Abstract
A new crystal structure of an anti–HIV-1 envelope antibody bound to an envelope–receptor complex shows the antibody binding both the HIV-1 envelope and the CD4 receptor, raising the question of what the role of antibody autoreactivity in host responses to HIV-1 may be.
The antibody response to HIV-1 seems to be peculiar in that, unlike influenza, neutralizing antibodies take a relatively long time (3–6 months) to develop against the transmitted/founder strain of HIV-1 (ref. 1); when they do first develop, HIV-1 envelope protein (Env) gp120 neutralizing antibodies that target the transmitted/founder virus are quite strain or type specific2,3. Broadly neutralizing antibodies can develop in the course of HIV-1 infection, but they do so only in a minority of subjects and only after several years of infection4. Passive protective trials with such rare broadly neutralizing antibodies have shown that these antibodies can prevent mucosal infection with simian immunodeficiency viruses expressing HIV-1 envelopes5. Thus, if broadly neutralizing antibodies could be routinely induced systemically and/or at mucosal sites, a practical HIV-1 vaccine would be in sight.
A recent HIV-1 vaccine trial in Thailand with a canarypox vector (called ALVAC) containing a clade AE_01 recombinant Env gp120 prime with a bivalent clade B and clade AE_01 gp120 boost showed 31% efficacy6. The lack of neutralization breadth induced by this type of vaccine has prompted the hypothesis that a type of non-neutralizing antibody might provide some protection, perhaps by blocking the movement of virions or virus-infected cells across mucosal barriers and/or mediating antibody-dependent cellular cytotoxicity7. Thus, it remains to be determined what antibodies are ‘good’ and capable of being induced by experimental vaccine candidates.
Many of the rare broadly neutralizing antibodies that have been isolated have unusual traits, such as long heavy chain complementarity-determining regions (HCDR3s), high levels of somatic mutations and polyreactivity with a variety of host molecules8. Antibody polyreactivity is the ability to react with more than one antigen and is a normal component of the immunoglobulin repertoire. Approximately 60% of the preselection immunoglobulin repertoire is autoreactive9; most of these autoreactive B cells are removed during B-cell development, but as many as 20% of postselection B cells make autoantibodies, of which ~5% are polyreactive. However, polyreactivity has not been observed among the commonly made anti–HIV-1 gp120 CD4-inducible (CD4i)8 antibodies (due to their ability to recognize conformations of gp120 that are induced by binding CD4) that recognize the highly conserved CCR5 binding site10. Some of these antibodies derive from restricted VH families VH1-69 and VH1-24 (for example, 21c)11. Although CD4i antibodies are reported to be induced by the type of vaccine used in the Thai trial10, they have not been considered to be strong candidates for induction by a preventive vaccine because whole CD4i antibodies do not neutralize HIV-1 well12, whereas CD4i antibody Fab fragments do, implying partial occlusion of the CD4i antibody binding site13.
In this issue, Diskin, Marcovecchio and Bjorkman present the structure of a HIV-1 clade C envelope protein bound by both soluble CD4 and by a CD4i monoclonal antibody (21c) against the gp120 CCR5 coreceptor site14. Whereas most of the residues of the 21c HCDR3 make contacts to the CCR5 binding site on gp120, a small part of the HCDR3 and the 21c light chain L1 region binds CD4 in the CD4–gp120 complex. Thus, this is the first crystal structure to our knowledge of an autoreactive antibody with its single Fab bound to more than one antigen at the same time. The antibody 21c has yet to be shown to react with host antigens other than CD48, but its simultaneous binding to both a viral and host antigen is reminiscent of previously reported polyreactive HIV-1 antibodies (2F5 and 4E10), which bind membrane-proximal HIV-1 Env gp41 in complex with lipids, making contacts with both gp41 and membrane lipids15–18.
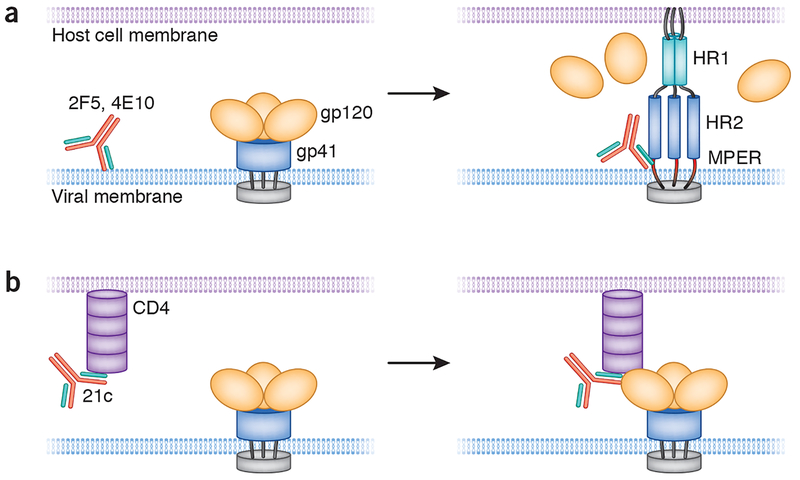
This study raises a number of critical questions regarding the significance of the auto-reactivity of anti–HIV-1 antibodies. First, is there functional significance behind the autoreactivity of the 21c and other HIV-1 antibodies? Both 2F5 and 4E10 have long hydrophobic HCDR3s, and mutation of those residues abrogates both lipid binding and HIV-1 neutralization while maintaining binding to gp41 (ref. 17). The binding of these mAbs to the gp41–lipid complex has been proposed as a sequential two-step process in which encountering the lipid membrane takes place first, presumably to help the antibody to dock with the transiently exposed gp41 intermediate neutralizing epitope during the virion–host cell fusion process17,18 (Fig. 1). The 21c antibody binds to some Envs in the absence of soluble CD4 (ref. 19), but it does not interact with the CAP210 clade C envelope unless soluble CD4 is present14. The 21c antibody reacts only weakly with soluble CD4 alone, raising the question of its biological relevance in vivo. It remains to be determined whether the initial binding of 21c to CD4 is required for gp120 binding or the interaction of 21c with CD4 is an event that occurs when antibody 21c encounters CD4 bound to gp120 (Fig. 1). In the first case, an initial interaction with CD4 could position antibody 21c for a more efficient subsequent encounter with gp120. In the second case, simultaneous interaction with CD4 and gp120 could enhance the overall binding avidity of antibody 21c. A third possibility is that CD4 binding to gp120 induces new 21c reactive epitopes not previously present on either molecule alone.
Figure 1.
Possible modes of gp41 neutralizing and 21c monoclonal antibodies binding to the HIV-1 envelope. (a) 2F5 and 4E10 antibodies may initially interact with viral lipid membrane, which renders them readily available to bind their epitopes within the transiently exposed membrane-proximal external region (MPER) of the gp41 intermediate during the fusion process. Depicted in the gp41 intermediate state (right) are gp41 heptad repeat (HR) regions 1 and 2 and the MPER. (b) The antibody 21c may initially interact with CD4, which would efficiently position the antibody for a subsequent encounter with gp120. Alternatively, 21c may bind a pre-existing CD4–gp120 complex (right panel in b), in which case binding avidity would be a consequence of the autoreactivity of antibody 21c.
Second, does antibody autoreactivity modulate the frequency of expression of antibodies like 21c? In human subjects infected with HIV-1, antibodies that react with the gp41 epitopes bound by antibodies 2F5 and 4E10 are rarely made. Because of their polyreactivity with lipids and other host proteins, it has been proposed that their expression is limited by central and peripheral tolerance mechanisms8. Indeed, in 2F5 and 4E10 VH knock-in mice, B-cell ontogeny analysis shows that a majority of these B cells are deleted during bone marrow B-cell development20 (Verkoczy, L. and B.F.H., unpublished data). In contrast, CD4i antibodies that map to the CCR5 coreceptor binding region are extremely common, and most HIV-1–infected subjects develop non-neutralizing CD4i antibody specificitie ~5–10 weeks after transmission1. However, it is not known whether any of the many CD4i antibodies produced are autoreactive for CD4, nor is it known what the functional significance of such antibodies might be if they are made. As is pointed out by Diskin and colleagues, other CD4i antibodies such as 17b, 412d and X5 do not react with CD4, and we did not find poly-reactivity in CD4i antibodies (including 21c) when screened against a series of host antigens8. Therefore, most types of CD4i antibodies are quite common and are unlikely to be regulated by tolerance mechanisms.
Third, are there any protective benefits derived from the induction of antibodies such as 21c by a preventive vaccine? The observation that CD4i antibodies do not neutralize transmitted/founder viruses, and that these antibodies are structurally constrained for access of whole antibody to the CCR5 binding site, have dampened enthusiasm for using the highly conserved CCR5 binding site as a vaccine target12,13. On the other hand, recent observations indicate that strategies to target this highly conserved site might be found. Gray and colleagues21 and Li and colleagues22 have found, for clades C and B, select patients whose broadly neutralizing antibodies map to or overlap the coreceptor binding site, respectively. CD4–gp120 complexes have been reported to induce neutralizing antibodies in monkeys23, and induced CD4i antibody levels have been correlated with protection from simian-human immunodeficiency virus mucosal challenge in monkeys24. If antibodies against CD4i epitopes can reach the surface of HIV-1 or HIV-1–infected cells, they could have salutary effects either by direct neutralization or by non-neutralizing mechanisms such as antibody-dependent cellular cytoxicity23.
Finally, the study of Diskin and colleagues is important because it raises the question of whether some of the commonly made antibodies induced by HIV-1 are autoreactive, as some of the rarely induced broadly neutralizing antibodies are. Autoreactive antibodies with low affinity for self antigens are commonly made in mice by B1 or marginal-zone B cells and are frequently derived by T cell–independent mechanisms. It will be critical for those working on HIV-1 vaccine development to determine if the specificities of antibodies with low-affinity self reactivity are important to induce for a successful preventive vaccine or, alternatively, if these types of antibody responses should instead be avoided.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Tomaras GD et al. J. Virol 82, 12449–12463 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei X et al. Nature 422, 307–312 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Richman DD et al. Proc. Natl. Acad. Sci. USA 100, 4144–4149 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stamatatos L et al. Nat. Med 15, 866–870 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Hessell AJ et al. Nature 449, 101–104 (2007). [DOI] [PubMed] [Google Scholar]
- 6.Rerks-Ngarm S et al. N. Engl. J. Med 361, 2209–2220 (2009). [DOI] [PubMed] [Google Scholar]
- 7.Letvin NL et al. Science 326, 1196–1198 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Haynes BF et al. Science 308, 1906 (2005).15860590 [Google Scholar]
- 9.Wardemann H et al. Science 301, 1374–1377 (2003). [DOI] [PubMed] [Google Scholar]
- 10.Decker JM et al. J. Exp. Med 201, 1407–1419 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang CC et al. Proc. Natl. Acad. Sci. USA 101, 2706–2711 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keele BF et al. Proc. Natl. Acad. Sci. USA 105, 7552–7557 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Labrijn AF et al. J. Virol 77, 10557–10565 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diskin R et al. Nat. Struct. Mol. Biol 17, 608–613 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ofek G et al. J. Virol 78, 10724–10737 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cardoso RM et al. J. Mol. Biol 365, 1533–1544 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Alam SM et al. Proc. Natl. Acad. Sci. USA 106, 20234–20239 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alam SM et al. J. Immunol 178, 4424–4435 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiang S-H et al. AIDS Res. Hum. Retroviruses 18, 1207–1217 (2002). [DOI] [PubMed] [Google Scholar]
- 20.Verkoczy L et al. Proc. Natl. Acad. Sci. USA 107, 181–186 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gray ES et al. J. Virol 83, 8925–8937 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y et al. J. Virol 83, 1045–1059 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fouts T et al. Proc. Natl. Acad. Sci. USA 99, 11842–11847 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeVico A et al. Proc. Natl. Acad. Sci. USA 104, 17477–17482 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]