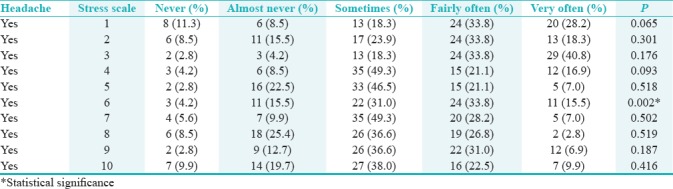
Table 6 shows that there was a statistically significant relation only between any headaches that included the temple areas of the head and “how often have you found that you could not cope with all the things that you had to do?” (P < 0.05).
Table 6.
Relationship between perceived stress scale and headache
TEMPOROMANDIBULAR JOINT DISORDER PAIN SCREENER AND PERCEIVED STRESS SCALE
Table 7 shows that those who reported pain or stiffness in their jaw on awakening were more likely to be upset when something happened unexpectedly (P = 0.022) but were confident in handling personal problems (P = 0.05).
Table 7.
Statistical significance via crosstab in relationship between temporomandibular joint disorders screener, symptom questionnaire, and perceived stress scale
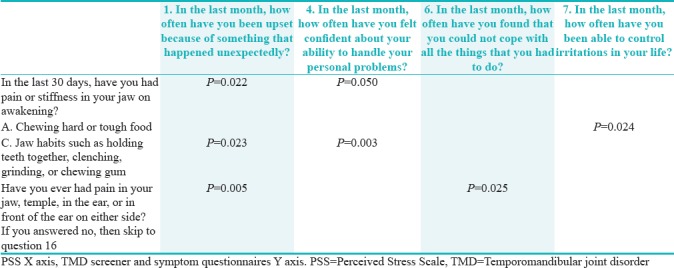
Those who reported pain in chewing hard or tough food were able to control irritations in their life (P = 0.024).
Those who had pain with jaw habits (holding teeth together, clenching, grinding, or chewing gum) have been more likely to get upset with something happening unexpectedly (P = 0.023) but were confident in handling personal problems (P = 0.003).
TEMPOROMANDIBULAR JOINT DISORDER SYMPTOM QUESTIONNAIRE AND PERCEIVED STRESS SCALE
Those who reported pain in their jaw, temple, ear, or in front of their ear on either side have been more likely to get upset with something happening unexpectedly (P = 0.005) and unable to cope with all the things they had to do (P = 0.025).
DISCUSSION
KEY FINDINGS
Primary outcome measures
The prevalence of DC/TMD screener and any headaches was 38% (n = 27) of the total 152 participants. They had headaches as well as pain and/or stiffness in their jaw upon awakening.
Furthermore, DC/TMD screener for those who responded to pain on chewing hard or tough food, pain following mouth excursions such as opening, lateral, and protrusive movements, as well as pain in jaw habits had a higher OR to headaches.
In addition, the prevalence between the DC/TMD screener and any headaches elaborated 66.2% (n = 47) of the total 152 recruits to experience headaches with pain in the jaw, temple, and in and around ear.
Moreover, there is an interesting association between DC/TMD and stress, illustrated by both DC/TMD screener and symptom questionnaire. The former shows a statistically significant association between “pain or stiffness in jaw on awakening” and “how often have you been upset because of something that happened unexpectedly” and also between “pain with jaw habits such as holding teeth together, clenching, grinding, or chewing gum” and “how often have you been upset because of something that happened unexpectedly.” The latter (DC/TMS symptom questionnaire) shows a statistically significant association between “have you ever had pain in your jaw, temple, ear, or in front of ear” and “how often have you been upset because of something that happened unexpectedly” as well as “how often have you found that you could not cope with all the things that you had to do.”
Secondary outcome measures
In the case of perceived stress and headaches, there was a statistically significant relationship between “how often have you found that you could not cope with all the things you had to?” and headache incidence.
Furthermore, on the PSS, 67% (n = 100) of the participants confided to feeling nervous and stressed as fairly often and often.
RESULTS AS RELATED TO PRIOR HYPOTHESIS
The aims and objectives of this study were to determine if there exists an association between the DC/TMD and report of headaches as well as stress. These aforementioned results all ascertain that headaches indeed occur with a report of stiffness or pain in the jaw, symptoms of pain in chewing hard or tough food, pain with mouth excursions, as well as pain with jaw habits.
Not only headaches but also stress is exacerbated with TMD, especially with those having symptoms of pain in their jaw, temple, ear, or in front of the ear they seem to have difficulty coping with the things they had to do.
STRENGTHS AND LIMITATIONS OF THE STUDY
There are many studies carried out on stress, but few that utilize DC/TMD together with PSS and headaches. A TMD tool for diagnoses is not clearly described in other studies; this study uses the DC/TMD symptom questionnaire and screener which has high validity and reliability together with the PSS. The robust statistical analysis used in this study helps elaborate the association between TMD, stress, and headache.
On the other hand, this study recruited a convenient sample and it is not representative of the Kingdom of Saudi Arabia let alone Riyadh. The age range of the participants is confined to a certain population; had it been a larger age group and equal gender distribution, it would have helped generalize a larger target. It is based on the DC/TMD screening and symptom questionnaire without any clinical examination involved. Time was an obstacle during the implementation of this research; had there been more time, there would have been more recruits and possibly a clinical examination. Future research should aim for random sampling, a TMD group together with age-matched controls ranging in the 20 and 40 years age group.
INTERPRETATIONS AND IMPLICATIONS IN THE CONTEXT OF TOTALITY OF EVIDENCE
The findings of our study are in agreement with the recent review of the OPPERA study,[24] which gained funding from the National Institute of Health on a mission to identify the risk factors affiliated with the development of TMD. To elaborate, this review article focuses on the results of the various articles pertaining to OPPERA study that was conducted at four US sites between 2006 and 2013 and included three study designs; it aims at highlighting the key findings. Most interestingly, self-reports of parafunctional jaw were considerably stauncher predictors of TMD than reciprocal examiner assessment. Report of parafunction in our study was statistically significantly associated with both headaches and stress.
Another recent review elaborated on the comorbidity between TMD and headaches as both share similar neuronal routes as well as central sensitization pathways and have generally become accepted as the fundamental determinants for their association.[25]
Likewise, this association between TMD and headaches was demonstrated in our cohort via DC/TMD screener and symptom questionnaires yielding higher OR to headaches.
An observational study conveyed in Brazil on university students found the preponderance of TMD and stress; the former being around 72% (n = 422) of the total 586 recruits and the latter being around 50% of the total recruits. Pearson correlation showed a significant correlation between the two. In our study, we had less TMD due to the DC/TMD criteria we used versus the Fonseca anamnestic index, and they also used the 14-item Brazilian PSS.[26] We had similar stress levels in our study.
CONTROVERSIES RAISED BY THIS STUDY
Around 75% of the participants were females, highlighting the compliance rate of females; and supported by the literature whereby TMD-related pain and other symptoms affect females more so than males.[27,28] This could be due to females possessing a lower pain threshold as dictated by neuropsychological and physiological factors making them more susceptible to the effects of stress as compared to their male counterparts.[29] Although conducted on adolescents, a recent study in Brazil recruiting a stratified random sample of 10–14-year-olds showed that of the 934 population representative participants, around 35% had TMD and around 21% complained of headaches. Girls were significantly more prone to TMD than the boys in this population (P = 0.001).[30]
Nearly 25.7% (n = 39) ticked yes on the TMD pain screener of the DC/TMD for pain or stiffness in their jaw on awakening, that is only a quarter of the total sample, which coincides with results of another study conducted on an older population of 40–50-year-olds who reported jaw/face pain as the most prevalent symptom.[31] In addition, around 39% (n = 59) ticked yes on the TMD pain screener of DC/TMD for increase in pain with parafunction, that is, jaw habits such as holding teeth together, clenching grinding, or chewing gum. Our study shows results not as dramatic as the Polish study where around 64% (n = 289) of the participants of a total of 456 exhibited occlusal parafunction.[13]
The concern is that painful TMD is on the rise, affecting more individuals and afflicting a conundrum of symptoms. The prevalence in the adolescent age alone ranges from 0.7% to 23.5%.[32,33] A recent study with similar subject phenotype conducted on Nepalese university students showed that 31% had TMD of the total 500 participants, i.e. 25% males and 75% females.[34] A Brazilian study comprising of 1094 adolescents showed that parafunctional habits were found to be strongly associated with painful TMDs. Those who had daytime bruxism, sleep bruxism, as well as parafunctional habits, were 4.5 times more likely to have a painful TMD condition.[35]
There was a statistically significant relationship between the presence of headaches in the temple area and TMD pain screener for the presence of pain or stiffness in jaw on awakening (P = 0.001). Furthermore, those reporting pain on the TMD pain screener for the following were at increased odds risk of experiencing headaches as follows; (1) pain with chewing hard or tough food was more than twice as likely to have headaches; (2) pain upon opening the mouth or moving the jaw forward or side to side was more than thrice as likely to experience headaches; (3) finally, those who had jaw habits of holding their teeth together, clenching, grinding, or chewing gum were more than 1.66 times likely to get a headache. These findings are interestingly similar to a study conducted on adolescent Japanese students (aged 11–15 years) where the OR of headache and neck pain was higher in the TMD group (n = 182) versus the controls (n = 1233, OR = 2.08).[36] Intriguingly, the Korean national survey conducted between 2010 and 2012 showed that of the 17,575 respondents who completed the TMD symptom questionnaire, those with migraine had a higher OR prevalence of TMD (OR = 1.44, CI = 1.26–1.65).[37] In fact, 44% of those participants in the aforementioned Nepalese university study complained of headaches (n = 170).[34]
In addition, in a cross-sectional study on 154 patients whom were divided into joint pain, myofascial pain (MFP), and mixed pain, there was a statistically significant Pearson correlation between chronic joint pain in TMD patients and impact of headaches. Further, those patients having mixed chronic pain (joint pain as well as MFP) were more likely to experience a higher headache impact.[38]
Likewise, a 2011 university study determining the annual presence of primary headaches elaborated the significance of the impact of headache on quality of life.[21]
Accordingly, in our study, the relationship between TMD symptom questionnaire (pain in jaw, temple, ear, or in front of the ear on either side) and headaches showed (P = 0.000) (OR = 2.98, CI = 1.92–4.63), so answering yes to that question meant you are nearly thrice as likely to experience headaches than a person that answered no.
With regard to stress on the PSS, there was a statistically significant relationship between any headaches that included the temple areas of your head and “how often have you found that you could not cope with all the things that you had to do?” (P < 0.05). This could be a red flag to indicate headaches and coping skills with stress. This is saturated in the literature, whereby a stressful event in life could aggravate daytime bruxism, which may ultimately initiate and predispose to TTHA. Stress is also a risk factor for parafunctional habits, which aggravate one's TMD condition. Parafunctional habits include lip biting, nail-biting, and tongue thrusting among others.[39,40,41] This was also displayed the Nepalese university student study which emphasized psychological stress in 42% of its participants.[34]
Moreover, a retrospective study analyzed the patients with TMD and found that 17% (n = 73) of a total of 426 patients had headaches.[42] In a Time Series Trial on 34 patients investigating the temporal association between TMD-related symptoms and headache, headaches were significantly reduced after physical therapy.[43] There are many studies showing the association between masticatory MFP and headaches, from its treatment via appliance therapy to its unique association with TMD.[44,45,46]
Conjointly, there was a statistically significant relationship between any headaches on the TMD symptom questionnaire and presence of pain in the jaw, in temple, and/or in front of the ear. Those who did have the pain in these structures were (P = 0.000) (OR = 2.98, CI = 1.92–4.63) nearly thrice more likely to experience headaches.
The relationship between TMD pain screener and symptom questionnaire with PSS is ever confirmed in the literature of the comorbidity of psychosocial stress with TMD. A study on 116 participants aged between 18 and 59 in Chapel Hill, North Carolina, found a statistical significance in those with TMD to perceive more stress than their controls.[47] It is of interest to clarify that although those who reported pain in the TMD pain screener were more likely to get upset with sudden unexpected requirements or incidents such as is the case with being a student be it a case assignment or an unplanned dental procedure for ones patient they were actually confident in handling their personal problems. However, those who had symptoms of pain in their jaw, temple, ear, and in front of their ears bilaterally could not cope with all the things they had to do.
Hence, pain that is present is different than that pain that is elicited, and pain that is actively present is different than that which is latent and is only evident once aggravated or induced. From this study, having positive results on the TMD symptom questionnaire is of interest and a red flag as those who elicit pain in their jaws, temples, ear, or in front of their ears are not able to handle their normal day-to-day life situations be it as a dental student, a spouse, a child, and or a parent.
FUTURE RESEARCH DIRECTIONS
From our research, we can use these predictors to headaches and stress. The DC/TMD symptom questionnaire and screener should be used for all patients to expedite their identification and guide the direction of management in those cases diagnosed with TMD. Headache and stress should be mentioned, and patients educated about the comorbid nature of these conditions. The devious interlacing of the clinical presentation of both TMD and headaches requires meticulous inspection and analysis to draw the appropriate diagnosis as erring in that might lead to inappropriate treatment. Both conditions share anatomic landmarks in proximity to peripheral and central sensitization prone areas; a poorly functioning descending pain inhibitory system due to chronicity of the condition, as well as referred pain from an active myofascial trigger point could all delude the clinician from a proper diagnosis. On the other hand, there are many similarities in therapeutic modalities for both TMD and headache, which majority of the time alleviates the complaint. It is however essential that a multidisciplinary approach involving both an orofacial pain specialist and a neurologist be secured to reach a mutual diagnosis for the most favorable efficient treatment.[48]
CONCLUSION
This study clarifies and reiterates the intertwined power of both stress and headaches; the former being a role player in TMD progression and the latter its product.
TMD affects females more so than males. Jaw habits including parafunction occurred in 40% of the sample, which was statistically significant and more likely to predispose one to headaches. Around half of the participants experienced headaches, and they were twice as likely to have painful TMD. In regard to stress on the PSS, those who had any form of TMD pain in the pain screener had better coping skills in stressful situations as opposed to those participants with ongoing pain (symptoms) who were not able to function as well during stressful situations.
To conclude, general dentists need to be aware of the telltale signs of TMD and their critical role and power in recognition and treatment. Treatment including and not limited to habit control – in the case of parafunctional behavior – sleep hygiene, diet, physical therapy exercises, and custom-made oral appliances – should be incorporated. When conventional therapy fails, it is the dentists ordained duty to make the adequate referral to an orofacial pain specialist, neurologist, psychiatrist, psychologist, and/or physical therapist; otherwise, its negligence could mean a future of patient's dependence on prescription medication and a lifetime commitment to chronic pain.
The silver lining TMD is on the rise, due to its multifactorial etiology; the progression of electronic entertainment and technology fueling its progression, the barrage of digitalization empowering our day-to-day lives, our stressful and fast-paced lifestyles being devoid and blanched dry from physical exercise, and old-fashioned socialization; the 21st century is amazing, but it is hitting us hard causing a cacophony of conditions. We need to increase the awareness and educate ourselves on the ripple effect of the “E,” instill discipline, and dedicate time for healthy physiologic behavior; walking, talking, socializing, normal head and neck posture as opposed to a “text neck” that ultimately play a role in TMD.
FINANCIAL SUPPORT AND SPONSORSHIP
Nil.
CONFLICTS OF INTEREST
There are no conflicts of interest.
REFERENCES
- 1.Stechman-Neto J, Porporatti AL, Porto de Toledo I, Costa YM, Conti PC, De Luca Canto G, et al. Effect of temporomandibular disorder therapy on otologic signs and symptoms: A systematic review. J Oral Rehabil. 2016;43:468–79. doi: 10.1111/joor.12380. [DOI] [PubMed] [Google Scholar]
- 2.Chisnoiu AM, Picos AM, Popa S, Chisnoiu PD, Lascu L, Picos A, et al. Factors involved in the etiology of temporomandibular disorders – A literature review. Clujul Med. 2015;88:473–8. doi: 10.15386/cjmed-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pedroni CR, De Oliveira AS, Guaratini MI. Prevalence study of signs and symptoms of temporomandibular disorders in university students. J Oral Rehabil. 2003;30:283–9. doi: 10.1046/j.1365-2842.2003.01010.x. [DOI] [PubMed] [Google Scholar]
- 4.LeResche L, Mancl L, Sherman JJ, Gandara B, Dworkin SF. Changes in temporomandibular pain and other symptoms across the menstrual cycle. Pain. 2003;106:253–61. doi: 10.1016/j.pain.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Warren MP, Fried JL. Temporomandibular disorders and hormones in women. Cells Tissues Organs. 2001;169:187–92. doi: 10.1159/000047881. [DOI] [PubMed] [Google Scholar]
- 6.Clark HM, Solomon NP. Age and sex differences in orofacial strength. Dysphagia. 2012;27:2–9. doi: 10.1007/s00455-011-9328-2. [DOI] [PubMed] [Google Scholar]
- 7.Park JS, You SJ, Kim JY, Yeo SG, Lee JH. Differences in orofacial muscle strength according to age and sex in East Asian healthy adults. Am J Phys Med Rehabil. 2015;94:677–86. doi: 10.1097/PHM.0000000000000230. [DOI] [PubMed] [Google Scholar]
- 8.Schiffman E, Ohrbach R, Truelove E, Look J, Anderson G, Goulet JP, et al. Diagnostic criteria for temporomandibular disorders (DC/TMD) for clinical and research applications: Recommendations of the International RDC/TMD Consortium Network* and orofacial pain special interest group†. J Oral Facial Pain Headache. 2014;28:6–27. doi: 10.11607/jop.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abe Y, Suganuma T, Ishii M, Yamamoto G, Gunji T, Clark GT, et al. Association of genetic, psychological and behavioral factors with sleep bruxism in a Japanese population. J Sleep Res. 2012;21:289–96. doi: 10.1111/j.1365-2869.2011.00961.x. [DOI] [PubMed] [Google Scholar]
- 10.Cavallo P, Carpinelli L, Savarese G. Perceived stress and bruxism in university students. BMC Res Notes. 2016;9:514. doi: 10.1186/s13104-016-2311-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salameh E, Alshaarani F, Hamed HA, Nassar JA. Investigation of the relationship between psychosocial stress and temporomandibular disorder in adults by measuring salivary cortisol concentration: A case-control study. J Indian Prosthodont Soc. 2015;15:148–52. doi: 10.4103/0972-4052.158075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ballegaard V, Thede-Schmidt-Hansen P, Svensson P, Jensen R. Are headache and temporomandibular disorders related? A blinded study. Cephalalgia. 2008;28:832–41. doi: 10.1111/j.1468-2982.2008.01597.x. [DOI] [PubMed] [Google Scholar]
- 13.Wieckiewicz M, Grychowska N, Wojciechowski K, Pelc A, Augustyniak M, Sleboda A, et al. Prevalence and correlation between TMD based on RDC/TMD diagnoses, oral parafunctions and psychoemotional stress in Polish university students. Biomed Res Int 2014. 2014 doi: 10.1155/2014/472346. 472346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohrbach R, Fillingim RB, Mulkey F, Gonzalez Y, Gordon S, Gremillion H, et al. Clinical findings and pain symptoms as potential risk factors for chronic TMD: Descriptive data and empirically identified domains from the OPPERA case-control study. J Pain. 2011;12:T27–45. doi: 10.1016/j.jpain.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calixtre LB, Grüninger BL, Chaves TC, Oliveira AB. Is there an association between anxiety/depression and temporomandibular disorders in college students? J Appl Oral Sci. 2014;22:15–21. doi: 10.1590/1678-775720130054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ciancaglini R, Radaelli G. The relationship between headache and symptoms of temporomandibular disorder in the general population. J Dent. 2001;29:93–8. doi: 10.1016/s0300-5712(00)00042-7. [DOI] [PubMed] [Google Scholar]
- 17.Cairns BE, Sessle BJ, Hu JW. Evidence that excitatory amino acid receptors within the temporomandibular joint region are involved in the reflex activation of the jaw muscles. J Neurosci. 1998;18:8056–64. doi: 10.1523/JNEUROSCI.18-19-08056.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woolf CJ. Central sensitization: Implications for the diagnosis and treatment of pain. Pain. 2011;152:S2–15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernández-de-las-Peñas C, Galán-del-Río F, Fernández-Carnero J, Pesquera J, Arendt-Nielsen L, Svensson P, et al. Bilateral widespread mechanical pain sensitivity in women with myofascial temporomandibular disorder: Evidence of impairment in central nociceptive processing. J Pain. 2009;10:1170–8. doi: 10.1016/j.jpain.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 20.Staud R. Evidence for shared pain mechanisms in osteoarthritis, low back pain, and fibromyalgia. Curr Rheumatol Rep. 2011;13:513–20. doi: 10.1007/s11926-011-0206-6. [DOI] [PubMed] [Google Scholar]
- 21.Souza-e-Silva HR, Rocha-Filho PA. Headaches and academic performance in university students: A cross-sectional study. Headache. 2011;51:1493–502. doi: 10.1111/j.1526-4610.2011.02012.x. [DOI] [PubMed] [Google Scholar]
- 22.Benamer HT, Deleu D, Grosset D. Epidemiology of headache in Arab countries. J Headache Pain. 2010;11:1–3. doi: 10.1007/s10194-009-0173-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lazarus RS, Folkman S. Stress, Appraisal, and Coping. New York: Springer; 1984. [Google Scholar]
- 24.Slade GD, Ohrbach R, Greenspan JD, Fillingim RB, Bair E, Sanders AE, et al. Painful temporomandibular disorder: Decade of discovery from OPPERA studies. J Dent Res. 2016;95:1084–92. doi: 10.1177/0022034516653743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Costa YM, Conti PC, de Faria FA, Bonjardim LR. Temporomandibular disorders and painful comorbidities: Clinical association and underlying mechanisms. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;123:288–97. doi: 10.1016/j.oooo.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Augusto VG, Perina KC, Penha DS, Dos Santos DC, Oliveira VA. Temporomandibular dysfunction, stress and common mental disorder in university students. Acta Ortop Bras. 2016;24:330–3. doi: 10.1590/1413-785220162406162873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.List T, Wahlund K, Wenneberg B, Dworkin SF. TMD in children and adolescents: Prevalence of pain, gender differences, and perceived treatment need. J Orofac Pain. 1999;13:9–20. [PubMed] [Google Scholar]
- 28.Nilsson IM, Drangsholt M, List T. Impact of temporomandibular disorder pain in adolescents: Differences by age and gender. J Orofac Pain. 2009;23:115–22. [PubMed] [Google Scholar]
- 29.Sena MF, Mesquita KS, Santos FR, Silva FW, Serrano KV. Prevalence of temporomandibular dysfunction in children and adolescents. Rev Paul Pediatr. 2013;31:538–45. doi: 10.1590/S0103-05822013000400018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bertoli FM, Bruzamolin CD, Pizzatto E, Losso EM, Brancher JA, de Souza JF, et al. Prevalence of diagnosed temporomandibular disorders: A cross-sectional study in Brazilian adolescents. PLoS One. 2018;13:e0192254. doi: 10.1371/journal.pone.0192254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anastassaki Köhler A, Hugoson A, Magnusson T. Prevalence of symptoms indicative of temporomandibular disorders in adults: Cross-sectional epidemiological investigations covering two decades. Acta Odontol Scand. 2012;70:213–23. doi: 10.3109/00016357.2011.634832. [DOI] [PubMed] [Google Scholar]
- 32.Drangsholt M, LeResche L. Temporomandibular disorder pain. In: Crombie I, Crombie IK, Croft PR, Linton SJ, LeResche L, VonKorff M, editors. Epidemiology of Pain. Seattle: IASP Press; 1999. pp. 203–33. [Google Scholar]
- 33.Ebrahimi M, Dashti H, Mehrabkhani M, Arghavani M, Daneshvar-Mozafari A. Temporomandibular disorders and related factors in a group of Iranian adolescents: A cross-sectional survey. J Dent Res Dent Clin Dent Prospects. 2011;5:123–7. doi: 10.5681/joddd.2011.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rokaya D, Suttagul K, Joshi S, Bhattarai BP, Shah PK, Dixit S, et al. An epidemiological study on the prevalence of temporomandibular disorder and associated history and problems in Nepalese subjects. J Dent Anesth Pain Med. 2018;18:27–33. doi: 10.17245/jdapm.2018.18.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernandes G, Franco-Micheloni AL, Siqueira JT, Gonçalves DA, Camparis CM. Parafunctional habits are associated cumulatively to painful temporomandibular disorders in adolescents. Braz Oral Res. 2016;30:pii: S1806-83242016000100214. doi: 10.1590/1807-3107BOR-2016.vol30.0015. [DOI] [PubMed] [Google Scholar]
- 36.Karibe H, Shimazu K, Okamoto A, Kawakami T, Kato Y, Warita-Naoi S, et al. Prevalence and association of self-reported anxiety, pain, and oral parafunctional habits with temporomandibular disorders in Japanese children and adolescents: A cross-sectional survey. BMC Oral Health. 2015;15:8. doi: 10.1186/1472-6831-15-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song HS, Shin JS, Lee J, Lee YJ, Kim MR, Cho JH, et al. Association between temporomandibular disorders, chronic diseases, and ophthalmologic and otolaryngologic disorders in Korean adults: A cross-sectional study. PLoS One. 2018;13:e0191336. doi: 10.1371/journal.pone.0191336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gil-Martínez A, Grande-Alonso M, López-de-Uralde-Villanueva I, López-López A, Fernández-Carnero J, La Touche R, et al. Chronic temporomandibular disorders: Disability, pain intensity and fear of movement. J Headache Pain. 2016;17:103. doi: 10.1186/s10194-016-0690-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Emodi-Perlman A, Eli I, Friedman-Rubin P, Goldsmith C, Reiter S, Winocur E, et al. Bruxism, oral parafunctions, anamnestic and clinical findings of temporomandibular disorders in children. J Oral Rehabil. 2012;39:126–35. doi: 10.1111/j.1365-2842.2011.02254.x. [DOI] [PubMed] [Google Scholar]
- 40.Carra MC, Huynh N, Morton P, Rompré PH, Papadakis A, Remise C, et al. Prevalence and risk factors of sleep bruxism and wake-time tooth clenching in a 7- to 17-yr-old population. Eur J Oral Sci. 2011;119:386–94. doi: 10.1111/j.1600-0722.2011.00846.x. [DOI] [PubMed] [Google Scholar]
- 41.Carlsson GE, Egermark I, Magnusson T. Predictors of bruxism, other oral parafunctions, and tooth wear over a 20-year follow-up period. J Orofac Pain. 2003;17:50–7. [PubMed] [Google Scholar]
- 42.Ungari C, Quarato D, Gennaro P, Riccardi E, Agrillo A, Mitro V, et al. A retrospective analysis of the headache associated with temporomandibular joint disorder. Eur Rev Med Pharmacol Sci. 2012;16:1878–81. [PubMed] [Google Scholar]
- 43.Hara K, Shinozaki T, Okada-Ogawa A, Matsukawa Y, Dezawa K, Nakaya Y, et al. Headache attributed to temporomandibular disorders and masticatory myofascial pain. J Oral Sci. 2016;58:195–204. doi: 10.2334/josnusd.15-0491. [DOI] [PubMed] [Google Scholar]
- 44.Ekberg EC, Nilner M. Treatment outcome of short- and long-term appliance therapy in patients with TMD of myogenous origin and tension-type headache. J Oral Rehabil. 2006;33:713–21. doi: 10.1111/j.1365-2842.2006.01659.x. [DOI] [PubMed] [Google Scholar]
- 45.Glaros AG, Urban D, Locke J. Headache and temporomandibular disorders: Evidence for diagnostic and behavioural overlap. Cephalalgia. 2007;27:542–9. doi: 10.1111/j.1468-2982.2007.01325.x. [DOI] [PubMed] [Google Scholar]
- 46.Gonçalves DA, Camparis CM, Speciali JG, Franco AL, Castanharo SM, Bigal ME, et al. Temporomandibular disorders are differentially associated with headache diagnoses: A controlled study. Clin J Pain. 2011;27:611–5. doi: 10.1097/AJP.0b013e31820e12f5. [DOI] [PubMed] [Google Scholar]
- 47.Lambert CA, Sanders A, Wilder RS, Slade GD, Van Uum S, Russell E, et al. Chronic HPA axis response to stress in temporomandibular disorder. J Dent Hyg. 2014;88(Suppl 1):5–12. [PMC free article] [PubMed] [Google Scholar]
- 48.Conti PC, Costa YM, Gonçalves DA, Svensson P. Headaches and myofascial temporomandibular disorders: Overlapping entities, separate managements? J Oral Rehabil. 2016;43:702–15. doi: 10.1111/joor.12410. [DOI] [PubMed] [Google Scholar]


