Abstract
Aims and Objective:
The aim of the study is to determine the effect of chewing gum containing xylitol and sorbitol on mutans streptococci and Lactobacilli count in saliva, plaque, and gingival health and to compare the efficacy of chewing gums.
Materials and Methods:
The study was designed as a double-blinded randomized uncontrolled clinical trial with two parallel arms. A total of 80 students consented and completed the study. The test group (X) received corresponding pellets with xylitol and the control group (S) was given pellets containing sorbitol and maltitol three times daily for 30 days. Clinical scoring and saliva samples were collected at three different intervals, at baseline, 15th, and 30th day of the study. The outcome measure was plaque index score, gingival index score, salivary mutans streptococci, and Lactobacilli counts. Data collected were analyzed using Statistical Package for the Social Sciences (SPSS version 19.0).
Results:
There was no statistically significant difference between the mean of mutans streptococci count of test and control group at baseline and 15th day, but there was statistically highly significant difference (P = 0.00) between the mean of mutans streptococci count in test and control group on the 30th day. The mean of Lactobacilli count, plaque index, and gingival index score between test and control group showed no statistically significant difference at baseline, 15th day, and 30th day.
Conclusion:
The results suggest that only xylitol gum may interfere with the mutans streptococci composition and reduce it after continuous use of 30 days effectively as compared to sorbitol gum, but both the gums are equally effective on salivary Lactobacilli, plaque, and gingiva at different intervals.
Keywords: Dental plaque, gingiva, Lactobacillus, sorbitol, Streptococcus mutans, xylitol
INTRODUCTION
The oral cavity provides a unique ecosystem in the human body. Its moist environment, temperature, and existence of endogenous and exogenous metabolic substrates make it an ideal medium for bacterial growth and poor oral hygiene is one of the reasons for accumulation of these microbes and their harmful activities. Hence, effective oral hygiene plays an important role in reducing dental plaque accumulation and maintaining oral health.[1]
A number of approaches through mechanical and chemical means for improving routine oral hygiene have been documented in the literature.[1] Sugar substitutes are one such agent that has been proposed to have anticarcinogenic properties. These include lactitol, maltitol, mannitol, sorbitol, isomalt, and xylitol and are commonly used in foods to replace sugars.[2] Frequent use of sugar-free chewing gum has been shown to have an inhibitory effect on dental caries. In fact, the incidence of dental caries has been reported to increase after chewing sucrose gum. Collectively, these observations suggest that the sweetening agent in chewing gum plays an important role in the cariogenicity of this products.[3]
Sorbitol and xylitol are the most commonly used sweeteners in chewing gum. Both polyols have proven to be nonacidogenic or hypoacidogenic in plaque telemetric studies.[4]
Sorbitol is a six-carbon, water-soluble polyhydric alcohol having humectancy and plasticizing property. It is metabolized at a slower rate than sucrose. It can be fermentated at a slower rate by mutans streptococci and Lactobacilli and can serve as a substrate for them.[2]
Xylitol, a naturally occurring sugar alcohol, is approved for use in food by the US Food and Drug Administration since 1963. Xylitol cannot be fermented by oral microorganisms and has shown to reduce mutans streptococcus levels in plaque and saliva and to markedly reduce tooth decays. It is unique among the sugar alcohols in its inhibitory effect on glycolysis. The inhibitory effect on glycolysis has been related to the uptake of xylitol through a constitutive fructose-specific phosphotransferase system and subsequent intracellular accumulation of xylitol-5-phosphate. This mechanism leads to reduced acid formation from glucose and a reduction in the Streptococcus mutans count in both plaque and saliva.[2]
As xylitol does not produce acid, it does not lower the pH of saliva. Xylitol lowers the temperature of the oral cavity slightly when it dissolves, which most people find refreshing. In contrast to other sugar alcohol, xylitol facilitates salivary secretion, thus immediately recovering a decline in pH. All these factors increase the amount of soluble calcium in dental plaque, which in turn facilitates remineralization of the enamel.[2] Today, most of the chewing gums and candies are sweetened with xylitol. One major obstacle with the use of both gums and candies for xylitol administration is high frequency and the rather large number of pellets that are required to deliver the therapeutic amounts. In addition, the costs for a long-term use could be a barrier. Therefore, novel low-cost delivery systems for xylitol are necessary which can be targeted to various ages.[2] The aim of the present study was to determine the effect of chewing gum containing xylitol and sorbitol on S. mutans and Lactobacillus count in saliva, plaque, and gingival health and to compare the efficacy and antimicrobial properties of xylitol-containing chewing gum with sorbitol-containing chewing gum among the study participants.
MATERIALS AND METHODS
Randomized double-blinded uncontrolled clinical trial with parallel study design was conducted among 18–24 years undergraduate BDS students of Darshan Dental College and Hospital during May and June in 2015. The study was to determine the effect of chewing gum containing xylitol and sorbitol on salivary S. mutans, Lactobacillus count, plaque, and gingiva. Before the start of the study, ethical clearance from ethical review board (letter no. DDCH/ADM/2011-12/1548-B) and informed concerned from all study participants was obtained.
Indices used in the study was,
Gingival index (GI, Loe and Sillness 1963) to assess the severity of gingivitis
Plaque index (PI, Turesky et al. 1970 modification of Quigley–Hein Plaque Index) to assess the plaque deposition.
TEST MATERIALS
The following chewing gums were used in the study:
Xylitol-containing chewing gum as test gum
Sorbitol-containing chewing gum as placebo control gum.
A pilot study was carried out on 16 students to check the feasibility and validity of the study and also to assess the acceptability of the chewing gum. The intra examiner validation was carried out before the study under the guidance of head of the institution, and the examination was carried out by four examiners who were doing their postgraduation and got training for the same. For the microbiological analysis, the microbiology faculty and the investigator after having detailed discussion of the methods involved in assessing the S. mutans and Lactobacillus count underwent a calibration session. Intraexaminer reliability (90%) was obtained.
Sample size determination was based on the expected minimum reduction in colonies of microorganisms, plaque, and gingivitis in the treated group, after intervention with the chewing gum for 4 weeks as observed in previous studies.[3]
From the pilot study, it was founded that the overall prevalence of plaque, gingivitis, and microbial count was 80%. Considering for the dropouts logistic and technical problems, the sample size was inflated by 10%, i.e. n = 8, hence the sample size was 80 + 8 = 88 with 44 participants in each group.
INCLUSION CRITERIA
Students belonging to 18–24 years
Students willing to participate and has given informed consent
No relevant medical history
No periodontal treatment during the past 3 months.
EXCLUSION CRITERIA
Students with orthodontic appliances
Receiving antibiotic therapy or medication within the past 6 months
The presence of any systemic illness
Students who availed oral prophylaxis in the past 6 months.
The participants received a brief instruction for the procedure; they had to perform, i.e., chewing 3 gums daily in addition to their routine toothbrushing. A participant was instructed to chew the gum at 8:00 am, 11:00 am, and 3:00 pm after lunch for 5 min daily. Each participant was instructed to follow their routine oral hygiene practices along with the assigned regimen and to maintain a reminder sheet on daily product use. Each one of the daily chewing gum was supervised on each weekday and also supervised by a daily recall message for reminding them to use the assigned chewing gum. The compliance was checked with the help of a reminder sheet by the examiner during surprise recall of the participants. Furthermore, the participant was recalled along with the chewing gum packet assigned to check for the chewing gum pallet count used by the participants. During the study, participants followed their usual oral hygiene and dietary habit and was instructed to refrain from using commercial chewing gum which is available in market.
COLLECTION OF SALIVA SAMPLE
On the day of saliva collection, participating student was instructed not to eat or drink anything for at least 1 hour before the collection of saliva sample. To control the Circadian variations, the sample was collected between 10:00 am and 11:00 am. The participant was asked to rinse their mouth with water before collection of saliva to avoid the contamination of food debris. Then, the participant was made to sit on a chair and resting; whole saliva was collected into the sterile graduated measuring cylinder with the help of a sterile funnel. The collected sample was transferred into 5-ml sterile disposable vials and carried in a vaccine carrier with freezing mixture to the laboratory, where analysis of the sample was done on the same day. The sample was collected at baseline at 15th day and at 30th-day interval after the use of assigned chewing gum.
MICROBIAL ANALYSIS OF SALIVA
The saliva sample was homogenized manually by stirring using a stirrer. Hundred microliter of saliva was diluted with 1 ml of sterile peptone water to obtain 1:10 dilution of saliva. About 100 μl of the diluted saliva was further added to 1 ml of sterile peptone water to obtain a dilution of 1:100. This procedure was repeated to obtain a dilution of 1:1000. This dilution of saliva was used for microbial analysis.
S. mutans was cultured on mitis salivarius-bacitracin (MSB) agar and Lactobacilli on Rogosa SL agar was the selective media for culture of these organisms. The media was prepared according to the manufacturer's instructions and poured into sterile disposable microbial culture plates and refrigerated till inoculation was done.
Using an inoculation loop (2 mm inner diameter), 5 μl of the 1:1000 dilution sample was streaked on MSB and Rogosa SL agar, under strict aseptic conditions. The MSB agar plate was incubated for 48 h at 37°C, anaerobically using candle jar. The Rogosa SL agar plate was incubated for 48 h at 37°C, aerobically in the incubator. After 48 h of incubation period, S. mutans appeared on the culture plate as small, rough, raised, and adherent colonies and Lactobacillus appeared as small white elevated round colonies. The S. mutans colony which was atypical was further confirmed by mannitol and sorbitol test. Colonies so identified were counted using an electronic colony counter. After incubation, plates with 30–300 colonies per standard-sized plate are counted to make the calculation of the number of colony-forming units/milliliter (CFU/ml) in the original samples easier; dilutions are designed to be easy to handle mathematically. The most common dilutions are 1/10, 1/100, and 1/1000. Looking first at the 1/10 dilution, it can be made by mixing 1 ml of sample with 9 ml of sterile dilution buffer.
DATA COMPILATION AND PRESENTATION
The obtained data were compiled systematically. A master table was prepared and the dataset was subdivided and distributed meaningfully and presented as individual tables along with graphs.
STATISTICAL ANALYSIS
Data collected were coded, computerized, and analyzed using Statistical Package for the Social Sciences (version 19.0) (xylitol: PERFETTI VAN MELLE’ HAPPYDENT XYLIT, sorbitol: WRIGLEYS ORBIT).
RESULTS
A 1-month follow-up study was conducted to assess the variation on salivary microbial counts, plaque score, and gingival score on administration of two different chewing gums among 90 undergraduate students, aged 18–24 years, residing in a hostel of Darshan Dental college, Udaipur city. The study participants were selected according to the inclusion and exclusion criteria. However, in spite of regular call and surprise visits, 7 students did not report whereas 3 students had to discontinue the study due to antibiotic coverage. Therefore, a total of 80 students was included in the main study with 40 students in each group [Table 1].
Table 1.
Distribution of chewing gum among the group at baseline, on 15th day, and 30th day of intervention according to colony-forming units/milliliter of Mutans streptococcus count
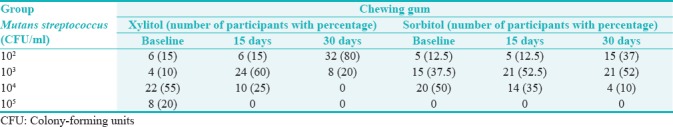
It was observed that a majority of the participants in xylitol group showed the mutans streptococcus count of 104 CFU/ml at baseline 22 (55%) which gradually changed to 103 CFU/ml on 15th day 24 (60%) and on 30th day it reduced to 102 CFU/ml 32 (80%). In sorbitol group, the baseline mutans streptococcus count of 20 (50%) participants was 104 CFU/ml at baseline which changed to 103 CFU/ml on 15th day 21 (52.5%) but remained constant on 30th day also. This shows that xylitol group was effective in reducing the mutans streptococcus count when compared to sorbitol chewing gum [Table 2].
Table 2.
Distribution of chewing gum among the group at baseline, on 15th day, and 30th day of intervention according to colony-forming units/milliliter of Lactobacilli count
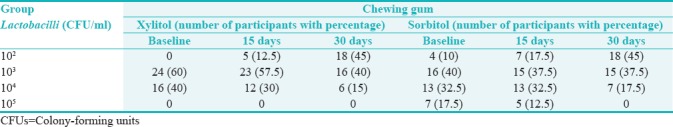
In xylitol group, colony count of majority of the participants (24, 60%) at baseline was 103 CFU/ml; on 15th day, for 23 (57.5%) participants, it was 103 CFU/ml; and on 30th day, for 18 (45%) participants, it was 102 CFU/ml showing that there was slight change at baseline and 15th day which gradually reduced on 30th day. In sorbitol group, the majority of participants (16, 40%) at baseline showed 103 CFU/ml; on 15th day, 15 (37.5%) participants showed 103 CFU/ml which was almost same; but on 30th day, it was seen that there was slight reduction in 18 (45%) participants to 102 CFU/ml. Therefore, xylitol and sorbitol chewing gums were almost equivalent in reducing Lactobacilli count [Table 3].
Table 3.
Comparisons of chewing gum at baseline, 15th day, and 30th day intervals for Mutans streptococcus and Lactobacilli count (mean log10 colony-forming units/milliliter)
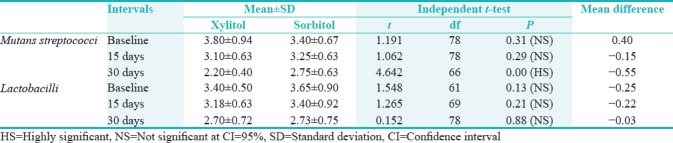
It was observed that the mean mutans streptococci count between xylitol and sorbitol group at baseline were 3.80 ± 0.94 and 3.40 ± 0.67, respectively. There was no statistically significant difference between the mean of xylitol and sorbitol group at baseline (P = 0.31) and 15th day (P = 0.29). The mean of mutans streptococci between xylitol (2.20 ± 0.40) and sorbitol (2.75 ± 0.63) showed statistically highly significant difference (P = 0.00) on the 30th day. The mean comparison of Lactobacilli count between xylitol (3.40 ± 0.50) and sorbitol (3.65 ± 0.90) group showed no statistically significant difference (P = 0.13) at baseline. Furthermore, there was no statistically significant difference on 15th day (P = 0.21) and 30th day (P = 0.88) [Table 4].
Table 4.
Comparisons of chewing gum at baseline, 15th day, and 30th day intervals for plaque index score and gingival index score
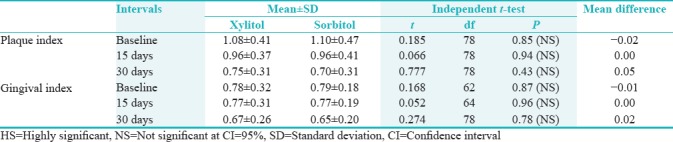
On comparing the mean plaque index score between xylitol and sorbitol group at baseline was 1.08 ± 0.41 and 1.10 ± 0.47, respectively, which reduced to 0.96 ± 0.37 in xylitol group and 0.96 ± 0.41 in sorbitol group on the 15th day. There was further reduction to 0.75 ± 0.31 in xylitol group and 0.70 ± 0.31 in sorbitol group on 30th day, but there was no statistically significant difference between two groups at all three intervals. In case of gingival index, the mean reduction of gingival scores was less and there was no statistically significant difference between xylitol and sorbitol groups at different study intervals.
DISCUSSION
In this study, the S. mutans counts and Lactobacillus counts were evaluated in saliva. S. mutans were cultured using MSB agar[5] and Lactobacilli in Rogosa agar,[6] which are the selective media for the growth of S. mutans and Lactobacilli, respectively. Studies reveal that the results obtained by this culture plate method, as used in this study, correlate well with the dip slide methods,[7,8] yet another method used for the same. All the study groups had similar salivary microbial count of S. mutans and Lactobacillus scores at baseline so that the changes seen after the administration of the test products can be attributed to the use of these products. Extensive exploration of the literature revealed no studies that compared all these products with each other.
Various plaque indices have been used for many years in epidemiological studies, clinical trials, and clinical practice to record oral hygiene. However, they have other applications; in particular, to assess tooth cleaning and plaque preventive actions of various mechanical devices and chemical agents.[9] In essence, plaque indices establish the oral hygiene status of dentition. The Turesky–Gilmore–Glickman modification of the Quigley–Hein Plaque index given in 1970 has been devised specifically to score the smooth surface plaque before and after toothbrushing and other plaque removal interventions.[10] It is a conventional index used in various chewing gum clinical trials and has been opted to be used in the present study. To score the severity of gingivitis, Loe and Silness Gingival index given in 1963 has been used.
The results in both the groups were expected and reinforced previous findings. Thus, for the test group, the null hypothesis could be rejected in case of mutans streptococci count. In xylitol group, the daily dose of xylitol equaled 6 g and the results were in harmony with previous findings with that amount.[11,12] The mechanisms of antibacterial action are basically different for both xylitol and sorbitol. Xylitol exerts its antibacterial action through hampering bacterial growth through metabolic reactions. Xylitol is incorporated into the cell with the help of the fructose-specific phosphotransferase system and phosphorylated to xylitol-5-phosphate,[13] which inhibits further intracellular metabolism of the bacterial cell and the process consumes energy. After exposure to xylitol, a shift toward xylitol-resistant mutans streptococci has been shown in saliva,[14] and it has been suggested that those strains have a reduced ability to adhere to the tooth surfaces.[15]
The total number of bacterial counts or mutans streptococci levels in saliva did not differ between the groups at either baseline or after 15 days. Nevertheless, the proportion of mutans streptococci decreased significantly in the xylitol gum group in contrast to the sorbitol gum group at 30th day. This result agrees with several other previous studies,[16] indicating a small advantage of xylitol over sorbitol. The xylitol influence was more pronounced here when compared to the previous report of lower daily xylitol doses from lozenges 3, which reinforces the findings of Milgrom et al.,[11] who suggested a dose–response relationship with a plateau effect for doses between 6 and 10 g.
Long-term[17] and frequent use of sorbitol-containing nicotine chewing gum did not induce notable changes in the number of salivary oral streptococci and Lactobacilli. This finding is in agreement with the results reported by Birkhed et al.[18] who observed no differences in the number of S. mutans or Lactobacilli when lozenges containing sorbitol had been used four times a day for 3 months. Lyn O’Brien[19] found no change in the S. mutans population in monkeys fed on a high-sorbitol diet over a long period. Loesche et al.[20] observed a tendency to an increase in S. mutans after 4 week consumption of sorbitol-mannitol-containing gums in humans, whereas Birkhed et al.[21] reported that persons who had consumed on the average 3–6 sorbitol-containing food items per day for at least 3 year, showed no change in the number of S. mutans and Lactobacilli in saliva. It has been shown that most strains of S. mutans and Lactobacilli can ferment and produce acid from sorbitol in vitro.[17] However, utilization of sorbitol is readily suppressed by low levels of glucose. The glucose level in parotid saliva is higher than the level needed for repressing the sorbitol pathway. This may imply that in persons with normal salivary flow the low utilization of sorbitol by S. mutans probably is too low to give an ecologic advantage for S. mutans. On the other hand, no decrease in the S. mutans population was observed after the sorbitol period, as was the case after chewing on xylitol. Possible reasons for this difference might be that the amount of salivary glucose is not enough to repress all enzyme activity in S. mutans when the individuals have an extensive exposure to sorbitol-containing gums between meals. Xylitol is not fermented by S. mutans[17] and when added to a glucose solution acid production was inhibited in plaque. Furthermore, recent studies show that intracellular accumulation of xylitol phosphate in S. mutans may be toxic for the bacteria.[22]
The significant decrease in the number of S. mutans noted after 2 months of frequent chewing of xylitol gums was lost after 3 months. This finding is in agreement with results presented both by Loesche et al.[20] who observed after 4 week lower prevalence of S. mutans in xylitol-consuming individuals and by Birkhed et al.[18] who found no changes in the salivary numbers of S. mutans and Lactobacilli after a 3-month period of frequent xylitol consumption. The reason for the return of S. mutans to baseline levels after 3 months is not clear, but it is not unlikely that bacterial adaptation might have occurred since xylitol-resistant strains of S. mutans have been isolated in high frequency in xylitol consumers.[23]
An obvious and clear finding from this study was that both chewing gum regimes reduced the amount of plaque and improve gingival health in saliva, and this was achieved on top of reinforced instructions in daily toothbrushing but that is not statistically significant. Thus, the null hypothesis could not be rejected for these selected endpoints. This showed that both the chewing gums affect plaque and gingival equally. On average, the students had relatively high plaque scores at baseline, and from a clinical point of view, they improved during the chewing period. Soderling et al.[16] indicated that the mechanism of plaque reduction may differ between xylitol and sorbitol which was not similar with present study. The latter performed equally well with respect to a reduction in the amount of plaque, but not the number of mutans streptococci. Since the reduction of plaque was not significant in both test groups. Furthermore, Olivera et al.[23] demonstrated that plaque formed during frequent use of xylitol contained less polysaccharide compared to sorbitol influenced plaque showed dissimilar result with the present study. The present study significantly evaluated the S. mutans and Lactobacillus counts in saliva, it was found that the xylitol- and sorbitol-containing chewing gums significantly reduced the amount of plaque and improved the gingival health. It was observed at the end of the study that xylitol-containing chewing gum significantly reduced the S. mutans levels in comparison to sorbitol. Both xylitol and sorbitol chewing gums were found effective in reducing Lactobacilli count. Xylitol-containing chewing gums can be recommended in those children or individuals who lack manual dexterity and when their brushing cannot be supervised.[13]
The series of studies as well as other retrospective studies and prospective intervention trials have established that xylitol in amount of 6 g/day is likely to be effective in reduction of S. mutans count during short-term use, and frequency of exposure of 3 times/day or more necessary for effectiveness. This knowledge is highly significant in considering the feasibility of public health prevention program using xylitol.
CONCLUSION
The conclusion suggests that for a short-term use, in comparison to sorbitol controls, xylitol-containing gum interfere with the microbial composition and decrease the proportion of salivary mutans streptococci. However, the chewing gum is not an effective adjunct for regular oral hygiene practice. Further exploration of the results to long-term clinical trials needs to be undertaken to confirm the effect.
FINANCIAL SUPPORT AND SPONSORSHIP
Nil.
CONFLICTS OF INTEREST
There are no conflicts of interest.
REFERENCES
- 1.Keijser BJ, van den Broek TJ, Slot DE, van Twillert L, Kool J, Thabuis C, et al. The impact of maltitol-sweetened chewing gum on the dental plaque biofilm microbiota composition. Front Microbiol. 2018;9:381. doi: 10.3389/fmicb.2018.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shwetha R, Vivek S. Effect of dentifrices containing sorbitol, combination of xylitol and sorbitol on salivary Streptococcus mutans and Lactobacillus counts in 14-15-year-old children: A randomized trial. Int J Clin Trials. 2017;4:184–90. [Google Scholar]
- 3.Dhingra S, Marya CM, Jnaneswar A, Kumar H, Dahiya N, Dahiya V, et al. Role of sugar free chewing gums in oral health. J Oral Health Comm Dent. 2015;9:35–9. [Google Scholar]
- 4.Tuncer D, Onen A, Yazici AR. Effect of chewing gums with xylitol, sorbitol and xylitol-sorbitol on the remineralization and hardness of initial enamel lesions in situ. Dent Res J (Isfahan) 2014;11:537–43. [PMC free article] [PubMed] [Google Scholar]
- 5.Freire PL, Albuquerque AJ, Sampaio FC, Galembeck A, Flores MA, Stamford TC, et al. AgNPs: The new allies against S. mutans biofilm – A pilot clinical trial and microbiological assay. Braz Dent J. 2017;28:417–22. doi: 10.1590/0103-6440201600994. [DOI] [PubMed] [Google Scholar]
- 6.Wasfi R, Abd El-Rahman OA, Zafer MM, Ashour HM. Probiotic Lactobacillus sp. Inhibit growth, biofilm formation and gene expression of caries-inducing Streptococcus mutans. J Cell Mol Med. 2018;22:1972–83. doi: 10.1111/jcmm.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J, Liu H, Liang X, Zhang M, Wang R, Peng G, et al. Investigation of salivary function and oral microbiota of radiation caries-free people with nasopharyngeal carcinoma. PLoS One. 2015;10:e0123137. doi: 10.1371/journal.pone.0123137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hemalatha N, Syamala N. Efficacy of dip slide test in assessing the asymptomatic bacteriuria in pregnancy. Int J Res Med Sci. 2016;4:1921–5. [Google Scholar]
- 9.Migliorati M, Isaia L, Cassaro A, Rivetti A, Silvestrini-Biavati F, Gastaldo L, et al. Efficacy of professional hygiene and prophylaxis on preventing plaque increase in orthodontic patients with multibracket appliances: A systematic review. Eur J Orthod. 2015;37:297–307. doi: 10.1093/ejo/cju044. [DOI] [PubMed] [Google Scholar]
- 10.Park SH, Cho SH, Han JY. Effective professional intraoral tooth brushing instruction using the modified plaque score: A randomized clinical trial. J Periodontal Implant Sci. 2018;48:22–33. doi: 10.5051/jpis.2018.48.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milgrom P, Ly KA, Roberts MC, Rothen M, Mueller G, Yamaguchi DK, et al. Mutans streptococci dose response to xylitol chewing gum. J Dent Res. 2006;85:177–81. doi: 10.1177/154405910608500212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holgerson PL, Sjöström I, Stecksén-Blicks C, Twetman S. Dental plaque formation and salivary Mutans streptococci in schoolchildren after use of xylitol-containing chewing gum. Int J Paediatr Dent. 2007;17:79–85. doi: 10.1111/j.1365-263X.2006.00808.x. [DOI] [PubMed] [Google Scholar]
- 13.Nayak PA, Nayak UA, Khandelwal V. The effect of xylitol on dental caries and oral flora. Clin Cosmet Investig Dent. 2014;6:89–94. doi: 10.2147/CCIDE.S55761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Decker EM, Klein C, Schwindt D, von Ohle C. Metabolic activity of Streptococcus mutans biofilms and gene expression during exposure to xylitol and sucrose. Int J Oral Sci. 2014;6:195–204. doi: 10.1038/ijos.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krzyściak W, Jurczak A, Kościelniak D, Bystrowska B, Skalniak A. The virulence of Streptococcus mutans and the ability to form biofilms. Eur J Clin Microbiol Infect Dis. 2014;33:499–515. doi: 10.1007/s10096-013-1993-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Söderling E, Mäkinen KK, Chen CY, Pape HR, Jr, Loesche W, Mäkinen PL, et al. Effect of sorbitol, xylitol, and xylitol/sorbitol chewing gums on dental plaque. Caries Res. 1989;23:378–84. doi: 10.1159/000261212. [DOI] [PubMed] [Google Scholar]
- 17.Philip N, Suneja B, Walsh LJ. Ecological approaches to dental caries prevention: Paradigm shift or shibboleth? Caries Res. 2018;52:153–65. doi: 10.1159/000484985. [DOI] [PubMed] [Google Scholar]
- 18.Birkhed D, Edwardsson S, Ahldén ML, Frostell G. Effects of 3 months frequent consumption of hydrogenated starch hydrolysate (Lycasin), maltitol, sorbitol and xylitol on human dental plaque. Acta Odontol Scand. 1979;37:103–15. doi: 10.3109/00016357909027577. [DOI] [PubMed] [Google Scholar]
- 19.Lyn O’Brien N, editor. Sorbitol and Mannitol. 3rd ed. Switzerland: Marcel Dekker AG; 2017. Alternative sweeteners; p. 317. [Google Scholar]
- 20.Loesche WJ, Grossman NS, Earnest R, Corpron R. The effect of chewing xylitol gum on the plaque and saliva levels of Streptococcus mutans. J Am Dent Assoc. 1984;108:587–92. doi: 10.14219/jada.archive.1984.0390. [DOI] [PubMed] [Google Scholar]
- 21.Birkhed D, Edwardsson S, Svensater G. Frequent sorbitol consumption and dental caries – A longitudinal clinical and bacteriological study. J Dent Res. 1981;60:1261–7. [Google Scholar]
- 22.Liu C, Niu Y, Zhou X, Zheng X, Wang S, Guo Q, et al. Streptococcus mutans copes with heat stress by multiple transcriptional regulons modulating virulence and energy metabolism. Sci Rep. 2015;5:12929. doi: 10.1038/srep12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krzysciak W, Jurezak A, Koscielniak D, Bystrowska B, Skalniak A. The virulence of streptococcus mutans and the ability to form biofilms. Eur J Clin Microbiol Infect Dis. 2014;33:499–515. doi: 10.1007/s10096-013-1993-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


