The incidence of hepatocellular carcinoma (HCC) in the United States is rising even as overall cancer rates are declining, and Latinos are disproportionately affected, especially in Texas. Our case-control study sought to determine relative etiologic contributions of lifestyle-related risk factors in South Texas.
Methods
Between October 2012 and July 2014, we identified, consented and interviewed in person 51 HCC adult cases (diagnosed within the past 12 months and residing in Bexar or any of the 7 surrounding counties) from clinics at University Hospital and the Cancer Therapy and Research Center at UT Health San Antonio; and 104 adult controls, 32 from a related study1,2 and 72 randomly selected residents in the study counties. From these 51 cases and 104 controls, we created 42 matched pairs, with exact matching on sex, ethnicity (Hispanic, Non-Hispanic), and age category (18–57 years, >57 years). Urine samples (10 mL each) and serum samples (150 μL each) were assessed for biomarkers of previous aflatoxin exposure using procedures previously described.3 The primary aflatoxin exposure serum biomarker was an aflatoxin B1 (AFB1)-lysine DNA adduct. We also assessed urinary aflatoxin M1. We used matched logistic regression to estimate odds ratios (OR) and 95% confidence intervals. The protocol was approved by a local Institutional Review Board.
Results
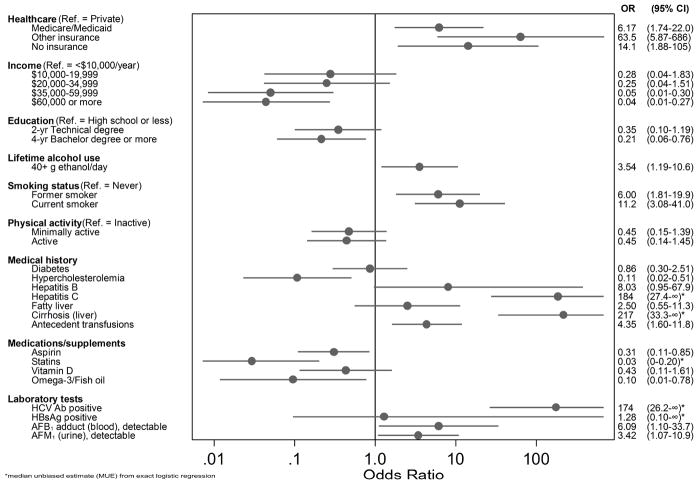
Most matched cases and controls were Latino (67%). Compared to controls, HCC cases were more likely to report Medicare or Medicaid, lower income, less education, more lifetime alcohol use, and more smoking (Figure 1). Cases were less likely to report hypercholesterolemia [0.11 (0.02–0.51)], more likely to report hepatitis C [183.74 (27.37–∞), cirrhosis 2.17 (33.3–∞), and transfusions [4.35 (1.60–11.84)], and less likely to be taking aspirin [0.31 (0.11–0.85)], statins [0.03 (0–0.20)], and omega-3/fish oil [0.10 (0.01–0.78)]. Cases did not differ significantly from controls with regard to reported consumption of products made from corn (data not shown). Relative to controls, cases were more likely to have HCV antibodies [174.3 (26.2–∞)] and have detectable aflatoxin levels in blood [6.09 (1.10–33.71)] and urine [3.42 (1.07–10.91)].
Figure 1.
Association between lifestyle and clinical factors and hepatocellular carcinoma risk in South Texas. Odds ratios (95% CI) derived from matched logistic regression.
Discussion
To our knowledge, this is the first epidemiologic study relating HCC and aflatoxin exposure in a U.S. population, particularly a Latino-majority study population. A recent review of non-U.S. studies4 reported that aflatoxin may play a causative role in 4.6% to 28.2% of all global HCC cases, and that the combined effect of AFB1 exposure and HBV infection appears additive rather than multiplicative. In the current study, serum (AFB1) and urine (AFM1) aflatoxin levels and HCV infection were significantly higher in cases than controls. Our results also support the observations of others that HCV infection is a major risk factor for HCC incidence in the U.S. Although HCV infection is a strong risk factor for development of HCC, rising trends in HCC incidence among Latinos cannot be attributed solely to HCV infection. Our data on a majority-Latino cohort suggest that HCC cases are poor and controls were more affluent, and smoked more with a higher prevalence of cirrhosis than controls. The lack of case-control differences for corn-based foods, a suspected risk factor for HCC, may be due to information bias from use of a 12-month dietary recall assessment. We hypothesized that contaminated corn products contributed to the increased risk of HCC and expected to observe increased consumption of corn-based foods in cases relative to controls; a possible reason we did not may be that cases and controls are consuming corn products differentially contaminated with aflatoxin (e.g., commercial vs. home-grown). Random assays of corn products purchased by cases and controls should be done in a future study to verify this. Study strengths include case-control matching on sex, ethnicity, and age; and in-person interviewing of subjects in their language of choice. A possible weakness is that cases and controls were not randomly selected with the same inclusion criteria; therefore, selection bias may exist. Cases were selected as a convenience sample from local medical facilities, whereas controls were selected from an existing prevention study and randomly from the surrounding county populations. A larger study will be needed to address sources of aflatoxin exposure as a risk factor for HCC.
Acknowledgments
Grant Support -- This project was supported by the Cancer Prevention Research Institute of Texas (CPRIT) RP120462; the Institute for Health Promotion Research (IHPR) U01 CA86117 and U54 CA153511; and the Cancer Therapy and Research Center (CTRC) at The University of Texas Health Science Center at San Antonio, an NCI-designated Cancer Center (P30 CA054174).
The authors thank Amanda Sintes for her assistance with interviews of participants, and Allen Trevino, Holiday Harris, and Norma Ketchum for their assistance with data management and their comments on an earlier version of the manuscript.
Footnotes
Disclosures -- The authors declare that there are no financial or personal conflicts of interest.
Author Contributions – AGR and BHP contributed to study concept and design; obtained funding; study supervision; and critical revision of the manuscript for important intellectual content. EM contributed to study concept and design; data acquisition, analysis and interpretation; statistical analysis; drafting and critical revision of the manuscript. DLP and TDP contributed to study concept and design; study supervision; data acquisition, analysis and interpretation; drafting and critical revision of the manuscript. JEM and AECH contributed to study concept and design; statistical analysis; and drafting and critical revision of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.ClinicalTrials.gov. [accessed 03-24-2015];Phase 2 Reduction of Dietary Mycotoxin Exposure by ACCS100 (RDMEACCS100) Available from URL: https://clinicaltrials.gov/ct2/show/NCT01677195?term=NCT01677195&rank=1.
- 2.Pollock BH, Elmore S, Romoser A, et al. Intervention trial with calcium montmorillonite clay in a south Texas population exposed to aflatoxin. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2016;33(8):1346–54. doi: 10.1080/19440049.2016.1198498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson NM, Qian G, Xu L, et al. Aflatoxin and PAH exposure biomarkers in a U.S. population with a high incidence of hepatocellular carcinoma. Sci Total Environ. 2010;408(23):6027–31. doi: 10.1016/j.scitotenv.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu HC, Santella R. The role of aflatoxins in hepatocellular carcinoma. Hepat Mon. 2012;12(10 HCC):e7238. doi: 10.5812/hepatmon.7238. [DOI] [PMC free article] [PubMed] [Google Scholar]