Abstract
To investigate the trophic transfer of nanomaterials along the food chain, we examine the potential trophic transfer and biomagnification of CdSe/ZnS quantum dots (QDs) in a simple freshwater food chain. Our results indicate that QDs can transfer from zooplankton to Danio rerio (zebrafish) by dietary exposure. No significant biomagnification of QDs was observed and the biomagnification factors for both adult and juvenile zebrafish were both less than one (0.04 and 0.004 respectively). The assimilation efficiency was 8% and 4% for adult and juvenile zebrafish respectively. This study is the first to examine the potential food chain transfer and biomagnification of QDs from zooplankton to zebrafish.
Keywords: nanoparticles, quantum dots, zebrafish, trophic transfer, bioaccumulation
Quantum dots (QDs) offer considerable benefits in several commercial sectors due to their unique optical properties. Their applications range from biomedical use as traceable therapeutic agents, to electronics use in LED flat panel displays, to alternative energy solutions including photovoltaic solar cells. As the number of commercially viable uses of QDs continues to rise, the production of QDs will shift from bench-scale to large-volume manufacturing, increasing the probability of QDs release into aquatic environments. Since most QDs contain heavy metals, such as cadmium in the case of CdSe/ZnS QDs, toxicity to aquatic organisms may result from heavy metal exposure in addition to any reactions associated with their nanoscale properties. Although there are an appreciable number of published studies on the ecotoxicity of nanoparticles (NPs), previous work has focused on establishing lethal concentration levels. There remains little information on potential bioaccumulation and trophic transfer of nanoparticles in aquatic organisms. Dietary exposure to nanoparticles can result in significantly higher total body concentrations in predatory organisms since prey organisms can accumulate nanoparticles. For example, Daphnia magna (daphnia) can have total body concentrations that are orders of magnitude greater than their surrounding water concentrations. To date, published dietary exposure studies of nanoparticles using fish examine only metal oxide and carbon nanoparticles.1–5 Comparing LC50 values for different metal-based nanoparticles in aquatic organisms revealed TiO2 to be harmful with the lowest LC50 value reported as 65.5 ppm.6 In contrast, a review of articles testing CdSe/ZnS QDs on aquatic organisms yields a lowest LC50 of 0.04 ppm making it extremely toxic to aquatic organisms.7
For QDs, trophic transfer studies have examined the transfer of QDs in invertebrate food webs and have suggested potential biomagnification to higher organisms.7–9 Our previous work demonstrated that the filter feeder, daphnia, accumulates QDs in their intestine. The gut passage time for daphnia exposed to metals has been reported as 8 hours or less, and our study showed QDs eliminated more slowly with around half of the initial dose remaining after 48 hr of clearance.10 Since our daphnia results suggested the possibility of biomagnification, this current study aims to investigate the trophic transfer of QDs from zooplankton to Danio rerio (zebrafish). Because zebrafish lack a stomach, no acid phase digestion is expected suggesting ingested quantum dots may experience little to no chemical degradation. Assuming the nanoparticles are indigestible, we hypothesized that the nanoparticles would be excreted from zebrafish with little accumulation. To test the potential effect on growing zebrafish, dietary exposure was conducted on juvenile fish in addition to adults. To date, no studies have been published on the biodistribution of QDs in adult or juvenile zebrafish.
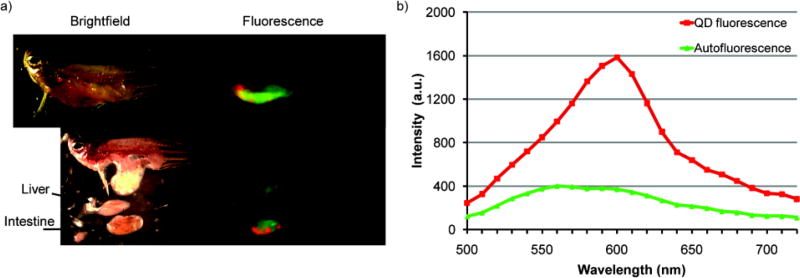
Since higher body burdens can result from dietary exposure versus aqueous exposure, dietary intake may constitute a major route of potential nanomaterial exposure for a higher trophic level of aquatic organisms. Our results show, similarly to studies on TiO2 NPs or Cd metal, that QD contaminated daphnia were readily eaten by the zebrafish and QDs accumulated in the intestines during the feeding period.5, 11 A representative image of an adult zebrafish after feeding is presented in Figure 1. Because of light scattering from the iridophores in the zebrafish skin, QD fluorescence could not be clearly observed non-invasively using either a fluorescence stereomicroscope or an in vivo whole animal fluorescence imaging system. However, upon removal of the skin, QD fluorescence was localized in the intestines with no distinguishable fluorescence in other organs (Figure 1).
Figure 1.
a) Representative whole animal brightfield (left) and fluorescence (right, false coloring) images taken with Maestro imaging system (CRi Inc., Woburn, MA) with b) corresponding spectra for QD (red, emission peak @ 600 nm) and endogenous (green) fluorescence signals.
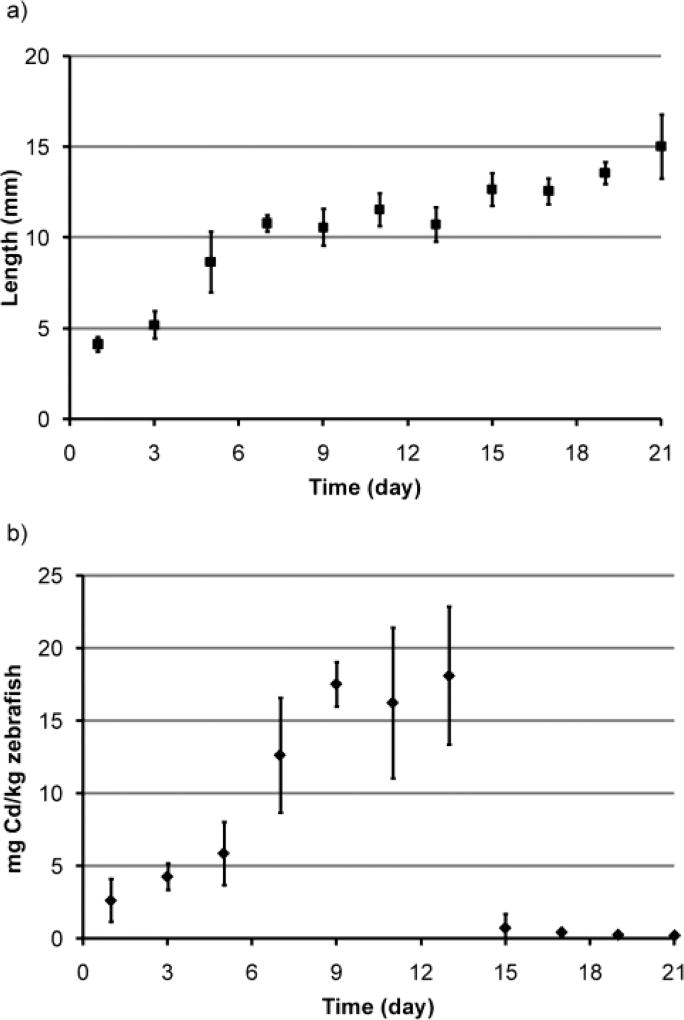
Both adult and juvenile zebrafish fed QD contaminated zooplankton exhibited no mortality, as predicted from acute embryo and adult zebrafish toxicity testing (see Supplemental Information), and no growth inhibition over the 21 day duration of the experiment. Chronic exposure to waterborne Cd or Zn has been shown to cause a variety of physiological and behavioral changes in fish including loss of appetite, reduced growth, and mortality.12 There were no signs of these effects during the entire experiment. In the adult zebrafish, growth was minimal with specific growth rates by length and by weight calculated to be 0.2% mm/day and 2% g/day respectively. Despite this small growth, size distribution influenced the uptake data. Specifically the body burden values for day 3 were skewed since larger zebrafish were inadvertently used at this time point. Normal growth was also observed in the juvenile zebrafish over the 21 days of the experiment as shown in Figure 2a. The specific growth rates by length and by weight for juvenile fish were calculated to be 7.1% mm/day and 10.2% g/day respectively, as compared to 6.8% mm/day and 14% g/day specific growth rates derived from published zebrafish growth data.13
Figure 2.
a) Growth curve based on length for juvenile fish. b) Uptake (day 1–14) and clearance (day 15–21) profile of QDs in juvenile zebrafish based on body burden. The elimination rate constant (ke) was 0.52. Error bars represent the standard deviation of the mean (n = 3).
The QD dose in the zooplankton was determined using ICP-MS before feeding. As previously reported, the average total Cd content from the QDs was 27 ± 6 ng Cd/daphnia or 208 ± 3 µg Cd/g daphnia.10 In the artemia, the average Cd content from the QDs was determined to be 2.14 ± 0.2 µg Cd/mL or 0.7 ± 0.1 ng Cd/artemia. During the uptake period, 95 ± 4% of the measured Cd total body concentration was associated with the intestines, 1 ± 0.5% with the liver, and 3.5 ± 1% with the remaining carcass. At the end of the uptake experiment, the QD body burdens in Cd equivalents were 8 ± 1 mg/kg and 18 ± 5 mg/kg and the biomagnification factors (BMF) were calculated to be 0.04 and 0.004 for the adult and juvenile zebrafish respectively. No biomagnification was observed for QDs since the BMF is much less than 1. Interestingly our BMF value was of the same order of magnitude reported for TiO2 nanoparticles (BMF = 0.024 and 0.009).5
Biphasic clearance, which involved a rapid elimination period during the first 24 hr followed by slower clearance over the following six days, was measured after resuming an uncontaminated diet. QDs were effectively cleared with > 90% of the initial dose removed after one day of clearance and ~ 99% clearance by day 7 for both adult and juvenile zebrafish as shown in Figures 3b and 2b respectively. Using a Michaelis-Menten kinetic model, the half-life was calculated to range between 26 to 38 hr. With the half-life greater than 24 hr, this could explain the gradual increase in QD body burden measured over the uptake period since complete clearance of the previous dose would not occur before the next feeding. The average total body burden of Cd increased by 34% from day 1 to 13, as shown in Figure 3.
Figure 3.
a) Uptake (day 1–14) and clearance (day 15–21) profile of QDs in adult zebrafish based on total body burden. The concentration at steady (Csat) was 7.5 mg/kg and the elimination rate constant (ke) was 0.6. b) Clearance profile of QDs in adult zebrafish based on dose remaining. Error bars represent the standard deviation of the mean (n = 3).
Elevated Cd levels were detected in the liver and carcass of the adult zebrafish starting at day 5, accounting for 1 ± 0.5% and 3.5 ± 1% of the total detected dose respectively. Although the liver Cd concentrations returned to baseline levels after one day of clearance, the carcass Cd levels remained elevated until two days after clearance conditions began. This suggests possible assimilation and the assimilation efficiency (AE) in adult zebrafish was calculated to be 8% after 24 hr of clearance. A smaller AE of 4% was calculated for the juvenile zebrafish. The AEs for QDs found in our study are similar to the AE reported for free Cd of 3–8%.11 For comparison, the AE for TiO2 NPs was calculated to be 26–38% from the Zhu et al. study using their depuration model equation.5, 11 Interestingly, the AEs differed significantly despite comparable elimination rate constants and elimination half-lives suggesting that AE is nanoparticle specific and has no correlation with the elimination kinetics.
Our study demonstrated that despite the high total body concentrations of QDs accumulated in the zebrafish over the two week dietary exposure period, a low percentage of QDs were absorbed and transported to other tissues. Results showed that zebrafish retained about 1% of the steady state QD-dose, given as QD labeled zooplankton, after the one week clearance period. This matches the findings from other dietary studies on TiO2 NPs.3, 5 While our study and others provide data to further understand the processes of nanoparticle transfer into fish, further research is required to identify the contributing factors that influence nanoparticle trophic transfer. In addition, although no bioaccumulation was observed in this study, further studies are needed to determine if dietary exposures may result in chronic effects such as reproductive toxicity or mutagenicity.
Methods
Preparation of Quantum Dot Contaminated Artemia and Daphnia
A description of the synthesis and characterization methods of the poly(acrylic acid)-octylamine copolymer (PAA) coated CdSe/ZnS QDs as well as the daphnia (Daphnia magna) exposure conditions were previously described.10,14 Artemia franciscana sp. (artemia) were chosen as the food source for the juvenile zebrafish since their small mouth opening prevented ingestion of daphnia neonates. Artemia eggs (0.5 g) were hatched overnight in synthetic sea water and after hatching were exposed to 7.7 nM water-solubilized CdSe/ZnS QDs (4.63×1012 particles/mL) with [Cd] = 0.6 ppm for a 24 hr duration. The artemia were then harvested, rinsed three times with clean culture media, and brought to a concentration of ~3000 artemia/mL.
Acclimation and training of zebrafish
Wild type zebrafish were raised on a recirculating aquarium system in an environmentally controlled room (28°C, 80% humidity, 14 hr light - 10 hr dark light cycle). Adult (> 3 months old) zebrafish were transferred to 1.8 L tanks (one fish per tank) containing 1 L of standard culture media. Juvenile (two weeks old) zebrafish were transferred to 1 L tanks (3 fish per tank) containing 0.8 L of standard culture media. All zebrafish were acclimated to static water conditions with daily water changes for three days before the experiment. The adult zebrafish were trained to eat live daphnia and the juvenile zebrafish continued to be fed an artemia diet, at a density of ~4 artemia/mL (1 mL stock artemia in 0.8 L). No abnormal behavior or death was observed during the training period. The Rice University Institutional Animal Care and Use Committee (IACUC) approved all animal protocols in this study.
Trophic transfer of QDs from zooplankton to zebrafish
After acclimation, the uptake experiment was initiated with the adult fish fed 10 QD contaminated daphnia (a 4% of body weight ration) and the juvenile zebrafish fed 1 mL of QD contaminated artemia (~3000 artemia/mL) daily for 14 days. This uptake period was followed by a clearance period with the remaining zebrafish fed uncontaminated zooplankton daily for 7 days to determine if assimilation and/or biomagnification of QDs can occur. One hour after feeding, any uneaten food was siphoned out and the culture media was renewed. During the experiment, samples (n = 3) were collected every other day, specifically zebrafish were taken on days 1, 3, 5, 7, 9, 11 and 13 during the exposure period, and on days 15, 17, 19, 21 during the clearance period. When collected, the zebrafish were euthanized with an overdose of tricaine methane sulfonate (10 mg/mL in culture media) and then frozen to ensure death before further processing.
Tissue Processing and Quantum Dot Quantification
The length of each fish was measured before dissection. Each zebrafish was first prepared for fluorescence imaging by removing the skin above the abdominal section. Following imaging, the intestines and liver were dissected from the carcass. Dissections were not performed on the juvenile fish due to their small size. Each sample was dried, weighed, and digested with 0.5 mL HNO3 under mild heating conditions (~60°C). Digested samples were diluted to 10 mL with 1% HNO3 and filtered through a 0.45 µm filter. The cadmium concentration in each sample was determined by inductively coupled plasma mass spectrometry (ICP-MS). Average cadmium concentrations for the 3 replicates were reported.
Calculations and Kinetic Model
From the length and mass data, the specific growth rate (SPR) was calculated as follows:
| (1) |
where xt=21 was either the length or mass of the fish at the end of the experiment (after 14 days contaminated food and 7 days of gut clearance) and xt=0 was the length or mass of the fish at the start of the experiment. Michaelis–Menten kinetics was used to analyze the Cd uptake data
| (2) |
where C is the whole body Cd concentration at time t, Csat is the saturated or maximum whole body concentration, and KM is the Michaelis-Menton constant, which is the amount of exposure time needed to reach half of the saturated concentration. First order kinetics were used to determine the Cd elimination profile
| (3) |
where ke is the elimination rate constant. By setting C to 0.5*Csat and rearranging Equation 3, the elimination half-life, or time needed to clear 50% of the total body content of QDs, can be calculated. To analyze the food chain transfer of QDs from daphnia or artemia to zebrafish, a biomagnification factor (BMF) was calculated as the ratio of the total QD concentration in zebrafish (mg/kg) to that in its zooplankton diet (mg/kg) at steady state.15 In addition, assimilation efficiency (AE) is a critical parameter in predicting bioaccumulation of substances in aquatic species. It is defined as the fraction of ingested material remaining in the gut after it has been emptied of undigested material and was calculated by dividing the body burden after 24 hr of clearance by the body burden at steady state.
Supplementary Material
Acknowledgments
We thank Mary Ellen Lane for her assistance in this zebrafish study. This study was supported in part by the Center for Biological and Environmental Nanotechnology (NSF Award EEC-0118007), in part by NIH Award RC2 GM092599 and in part by a NSF Graduate Research Fellowship to N.A.L.
References
- 1.Fraser T, Reinardy H, Shaw B, Henry TB, Handy R. Nanotoxicology. 2010;5:98–108. doi: 10.3109/17435390.2010.502978. [DOI] [PubMed] [Google Scholar]
- 2.Johnston BD, Scown TM, Moger J, Cumberland SA, Baalousha M, Linge K, van Aerle R, Jarvis K, Lead JR, Tyler CR. Environ Sci Technol. 2010;44:1144–1151. doi: 10.1021/es901971a. [DOI] [PubMed] [Google Scholar]
- 3.Ramsden CS, Smith TJ, Shaw BJ, Handy RD. Ecotoxicology. 2009;18:939–951. doi: 10.1007/s10646-009-0357-7. [DOI] [PubMed] [Google Scholar]
- 4.Zhou XX, Wang YB, Gu Q, Li WF. Aquaculture. 2009;291:78–81. [Google Scholar]
- 5.Zhu XS, Wang JX, Zhang XZ, Chang Y, Chen YS. Chemosphere. 2010;79:928–933. doi: 10.1016/j.chemosphere.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 6.Kahru A, Dubourguier HC. Toxicology. 2010;269:105–119. doi: 10.1016/j.tox.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 7.Bouldin J, Ingle T, Sengupta A, Alexander R, Hannigan R, Buchanan R. Environmental Toxicology and Chemistry. 2008;27:1958–1963. doi: 10.1897/07-637.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holbrook RD, Murphy KE, Morrow JB, Cole KD. Nat Nanotechnol. 2008;3:352–355. doi: 10.1038/nnano.2008.110. [DOI] [PubMed] [Google Scholar]
- 9.Werlin R, Priester JH, Mielke RE, Kramer S, Jackson S, Stoimenov PK, Stucky GD, Cherr GN, Orias E, Holden PA. Nat Nanotechnol. 2011;6:65–71. doi: 10.1038/nnano.2010.251. [DOI] [PubMed] [Google Scholar]
- 10.Lewinski NA, Zhu HG, Jo HJ, Pham D, Kamath RR, Ouyang CR, Vulpe CD, Colvin VL, Drezek RA. Environ Sci Technol. 2010;44:1841–1846. doi: 10.1021/es902728a. [DOI] [PubMed] [Google Scholar]
- 11.Liu XJ, Ni IH, Wang WX. Water Res. 2002;36:4563–4569. doi: 10.1016/s0043-1354(02)00180-x. [DOI] [PubMed] [Google Scholar]
- 12.McGeer JC, Szebedinszky C, McDonald DG, Wood CM. Aquat Toxicol. 2000;50:231–243. doi: 10.1016/s0166-445x(99)00105-8. [DOI] [PubMed] [Google Scholar]
- 13.Gomez-Requeni P, Conceicao L, Olderbakk Jordal AE, Ronnestad I. Fish Physiology and Biochemistry. 2010;36:1199–1215. doi: 10.1007/s10695-010-9400-0. [DOI] [PubMed] [Google Scholar]
- 14.Zhu HG, Prakash A, Benoit DN, Jones CJ, Colvin VL. Nanotechnology. 2010;21 doi: 10.1088/0957-4484/21/25/255604. [DOI] [PubMed] [Google Scholar]
- 15.Arnot JA, Gobas FAPC. Environ Rev. 2006;14:257–297. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.